Sensitivity Assessment of Varieties, Effectiveness of Weed Control by Selected Herbicides, and Infection of the Fusarium in Maize (Zea mays L.) Cultivation
Abstract
1. Introduction
2. Materials and Methods
2.1. Greenhouse Experiments
2.1.1. Experiment I
2.1.2. Experiment II
2.1.3. Experiment III
2.2. Laboratory Experiments
2.2.1. Methodology—Genetic Similarity of Varieties
2.2.2. Identification of Fusarium Species
- 1.
- DNA extraction
- 2.
- Species-specific PCR
2.3. Statistical Analysis
3. Results
3.1. Greenhouse Experiments
3.1.1. Experiment I
3.1.2. Experiment II
3.1.3. Experiment III
3.2. Laboratory Experiments
3.2.1. Genetic Similarity of Varieties
3.2.2. Identification of Fusarium Species
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Owen, M.D.K. Diverse Approaches to Herbicide-Resistant Weed Management. Weed Sci. 2016, 64, 570–584. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Mohsin, S.M.; Borhannuddin Bhuyan, M.H.M.; Farha Bhuiyan, T.; Anee, T.I.; Awal, A.; Masud, C.; Nahar, K. Phytotoxicity, environmental and health hazards of herbicides: Challenges and ways forward. In Agrochemicals Detection, Treatment and Remediation. Pesticides and Chemical Fertilizers; Vara Prasad, M.N., Ed.; Butterworth Heinemann: Hyderabad, India, 2020; pp. 55–99. [Google Scholar]
- Bountin, C.; Aya, K.L.; Carpenter, D.; Thomas, P.J.; Rowland, O. Phytotoxicity testing for herbicide 451 regulation: Shortcomings in relation to biodiversity and ecosystem services in agrarian systems. Sci. Total Environ. 2012, 415, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, K. Auxin herbicides: Current status of mechanism and mode of action. Pest Manag. Sci. 2010, 66, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Vink, J.P.; Soltani, N.; Robinson, D.E.; Tardif, F.J.; Lawton, M.B.; Sikkema, P.H. Glyphosate-resistant giant ragweed (Ambrosia trifida) control in dicamba-tolerant soybean. Weed Technol. 2012, 26, 422–428. [Google Scholar] [CrossRef]
- Peterson, M.A.; McMaster, S.A.; Riechers, D.E.; Skelton, J.; Stahlman, P.W. 2,4-D past, present, and future: A review. Weed Technol. 2016, 30, 303–345. [Google Scholar] [CrossRef]
- Sutton, P.; Richards, C.; Buren, L.; Glasgow, L. Activity of mesotrione on resistant weeds in maize. Pest Manag. Sci. 2002, 58, 981–984. [Google Scholar] [CrossRef]
- Moran, G.R. 4-Hydroxyphenylpyruvate dioxygenase. Arch. Biochem. Biophys. 2005, 433, 117–128. [Google Scholar] [CrossRef]
- Ahrens, H.; Lange, G.; Müller, T.; Rosinger, C.; Willms, L.; van Almsick, A. 4-Hydroxyphenylpyruvate Dioxygenase Inhibitors in Combination with Safeners: Solutions for Modern and Sustainable Agriculture. Angew. Chem. Int. Ed. 2013, 52, 9388–9398. [Google Scholar] [CrossRef]
- Mitchell, G.; Bartlett, D.W.; Fraser, T.E.M.; Hawkes, T.R.; Holt, D.C.; Townson, J.K.; Wichert, R.A. Mesotrione: A new selective herbicide for use in maize. Pest Manag. Sci. 2001, 57, 120–128. [Google Scholar] [CrossRef]
- Abendroth, J.A.; Martin, A.R.; Roeth, F.W. Plant Response to Combinations of Mesotrione and Photosystem II Inhibitors. Weed Technol. 2006, 20, 267–274. [Google Scholar] [CrossRef]
- Hess, F.D. Light-dependent herbicides: An overview. Weed Sci. 2000, 48, 160–170. [Google Scholar] [CrossRef]
