Functional Characterization and in Silico Analysis of Phytoene Synthase Family Genes Responsible for Carotenoid Biosynthesis in Watermelon (Citrullus lanatus L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Lycopene Measurement
2.2. Isolation of PSY cDNA Sequences and Corresponding Genomic Sequences
2.3. Protein Sequence Analysis
2.4. Real-Time PCR Analysis
2.5. Plasmids and Functional Complementation
2.6. In Silico Analysis of ClPSY1 Promoter Elements and Related Transcription Factors in Watermelon
3. Results
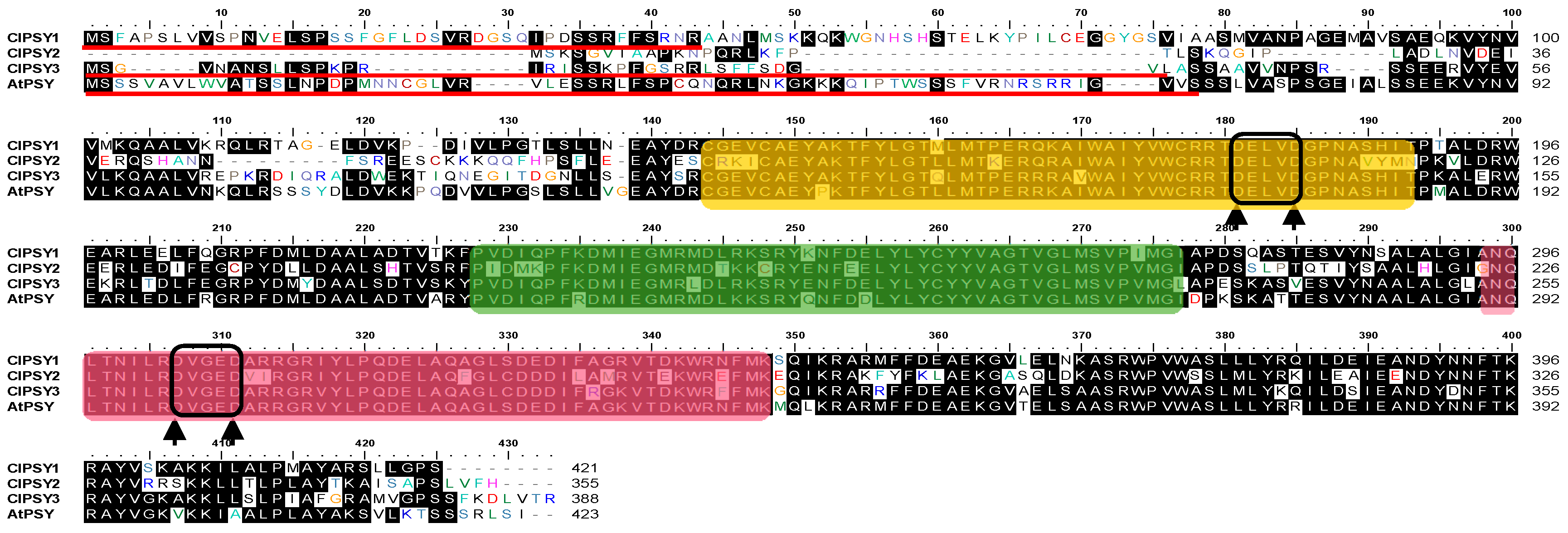
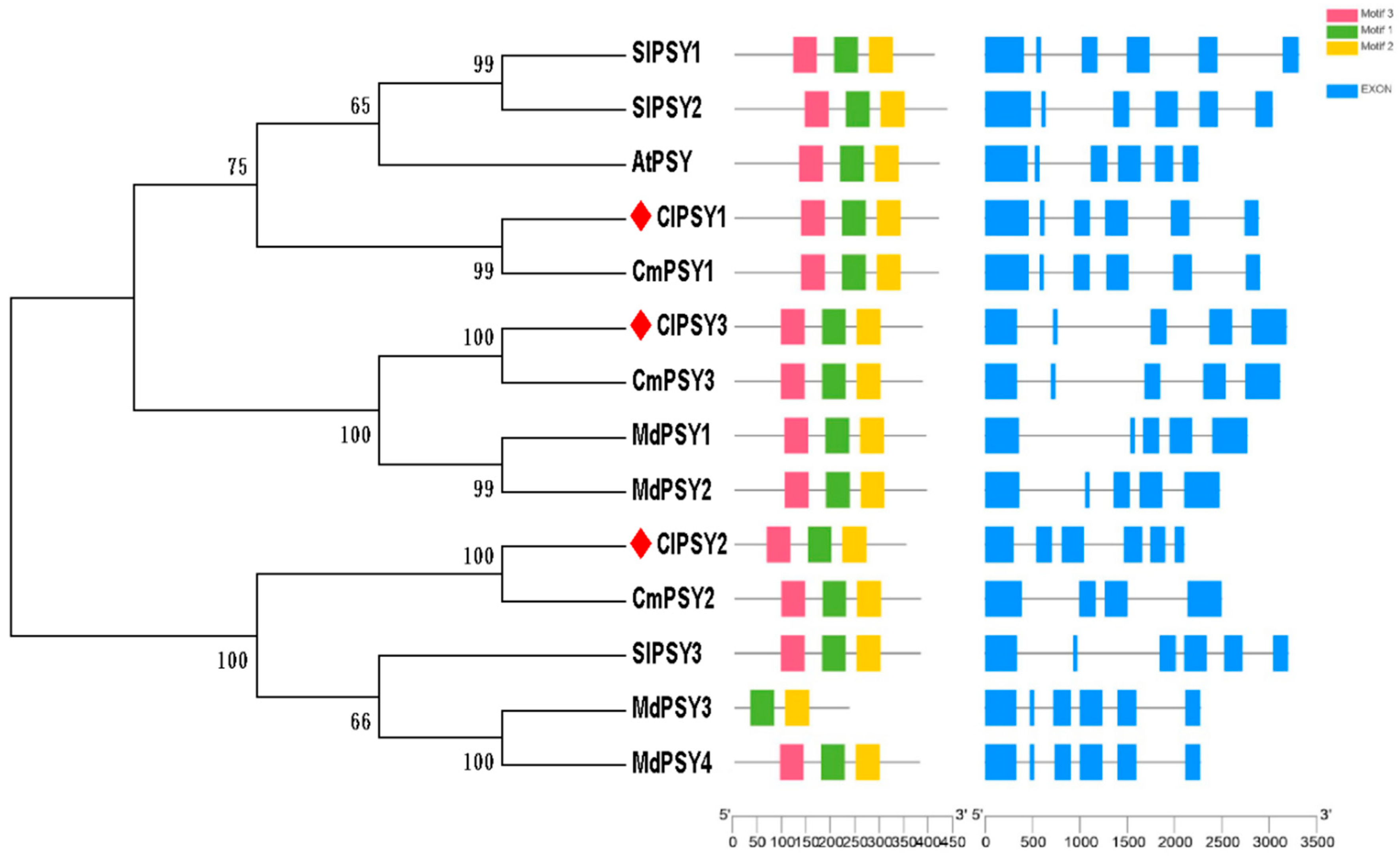
3.1. Characterization of Watermelon PSY Gene and Protein Sequences
3.2. Functional Complementation
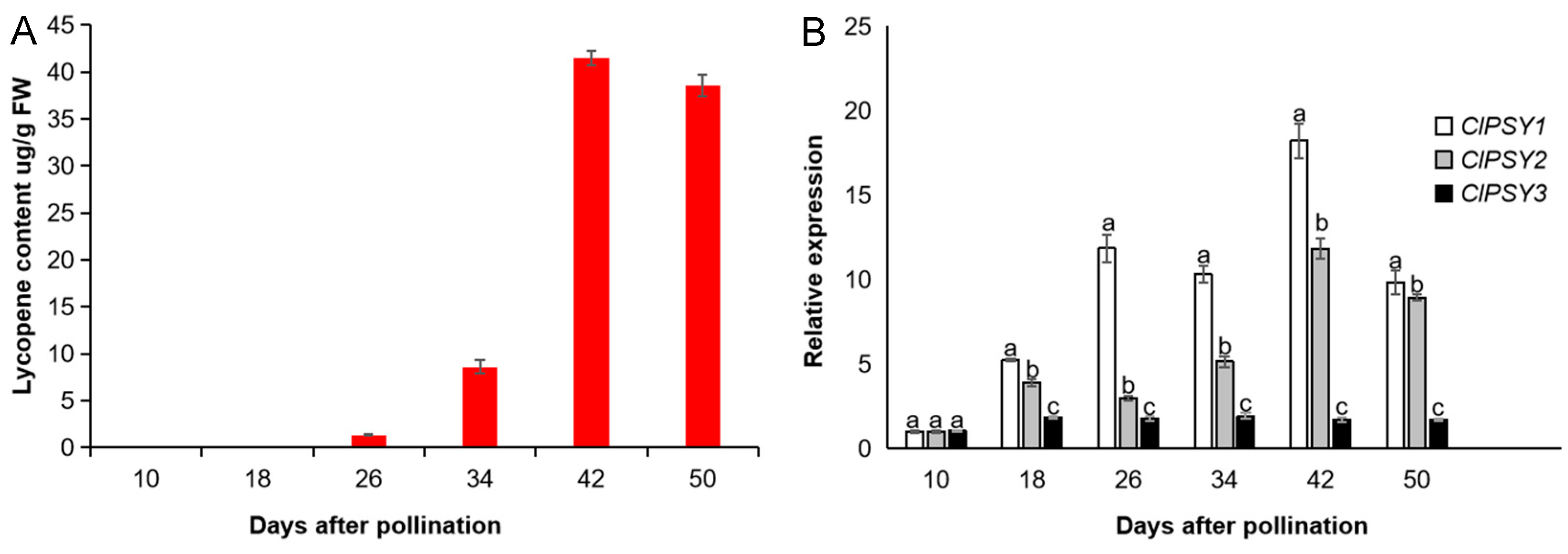
3.3. Carotenoid Synthesis and Accumulation in Watermelon Fruit
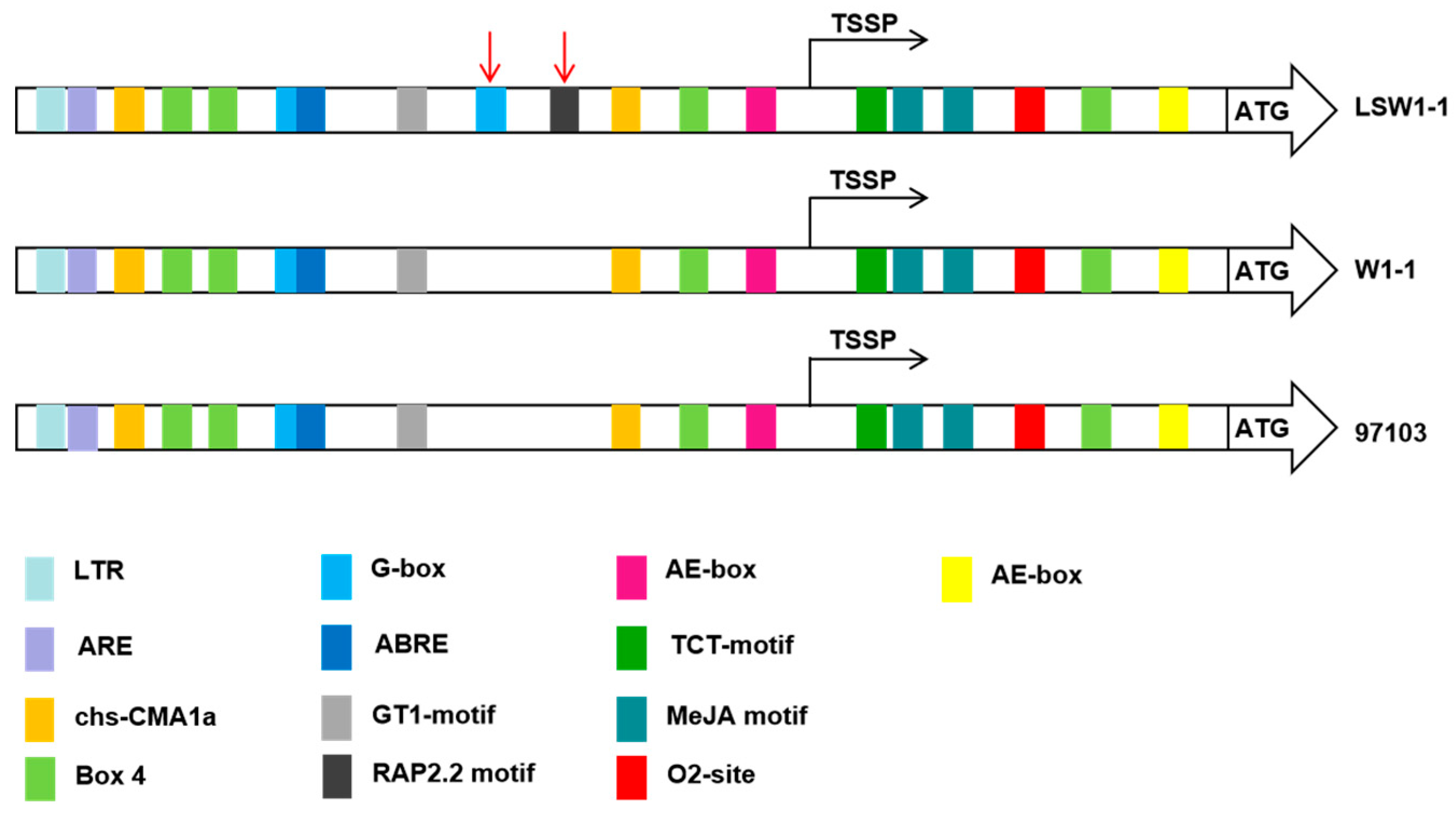
3.4. Cis-Regulatory Structures of ClPSY1 Promoter
3.5. WD40-Like and bZIP Regulate ClPSY1 Gene Expression during Watermelon Fruit Ripening
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Perkins-Veazie, P.; Collins, J.K.; Davis, A.R.; Roberts, W. Carotenoid Content of 50 Watermelon Cultivars. J. Agric. Food Chem. 2006, 54, 2593–2597. [Google Scholar] [CrossRef]
- Botella-Pavía, P.; Rodríguez-Concepción, M. Carotenoid biotechnology in plants for nutritionally improved foods. Physiol. Plant 2006, 126, 369–381. [Google Scholar] [CrossRef]
- Yabuzaki, J. Carotenoids Database: Structures, chemical fingerprints and distribution among organisms. Database 2017, 2017, bax004. [Google Scholar] [CrossRef]
- Frank, H.A.; Cogdell, R.J. Carotenoids in photosynthesis. Photochem. Photobiol. 1996, 63, 257–264. [Google Scholar] [CrossRef]
- Niyogi, K.K. Photoprotection revisited: Genetic and molecular approaches. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 333–359. [Google Scholar] [CrossRef]
- Clagett-Dame, M.; Knutson, D. Vitamin A in reproduction and development. Nutrients 2011, 3, 385–428. [Google Scholar] [CrossRef]
- Cutler, S.R.; Rodriguez, P.L.; Finkelstein, R.R.; Abrams, S.R. Abscisic acid: Emergence of a core signaling network. Annu. Rev. Plant Biol. 2010, 61, 651–679. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Rivers, J.; León, P.; McQuinn, R.P.; Pogson, B.J. Synthesis and function of apocarotenoid signals in plants. Trends Plant Sci. 2016, 21, 792–803. [Google Scholar] [CrossRef] [PubMed]
- Loreto, F.; Pollastri, S.; Fineschi, S.; Velikova, V. Volatile isoprenoids and their importance for protection against environmental constraints in the Mediterranean area. Env. Exp. Bot. 2014, 103, 99–106. [Google Scholar] [CrossRef]
