Organic Plant Biostimulants and Fruit Quality—A Review
Abstract
1. Introduction
2. Plant Biostimulants
3. Plant Biostimulants and Fruit Quality
3.1. Banana (Musa Spp.)
3.2. Eggplant (Solanum Melongena)
3.3. Capsicum Spp.
3.4. Cherry (Prunus Avium)
3.5. Citrus Spp.
3.6. Apricot (Prunus Armeniaca)
3.7. Kiwi (Actinidia Delicious)
3.8. Apple (Malus Domestica)
3.9. Strawberry (Fragaria × Ananassa)
3.10. Pomegranate (Punica Granatum)
3.11. Tomato (Solanum Lycopersicum)
3.12. Grape (Vitis Vinifera)
4. Sources of Variation in Results with Plant Biostimulants
5. Mode and Mechanisms of Action of Plant Biostimulants
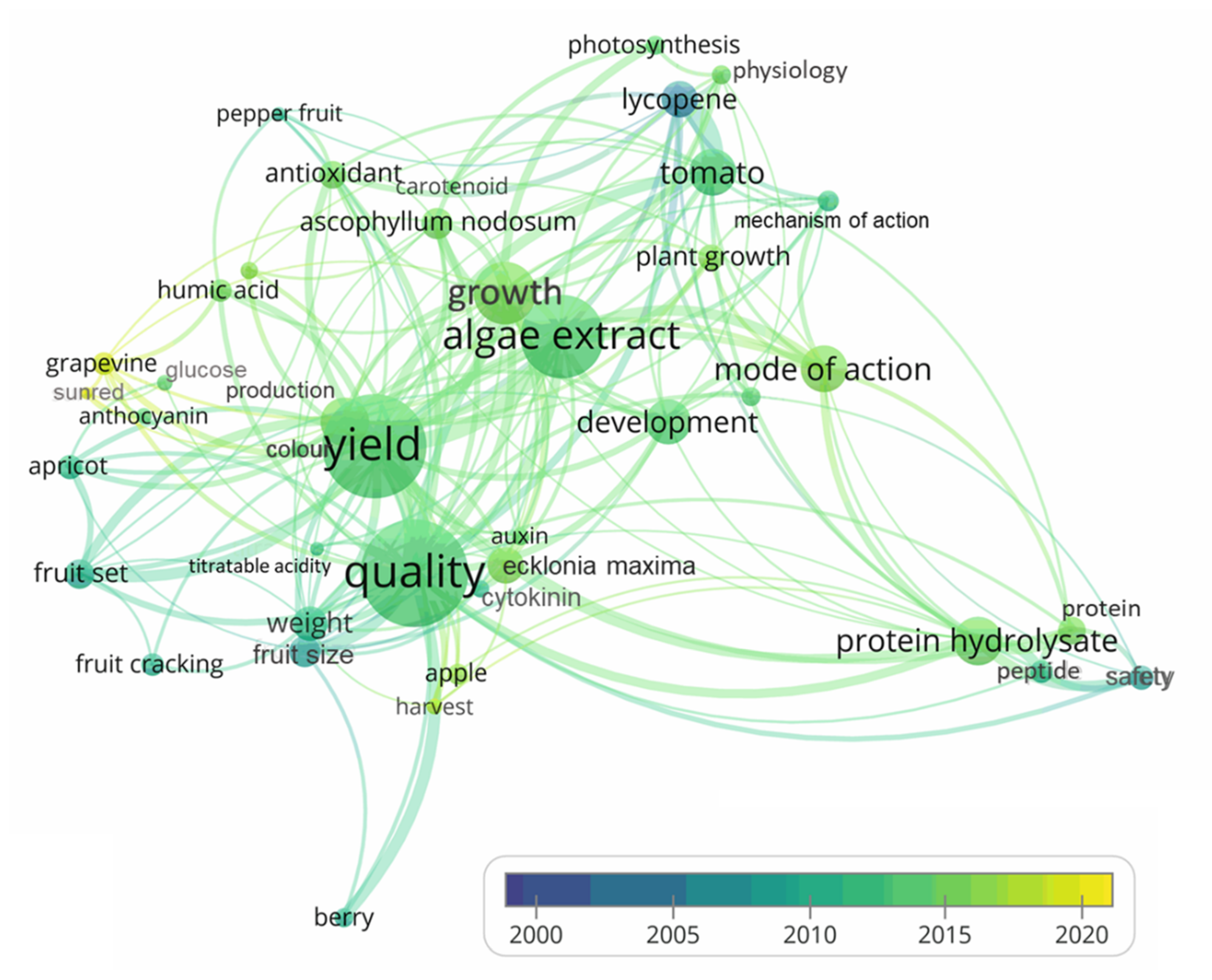
6. Bibliometric Analysis
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mratinić, E.; Popovski, B.; Milošević, T. Evaluation of apricot fruit quality and correlations between physical and chemical attributes. Czech. J. Food Sci. 2011, 29, 161–170. [Google Scholar] [CrossRef]
- Tarantino, A.; Lops, F.; Disciglio, G.; Lopriore, G. Effects of plant biostimulants on fruit set, growth, yield and fruit quality attributes of ‘Orange rubis®’ apricot (Prunus armeniaca L.) cultivar in two consecutive years. Sci. Hortic. 2018, 239, 26–34. [Google Scholar] [CrossRef]
- Camelo, A.F.L. The quality in fruits and vegetables. In Manual for the Preparation and Sale of Fruits and Vegetables: From Field to Market; Instituto Nacional de Tecnología Agropecuaria Estación Experimental Agropecuaria Balcarce: Balcarce, Argentina, 1981; Volume 12, pp. 87–104. [Google Scholar]
- Kyriacou, M.C.; Rouphael, Y. Towards a new definition of quality for fresh fruits and vegetables. Sci. Hortic. 2018, 234, 463–469. [Google Scholar] [CrossRef]
- Di Vittori, L.; Mazzoni, L.; Battino, M.; Mezzetti, B. Pre-harvest factors influencing the quality of berries. Sci. Hortic. 2018, 233, 310–322. [Google Scholar] [CrossRef]
- Kader, A.A. Postharvest Technology of Horticultural Crops, 3rd ed.; Kader, A.A., Ed.; University of California: Richmond, CA, USA, 2002. [Google Scholar]