- Muhammad, K.; Irshad, A.; Haiqi, W.; Xiaorong, W.; Jing, X.; Tiening, L.; Ruixia, D.; Qingfang, H. Mepiquat chloride application increases lodging resistance of maize by enhancing stem physical strength and lignin biosynthesis. Field Crops Res. 2018, 224, 148–159. [Google Scholar]
- Muthukumar, V.B.; Velayudham, K.; Thavaprakaash, N. Growth and Yield of Baby Corn (Zea mays L.) as Influenced by Plant Growth Regulators and Different Time of Nitrogen Application. Res. J. Agric. Biol. Sci. 2005, 1, 303–307. [Google Scholar]
- Miziniak, W.; Matysiak, K. Interaction of herbicides with mepiquat chloride and prohexadione calcium in winter wheat. J. Plant Prot. Res. 2019, 59, 494–502. [Google Scholar]
- Logrieco, A.; Mulé, G.; Moretti, A.; Bottalico, A. Toxigenic Fusarium species and mycotoxins associated with maize ear rot in Europe. Eur. J. Plant Pathol. 2002, 108, 597–609. [Google Scholar] [CrossRef]
- Szabó, B.; Varga, M.; György, A.; Mesterházy, Á.; Tóth, B. Role of Fusarium species in mycotoxin contamination of maize. J. Agric. Ext. Rural Dev. 2016, 5, 104–108. [Google Scholar]
- Nowosad, K.; Bocianowski, J.; Szulc, P. Analysis of molecular variance and genetic similarity between selected cultivars of maize (Zea mays L.) revealed by SSR markers. Fragm. Agron. 2017, 34, 134–144. [Google Scholar]
- Available online: www.maizegdb.org (accessed on 25 May 2020).
- Nei, M.; Li, W. Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc. Natl. Acad. Sci. USA 1979, 76, 5269–5273. [Google Scholar] [CrossRef]
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual; Blackwell Publishing Professional: Ames, IA, USA, 2006; p. 388. [Google Scholar]
- Gerlach, W.; Nirenberg, H. The Genus Fusarium: A Pictorial Atlas; Mitteilungen aus der Biologischen Bundesanstalt für Land-und Forstwirtschaft Berlin-Dahlem; Kommissionsverlag P. Parey: Berlin, Germany, 1982; p. 406. [Google Scholar]
- Mulé, G.; Susca, A.; Stea, G.; Moretti, A. A species-specific PCR assay based on the calmodulin partial gene for identification of Fusarium varticillioides, F. proliferatum and F. subglutinans. Eur. J. Plant Pathol. 2004, 110, 495–502. [Google Scholar] [CrossRef]
- Filipek-Mazur, B.; Lepiarczyk, A.; Tabak, M. Wpływ nawożenia azotem i siarką na plonowanie oraz skład chemiczny ziarna kukurydzy część II. Zawartość azotu i siarki. Fragm. Agron. 2013, 30, 29–35. [Google Scholar]
- Adamczyk, J. Znaczenie doboru odmian w uprawie kukurydzy na ziarno i kiszonkę. Biul. Inf. Zoot. 2001, 39, 29–35. [Google Scholar]
- Qasem, J.R. Herbicides applications: Problems and considerations. In Herbicides and Environment; Kortekamp, A., Ed.; InTech: London, UK, 2011; pp. 643–664. [Google Scholar]
- Jhala, A.J.; Knezevic, S.Z.; Ganie, Z.A.; Singh, M. Integrated weed management in corn (Zea mays L.). In Recent Advances in Weed Management; Chauhan, B., Mahajan, G., Eds.; Springer: New York, NY, USA, 2014; pp. 177–196. [Google Scholar]
- Rola, H. Oddziaływanie fitotoksyczne niektórych herbicydów na odmiany kukurydzy. Prog. Plant Prot. 2003, 43, 176–178. [Google Scholar]
- Gołębiowska, H.; Rola, H. The influence of weather conditions on selectivity of sylfonylourea herbicides to the selected maize varieties. J. Plant Prot. Res. 2003, 43, 219–224. [Google Scholar]
- Available online: www.regional.org.au/au/allelopathy/2005/2/7/2636_cornesd.htm (accessed on 25 May 2020).
