Arbuscular Mycorrhizas Traits and Yield of Winter Wheat Profiled by Mineral Fertilization
Abstract
1. Introduction
2. Materials and Methods
3. Results
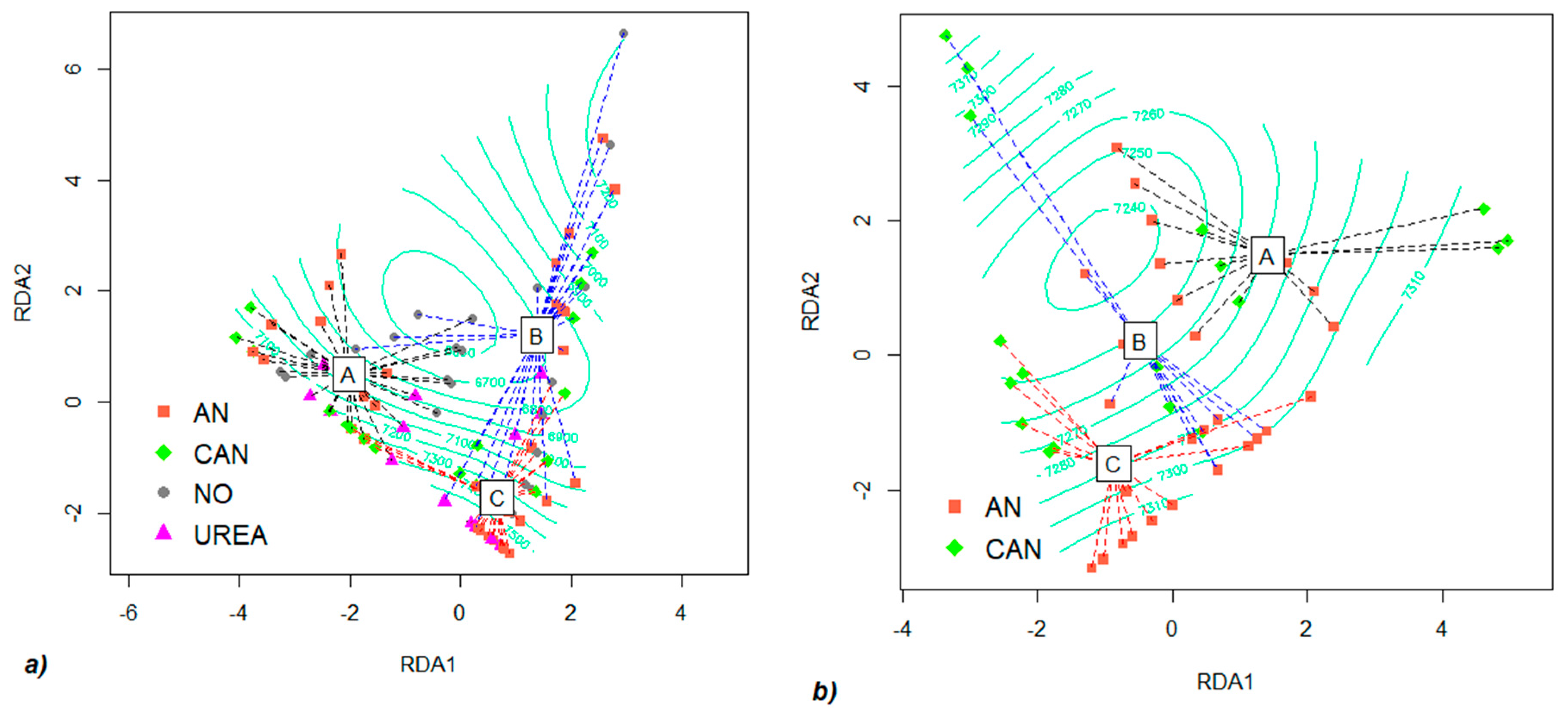
3.1. Global Impact of Fertilization on Arbuscular Mycorrhiza Colonization and Wheat Yield
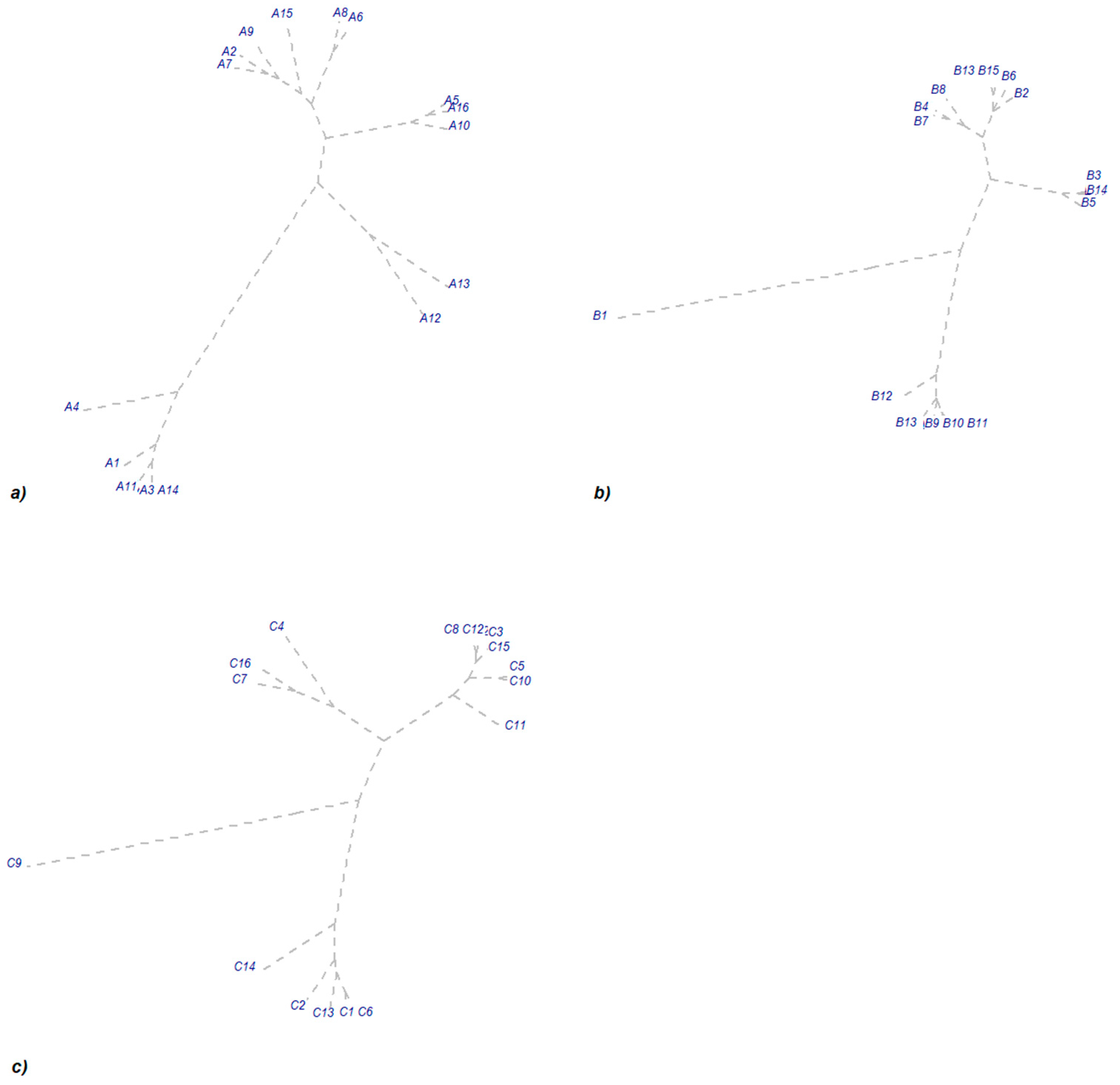
3.2. Inter-Annual Dynamics of Arbuscular Mycorrhiza Colonization
3.3. Impact of Year and Phased Fertilizer on Colonization Potential
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Academic Press: Cambridge, MA, USA, 2010. [Google Scholar]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ahmed, N.; Ashraf, M.; Zhang, L. Role of arbuscular mycorrhizal fungi in plant growth regulation: Implications in abiotic stress tolerance. Front. Plant Sci. 2019, 10, 1068. [Google Scholar] [CrossRef]
- Bitterlich, M.; Rouphael, Y.; Graefe, J.; Franken, P. Arbuscular mycorrhizas: A promising component of plant production systems provided favorable conditions for their growth. Front. Plant Sci. 2018, 9, 1329. [Google Scholar] [CrossRef]
- Dörmann, P.; Kim, H.; Ott, T.; Schulze-Lefert, P.; Trujillo, M.; Wewer, V.; Hückelhoven, R. Cell-autonomous defense, re-organization and trafficking of membranes in plant-microbe interactions. New Phytol. 2014, 204, 815–822. [Google Scholar] [CrossRef]
- Ehrmann, J.; Ritz, K. Plant: Soil interactions in temperate multi-cropping production systems. Plant Soil 2014, 376, 1–29. [Google Scholar] [CrossRef]
- Goh, C.H.; Vallejos, D.F.V.; Nicotra, A.B.; Mathesius, U. The impact of beneficial plant-associated microbes on plant phenotypic plasticity. J. Chem. Ecol. 2013, 39, 826–839. [Google Scholar] [CrossRef]
