The Contribution of Root Turnover on Biological Nitrification Inhibition and Its Impact on the Ammonia-Oxidizing Archaea under Brachiaria Cultivations
Abstract
1. Introduction
2. Materials and Methods
2.1. Investigated Brachiaria Lines
2.2. Research Site and Experimental Design
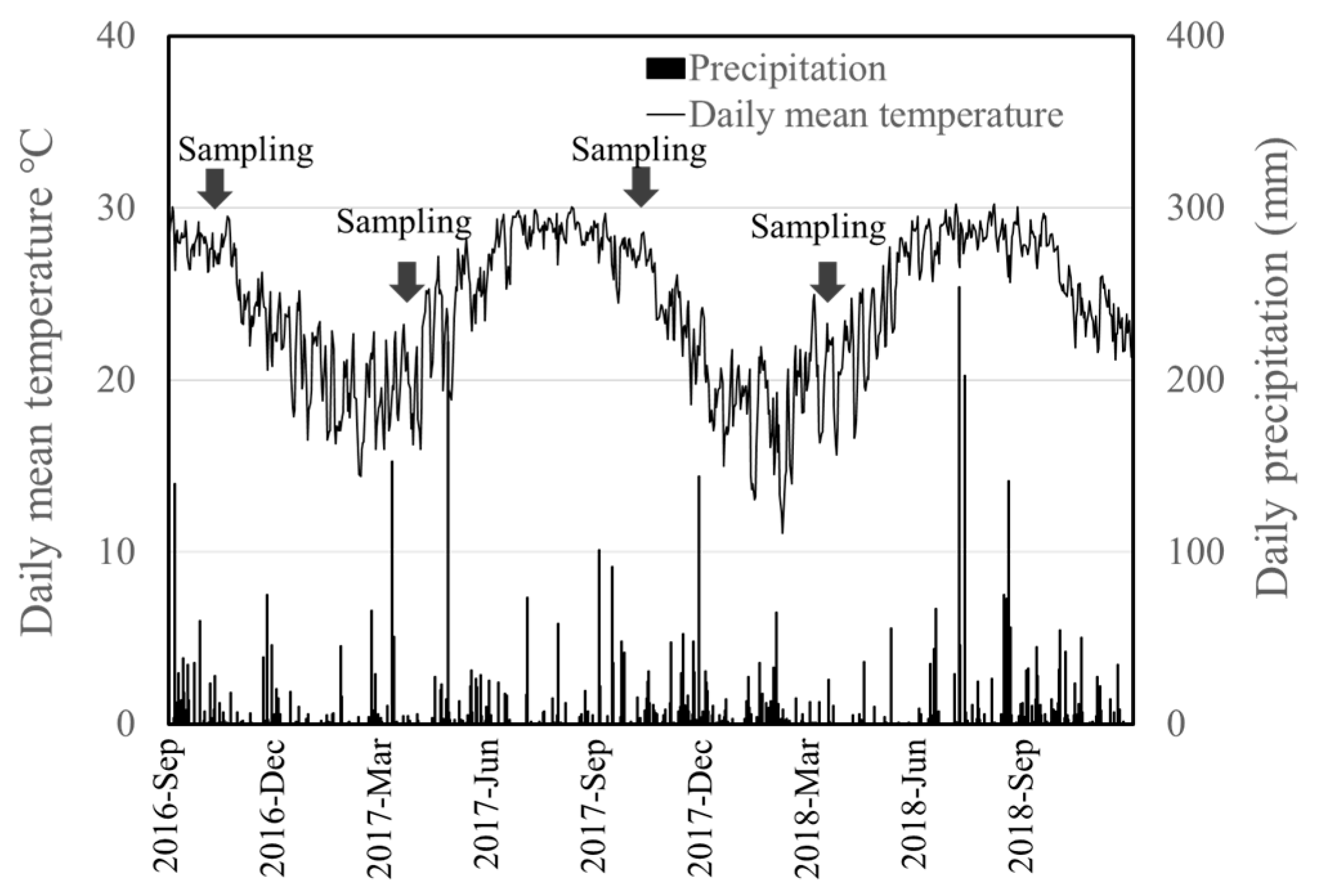
2.3. Soil Sampling
2.4. Chemical and Biological Analysis
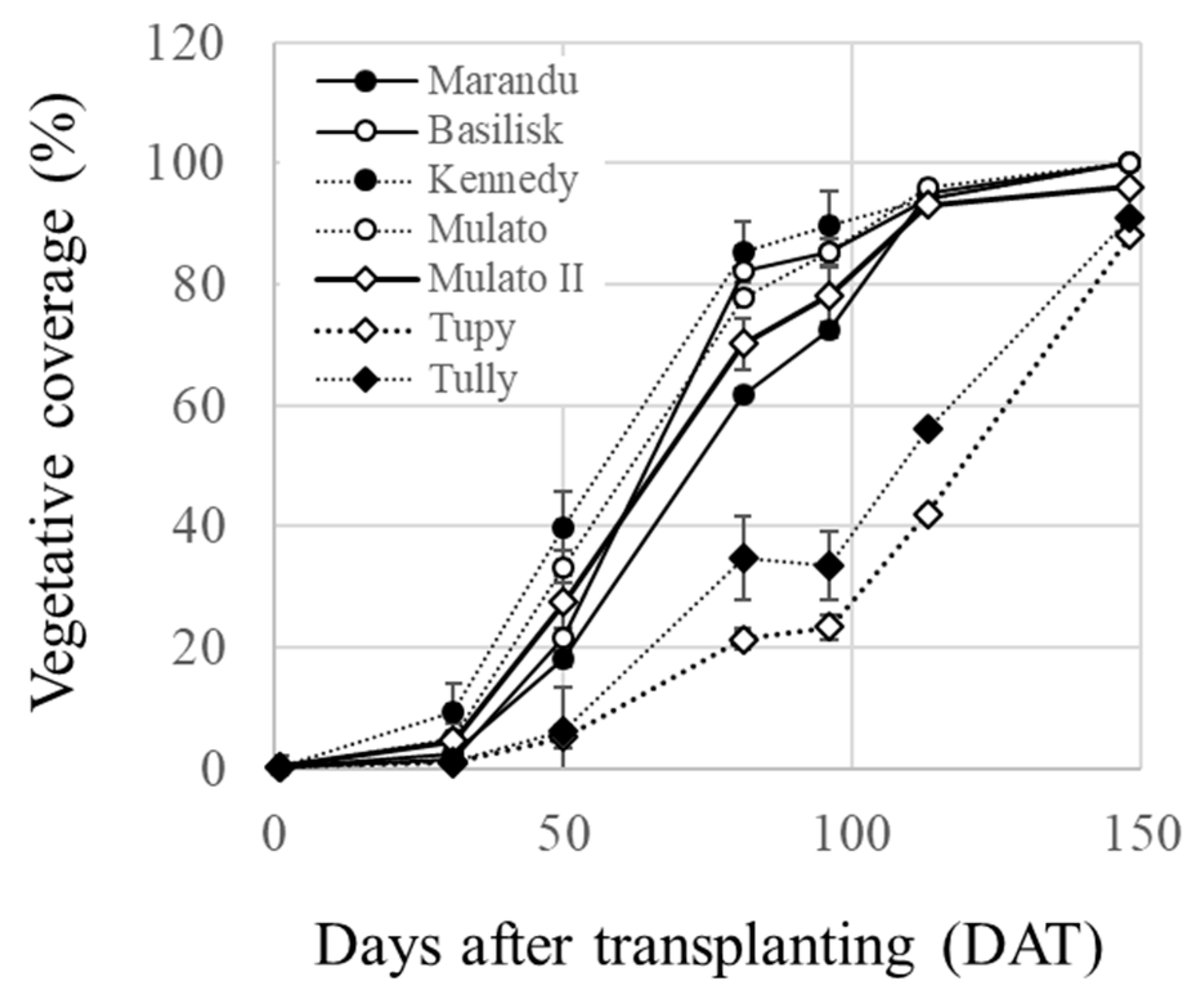
2.5. Plant Growth Monitoring
2.6. Evaluation of BNI Activities in Plant Root Tissue
2.7. Statistical Analysis
3. Results and Discussion
3.1. Effect of Brachiaria Cultivation on General Soil Chemical Properties and Potential Nitrification Rate
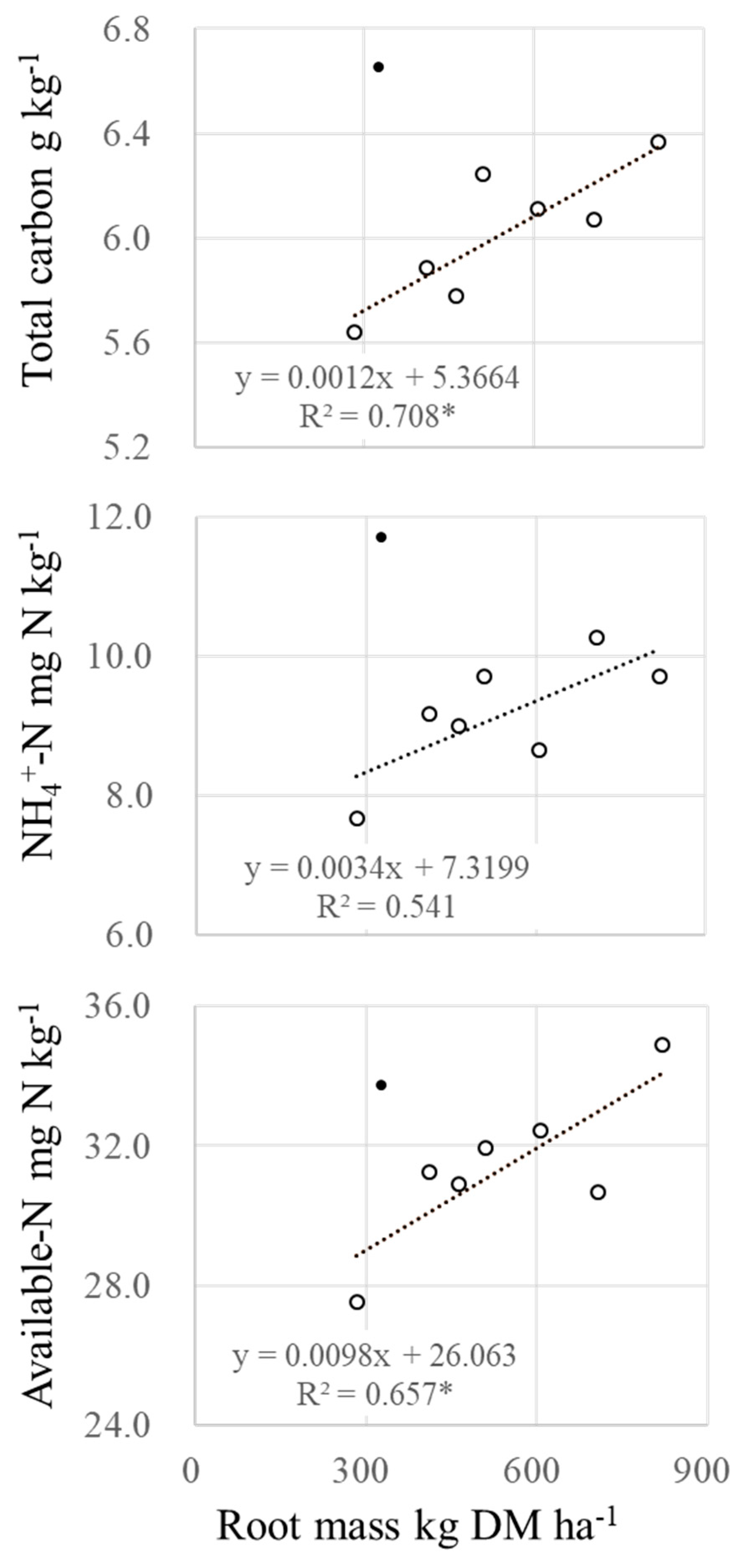
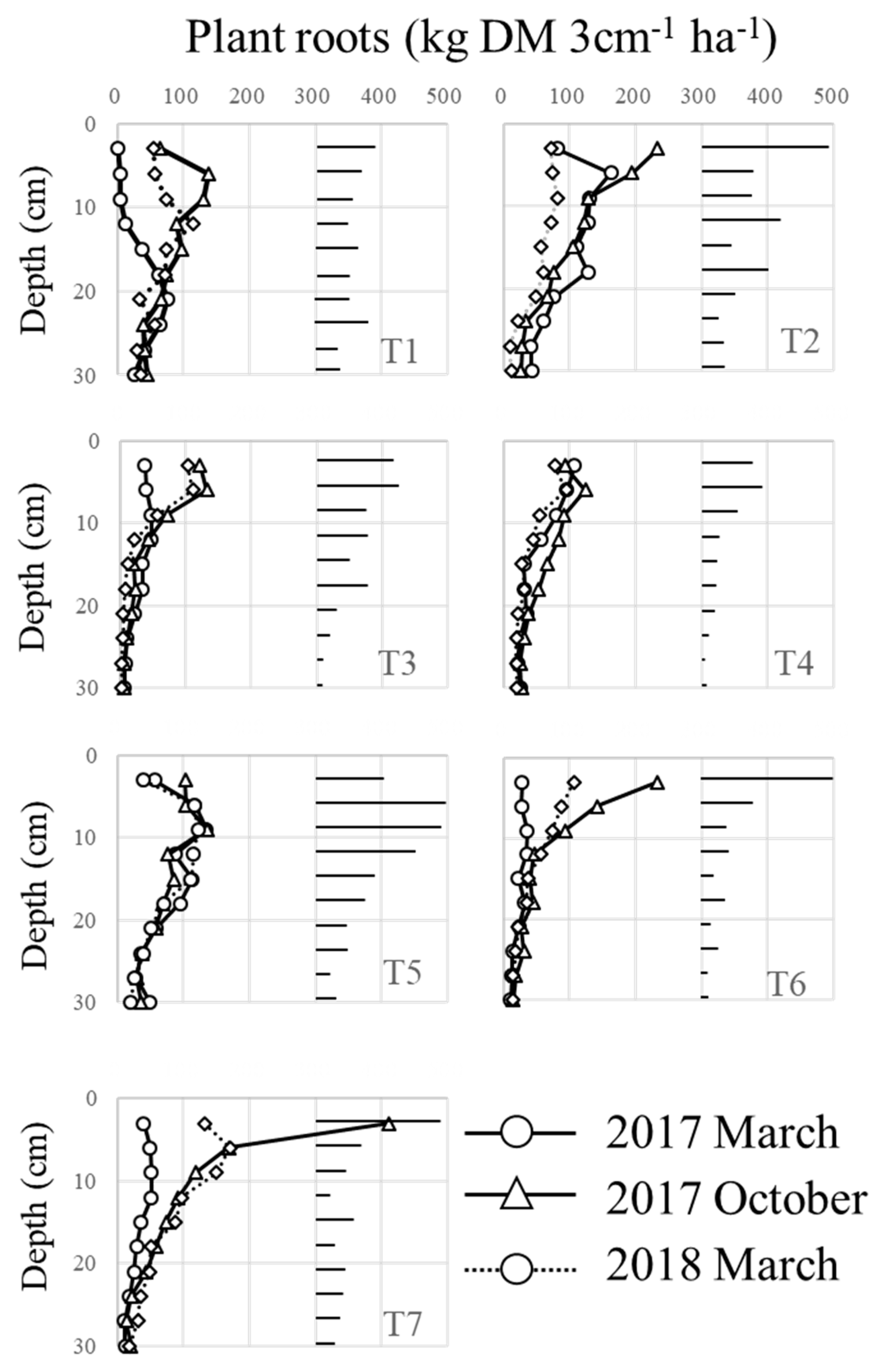
3.2. Changes of Plant Root Abundance under Brachiaria Cultivation and Its BNI Activities
3.3. Seasonal and Profile Changes in AOB and AOA under Brachiaria Cultivation
3.4. Relationships between Ammonium Oxidizers and Potential Nitrification
3.5. Effect of BNI Activity in Root Exudate and Root Tissue Decomposition