- Fiedor, J.; Burda, K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Zhang, J.; Nageswaran, D.; Li, L. Carotenoid metabolism and regulation in horticultural crops. Hortic. Res. 2015, 2, 15036. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Sola, M.A.; Rodríguez-Concepción, M. Carotenoid biosynthesis in Arabidopsis: A colorful pathway. Arabidopsis Book 2012, 10, e0158. [Google Scholar] [CrossRef] [PubMed]
- Enfissi, E.M.A.; Nogueira, M.; Bramley, P.M.; Fraser, P.D. The regulation of carotenoid formation in tomato fruit. Plant J. 2017, 89, 774–788. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Zhang, Y.; Zhu, K.; Yang, W.; Ye, J.; Chai, L.; Xu, Q.; Deng, X. The citrus transcription factor CsMADS6 modulates carotenoid metabolism by directly regulating carotenogenic genes. J. Plant Physiol. 2018, 176, 2657–2676. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Villalon, A.; Gas, E.; Rodriguez-Concepcion, M. Phytoene synthase activity controls the biosynthesis of carotenoids and the supply of their metabolic precursors in dark-grown Arabidopsis seedling. Plant J. 2009, 60, 424–435. [Google Scholar] [CrossRef] [PubMed]
- Welsch, R.; Beyer, P.; Hugueney, P.; Kleinig, H.; Lintig, V.J. Regulation and activation of phytoene synthase, a key enzyme in carotenoid biosynthesis, during photomorphogenesis. Planta 2000, 211, 846–854. [Google Scholar] [CrossRef]
- Clotault, J.; Peltier, D.; Berruyer, R.; Thomas, M.; Briard, M.; Geoffriau, E. Expression of carotenoid biosynthesis genes during carrot root development. J. Exp. Bot. 2008, 59, 3563–3573. [Google Scholar] [CrossRef]
- Fantini, E.; Falcone, G.; Frusciante, S.; Giliberto, L.; Giuliano, G. Dissection of Tomato Lycopene Biosynthesis through Virus-Induced Gene Silencing. Plant Physiol. 2013, 163, 986–998. [Google Scholar] [CrossRef]
- Wang, H.; Ou, C.; Zhuang, F.; Ma, Z. The dual role of phytoene syntehase genes in carotenogenesis in carrot roots and leaves. Mol. Breed. 2014, 34, 2065–2079. [Google Scholar] [CrossRef]
- Giorio, G.; Stigliani, A.L.; D’Ambrosio, C. Phytoene synthase genes in tomato (Solanum lycopersicum L.)—New data on the structures, the deduced amino acid sequences and the expression patterns. FEBS J. 2008, 275, 527–535. [Google Scholar] [CrossRef]
- Arango, J.; Wust, F.; Beyer, P.; Welsch, R. Characterization of phytoene synthases from cassava and their involvement in abiotic stress-mediated responses. Planta 2010, 232, 1251–1262. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, N.; Nijhawan, A.; Khurana, J.P.; Khurana, P. Carotenoid Biosynthesis Genes in Rice: Structural Analysis, Genome-Wide Expression Profiling and Phylogenetic Analysis. Mol. Genet. Genomics. 2010, 283, 13–33. [Google Scholar] [CrossRef] [PubMed]