- Hodge, A.; Storer, K. Arbuscular mycorrhiza and nitrogen: Implications for individual plants through to ecosystems. Plant Soil 2015, 386, 1–19. [Google Scholar] [CrossRef]
- Javaid, A. Arbuscular mycorrhizal mediated nutrition in plants. J. Plant Nutr. 2009, 32, 1595–1618. [Google Scholar] [CrossRef]
- Smith, S.E.; Smith, F.A. Fresh perspectives on the roles of arbuscular mycorrhizal fungi in plant nutrition and growth. Mycologia 2012, 104, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Koltai, H.; Kapulnik, Y. Arbuscular Mycorrhizas: Physiology and Function; Springer Science Business Media: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Hu, J.; Cui, X.; Wang, J.; Lin, X. The non-simultaneous enhancement of phosphorus acquisition and mobilization respond to enhanced arbuscular mycorrhization on maize (Zea mays L.). Microorganisms 2019, 7, 651. [Google Scholar] [CrossRef] [PubMed]
- Talboys, P.J.; Healey, J.R.; Withers, P.J.A.; Jones, D.L. Phosphate depletion modulates auxin transport in Triticum aestivum leading to altered root branching. J. Exp. Bot. 2014, 65, 5023–5032. [Google Scholar] [CrossRef]
- Wang, W.; Shi, J.; Xie, Q.; Jiang, Y.; Yu, N.; Wang, E. Nutrient exchange and regulation in arbuscular mycorrhizal symbiosis. Mol. Plant 2017, 10, 1147–1158. [Google Scholar] [CrossRef] [PubMed]
- Keymer, A.; Gutjahr, C. Cross-kingdom lipid transfer in arbuscular mycorrhiza symbiosis and beyond. Curr. Opin. Plant Biol. 2018, 44, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Bever, J.D.; Dickie, I.A.; Facelli, E.; Facelli, J.M.; Klironomos, J.; Moora, M.; Rillig, M.C.; Stock, W.D.; Tibbett, M.; Zobel, M. Rooting theories of plant community ecology in microbial interactions. Trends Ecol. Evol. 2010, 25, 468–478. [Google Scholar] [CrossRef] [PubMed]