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Subbarao, G.V.; Nakahara, K.; Hurtado, M.P.; Ono, H.; Moreta, D.E.; Salcedo, A.F.; Yoshihashi, T.; Ishikawa, T.; Ishitani, M.; Ohnishi-Kameyama, M.; et al. Evidence for biological nitrification inhibition in Brachiaria pastures. Proc. Natl. Acad. Sci. USA 2009, 106, 17302–17307. [Google Scholar] [CrossRef] [PubMed]
- Subbarao, G.V.; Arango, J.; Masahiro, K.; Hooper, A.M.; Yoshihashi, T.; Ando, Y.; Nakahara, K.; Deshpande, S.; Ortiz-Monasterio, I.; Ishitani, M.; et al. Genetic mitigation strategies to tackle agricultural GHG emissions: The case for biological nitrification inhibition technology. Plant Sci. 2017, 262, 165–168. [Google Scholar] [CrossRef]
- Coskun, D.; Britto, D.T.; Shi, W.; Kronzucker, H.J. Nitrogen transformations in modern agriculture and the role of biological nitrification inhibition. Nat. Plants 2017, 3, 17074. [Google Scholar] [CrossRef] [PubMed]
- Brynes, R.C.; Nùñez, J.; Arenas, L.; Rao, I.; Trujillo, C.; Alvarez, C.; Arango, J.; Rasche, F.; Chirinda, N. Biological nitrification inhibition by Brachiaria grasses mitigates soil nitrous oxide emissions from bovine urine patches. Soil Biol. Biochem. 2017, 107, 156–163. [Google Scholar] [CrossRef]
- Karwat, H.; Moreta, D.; Arango, J.; Núñez, J.; Rao, I.; Rincón, Á.; Rasche, F.; Cadisch, G. Residual effect of BNI by Brachiaria humidicola pasture on nitrogen recovery and grain yield of subsequent maize. Plant Soil 2017, 420, 389–406. [Google Scholar] [CrossRef]