- Dibari, B.; Murat, F.; Chosson, A.; Gautier, V.; Poncet, C.; Lecomte, P.; Mercier, I.; Bergès, H.; Pont, C.; Blanco, A.; et al. Deciphering the genomic structure, function and evolution of carotenogenesis related phytoene synthases in grasses. BMC Genomics 2012, 13, 221. [Google Scholar] [CrossRef] [PubMed]
- Ampomah-Dwamena, C.; Driedonks, N.; Lewis, D.; Shumskaya, M.; Chen, X.; Wurtzel, E.; Espley, R.; Allan, A. The Phytoene synthase gene family of apple (Malus x domestica) and its role in controlling fruit carotenoid content. BMC Plant Biol. 2015, 15, 185. [Google Scholar] [CrossRef]
- Cerda, A.; Moreno, J.C.; Acosta, D.; Godoy, F.; Cáceres, J.C.; Cabrera, R.; Stange, C. Functional characterization and in silico modelling of MdPSY2 variants and MdPSY5 phytoene synthases from Malus domestica. J. Plant Physiol. 2020, 249, 153166. [Google Scholar] [CrossRef] [PubMed]
- López-Emparán, A.; Quezada-Martinez, D.; Zúñiga-Bustos, M.; Cifuentes, V.; Iñiguez-Luy, F.; Federico, M.L. Functional Analysis of the Brassica napus L. Phytoene Synthase (PSY) Gene Family. PLoS ONE 2014, 9, e114878. [Google Scholar] [CrossRef]
- Shumskaya, M.; Wurtzel, E. The carotenoid biosynthetic pathway: Thinking in all dimensions. Plant Sci. 2013, 208, 58–63. [Google Scholar] [CrossRef]
- Qin, X.Q.; Coku, A.; Inoue, K.; Tian, L. Expression, subcellular localization, and cis-regulatory structure of duplicated phytoene synthase genes in melon (Cucumis melo L.). Planta 2011, 234, 737–748. [Google Scholar] [CrossRef]
- Li, F.; Vallabhaneni, R.; Yu, J.; Rocheford, T.; Wurtzel, E.T. The maize phytoene synthase gene family: Overlapping roles for carotenogenesis in endosperm, photomorphogenesis, and thermal stress tolerance. Plant Physiol. 2008, 147, 1334–1346. [Google Scholar] [CrossRef]
- Dumas, Y.; Dadomo, M.; Lucca, G.D.; Grolier, P. Effects of environmental factors and agricultural techniques on antioxidant content of tomatoes. J. Sci. Food Agric. 2003, 83, 369–382. [Google Scholar] [CrossRef]
- Toledo-Ortiz, G.; Huq, E.; Rodríguez-Concepción, M. Direct regulation of phytoene synthase gene expression and carotenoid biosynthesis by phytochrome-interacting factors. Proc. Natl. Acad. Sci. USA 2010, 107, 11626. [Google Scholar] [CrossRef] [PubMed]