- Tkacz, A.; Poole, P. Role of root microbiota in plant productivity. J. Exp. Bot. 2015, 66, 2167–2175. [Google Scholar] [CrossRef]
- Venkateshwaran, M.; Volkening, J.D.; Sussman, M.R.; Ané, J.M. Symbiosis and the social network of higher plants. Curr. Opin. Plant Biol. 2013, 16, 118–127. [Google Scholar] [CrossRef]
- Kahiluoto, H.; Ketoja, E.; Vestberg, M. Contribution of arbuscular mycorrhiza to soil quality in contrasting cropping systems. Agric. Ecosyst. Environ. 2009, 134, 36–45. [Google Scholar] [CrossRef]
- Ramaekers, L.; Remans, R.; Rao, I.M.; Blair, M.W.; Vanderleyden, J. Strategies for improving phosphorus acquisition efficiency of crop plants. Field Crop. Res. 2010, 117, 169–176. [Google Scholar] [CrossRef]
- Del Mar-Alguacil, M.; Lozano, Z.; Campoy, M.J.; Roldán, A. Phosphorus fertilisation management modifies the biodiversity of AM fungi in a tropical savanna forage system. Soil Biol. Biochem. 2010, 42, 1114–1122. [Google Scholar] [CrossRef]
- Wu, F.; Dong, M.; Liu, Y.; Ma, X.; An, L.; Young, J.P.W.; Feng, H. Effects of long-term fertilization on AM fungal community structure and glomalin-related soil protein in the Loess Plateau of China. Plant Soil 2011, 342, 233–247. [Google Scholar] [CrossRef]
- Mao, L.; Liu, Y.; Shi, G.; Jiang, S.; Cheng, G.; Li, X.; An, L.; Feng, H. Wheat cultivars form distinctive communities of root-associated arbuscular mycorrhiza in a conventional agroecosystem. Plant Soil 2014, 374, 949–961. [Google Scholar] [CrossRef]
- Rai, A.; Rai, S.; Rakshit, A. Mycorrhiza-mediated phosphorus use efficiency in plants. Environ. Exp. Biol. 2013, 11, 107–117. [Google Scholar]