- Subbarao, G.V.; Wang, H.Y.; Ito, O.; Nakahara, K.; Berry, W.L. NH4+ triggers the synthesis and release of biological nitrification inhibition compounds in Brachiaria humidicola roots. Plant Soil 2007, 290, 1–2. [Google Scholar] [CrossRef]
- Moreta, D.E.; Arango, J.; Sotelo, M.; Vergara, D.; Rincon, A.; Ishitani, M.; Castro, A.; Miles, J.; Peters, M.; Tohme, J.; et al. Biological nitrification inhibition (BNI) in Brachiaria pastures: A novel strategy to improve eco-efficiency of crop-livestock systems and to mitigate climate change. Trop. Grassl. 2014, 2, 88–91. [Google Scholar] [CrossRef]
- Subbarao, G.V.; Nakahara, K.; Ishikawa, T.; Ono, H.; Yoshida, M.; Yoshihashi, T.; Sahrawat, K.L. Biological nitrification inhibition (BNI) activity in sorghum and its characterization. Plant Soil 2013, 366, 243–259. [Google Scholar] [CrossRef]
- Zhang, M.; Fan, C.H.; Li, Q.L.; Li, B.; Zhu, Y.Y.; Xiong, Z.Q. A 2-yrfield assessment of the effects of chemical and biological nitrification inhibitors on nitrous oxide emissions and nitrogen use efficiency in an intensively managed vegetable cropping system. Agric. Ecosyst. Environ. 2015, 201, 43–50. [Google Scholar] [CrossRef]