- Llorente, B.; D’Andrea, L.; Ruiz-Sola, M.A.; Botterweg, E.; Pulido, P.; Andilla, J.; Loza-Alvarez, P.; Rodriguez-Concepcion, M. Tomato fruit carotenoid biosynthesis is adjusted to actual ripening progression by a light-dependent mechanism. Plant J. 2016, 85, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Welsch, R.; Maass, D.; Voegel, T.; DellaPenna, D.; Beyer, P. Transcription factor RAP2.2 and its interacting partner SINAT2: Stable elements in the carotenogenesis of Arabidopsis leaves. Plant Physiol. 2007, 145, 1073–1085. [Google Scholar] [CrossRef] [PubMed]
- Vrebalov, J.; Pan, I.; Arroyo, A.J.; Mcquinn, R.; Chung, M.; Poole, M.; Rose, J.; Seymour, G.; Grandillo, S.; Giovannoni, J.; et al. Fleshy fruit expansion and ripening are regulated by the tomato SHATTERPROOF gene TAGL1. Plant Cell. 2009, 21, 3041–3062. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.; Wang, Y.; Cao, B.; Wang, W.; Tian, S. Unraveling the regulatory network of the MADS box transcription factor RIN in fruit ripening. Plant J. 2012, 70, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Bemer, M.; Karlova, R.; Ballester, A.; Tikunov, Y.; Bovy, A.; Wolters-Arts, M.; de Barros Rossetto, P.; Angenent, G.C.; de Maagd, R.A. The tomato FRUITFULL homologs TDR4/FUL1 and MBP7/FUL2 regulate ethylene-independent aspects of fruit ripening. Plant Cell. 2012, 24, 4437–4451. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.; Hu, Z.; Deng, L.; Wang, Y.; Zhu, M.; Zhang, J.; Chen, G. A tomato MADS-box transcription factor, SlMADS1, acts as a negative regulator of fruit ripening. J. Plant Physiol. 2013, 163, 1026–1036. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Hu, Z.; Zhu, Z.; Dong, T.; Zhao, Z.; Cui, B.; Chen, G. Overexpression of a novel MADS-box gene SlFYFL delays senescence, fruit ripening and abscission in tomato. Sci. Rep. 2014, 4, 4367. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, Z.; Yao, Q.; Guo, X.; Nguyen, V.; Li, F.; Chen, G. A tomato MADS-box protein, SlCMB1, regulates ethylene biosynthesis and carotenoid accumulation during fruit ripening. Sci. Rep. 2018, 8, 3413. [Google Scholar] [CrossRef]
- Zhu, M.; Chen, G.; Zhou, S.; Tu, Y.; Wang, Y.; Dong, T.; Hu, Z. A new tomato NAC (NAM ATAF1/2/CUC2) transcription factor, SlNAC4, functions as a positive regulator of fruit ripening and carotenoid accumulation. Plant Cell Physiol. 2014, 55, 119–135. [Google Scholar] [CrossRef]