- Frioni, T.; Sabbatini, P.; Tombesi, S.; Norrie, J.; Poni, S.; Gatti, M.; Palliotti, A. Effects of a biostimulant derived from the brown seaweed Ascophyllum nodosum on ripening dynamics and fruit quality of grapevines. Sci. Hortic. 2018, 232, 97–106. [Google Scholar] [CrossRef]
- Ubi, B.E. External stimulation of anthocyanin biosynthesis in apple fruit. Food Agric. Environ. 2004, 2, 65–70. [Google Scholar]
- Dorais, M.; Ehret, D.L. Agronomy and the nutritional quality of fruit. In Improving the Health-Promoting Properties of Fruit and Vegetable Products; Woodhead Publishing Limited: Cambridge, UK, 2008; pp. 346–391. ISBN 9781845691844. [Google Scholar]
- Ledbetter, C.; Peterson, S.; Jenner, J. Modification of sugar profiles in California adapted apricots (Prunus armeniaca L.) through breeding with Central Asian germplasm. Euphytica 2006, 148, 251–259. [Google Scholar] [CrossRef]
- Ruiz, D.; Egea, J. Phenotypic diversity and relationships of fruit quality traits in apricot (Prunus armeniaca L.) germplasm. Euphytica 2008, 163, 143–158. [Google Scholar] [CrossRef]
- Shi, J.; Le Maguer, M. Lycopene in tomatoes: Chemical and physical properties affected by food processing. Crit. Rev. Food Sci. Nutr. 2000, 40, 1–42. [Google Scholar] [CrossRef]
- Carvalho, M.E.A.; Castro, P.R.C. Extratos de algas e suas aplicações na agricultura. Série Prod. Rural 2014, 58. [Google Scholar] [CrossRef]
- Looney, N.E. Improving Fruit Size, Appearance, and Other Aspects of Fruit Crop “Quality” With Plant Bioregulating Chemicals. Acta Hortic. 1993, 120–127. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant. Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in plant science: A global perspective. Front. Plant. Sci. 2017, 7. [Google Scholar] [CrossRef]
- EBIC European Biostimulant Industry Council. Available online: http://www.biostimulants.eu2013 (accessed on 20 May 2020).