- Pannacci, E.; Covarelli, G. Efficacy of mesotrione used at reduced doses for post-emergence weed control in maize (Zea mays L.). Crop Prot. 2009, 28, 57–61. [Google Scholar] [CrossRef]
- Clay, D.; Reitsma, K.D.; Clay, S. Best Management Practices for Corn Production in South Dakota; South Dakota State University, College of Agriculture and Biological Sciences, AgBio Communications Unit, Box 2218A: Brookings, SD, USA, 2009. [Google Scholar]
- Keane, P.; Kerr, A. Factors affecting disease development. In Plant Pathogens and Plant Diseases; Brown, J.F., Ogle, H.J., Eds.; Rockvale Publishing: Armidale, Australia, 1997; pp. 287–298. [Google Scholar]
- Redwitz, C.; Gerowitt, B. Welche Faktoren fördern das Auftreten von Chenopodium album auf norddeutschen Maisflächen? In Proceedings of the 26th German Conference on weed Biology an Weed Control, Braunschweig, Germany, 11–13 March 2014. [Google Scholar]
- Weber, E.A.; Gruber, S.; Claupein, W. Emergence and performance of volunteer oilseed rape (Brassica napus) in different crops. Europ. J. Agron. 2014, 60, 33–40. [Google Scholar] [CrossRef]
- Streit, B.; Rieger, S.B.; Stamp, P.; Richner, W. The effect of tillage intensity and time of herbicide application on weed communities and populations in maize in central Europe. Agric. Ecosyst. Environ. 2002, 92, 211–224. [Google Scholar] [CrossRef]
- Majchrzak, L.; Idziak, R.; Pudełko, J.; Piechota, T.; Sobiech, Ł. Skuteczność i selektywność różnych soli MCPA do odchwaszczania jęczmienia jarego. Fragm. Agron. 2012, 29, 120–126. [Google Scholar]