- Meng, C.; Yang, D.; Ma, X.; Zhao, W.; Liang, X.; Ma, N.; Meng, Q. Suppression of tomato SlNAC1 transcription factor delays fruit ripening. J. Plant Physiol. 2016, 193, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Endo, T.; Fujii, H.; Sugiyama, A.; Nakano, M.; Nkajima, N.; Ikoma, Y.; Omura, M.; Shimada, T. Overexpression of a citrus basic helix-loop-helix transcription factor (CubHLH1), which is homologous to Arabidopsis activation-tagged bri1 suppressor 1 interacting factor genes, modulates carotenoid metabolism in transgenic tomato. Plant Sci. 2016, 243, 35–48. [Google Scholar] [CrossRef]
- Zhou, X.; Welsch, R.; Yang, Y.; Álvarez, D.; Riediger, M.; Yuan, H.; Fish, T.; Liu, J.; Thannhauser, T.; Li, L. Arabidopsis OR proteins are the major posttranscriptional regulators of phytoene synthase in controlling carotenoid biosynthesis. Proc. Natl. Acad. Sci. USA 2015, 112, 3558–3563. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, H.; Jung, Y.; Kim, S.; Ji, C.Y.; Wang, Z.; Jeong, J.; Lee, H.; Kwak, S. Orange Protein Has a Role in Phytoene Synthase Stabilization in Sweetpotato. Sci. Rep. 2016, 6, 33563. [Google Scholar] [CrossRef]
- Welsch, R.; Zhou, X.; Yuan, H.; Álvarez, D.; Sun, T.; Schlossarek, D.; Yang, Y.; Shen, G.; Zhang, H.; Rodriguez-Concepcion, M.; et al. Clp Protease and OR Directly Control the Proteostasis of Phytoene Synthase, the Crucial Enzyme for Carotenoid Biosynthesis in Arabidopsis. Mol. Plant 2017, 11, 149–162. [Google Scholar] [CrossRef]
- Liu, S.; Gao, P.; Wang, X.; Davis, A.; Baloch, M.; Luan, F. Mapping of quantitative trait loci for lycopene content and fruit traits in Citrullus lanatus. Euphytica 2015, 202, 411–426. [Google Scholar] [CrossRef]
- Wang, C.; Qiao, A.; Fang, X.; Sun, L.; Gao, P.; Davis, A.; Liu, L.; Luan, F. Fine Mapping of Lycopene Content and Flesh Color Related Gene and Development of Molecular Marker-Assisted Selection for Flesh Color in Watermelon (Citrullus lanatus). Front. Plant Sci. 2019, 10, 1240. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, H.; Guo, S.; Ren, Y.; Li, M.; Wang, J.; Zhang, H.; Gong, G.; Xu, Y. Decreased Protein Abundance of Lycopene β-Cyclase Contributes to Red Flesh in Domesticated Watermelon. Plant Physiol. 2020, 22, 01409. [Google Scholar] [CrossRef]
- Zhu, Q.; Gao, P.; Liu, S.; Zhu, Z.; Amanullah, S.; Davis, A.; Luan, F. Comparative transcriptome analysis of two contrasting watermelon genotypes during, fruit development and ripening. BMC Genomics 2017, 18, 3. [Google Scholar] [CrossRef]
- Thompson, J.; Gibson, T.; Plewniak, F.; Jeanmougin, F.; Higgins, D. The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for biggerdatasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Xia, R.; Chen, H.; He, Y. TBtools, a Toolkit for Biologists integrating various HTS-data handling tools with a user-friendly interface. bioRxiv 2018. [Google Scholar] [CrossRef]