- Ganugi, P.; Masoni, A.; Pietramellara, G.; Benedettelli, S. A review of studies from the last twenty years on plant-arbuscular mycorrhizal fungi associations and their uses for wheat crops. Agronomy 2019, 9, 840. [Google Scholar] [CrossRef]
- Brito, I.; Goss, M.J.; De Carvalho, M. Effect of tillage and crop on arbuscular mycorrhiza colonization of winter wheat and triticale under Mediterranean conditions. Soil Manag. 2012, 28, 202–208. [Google Scholar] [CrossRef]
- Castillo, C.G.; Rubio, R.; Rouanet, J.L.; Borie, F. Early effects of tillage and crop rotation on arbuscular mycorrhizal fungal propagules in an Ultisol. Biol. Fert. Soils 2006, 43, 83–92. [Google Scholar] [CrossRef]
- Gosling, P.; Hodge, A.; Goodlass, G.; Bending, G.D. Arbuscular mycorrhizal fungi and organic farming. Agric. Ecosyst. Environ. 2006, 113, 17–35. [Google Scholar] [CrossRef]
- Graham, J.H.; Abbott, L.K. Wheat responses to aggressive and non-aggressive arbuscular mycorrhizal fungi. Plant Soil 2000, 220, 207–218. [Google Scholar] [CrossRef]
- Oehl, F.; Sieverding, E.; Ineichen, K.; Ris, E.A.; Boller, T.; Wiemken, A. Community structure of arbuscular mycorrhizal fungi at different soil depths in extensively and intensively managed agroecosystems. New Phytol. 2005, 165, 273–283. [Google Scholar] [CrossRef]
- Manufacturer of Fertilizers Mineral for Agriculture. Available online: https://www.azomures.com/ (accessed on 6 June 2020).
- Stoian, V.; Florian, V. Mycorrhiza-benefits, influence, diagnostic method. Bulletin of University of agricultural sciences and veterinary medicine Cluj-Napoca. Agriculture 2009, 66. [Google Scholar] [CrossRef]
- Arbuscular Mycorrhizal Fungi in Plant Production Systems: Detection, Taxonomy, Conservation and Ecophysiology. Available online: https://www2.dijon.inrae.fr/mychintec/Protocole/protoframe.html (accessed on 6 June 2020).