- Roskamp, J.M.; Chahal, G.S.; Johnson, W.G. The Effect of Cations and Ammonium Sulfate on the Efficacy of Dicamba and 2,4-D. Weed Technol. 2013, 27, 72–77. [Google Scholar] [CrossRef]
- Baghbani, F.; Lotfi, R.; Moharramnejad, S.; Bandehagh, A.; Roostaei, M.; Rastogi, A.; Kalaji, H.M. Impact of Fusarium verticillioides on chlorophyll fluorescence parameters of two maize lines. Eur. J. Plant Pathol. 2019, 154, 337–346. [Google Scholar] [CrossRef]
- Jurado, M.; Marín, P.; Callejas, C.; Moretti, A.; Vázquez, C.; González-Jaén, M.T. Genetic variability and Fumonisin production by Fusarium proliferatum. Food Microbiol. 2010, 27, 50–57. [Google Scholar] [CrossRef]
- Scauflaire, J.; Gourgue, M.; Munaut, F. Fusarium temperatum sp. nov. from maize, an emergent species closely related to Fusarium subglutinans. Mycologia 2011, 103, 586–597. [Google Scholar] [CrossRef] [PubMed]
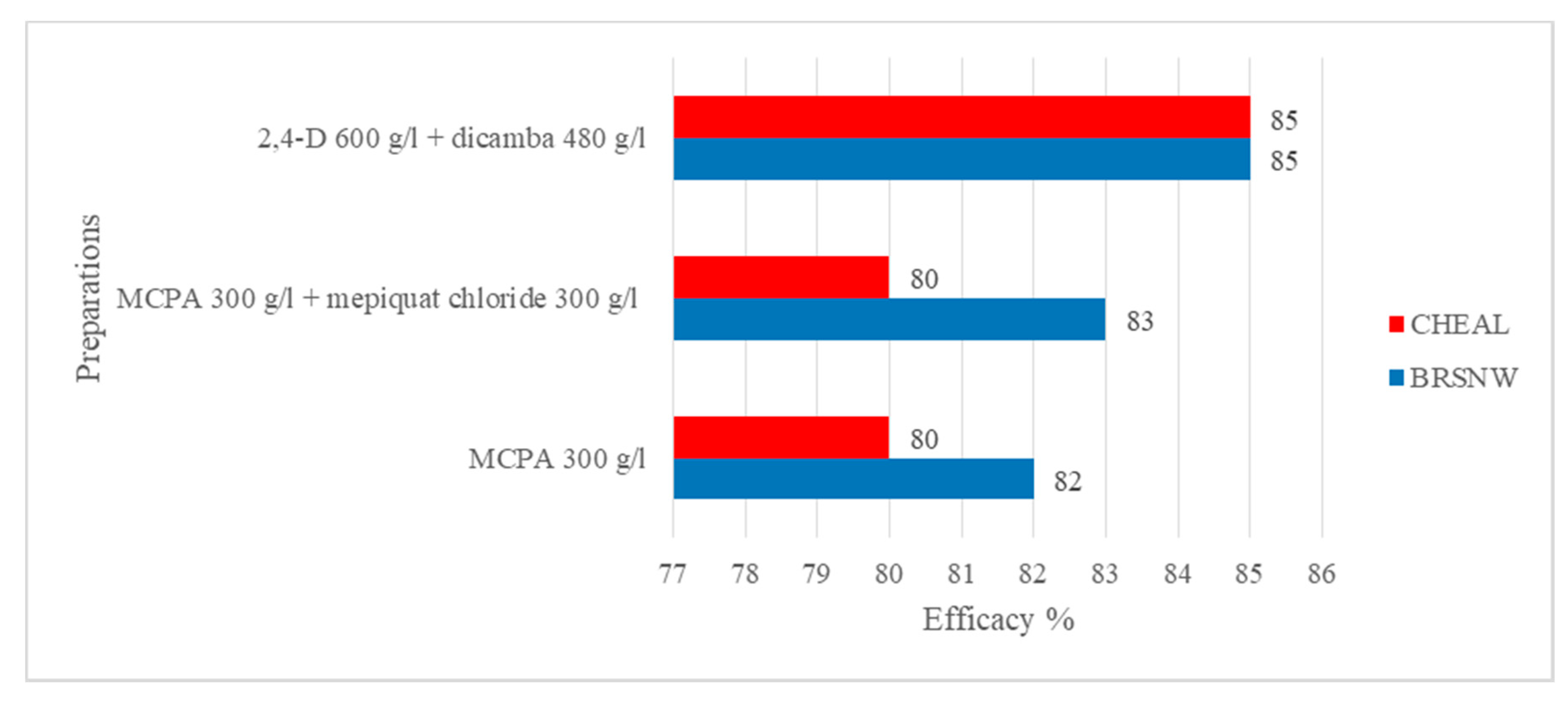
| No. | Plant Protection Products | Producer | Active Ingredient | Dose (L/ha) | Dose (g a.i./ha) |
|---|---|---|---|---|---|
| 1 | Chwastox Extra 300 SL | CIECH Sarzyna S.A., Poland | MCPA (300 g/L) | 3 | 900 |
| 2 | Chwastox Extra 300 SL + Mepik 300 SL | CIECH Sarzyna S.A., Polska + Innvigo Sp. z o.o., Poland | MCPA (300 g/L) + mepiquat chloride (300 g/L) | 3 + 1.3 | 900 + 390 |
| 3 | Aminopielik Standard 600 SL + Dicash | ADAMA Manufacturing Poland S.A. + Sharda Europe b.v.b.a., Kingdom of Belgium | 2,4-D (600 g/L) + dicamba (480 g/L) | 0.5 + 0.5 | 300 + 240 |