- Gopalakrishnan, S.; Subbarao, G.V.; Nakahara, K.; Yoshihashi, T.; Ito, O.; Maeda, I.; Ono, H.; Yoshida, M. Nitrification Inhibitors from the Root Tissues of Brachiaria humidicola, a Tropical Grass. J. Agric. Food Chem. 2007, 55, 1385–1388. [Google Scholar] [CrossRef]
- Kanno, T.; Macedo, M.C.; Euclides, V.P.B.; Bono, J.A.; Santos, J.D.G.; Rocha, M.C.; Bereta, L.G. Root biomass of five tropical grass pastures under continuous grazing in Brazilian savannas. J. Jpn. Grassl. Sci. 1999, 45, 9–14. [Google Scholar]
- Santos, F.L.F. Seed production: Perspective from the Brazilian private sector. In Brachiaria: Biology, Agronomy and Improvement; Miles, J.W., Maass, B.L., do Valle, C.B., Eds.; CIAT, Cali, Colombia and CNPGC/EMBRAPA: Campo Grande, MS, Brazil, 1996; pp. 141–146. [Google Scholar]
- Kouki, K.; Ebina, M. Breeding and forage utilization of Brachiaria grass (Brachiaria spp.). J. Jpn. Grassl. Sci. 2009, 55, 179–187. (In Japanese) [Google Scholar]
- Argel, P.J.; Miles, J.W.; Guiot, J.D.; Carolos, E.; Lascano, C.E. Cultivar Mulato (Brachiaria hibrido CIAT36061). 2001. Available online: https://core.ac.uk/download/pdf/132682261.pdf (accessed on 1 March 2020).