- Stoian, V.; Vidican, R.; Crișan, I.; Puia, C.; Șandor, M.; Stoian, V.A.; Păcurar, F.; Vaida, I. Sensitive approach and future perspectives in microscopic patterns of mycorrhizal roots. Sci. Rep. 2019, 15, 10233. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development Environment for R; RStudio, Inc.: Boston, MA, USA, 2019. [Google Scholar]
- Revelle, W. Psych: Procedures for Psychological, Psychometric, and Personality Research, R package version 1.9.12.; Northwestern University: Evanston, IL, USA, 2019. [Google Scholar]
- De Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research, R package version 1.3-2.24; R Foundation Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Paradis, E.; Schliep, K. Ape 5.0: An environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 2018, 35, 526–528. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package, R package version 2.5-6; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002; ISBN 0-387-95457-0. [Google Scholar]
- Bărdaș, M.; Ignea, M.; Deac, V. Research on assimilation and some elements of productivity concerning crops, corn and soybeans, treated with the foliar fertilizer “agro argentum forte” under the agricultural development research at station Turda. Bull. Univ. Agric. Sci. Vet. Med. Cluj Napoca. Agric. 2015, 72, 347–351. [Google Scholar] [CrossRef][Green Version]
- Bărdaș, M.; Chețan, F.; Imon, Ș.A.; Deac, V.; Popa, A.; Oltean, V. Influence of classical and conservative tillage systems on physiological parameters and yield on Soybean culture in the Transylvanian plain. Proenviron. Promediu 2019, 12, 208–215. [Google Scholar]
- Bărdaș, M.; Chețan, F.; Kadar, F.; Racz, I.; Imon, Ș.A.; Deac, V.; Popa, A.; Chețan, C. research regarding the influence of folliar fertilization on plant assimilation, grain yield and quality of wheat, in the Transylvanian field conditions. Bull. Univ. Agric. Sci. Vet. Med. Cluj Napoca. Agric. 2019, 76, 1–10. [Google Scholar]
- Șimon, A.; Chețan, F.; Chețan, C.; Bărdaș, M.; Deac, V. The Influence of the tillage system and fertilization on Soybean yield at ARDS Turda, 2015–2017. Bull. Univ. Agric. Sci. Vet. Med. Cluj Napoca. Agric. 2019, 76, 93–98. [Google Scholar]
- Al-Karaki, G.; McMichael, B.Z.A.K.J.; Zak, J. Field response of wheat to arbuscular mycorrhizal fungi and drought stress. Mycorrhiza 2004, 14, 263–269. [Google Scholar] [CrossRef]
- Mohammad, M.J.; Pan, W.L.; Kennedy, A.C. Seasonal mycorrhizal colonization of winter wheat and its effect on wheat growth under dryland field conditions. Mycorrhiza 1998, 8, 139–144. [Google Scholar]
- Soudzilovskaia, N.A.; Douma, J.C.; Akhmetzhanova, A.A.; van Bodegom, P.M.; Cornwell, W.K.; Moens, E.J.; Treseder, K.K.; Tibbett, M.; Wang, Y.; Cornelissen, J.H.C. Global patterns of plant root colonization intensity by mycorrhizal fungi explained by climate and soil chemistry. Glob. Ecol. Biogeogr. 2015, 24, 371–382. [Google Scholar] [CrossRef]
- Zhu, X.C.; Song, F.B.; Xu, H.W. Arbuscular mycorrhizae improves low temperature stress in maize via alterations in host water status and photosynthesis. Plant Soil 2010, 331, 129–137. [Google Scholar] [CrossRef]