| Phytotoxicity (%) | ||||
|---|---|---|---|---|
| No. | Variety | Callisto 3.0 L/ha | ||
| 7 DAT | 14 DAT | 21 DAT | ||
| 2 | NK Jasmic | 0 | 0 | 0 |
| 3 | NK Ravello SG | 0 | 0 | 0 |
| 4 | Exapic | 3 | 4 | 0 |
| 5 | Drim SG | 0 | 0 | 0 |
| 6 | SY Cooky | 0 | 0 | 0 |
| 7 | Delitop SG | 6 | 6 | 0 |
| 8 | NK Cooler SG | 4 | 3 | 0 |
| 9 | SY Contract | 11 | 10 | 0 |
| 10 | SY Multitop | 11 | 12 | 0 |
| 11 | SY Consistent SG | 0 | 0 | 0 |
| 12 | Arobase | 10 | 6 | 0 |
| 13 | NK Nekta SG | 6 | 6 | 0 |
| 14 | SY Counter SG | 1 | 1 | 0 |
| 15 | SY Enigma | 0 | 1 | 0 |
| 16 | Nebora | 9 | 6 | 0 |
| 17 | NK Magitop SG | 3 | 3 | 0 |
| 18 | SY Ondina SG | 0 | 0 | 0 |
| 19 | Longitop | 3 | 3 | 0 |
| 20 | Kardona | 13 | 9 | 0 |
| 21 | Kaivo | 0 | 0 | 0 |
| LSD 0.05 | 4.7 | 5.1 | 0.0 | |
| Phytotoxicity (%) | ||||
|---|---|---|---|---|
| No. | Variety | Callisto 3.0 L/ha | ||
| 7 DAT | 14 DAT | 21 DAT | ||
| 2 | LG 30.260 SG | 5 | 8 | 0 |
| 3 | LG 30.240 | 3 | 1 | 0 |
| 4 | LG 32.16 | 2 | 6 | 0 |
| 5 | LG 30.275 SG | 0 | 1 | 0 |
| 6 | LG 32.32 | 2 | 6 | 0 |
| 7 | LG 22.44 | 8 | 9 | 0 |
| 8 | LG 32.15 | 16 | 12 | 0 |
| 9 | LG 22.43 | 9 | 11 | 0 |
| 10 | LG 32.52 | 13 | 8 | 0 |
| 11 | LG 30.238 | 2 | 0 | 0 |
| 12 | LG 32.58 | 4 | 1 | 0 |
| 13 | Laureen | 21 | 15 | 0 |
| LSD 0.05 | 5.3 | 6.2 | 0.0 | |
| Phytotoxicity (%) | ||||
|---|---|---|---|---|
| No. | Variety | Esteron 2.0 L/ha | ||