- Ricci, M.; Tilbury, L.; Daridon, B.; Sukalac, K. General principles to justify plant biostimulant claims. Front. Plant. Sci. 2019, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, Y.; Franken, P.; Schneider, C.; Schwarz, D.; Giovannetti, M.; Agnolucci, M.; De Pascale, S.; Bonini, P.; Colla, G. Arbuscular mycorrhizal fungi act as biostimulants in horticultural crops. Sci. Hortic. 2015, 196, 91–108. [Google Scholar] [CrossRef]
- Nardi, S.; Cardarelli, M.; Ertani, A.; Lucini, L.; Canaguier, R. Protein hydrolysates as biostimulants in horticulture. Sci. Hortic. (Amsterdam) 2015, 196, 28–38. [Google Scholar]
- Ruzzi, M.; Aroca, R. Plant growth-promoting rhizobacteria act as biostimulants in horticulture. Sci. Hortic. 2015, 196, 124–134. [Google Scholar] [CrossRef]
- Soppelsa, S.; Kelderer, M.; Casera, C.; Bassi, M.; Robatscher, P.; Andreotti, C. Use of biostimulants for organic apple production: Effects on tree growth, yield, and fruit quality at harvest and during storage. Front. Plant. Sci. 2018, 9. [Google Scholar] [CrossRef]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Rengasamy, K.R.R.; Kulkarni, M.G.; Stirk, W.A.; Van Staden, J. Eckol – A new plant growth stimulant from the brown seaweed Ecklonia maxima. J. Appl. Phycol. 2014, 27, 581–587. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G.; Giordano, M.; El-Nakhel, C.; Kyriacou, M.C.; De Pascale, S. Foliar applications of a legume-derived protein hydrolysate elicit dose-dependent increases of growth, leaf mineral composition, yield and fruit quality in two greenhouse tomato cultivars. Sci. Hortic. 2017, 226, 353–360. [Google Scholar] [CrossRef]
- Kulkarni, M.G.; Rengasamy, K.R.R.; Pendota, S.C.; Gruz, J.; Plačková, L.; Novák, O.; Doležal, K.; Van Staden, J. Bioactive molecules derived from smoke and seaweed Ecklonia maxima showing phytohormone-like activity in Spinacia oleracea L. N. Biotechnol. 2019, 48, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Schaafsma, G. Safety of protein hydrolysates, fractions thereof and bioactive peptides in human nutrition. Eur. J. Clin. Nutr. 2009, 63, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Parrado, J.; Escudero-Gilete, M.L.; Friaza, V.; García-Martínez, A.; González-Miret, M.L.; Bautista, J.D.; Heredia, F.J. Enzymatic vegetable extract with bio- active components: Influence of fertilizer on the colour and anthocyanins of red grapes. J. Sci. Food Agric. 2007, 87, 2310–2318. [Google Scholar] [CrossRef]
- Paradiković, N.; Vinković, T.; Vinković Vrček, I.; Žuntar, I.; Bojić, M.; Medić-Šarić, M. Effect of natural biostimulants on yield and nutritional quality: An example of sweet yellow pepper (Capsicum annuum L.) plants. J. Sci. Food Agric. 2011, 91, 2146–2152. [Google Scholar] [CrossRef]
- Barrajón-Catalán, E.; Álvarez-Martínez, F.J.; Borrás, F.; Pérez, D.; Herrero, N.; Ruiz, J.J.; Micol, V. Metabolomic analysis of the effects of a commercial complex biostimulant on pepper crops. Food Chem. 2020, 310, 125818. [Google Scholar] [CrossRef]
- Grabowska, A.; Kunicki, K.; Slękara, A.; Kalisz, A.; Jezdinský, A.; Gintro-Wicz, K. The effect of biostimulants on the quality parameters of tomato grown for the processing industry. Agrochimica 2015, 59, 203–217. [Google Scholar]