- Argel, P.J.; Miles, J.W.; Guiot, J.D.; Cuadrado, H.; Lascano, C.E. Cultivar Mulato II (Brachiaria Hybrid CIAT 36087): A High Quality Forage Grass, Resistant to Spittlebugs and Adapted to Well-Drained, Acid Tropical Soils; International Center for Tropical Agriculture (CIAT): Cali, Colombia, 2007; p. 21. [Google Scholar]
- Subbarao, G.V.; Ishikawa, T.; Ito, O.; Nakahara, K.; Wang, H.Y.; Berry, W.L. A bioluminescence assay to detect nitrification inhibitors released from plant roots: A case study with Brachiaria humidicola. Plant Soil 2006, 288, 101–112. [Google Scholar] [CrossRef]
- Loughran, R.J.; Wallbrink, P.J.; Walling, D.E.; Appleby, P.G. Sampling methods. In Handbook for the Assessment of Soil Erosion and Sedimentation Using Environmental Radionuclides; Springer: Berlin/Heidelberg, Germany, 2002; Chapter 3; pp. 41–57. [Google Scholar]
- Matsuda, N.; Mikami, S.; Shimoura, S.; Takahashi, J.; Nakano, M.; Shimada, K.; Uno, K.; Hagiwara, S.; Saito, K. Depth profiles of radioactive cesium in soil using a scraper plate over a wide area surrounding the Fukushima Dai-ichi Nuclear Power Plant. Jpn. J. Environ. Radioact. 2015, 139, 427–434. [Google Scholar] [CrossRef]
- Ma, L.; Oshima, M.; Motomizu, S.; Hattori, T. Simultaneous determination of nitrate and nitrite ion by micro-flow injection analysis. Jpn. Analyst. 1998, 47, 375–380. [Google Scholar] [CrossRef][Green Version]
- Nelson, D.W. Determination of ammonium in KCl extracts of soils by the salicylate method. Commun. Soil Sci. Plant Anal. 1983, 14, 1051–1062. [Google Scholar] [CrossRef]
- Uezono, I.; Kano, N.; Moriizumi, M. Applicability of rapid analysis by 80 °C-16 h hot water extraction for estimating available nitrogen in upland soil in Japan. Jpn. J. Soil Sci. Plant Nutr. 2010, 81, 39–43. [Google Scholar]
- Belser, L.W.; Mays, E.L. Specific inhibition of nitrite oxidation by chlorate and its use in assessing nitrification in soils and sediments. Appl. Environ. Microbiol. 1980, 39, 505–510. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Tsujimoto, Y.; Pedro, A.J.; Boina, G.; Murracama, V.M.; Tobita, S.; Oya, T.; Nakamura, S.; Cuambe, E.C.; Martinho, C. An application of digital imagery analysis to understand the effect of N application on light interception, radiation use efficiency, and grain yield of maize under various agro-environments in Northern Mozambique. Plant Prod. Sci. 2017, 20, 12–23. [Google Scholar] [CrossRef]