- Schmittgen, T.; Livak, K. Analyzing Real-Time PCR Data by the Comparative C(T) Method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Kong, Q.; Yuan, J.; Gao, L.; Zhao, S.; Jiang, W.; Huang, Y.; Bie, Z. Identification of Suitable Reference Genes for Gene Expression Normalization in qRT-PCR Analysis in Watermelon. PLoS ONE 2014, 9, e90612. [Google Scholar] [CrossRef] [PubMed]
- Misawa, N.; Nakagawa, M.; Kobayashi, K.; Yamano, S.; Izawa, Y.; Nakamura, K.; Harashima, K. Elucidation of the Erwinia uredovora carotenoid biosynthetic pathway by functional analysis of gene products expression in Escherichia coli. J. Bacteriol. 1990, 172, 6704–6712. [Google Scholar] [CrossRef]
- Castillo, R.; Fernandez, J.A.; Gomez-Gomez, L. Implications of carotenoid biosynthetic genes in apocarotenoid formation during the stigma development of Crocus sativus and its closer relatives. Plant Physiol. 2005, 139, 674–689. [Google Scholar] [CrossRef] [PubMed]
- Lescot, M.; Dehais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Higo, K.; Ugawa, Y.; Iwamoto, M.; Higo, H. PLACE: A database of plant cis-acting regulatory DNA elements. Nucleic Acids Res. 1998, 26, 358–359. [Google Scholar] [CrossRef]
- Shahmuradov, I.; Gammerman, A.; Hancock, J.; Bramley, P.; Solovyev, V. PlantProm: A database of plant promoter sequences. Nucleic Acids Res. 2003, 31, 114–117. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, Y.S.; Cui, H.N.; Zhang, L.P.; Sha, T.Y.; Wang, C.N.; Fan, C.; Luan, F.S.; Wang, X.Z. Linkage Mapping and Comparative Transcriptome Analysis of Firmness in Watermelon (Citrullus lanatus). Front. Plant Sci. 2020, 11, 831. [Google Scholar] [CrossRef]
- Wang, N.; Liu, S.; Gao, P.; Luan, F.; Davis, A. Developmental Changes in Gene Expression Drive Accumulation of Lycopene and β-Carotene in Watermelon. J. Am. Soc. Hortic. Sci. 2016, 141, 434–443. [Google Scholar] [CrossRef]
- Dwivedi, K.K.; Roche, D.; Carman, J.G. Expression in Arabidopsis of a nucellus-specific promoter from watermelon (Citrullus lanatus). Plant Sci. 2010, 179, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Conforte, A.J.; Guimarães-Dias, F.; Neves-Borges, A.C.; Bencke-Malato, M.; Felix-Whipps, D.; Alves-Ferreira, M. Isolation and characterization of a promoter responsive to salt, osmotic and dehydration stresses in soybean. Genet. Mol. Biol. 2017, 40, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Tadmor, Y.; King, S.; Levi, A.; Davis, A.; Meir, A.; Wasserman, B.; Hirschberg, J.; Lewinsohn, E. Comparative fruit colouration in watermelon and tomato. Food Res. Int. 2005, 38, 837–841. [Google Scholar] [CrossRef]