- Colla, G.; Cardarelli, M.; Bonini, P.; Rouphael, Y. Foliar applications of protein hydrolysate, plant and seaweed extracts increase yield but differentially modulate fruit quality of greenhouse tomato. HortScience 2017, 52, 1214–1220. [Google Scholar] [CrossRef]
- Aziz, R.A.; Naira, A.; Moieza, A. Effect of plant biostimulants on fruit cracking and quality attributes of pomegranate cv. Kandhari kabuli. Sci. Res. Essays 2013, 8, 2171–2175. [Google Scholar] [CrossRef]
- Fenili, C.L.; Petri, J.L.; Steffens, C.A.; De Martin, M.S.; Do Amarante, C.V.T.; Heinzen, A.S. Alternatives to increase the red color of the peel in ‘daiane’ and ‘venice’ apples. Rev. Bras. Frutic. 2019, 41, 1–11. [Google Scholar] [CrossRef]
- Deng, Q.; Xia, H.; Lin, L.; Wang, J.; Yuan, L.; Li, K.; Zhang, J.; Lv, X.; Liang, D. SUNRED, a natural extract-based biostimulant, application stimulates anthocyanin production in the skins of grapes. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gurav, R.G.; Jadhav, J.P. A novel source of biofertilizer from feather biomass for banana cultivation. Environ. Sci. Pollut. Res. 2013, 20, 4532–4539. [Google Scholar] [CrossRef] [PubMed]
- Pohl, A.; Grabowska, A.; Kalisz, A.; Sȩkara, A. The eggplant yield and fruit composition as affected by genetic factor and biostimulant application. Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 929–938. [Google Scholar] [CrossRef]
- Ali, M.; Cheng, Z.H.; Hayat, S.; Ahmad, H.; Ghani, M.I.; Liu, T. Foliar spraying of aqueous garlic bulb extract stimulates growth and antioxidant enzyme activity in eggplant (Solanum melongena L.). J. Integr. Agric. 2019, 18, 1001–1013. [Google Scholar] [CrossRef]
- Ertani, A.; Pizzeghello, D.; Francioso, O.; Sambo, P.; Sanchez-Cortes, S.; Nardi, S. Capsicum chinensis L. growth and nutraceutical properties are enhanced by biostimulants in a long-term period: Chemical and metabolomic approaches. Front. Plant. Sci. 2014, 5, 375. [Google Scholar] [CrossRef] [PubMed]
- Unlu, H.; Karakurt, Y. Influence of humic acid on the antioxidant compounds in pepper fruit. J. Food Agric. Environ. 2010, 8, 434–438. [Google Scholar]
- Correia, S.; Oliveira, I.; Queirós, F.; Ribeiro, C.; Ferreira, L.; Luzio, A.; Silva, A.P.; Gonçalves, B. Preharvest Application of Seaweed Based Biostimulant Reduced Cherry (Prunus Avium L.) Cracking. Procedia Environ. Sci. 2015, 29, 251–252. [Google Scholar] [CrossRef]
- Gonçalves, B.; Morais, M.C.; Sequeira, A.; Ribeiro, C.; Guedes, F.; Silva, A.P.; Aires, A. Quality preservation of sweet cherry cv. “Staccato” by using glycine-betaine or Ascophyllum nodosum. Food Chem. 2020, 126713. [Google Scholar] [CrossRef]
- Atawia, A.A.R.; El-Desouqi, S.A. Trials for improving fruit set, yield and fruit quality of Washington navel orange by application of some growth regulators and yeast extract as a natural source of phytohormones. Ann. Agric. Sci. 1997, 39, 1613–1632. [Google Scholar]
- Fornes, F.; Sánchez-Perales, M.; Guardiola, J.L. Effect of a Seaweed Extract on Citrus Fruit Maturation. Acta Hortic. 1995, 75–82. [Google Scholar] [CrossRef]
- Fornes, F.; Sánchez-Perales, M.; Guardiola, J.L. Effect of a seaweed extract on the productivity of “de Nules” clementine mandarin and Navelina orange. Bot. Mar. 2002, 45, 486–489. [Google Scholar] [CrossRef]