- Van Der Heijden, M.G.A.; Martin, F.M.; Selosse, M.; Sanders, I.R. Mycorrhizal ecology and evolution: The past, the present, and the future. New Phytol. 2015, 205, 1406–1423. [Google Scholar] [CrossRef]
- Smith, S.E.; Jakobsen, I.; Grønlund, M.; Smith, F.A. Roles of arbuscular mycorrhizas in plant phosphorus nutrition: Interactions between pathways of phosphorus uptake in arbuscular mycorrhizal roots have important implications for understanding and manipulating plant phosphorus acquisition. Plant Physiol. 2011, 156, 1050–1057. [Google Scholar] [CrossRef]
- Chen, M.; Arato, M.; Borghi, L.; Nouri, E.; Reinhardt, D. Beneficial services of arbuscular mycorrhizal fungi-from ecology to application. Front. Plant Sci. 2018, 9, 1270. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.C.; Gehring, C.; Jansa, J. Mycorrhizal Mediation of Soil: Fertility, Structure, and Carbon Storage; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Bakhshandeh, S.; Corneo, P.E.; Mariotte, P.; Kertesz, M.A.; Dijkstra, F.A. Effect of crop rotation on mycorrhizal colonization and wheat yield under different fertilizer treatments. Agric. Ecosyst. Environ. 2017, 247, 130–136. [Google Scholar] [CrossRef]
- Verzeaux, J.; Hirel, B.; Dubois, F.; Lea, P.J.; Tétu, T. Agricultural practices to improve nitrogen use efficiency through the use of arbuscular mycorrhizae: Basic and agronomic aspects. Plant Sci. 2017, 264, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.P.; Reddy, U.G.; Adholeya, A. Response of arbuscular mycorrhizal fungi on wheat (Triticum aestivum L.) grown conventionally and on beds in a sandy loam soil. Indian J. Microbiol. 2011, 51, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Campos, P.; Borie, F.; Cornejo, P.; López-Ráez, J.A.; López-García, J.A.; Seguel, A. Phosphorus acquisition efficiency related to root traits: Is mycorrhizal symbiosis a key factor to wheat and barley cropping? Front. Plant Sci. 2018, 9, 752. [Google Scholar] [CrossRef]
- Grant, C.; Bittman, S.; Montreal, M.; Plenchette, C.; Morel, C. Soil and fertilizer phosphorus: Effects on plant P supply and mycorrhizal development. Can. J. Plant Sci. 2005, 85, 3–14. [Google Scholar] [CrossRef]
- Saia, S.; Rappa, V.; Ruisi, P.; Abenavoli, M.R.; Sunseri, F.; Giambalvo, D.; Frenda, A.S.; Martinelli, F. Soil inoculation with symbiotic microorganisms promotes plant growth and nutrient transporter genes expression in durum wheat. Front. Plant Sci. 2015, 6, 815. [Google Scholar] [CrossRef]
- Yuan, L.; Bao, D.J.; Jin, Y.; Yang, Y.H.; Huang, J.G. Influence of fertilizers on nitrogen mineralization and utilization in the rhizosphere of wheat. Plant Soil 2011, 343, 187–193. [Google Scholar] [CrossRef]
- Assainar, S.K.; Abbott, L.K.; Mickan, B.S.; Whiteley, A.S.; Siddique, K.H.M.; Solaiman, Z.M. Response of wheat to a multiple species microbial inoculant compared to fertilizer application. Front. Plant Sci. 2018, 9, 1601. [Google Scholar] [CrossRef]