- Cardoso, J.A.; Jimenez, J.C.; Gichangi, E.; Njarui, D.M.G.; Mutimura, M.; Rao, I.M. Climate-smart Brachiaria grasses: Progress in identifying the role of root traits in soil carbon accumulation. In Proceedings of the Poster presentation in 9th International Symposium of Root Research, Canberra, Australia, 5–9 October 2015. [Google Scholar]
- Sarr, P.S.; Ando, Y.; Nakamura, S.; Deshpande, S.; Subbarao, G.V. Sorgoleone release from sorghum roots shapes the composition of nitrifying populations, total bacterial and archaea and determines the level of nitrification. Biol. Fertil. Soils 2020, 56, 145–166. [Google Scholar] [CrossRef]
- Rao, I.M.; Plazas, C.; Ricaurte, J. Root turnover and nutrient cycling in native and introduced pastures in tropical savannas. In Plant Nutrition; Developments in Plant and Soil Sciences; Horst, W.J., Schenk, M.K., Bürkert, A., Claassen, N., Flessa, H., Frommer, W.B., Goldbach, H., Olfs, H.W., Römheld, V., Sattelmacher, B., et al., Eds.; Springer: Dordrecht, The Netherlands, 2001; Volume 92, pp. 976–977. [Google Scholar] [CrossRef]
- Horrocks, C.A.; Arango, J.; Arevalo, A.; Nuñez, J.; Cardoso, J.A.; Dungait, J.A.J. Smart forage selection could significantly improve soil health in the tropics. Sci. Total. Environ. 2019, 688, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Gichangi, E.M.; Njarui, D.M.G.; Gatheru, M. Plant shoots and roots biomass of Brachiaria grasses and their effects on soil carbon in the semi-arid tropics of Kenya. Trop. Subtrop. Agroecosyst. 2017, 20, 65–74. [Google Scholar]
- Di, H.; Cameron, K.; Shen, J.; He, J. Nitrification driven by bacteria and not archaea in nitrogen-rich grassland soils. Nat. Geosci. 2009, 2, 621–624. [Google Scholar] [CrossRef]
- Banning, N.; Maccarone, L.; Fisk, L.; Murphy, D.V. Ammonia-oxidising bacteria not archaea dominate nitrification activity in semi-arid agricultural soil. Sci. Rep. 2015, 5, 11146. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, H.; Shen, J.; He, J. Ammonia-oxidizing archaea have more important role than ammonia-oxidizing bacteria in ammonia oxidation of strongly acidic soils. ISME J. 2012, 6, 1032–1045. [Google Scholar] [CrossRef]
- Hu, H.; Xu, Z.; He, J. Ammonia-Oxidizing Archaea play a predominant role in acid soil nitrification. Adv. Agron. 2014, 125, 261–294. [Google Scholar] [CrossRef]
- Taylor, A.E.; Zeglin, L.H.; Wanzek, T.A.; Myrold, D.D.; Bottomley, P.J. Dynamics of ammonia-oxidizing archaea and bacteria populations and contributions to soil nitrification potentials. ISME J. 2012, 6, 2024–2032. [Google Scholar] [CrossRef]