- Koo, R.C.J. Response of citrus to seaweed-based nutrient sprays. Proceed. Fla. State Hort. Soc. 1988, 101, 26–28. [Google Scholar]
- Koo, R.C.J.; Alfred, L. Effects of Seaweed Sprays on Citrus Fruit Production. Proceed. Fla. State Hort. Soc. 1994, 107, 82–85. [Google Scholar]
- Fathy, M.A.; Gabr, M.A.; El Shall, S.A. Effect of Humic Acid Treatments on “Canino” Apricot Growth, Yield and Fruit Quality. N. Y. Sci. J. 2010, 3, 109–115. [Google Scholar]
- Eissa, F.M.; Fathi, M.A.; El Shall, S.A. Response of peach and apricot seedlings to humic acid treatments under salinity condition. J. Agric. Sci. Mansoura Univ. 2007, 32, 3605–3620. [Google Scholar]
- Donno, D.; Beccaro, G.L.; Mellano, M.G.; Canterino, S.; Cerutti, A.K.; Bounous, G. Improving the nutritional value of kiwifruit with the application of agroindustry waste extracts. J. Appl. Bot. Food Qual. 2013, 86, 11–15. [Google Scholar]
- Weber, N.; Schmitzer, V.; Jakopic, J.; Stampar, F. First fruit in season: seaweed extract and silicon advance organic strawberry (Fragaria × ananassa Duch.) fruit formation and yield. Sci. Hortic. 2018, 242, 103–109. [Google Scholar]
- Soppelsa, S.; Kelderer, M.; Casera, C.; Bassi, M.; Robatscher, P.; Matteazzi, A.; Andreotti, C. Foliar applications of biostimulants promote growth, yield and fruit quality of strawberry plants grown under nutrient limitation. Agronomy 2019, 9, 483. [Google Scholar] [CrossRef]
- Aghaeifard, F.; Babalar, M.; Fallahi, E. Influence of Humic Acid and Salicylic Acid on Yield, Fruit Quality, And Leaf Mineral Elements of Strawberry (Fragaria ananassa Duch.) Cv. Camarosa. J. Plant. Nutr. 2015, 39, 1821–1829. [Google Scholar] [CrossRef]
- Byers, R.E.; Carbaugh, D.H.; Presley, C.N. “Stayman” fruit cracking as affected by surfactants, plant growth regulators, and other chemicals. J. Am. Soc. Hortic. Sci. 1990, 115, 405–411. [Google Scholar] [CrossRef]
- Zhang, C.; Whiting, M.D. Improving ‘Bing’ sweet cherry fruit quality with plant growth regulators. Sci. Hortic. 2011, 127, 341–346. [Google Scholar] [CrossRef]
- Lal, S.; Ahmed, N.; Mir, J.I. Effect of different chemicals on fruit cracking in pomegranate under Karewa condition of Kashmir valley. Indian J. Plant. Physiol. 2011, 16, 326–330. [Google Scholar]
- Dorais, M.; Papadopoulos, A.P.; Gosselin, A. Greenhouse Tomato Fruit Quality. Hortic. Rev. 2000, 239–319. [Google Scholar] [CrossRef]
- Zodape, S.T.; Gupta, A.; Rawat, U.S.; Chaudhary, D.R.; Eswaran, K.; Chikara, J. Foliar application of seaweed sao as biostimulant for enhancement of yield and quality of tomato (Lycopersicon esculentum Mill.). J. Sci. Ind. Res. 2011, 70, 215–219. [Google Scholar]
- Chanthini, K.M.-P.; Stanley-Raja, V.; Thanigaivel, A.; Karthi, S.; Palanikani, R.; Shyam Sundar, N.; Sivanesh, H.; Soranam, R.; Senthil-Nathan, S. Sustainable agronomic strategies for enhancing the yield and nutritional quality of wild tomato, Solanum lycopersicum (L.) var ‘Cerasiforme Mill’. Agronomy 2019, 9, 311. [Google Scholar] [CrossRef]
- Abou Chehade, L.; Al Chami, Z.; De Pascali, S.A.; Cavoski, I.; Fanizzi, F.P. Biostimulants from food processing by-products: Agronomic, quality and metabolic impacts on organic tomato (Solanum lycopersicum L.). J. Sci. Food Agric. 2018, 98, 1426–1436. [Google Scholar] [CrossRef]