| 7 DAT | 14 DAT | 21 DAT | ||
| 2 | LG 30.260 SG | 10 | 15 | 0 |
| 3 | LG 30.240 | 10 | 15 | 0 |
| 4 | LG 32.16 | 10 | 11 | 0 |
| 5 | LG 30.275 SG | 10 | 9 | 0 |
| 6 | LG 32.32 | 8 | 8 | 0 |
| 7 | LG 22.44 | 5 | 8 | 0 |
| 8 | LG 32.15 | 10 | 8 | 0 |
| 9 | LG 22.43 | 10 | 13 | 0 |
| 10 | LG 32.52 | 1 | 2 | 0 |
| 11 | LG 30.238 | 0 | 2 | 0 |
| 12 | LG 32.58 | 10 | 8 | 0 |
| 13 | Laureen | 18 | 20 | 0 |
| LSD 0.05 | 1 | 1.9 | 0.0 | |
| Phytotoxicity (%) | ||||
|---|---|---|---|---|
| No. | Variety | Bromotril 2.0 L/ha | ||
| 7 DAT | 14 DAT | 21 DAT | ||
| 2 | LG 30.260 SG | 0 | 0 | 0 |
| 3 | LG 30.240 | 0 | 0 | 0 |
| 4 | LG 32.16 | 0 | 0 | 0 |
| 5 | LG 30.275 SG | 5 | 0 | 0 |
| 6 | LG 32.32 | 0 | 0 | 0 |
| 7 | LG 22.44 | 0 | 0 | 0 |
| 8 | LG 32.15 | 0 | 0 | 0 |
| 9 | LG 22.43 | 0 | 0 | 0 |
| 10 | LG 32.52 | 0 | 0 | 0 |
| 11 | LG 30.238 | 0 | 0 | 0 |
| 12 | LG 32.58 | 0 | 0 | 0 |
| 13 | Laureen | 4 | 0 | 0 |
| LSD 0.05 | 0.6 | 0 | 0 | |
| Phytotoxicity (%) | |||||
|---|---|---|---|---|---|
| No. | Active Ingredient | Dose (L/ha) | Dose (g a.i./ha) | ZEAMX—SY Cooky | ZEAMX—NK Ravello |
| 14 DAT | 14 DAT | ||||
| 1 | Untreated * | - | - | 0 | 0 |
| 2 | MCPA | 3 | 900 | 0 | 0 |
| 3 | MCPA + mepiquat chloride | 3 + 1.3 | 900 + 390 | 0 | 0 |
| 4 | 2,4 D + dicamba | 0.5 + 0.5 | 300 + 240 | 0 | 0 |
| LSD 0.05 | 0 | 0 | |||
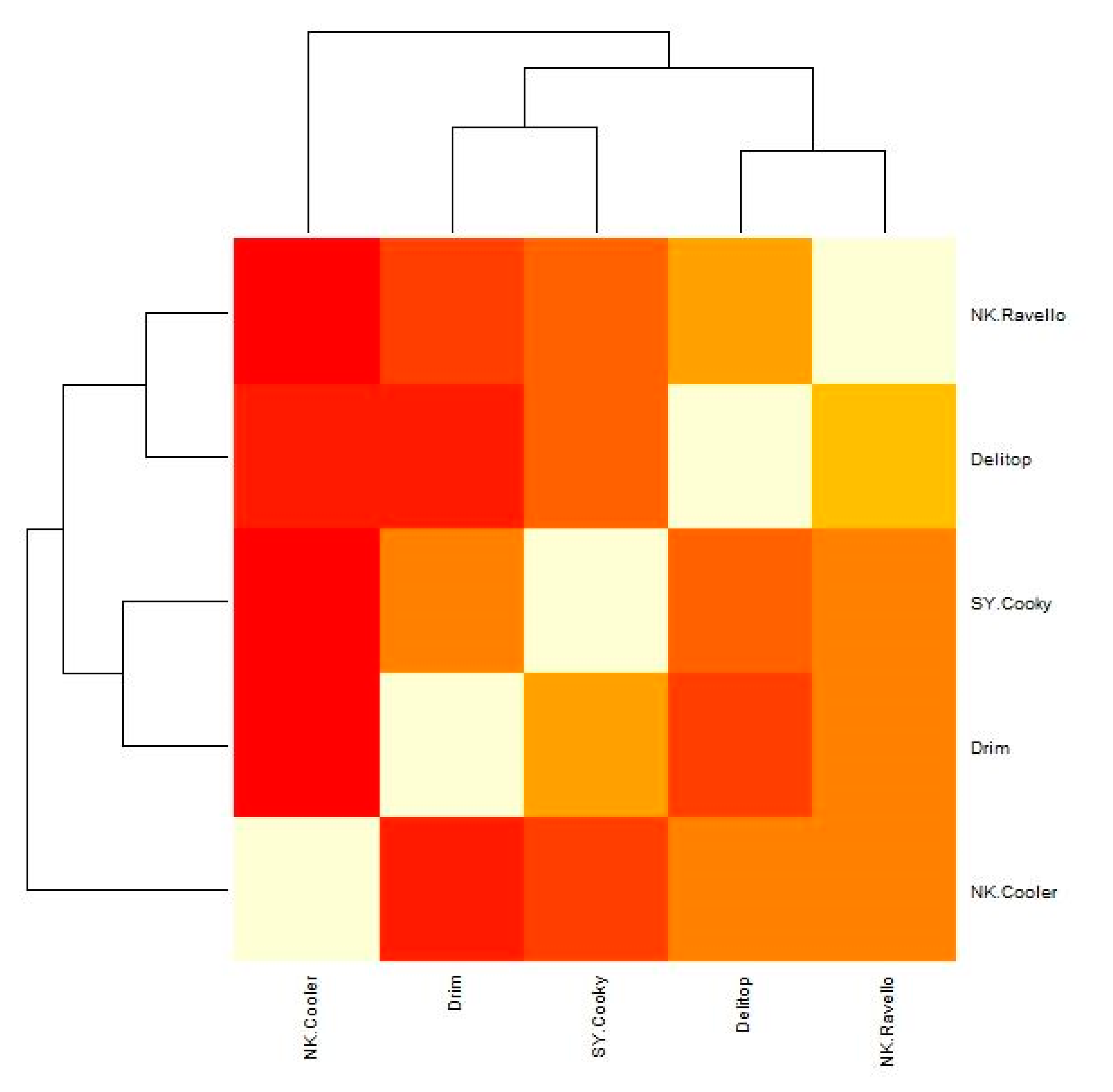
| Cultivar | NK Cooler | Delitop | NK Ravello | SY Cooky | Drim |
|---|---|---|---|---|---|
| NK Cooler | 1 | ||||