- Grassi, S.; Piro, G.; Lee, J.; Zheng, Y.; Fei, Z.J.; Dalessandro, G.; Giovannoni, J.J.; Lenucci, M.S. Comparative genomics reveals candidate carotenoid pathway regulators of ripening watermelon fruit. BMC Genomics 2013, 14, 781. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Sun, H.; Zhang, H.; Liu, J.; Ren, Y.; Gong, G.; Jiao, C.; Zheng, Y.; Yang, W.; Fei, Z.; et al. Comparative Transcriptome Analysis of Cultivated and Wild Watermelon during Fruit Development. PLoS ONE 2015, 10, e0130267. [Google Scholar] [CrossRef]
- Gallagher, C.E.; Matthews, P.D.; Li, F.; Wurtzel, E.T. Gene duplication in the carotenoid biosynthetic pathway preceded evolution of the grasses. Plant Physiol. 2004, 135, 1776–1783. [Google Scholar] [CrossRef]
- Shewmaker, C.K.; Sheehy, J.A.; Daley, M.; Colburn, S.; Ke, D.Y. Seed specific overexpression of phytoene synthase:increase in carotenoids and other metabolic effects. Plant J. 1999, 20, 401–412. [Google Scholar] [CrossRef]
- Fu, X.; Feng, C.; Wang, C.; Yin, X.; Lu, P.; Grierson, D.; Xu, C.; Chen, K. Involvement of multiple phytoene synthase genes in tissue- and cultivar-specific accumulation of arotenoids in loquat. J. Exp. Bot. 2014, 65, 4679–4689. [Google Scholar] [CrossRef]
- Alquezar, B.; Zacarias, L.; Rodrigo, M.J. Molecular and functional characterization of a novel chromoplast-specific lycopene β-cyclase from Citrus and its relation to lycopene accumulation. J. Exp. Bot. 2009, 60, 1783–1797. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Y.; Shan, W.; Cai, Y.; Liang, S.; Chen, J.; Lu, W.; Kuang, J. Identification of Two Transcriptional Activators MabZIP4/5 in Controlling Aroma Biosynthetic Genes during Banana Ripening. J. Agric. Food Chem. 2018, 66, 6142–6150. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guo, S.; Tian, S.; Zhang, J.; Ren, Y.; Sun, H.; Gong, G.; Xu, Y. Abscisic Acid Pathway Involved in the Regulation of Watermelon Fruit Ripening and Quality Trait Evolution. PLoS ONE 2017, 12, e0179944. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Hong, J.; Ha, J.; Kang, J.; Kim, S.Y. ABFs, a family of ABA-responsive element binding factors. J. Biol. Chem. 2000, 275, 1723–1730. [Google Scholar] [CrossRef] [PubMed]
- Uno, Y.; Furihata, T.; Abe, H.; Yoshida, R.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Arabidopsis basic leucine zipper transcription factors involved in an abscisic acid-dependent signal transduction pathway under drought and high-salinity conditions. Proc. Natl. Acad. Sci. USA. 2000, 97, 11632–11637. [Google Scholar] [CrossRef] [PubMed]