- Norrie, J.; Branson, T.; Keathley, P.E. Marine plant extracts impact on grape yield and quality. Acta Hortic. 2002, 594, 315–319. [Google Scholar] [CrossRef]
- Boselli, M.; Bahouaoui, M.A.; Lachhab, N.; Sanzani, S.M.; Ferrara, G.; Ippolito, A. Protein hydrolysates effects on grapevine (Vitis vinifera L., cv. Corvina) performance and water stress tolerance. Sci. Hortic. 2019, 258, 108784. [Google Scholar] [CrossRef]
- Popescu, G.C.; Popescu, M. Yield, berry quality and physiological response of grapevine to foliar humic acid application. Bragantia 2018, 77, 273–282. [Google Scholar] [CrossRef]
- Khan, W.; Rayirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Critchley, A.T.; Craigie, J.S.; Norrie, J.; Prithiviraj, B. Seaweed extracts as biostimulants of plant growth and development. J. Plant. Growth Regul. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- Craigie, J.S. Seaweed extract stimuli in plant science and agriculture. J. Appl. Phycol. 2011, 23, 371–393. [Google Scholar] [CrossRef]
- Michalak, I.; Chojnacka, K.; Saeid, A. Plant Growth Biostimulants, Dietary Feed Supplements and Cosmetics Formulated with Supercritical CO2 Algal Extracts. Molecules 2017, 22, 66. [Google Scholar] [CrossRef] [PubMed]
- Buschmann, A.H.; Camus, C.; Infante, J.; Neori, A.; Israel, Á.; Hernández-González, M.C.; Pereda, S.V.; Gomez-Pinchetti, J.L.; Golberg, A.; Tadmor-Shalev, N.; et al. Seaweed production: Overview of the global state of exploitation, farming and emerging research activity. Eur. J. Phycol. 2017, 52, 391–406. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, P. Effect of temperature and light on the growth of algae species: A review. Renew. Sustain. Energy Rev. 2015, 50, 431–444. [Google Scholar] [CrossRef]
- Gonçalves, A.; De Figueiredo, D.; Pereira, M. The effects of different salinity concentrations on growth of three freshwater green algae. Fresenius Environ. Bull. 2006, 15, 1382–1386. [Google Scholar]
- Nitschke, U.; Karsten, U.; Eggert, A. Physiological performance of the red alga Stylonema alsidii (Stylonematophyceae) under varying salinities. J. Exp. Mar. Biol. Ecol. 2014, 460, 170–176. [Google Scholar] [CrossRef]
- Connan, S.; Stengel, D.B. Impacts of ambient salinity and copper on brown algae: 1. Interactive effects on photosynthesis, growth, and copper accumulation. Aquat. Toxicol. 2011, 104, 94–107. [Google Scholar] [CrossRef]
- Halpern, M.; Bar-Tal, A.; Ofek, M.; Minz, D.; Muller, T.; Yermiyahu, U. The Use of Biostimulants for Enhancing Nutrient Uptake; Elsevier Inc.: Amsterdam, The Netherlands, 2015; Volume 130, ISBN 9780128021378. [Google Scholar]
- EL Boukhari, M.E.M.; Barakate, M.; Bouhia, Y.; Lyamlouli, K. Trends in Seaweed Extract Based Biostimulants: Manufacturing Process and Beneficial Effect on Soil-Plant Systems. Plants 2020, 9, 359. [Google Scholar] [CrossRef]
- Pasupuleti, V.K.; Demain, A.L. Protein hydrolysates in biotechnology. Protein Hydrolysates Biotechnol. 2010, 1–229. [Google Scholar] [CrossRef]
- Goñi, O.; Fort, A.; Quille, P.; McKeown, P.C.; Spillane, C.; O’Connell, S. Comparative Transcriptome Analysis of Two Ascophyllum nodosum Extract Biostimulants: Same Seaweed But Different. J. Agric. Food Chem. 2016, 64, 2980–2989. [Google Scholar] [CrossRef]