- Di, H.J.; Cameron, K.C.; Shen, J.; Winefield, C.S.; O’Callaghan, M.; Bowatte, S.; He, J. Ammonia-oxidizing bacteria and archaea grow under contrasting soil nitrogen conditions. FEMS Microbiol. Ecol. 2010, 72, 386–394. [Google Scholar] [CrossRef]
- Verhamme, D.T.; Prosser, J.I.; Nicol, G.W. Ammonia concentration determines differential growth of ammonia-oxidising archaea and bacteria in soil microcosms. ISME J. 2011, 5, 1067–1071. [Google Scholar] [CrossRef]


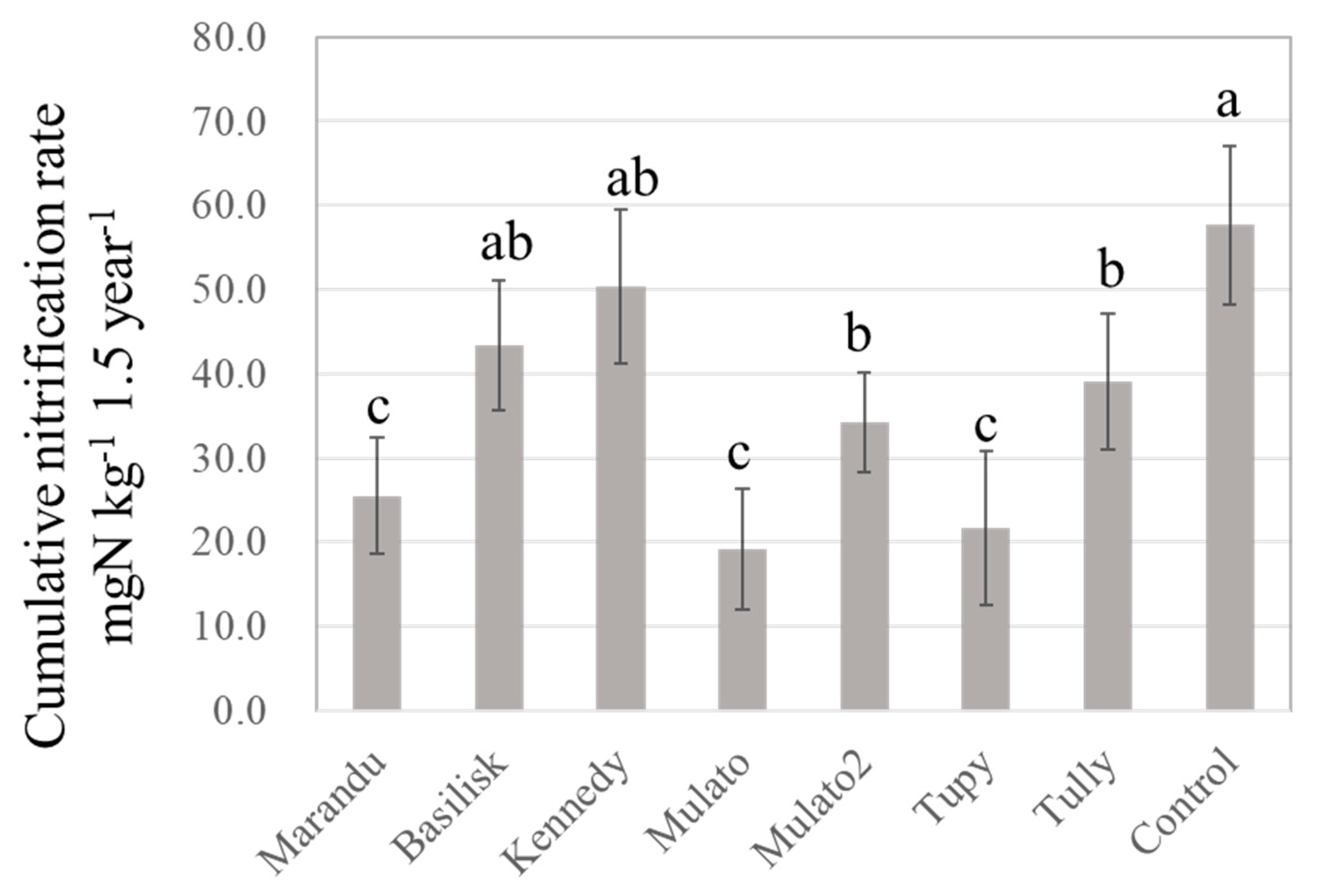
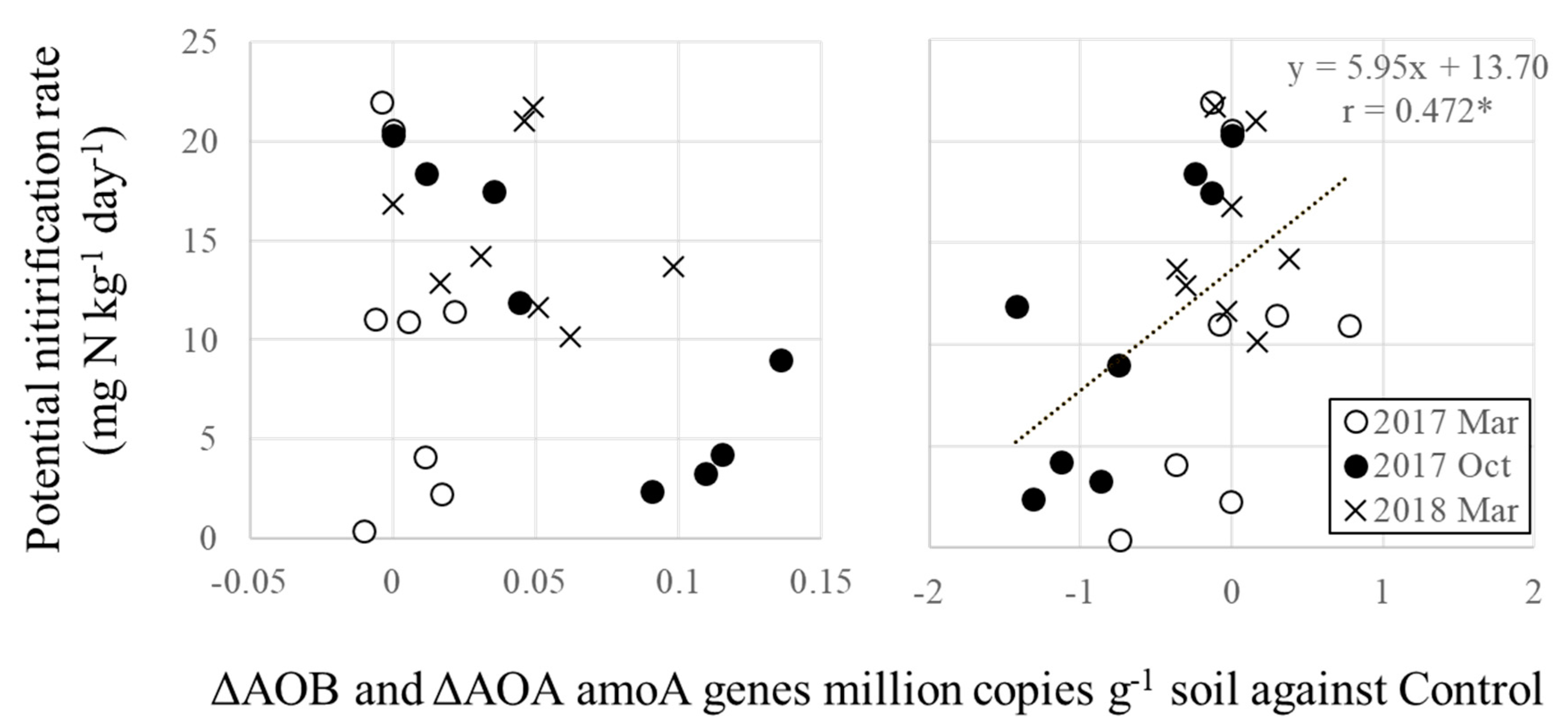
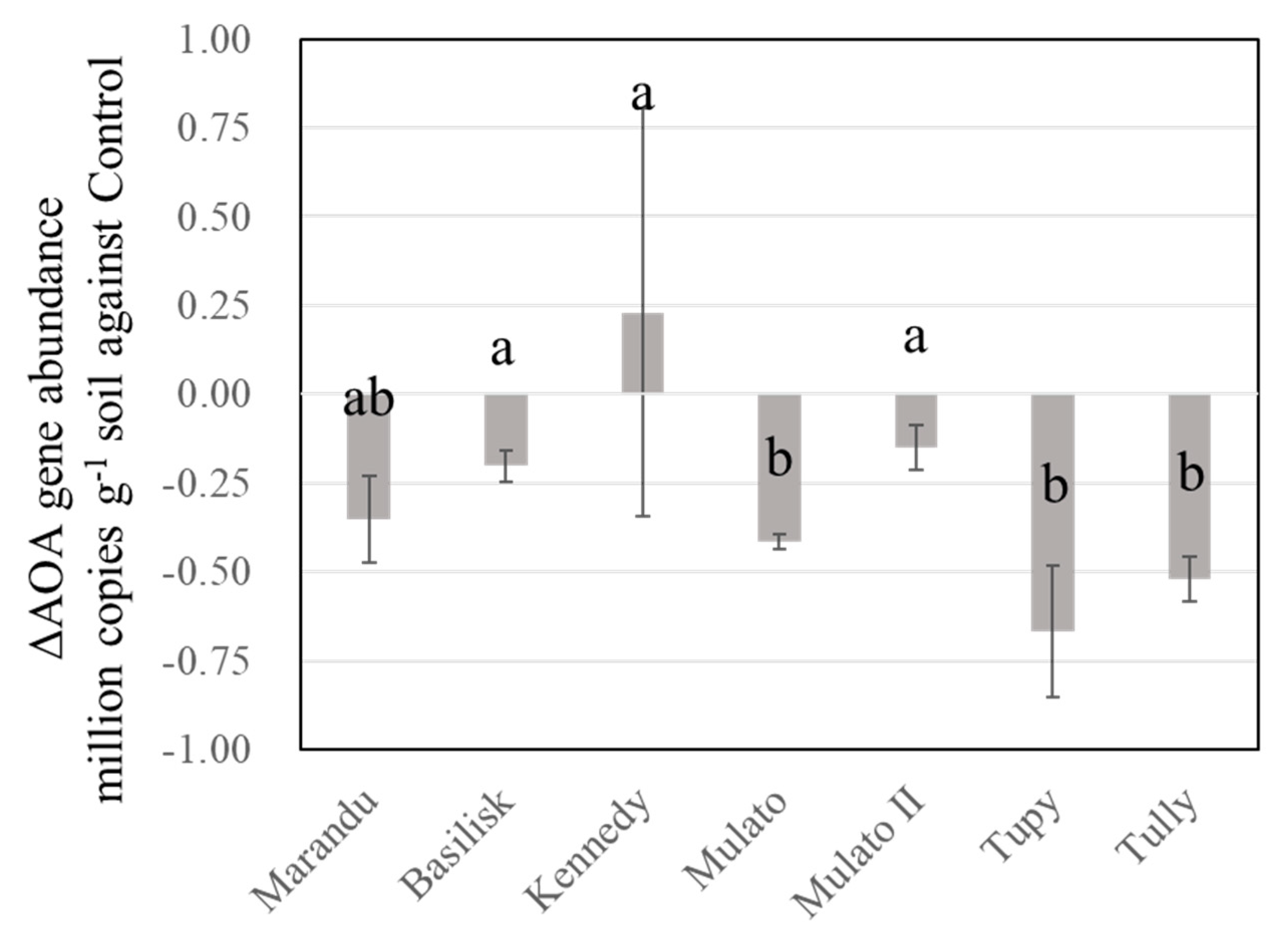
| Variety | CIAT † No. | BNI Activity in Root Exudate ATU †† g−1 Root DM d−1 (year−1) | References | ||
|---|---|---|---|---|---|
| T1 | Brachiaria brizantha cv. | Marandu | CIAT6294 | 2.0 (730) | [6] |
| T2 | B. decumbens cv. | Basirisk | CIAT606 | 18.3 (6680) | [6] |
| T3 | B. ruziziensis cv. | Kennedy | CIAT605 | 24.4 (8906) | Subbarao (unpublished data) |
| T4 | B. hybrid cv. | Mulato | CIAT36061 | 3.0 to 10.0 (1095 to 3650) | [1] |
| T5 | B. hybrid cv. | Mulato II | CIAT36087 | N.D. | |
| T6 | B. humidicola cv. | Tupy | CIAT26159 | 46.3 (16900) | [6] |
| T7 | B. humidicola cv. | Tully | CIAT679 | 17.5 (6388) | [6] |
| T8 | Bare soil as control | ||||
| Lab ID | Cultivar | pH (H2O) | T-N | T-C | C/N | NO3−-N | NH4+-N | Available N |
|---|---|---|---|---|---|---|---|---|
| g kg−1 | mg N kg−1 | |||||||
| T1 | Marandu | 6.83 b | 0.779 | 6.11 ab | 7.81 ab | 1.09 bc | 8.66 b | 32.4 ab |
| T2 | Basirisk | 6.80 b | 0.771 | 6.24 ab | 8.07 ab | 1.20 b | 9.70 ab | 31.9 ab |
| T3 | Kennedy | 6.77 b | 0.791 | 6.65 a | 8.34 a | 0.97 bc | 11.72 a | 33.8 ab |
| T4 | Mulato | 6.69 b | 0.769 | 5.89 b | 7.63 ab | 1.03 bc | 9.17 b | 31.2 ab |
| T5 | Mulato II | 6.81 b | 0.782 | 6.07 ab | 7.74 ab | 0.95 bc | 10.27 a | 30.7 ab |
| T6 | Tupy | 7.17 a | 0.741 | 5.78 b | 7.76 ab | 0.77 bc | 8.99 b | 30.9 ab |
| T7 | Tully | 6.73 b | 0.774 | 6.37 ab | 8.17 ab | 0.60 c | 9.71 ab | 34.9 a |
| T8 | Control | 6.67 b | 0.750 | 5.64 b | 7.51 b | 1.69 a | 7.68 b | 27.5 b |
| ANOVA | p-value | *** | n.s. | ** | ** | ** | *** | * |
| Root Mass | BNI Activity in Root Tissue | Estimated Root Length Turnover | Estimated Annual BNI Activity | |||||
|---|---|---|---|---|---|---|---|---|
| Treatment | 0–30 cm | 0–9 cm | Root Exudate (A) | Root Tissue (B) | Total BNI (A + B) | |||
| kg DM ha−1 | ATU g−1 Root DM | Year−1 | Million ATU ha−1 year−1 | |||||