- Rouster, J.; Leah, R.; Mundy, J.; Cameron-Mills, V. Identification of a Methyl Jasmonate-Responsive Region in the Promoter of a Lipoxygenase 1 Gene Expressed in Barley Grain. Plant J. 1997, 11, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.; Gaitatzes, C.; Saxena, K.; Neer, E. The WD repeat: A common architecture for diverse functions. Trends Biochem. 1999, 24, 181–185. [Google Scholar] [CrossRef]
- Jain, B.; Pandey, S. WD40 Repeat Proteins: Signalling Scaffold with Diverse Functions. Protein J. 2018, 37, 391–406. [Google Scholar] [CrossRef]
- Feng, R.; Zhang, C.; Ma, R.; Cai, Z.; Yu, M. Identification and characterization of WD40 superfamily genes in peach. Genes 2019, 710, 291–306. [Google Scholar] [CrossRef]
- Zhao, M.; Li, J.; Zhu, L.; Chang, P.; Li, L.; Zhang, L. Identification and Characterization of MYB-bHLH-WD40 Regulatory Complex Members Controlling Anthocyanidin Biosynthesis in Blueberry Fruits Development. Genes 2019, 10, 496. [Google Scholar] [CrossRef]
- Li, G.; Zhao, J.; Qin, B.; Yin, Y.; An, W.; Mu, Z.; Cao, Y. ABA Mediates Development-Dependent Anthocyanin Biosynthesis and Fruit Coloration in Lycium Plants. BMC Plant Biol. 2019, 19, 317. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.; Sun, L.; Lv, Y.; Cui, H.; Wang, X.; Gao, P.; Luan, F. Functional Characterization and in Silico Analysis of Phytoene Synthase Family Genes Responsible for Carotenoid Biosynthesis in Watermelon (Citrullus lanatus L.). Agronomy 2020, 10, 1077. https://doi.org/10.3390/agronomy10081077
Wu C, Sun L, Lv Y, Cui H, Wang X, Gao P, Luan F. Functional Characterization and in Silico Analysis of Phytoene Synthase Family Genes Responsible for Carotenoid Biosynthesis in Watermelon (Citrullus lanatus L.). Agronomy. 2020; 10(8):1077. https://doi.org/10.3390/agronomy10081077
Chicago/Turabian StyleWu, Chuan, Lei Sun, Yuanzuo Lv, Haonan Cui, Xuezheng Wang, Peng Gao, and Feishi Luan. 2020. "Functional Characterization and in Silico Analysis of Phytoene Synthase Family Genes Responsible for Carotenoid Biosynthesis in Watermelon (Citrullus lanatus L.)" Agronomy 10, no. 8: 1077. https://doi.org/10.3390/agronomy10081077
APA StyleWu, C., Sun, L., Lv, Y., Cui, H., Wang, X., Gao, P., & Luan, F. (2020). Functional Characterization and in Silico Analysis of Phytoene Synthase Family Genes Responsible for Carotenoid Biosynthesis in Watermelon (Citrullus lanatus L.). Agronomy, 10(8), 1077. https://doi.org/10.3390/agronomy10081077