- Sharma, S.H.S.; Lyons, G.; McRoberts, C.; McCall, D.; Carmichael, E.; Andrews, F.; Swan, R.; McCormack, R.; Mellon, R. Biostimulant activity of brown seaweed species from Strangford Lough: Compositional analyses of polysaccharides and bioassay of extracts using mung bean (Vigno mungo L.) and pak choi (Brassica rapa chinensis L.). J. Appl. Phycol. 2012, 24, 1081–1091. [Google Scholar] [CrossRef]
- Goñi, O.; Quille, P.; O’Connell, S. Ascophyllum nodosum extract biostimulants and their role in enhancing tolerance to drought stress in tomato plants. Plant. Physiol. Biochem. 2018, 126, 63–73. [Google Scholar] [CrossRef] [PubMed]
- MAPA. Regulation of Law No. 6,894, of December 16, 1980; MAPA-Ministry of Agriculture, Livestock and Supply: Brasília, Brazil, 2004. Available online: http://www.planalto.gov.br/ccivil_03/_ato2011-2014/2014/decreto/D8384.htm (accessed on 20 May 2020).
- Grant, R.L.; Combs, A.B.; Acosta, D. Experimental Models for the Investigation of Toxicological Mechanisms. Compr. Toxicol. Second Ed. 2010, 1–14, 203–224. [Google Scholar]
- Stirk, W.A.; Tarkowská, D.; Turečová, V.; Strnad, M.; Van Staden, J. Abscisic acid, gibberellins and brassinosteroids in Kelpak®, a commercial seaweed extract made from Ecklonia maxima. J. Appl. Phycol. 2014, 26, 561–567. [Google Scholar] [CrossRef]
- De Saeger, J.; Van Praet, S.; Vereecke, D.; Park, J.; Jacques, S.; Han, T.; Depuydt, S. Toward the molecular understanding of the action mechanism of Ascophyllum nodosum extracts on plants. J. Appl. Phycol. 2020, 32, 573–597. [Google Scholar] [CrossRef]
- Ertani, A.; Francioso, O.; Tinti, A.; Schiavon, M.; Pizzeghello, D.; Nardi, S. Evaluation of seaweed extracts from laminaria and Ascophyllum nodosum spp. As biostimulants in Zea mays L. using a combination of chemical, biochemical and morphological approaches. Front. Plant. Sci. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Ertani, A.; Schiavon, M.; Nardi, S. Transcriptome-wide identification of differentially expressed genes in Solanum lycopersicon L. In response to an Alfalfa-protein hydrolysate using microarrays. Front. Plant Sci. 2017, 8, 1–19. [Google Scholar] [CrossRef]
- Casadesús, A.; Polo, J.; Munné-Bosch, S. Hormonal effects of an enzymatically hydrolyzed animal protein-based biostimulant (pepton) in water-stressed tomato plants. Front. Plant. Sci. 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Van Eck, N.J.; Waltman, L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef]
- Van Eck, N.J.; Waltman, L. VOSviewer Manual; Univeristeit Leiden: Leiden, The Netherlands, 2013; Volume 1, pp. 1–53. [Google Scholar]
- Brizzolara, S.; Manganaris, G.A.; Fotopoulos, V.; Watkins, C.B.; Tonutti, P. Primary Metabolism in Fresh Fruits during Storage. Front. Plant. Sci. 2020, 11, 1–16. [Google Scholar] [CrossRef]
- Decros, G.; Baldet, P.; Beauvoit, B.; Stevens, R.; Flandin, A.; Colombié, S.; Gibon, Y.; Pétriacq, P. Get the Balance Right: ROS Homeostasis and Redox Signalling in Fruit. Front. Plant. Sci. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
| Plant Biostimulant | Composition | References |
|---|---|---|
| Based on amino acids | ||
| Ergostim® XL | Two carboxylic acids (N-acetiltiazolidin-4-carboxylic acid (AATC) 2.5%, triazolidine-carboxylic acid (ATC) 2%) activate the metabolism of proline and cysteine in plants, which in turn increases stress tolerance | [2] |