| T1 | Marandu | 849 | 334 | 174.9 a | 1.39 | 619 | 206 | 826 |
| T2 | Basirisk | 1147 | 557 | 174.5 a | 1.44 | 7661 | 289 | 7951 |
| T3 | Kennedy | 488 | 326 | 207.0 b | 1.43 | 4343 | 144 | 4486 |
| T4 | Mulato | 648 | 309 | 200.5 b | 1.41 | 2366 | 183 | 2550 |
| T5 | Mulato II | 855 | 341 | 202.8 b | 1.43 | 3121 | 247 | 3369 |
| T6 | Tupy | 699 | 239 | 208.6 b | 1.31 | 11,804 | 192 | 11,996 |
| T7 | Tully | 1109 | 698 | 183.2 a | 1.32 | 7082 | 269 | 7351 |
| Explanatory Variables | 1.5 year (0–18 Months) | 2017 March (0–6 Months) | 2017 October (6–12 Months) | 2018 March (12–18 Months) | ||||
|---|---|---|---|---|---|---|---|---|
| Partial † | Standard †† | Partial | Standard | Partial | Standard | Partial | Standard | |
| b0 | 0.042 * | 0.126 *** | 0.086 ** | −0.036 n.s. | ||||
| b1: (Total BNI in root tissue) | 0.001 *** | 0.261 | 0.003 *** | 0.402 | 0.002 *** | 0.527 | 0.002 ** | 0.362 |
| b2: (Total BNI in root exudate) | 0.000 n.s. | 0.109 | 0.000 n.s. | −0.139 | 0.000 * | 0.270 | 0.000 n.s. | 0.163 |
| b3: (Available N) | −0.003 ** | −0.205 | −0.007 ** | −0.389 | −0.007 *** | −0.618 | 0.001 n.s. | 0.050 |
| R2 | 0.091 *** | 0.245 *** | 0.324 *** | 0.193** | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakamura, S.; Saliou, P.S.; Takahashi, M.; Ando, Y.; Subbarao, G.V. The Contribution of Root Turnover on Biological Nitrification Inhibition and Its Impact on the Ammonia-Oxidizing Archaea under Brachiaria Cultivations. Agronomy 2020, 10, 1003. https://doi.org/10.3390/agronomy10071003
Nakamura S, Saliou PS, Takahashi M, Ando Y, Subbarao GV. The Contribution of Root Turnover on Biological Nitrification Inhibition and Its Impact on the Ammonia-Oxidizing Archaea under Brachiaria Cultivations. Agronomy. 2020; 10(7):1003. https://doi.org/10.3390/agronomy10071003
Chicago/Turabian StyleNakamura, Satoshi, Papa Sarr Saliou, Minako Takahashi, Yasuo Ando, and Guntur Venkata Subbarao. 2020. "The Contribution of Root Turnover on Biological Nitrification Inhibition and Its Impact on the Ammonia-Oxidizing Archaea under Brachiaria Cultivations" Agronomy 10, no. 7: 1003. https://doi.org/10.3390/agronomy10071003
APA StyleNakamura, S., Saliou, P. S., Takahashi, M., Ando, Y., & Subbarao, G. V. (2020). The Contribution of Root Turnover on Biological Nitrification Inhibition and Its Impact on the Ammonia-Oxidizing Archaea under Brachiaria Cultivations. Agronomy, 10(7), 1003. https://doi.org/10.3390/agronomy10071003