| Delitop | 0.478 | 1 | |||
| NK Ravello | 0.481 | 0.719 | 1 | ||
| SY Cooky | 0.388 | 0.597 | 0.612 | 1 | |
| Drim | 0.310 | 0.441 | 0.553 | 0.652 | 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jagła, M.; Sobiech, Ł.; Szulc, P.; Nowosad, K.; Bocianowski, J.; Grzanka, M. Sensitivity Assessment of Varieties, Effectiveness of Weed Control by Selected Herbicides, and Infection of the Fusarium in Maize (Zea mays L.) Cultivation. Agronomy 2020, 10, 1115. https://doi.org/10.3390/agronomy10081115
Jagła M, Sobiech Ł, Szulc P, Nowosad K, Bocianowski J, Grzanka M. Sensitivity Assessment of Varieties, Effectiveness of Weed Control by Selected Herbicides, and Infection of the Fusarium in Maize (Zea mays L.) Cultivation. Agronomy. 2020; 10(8):1115. https://doi.org/10.3390/agronomy10081115
Chicago/Turabian StyleJagła, Małgorzata, Łukasz Sobiech, Piotr Szulc, Kamila Nowosad, Jan Bocianowski, and Monika Grzanka. 2020. "Sensitivity Assessment of Varieties, Effectiveness of Weed Control by Selected Herbicides, and Infection of the Fusarium in Maize (Zea mays L.) Cultivation" Agronomy 10, no. 8: 1115. https://doi.org/10.3390/agronomy10081115
APA StyleJagła, M., Sobiech, Ł., Szulc, P., Nowosad, K., Bocianowski, J., & Grzanka, M. (2020). Sensitivity Assessment of Varieties, Effectiveness of Weed Control by Selected Herbicides, and Infection of the Fusarium in Maize (Zea mays L.) Cultivation. Agronomy, 10(8), 1115. https://doi.org/10.3390/agronomy10081115