| Benefit® | Organic carbon, amino acids (glycine, alanine, aspartic acid and glutamic acid), nucleotides, free enzymatic proteins and vitamins | [29] |
| Megafol® | Amino acids (proline and tryptophan), glycosides, polysaccharides, organic nitrogen and organic carbon | [29] |
| Radifarm® | Amino acids (asparagine, arginine and tryptophan), glycosides, polysaccharides and organic acids | [29] |
| Actium ® | Mix of polysaccharides, polypeptides and vitamins (40%), potassium oxide (5%) and amino acids (2%) | [30] |
| Based on plant extracts | ||
| Biozyme® | Macro and micronutrients combined with hydrolyzed plant extracts | [31] |
| Auxym® | A complex of natural plant extracts from which the auxin-like effects are derived from the concentration of biologically active natural substances such as amino acids, vitamins, enzymes, phytochelatins, auxins, cytokinins, humic acid, macro and microelements | [32] |
| Vipul® | Is a commercial formulation of triacontanol which is a long chain 30 carbon primary alcohol and occurs in nature as a natural constituent of bees wax and plant waxes | [33] |
| Sunred® | Plant extracts, methionine, phenylalanine, monosaccharides | [34] |
| [35] | ||
| Based on algae extracts | ||
| Goëmar BM 86® | Gibberellic acid (GA14) seaweed cream + elements Molybdenum (Mo) 0.2 g/kg Boron (B) 21 g/kg Magnesium (Mg) 29 g/kg Sulphur (S) 38 g/kg Contains auxin, cytokinin and betaine | [31] |
| Kelpak® | Liquid seaweed concentrate | [32] |
| Biozyme crop plus® | Is a commercial formulation of AE (A. nodosum), enzymes and protein hydrolysate | [33] |
| Citocina spic® | Gibberellic acid, auxins, cytokinins, algae extract (A. nodosum), PH and trace elements | [33] |
| Based on humic and fulvic acids | ||
| Radicon® | Humic and fulvic acids, obtained from the compost of worm (night crawled), containing total organic matter: 4%, humified organic substance 90% of total organic matter | [2] |
| Viva® | 33% organic matter in dry matter, folic acid, vitamins B1, B2, B6 and PP, inositol and humic acids | [29] |
| Protein hydrolysates from animals and plants | ||
| Trainer® | It is derived from 100% plant protein hydrolysate | [25] |
| [32] | ||
| Others | ||
| Hendophyt® PS | 60% biopolymers of polysaccharides (polyglucosamine—A low-molecular weight chitosan), 35% containing carbon, 4% organic nitrogen, 0.25% boron | [2] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, M.; Baptistella, J.L.C.; Horz, D.C.; Bortolato, L.M.; Mazzafera, P. Organic Plant Biostimulants and Fruit Quality—A Review. Agronomy 2020, 10, 988. https://doi.org/10.3390/agronomy10070988
Rodrigues M, Baptistella JLC, Horz DC, Bortolato LM, Mazzafera P. Organic Plant Biostimulants and Fruit Quality—A Review. Agronomy. 2020; 10(7):988. https://doi.org/10.3390/agronomy10070988
Chicago/Turabian StyleRodrigues, Mayara, João Leonardo Corte Baptistella, Daniele Caroline Horz, Laura Minatel Bortolato, and Paulo Mazzafera. 2020. "Organic Plant Biostimulants and Fruit Quality—A Review" Agronomy 10, no. 7: 988. https://doi.org/10.3390/agronomy10070988
APA StyleRodrigues, M., Baptistella, J. L. C., Horz, D. C., Bortolato, L. M., & Mazzafera, P. (2020). Organic Plant Biostimulants and Fruit Quality—A Review. Agronomy, 10(7), 988. https://doi.org/10.3390/agronomy10070988