- Kirk, A.; Entz, M.; Fox, S.; Tenuta, M. Mycorrhizal colonization, P uptake and yield of older and modern wheats under organic management. Can. J. Plant Sci. 2011, 91, 663–667. [Google Scholar] [CrossRef]
- Peterson, R.L.; Massicotte, H.B.; Melville, L.H. Mycorrhizas: Anatomy and Cell Biology; NRC Research Press: Ottawa, ON, Canada, 2004. [Google Scholar]
- Zhang, S.; Lehmann, A.; Zheng, W.; You, Z.; Rillig, M.C. Arbuscular mycorrhizal fungi increase grain yields: A meta-analysis. New Phytol. 2019, 222, 543–555. [Google Scholar] [CrossRef] [PubMed]

| Autumn Fertilizer | Phased Fertilizer | |||||||
|---|---|---|---|---|---|---|---|---|
| Treat. | Commercial Formula | Active Ingredient (kg ha−1) | Commercial Formula | Active Ingredient (kg ha−1) | Total N (kg ha−1) | Total P (kg ha−1) | ||
| Na | Pa | Nv, S1 | Nv, S2 | |||||
| V1 | N20P20 | 40 | 40 | 40 | 40 | |||
| V2 | N20P20 | 80 | 80 | 80 | 80 | |||
| V3 | N20P20 | 120 | 120 | 120 | 120 | |||
| V4 | N18P46 | 15 | 40 | AN | 25 | 40 | 40 | |
| V5 | N20P20 | 80 | 80 | AN | 67 | 147 | 80 | |
| V6 | N20P20 | 80 | 80 | CAN | 67 | 147 | 80 | |
| V7 | N20P20 | 80 | 80 | Urea | 67 | 147 | 80 | |
| V8 | N20P20 | 80 | 80 | AN | 33.5 | 33.5 | 147 | 80 |
| V9 | N20P20 | 80 | 80 | CAN | 33.5 | 33.5 | 147 | 80 |
| V10 | N20P20 | 80 | 80 | Urea | 33.5 | 33.5 | 147 | 80 |
| V11 | N18P46 | 31 | 80 | AN | 116 | 147 | 80 | |
| V12 | N18P46 | 31 | 80 | AN | 58 | 58 | 147 | 80 |
| V13 | N18P46 | 31 | 80 | CAN | 116 | 147 | 80 | |
| V14 | N18P46 | 31 | 80 | CAN | 58 | 58 | 147 | 80 |
| V15 | N20P20 | 120 | 120 | AN | 67 | 187 | 120 | |
| V16 | N20P20 | 120 | 120 | AN | 33.5 | 33.5 | 187 | 120 |
| Treat. | Freq (%) | Int (%) | Arb (%) | Cdeg (%) | Yield (kg ha−1) |
|---|---|---|---|---|---|
| V1 | 88.9 ± 1.1 a–e | 28.4 ± 4.5 a,b | 0.9 ± 0.5 b,c | 25.4 ± 4.2 a–c | 6133.6 ± 72.1 g |
| V2 | 88.2 ± 3. 9 b–e | 27.1 ± 7.6 a–c | 0.8 ± 0.3 b,c | 25.6 ± 7.8 a–c | 6828.7 ± 73.3 e,f |
| V3 | 80.7 ± 6.1 e,f | 16.9 ± 3.5 b,c | 0.1 ± 0.0 c | 14.8 ± 3.3 b,c | 7088.6 ± 83.3 d,e |
| V4 | 87.4 ± 3.6 c–e | 21.1 ± 2.2 b,c | 0.3 ± 0.1 c | 18.7 ± 2.4 b,c | 6376.7 ± 60.7 f,g |
| V5 | 87.4 ± 5.8 c –e | 24.1 ± 5.4 a–c | 0.5 ± 0.2 c | 23.0 ± 5.7 a–c | 7717.8 ± 74.4 a |
| V6 | 87.0 ± 3.9 d,e | 28.2 ± 9.3 a,b | 0.5 ± 0.2 c | 26.9 ± 9.5 a,b | 7176.9 ± 46.0 b–e |
| V7 | 94.5 ± 2.1 a–d | 28.5 ± 5.8 a,b | 0.5 ± 0.2 c | 27.5 ± 5.9 a,b | 7170.8 ± 45.7 b–e |
| V8 | 96.3 ± 1.4 a–d | 29.5 ± 8.3 a,b | 0.1 ± 0.0 c | 28.9 ± 8.3 a,b | 7575.0 ± 124.4 a–c |
| V9 | 98.2 ± 0.8 ab | 38.4 ± 3.6 a | 1.1 ± 0.4 a–c | 37.8 ± 3.7 a | 7458.7 ± 129.8 a–d |
| V10 | 98.5 ± 0.8 a | 26.8 ± 2.7 a–c | 0.7 ± 0.3 c | 26.3 ± 2.6 a,b | 7565.6 ± 73.6 a–c |
| V11 | 94.8 ± 2.2 a–d | 19.0 ± 2.2 b,c | 0.3 ± 0.1 c | 17.8 ± 2.0 b,c | 7465.9 ± 94.0 a–d |
| V12 | 97.4 ± 1.2 a–c | 25.9 ± 3.3 a–c | 1.9 ± 0.8 a,b | 25.1 ± 3.1 a–c | 7756.2 ± 70.0 a |
| V13 | 93.7 ± 1.6 a–d | 27.62 ± 7.46 a–c | 2.1 ± 1.0 a | 26.8 ± 7.5 a,b | 7147.0 ± 96.1 c–e |
| V14 | 76.3 ± 4.2 f | 12.53 ± 4.13 c | 0.1 ± 0.0 c | 10.4 ± 3.7 c | 7611.4 ± 66.0 a,b |
| V15 | 88.5 ± 3.0 a–e | 27.45 ± 6.65 a–c | 0.4 ± 0.2 c | 24.7 ± 6.1 a–c | 6874.3 ± 104.2 e |
| V16 | 90.7 ± 3.9 a–e | 24.95 ± 4.47 a–c | 0.8 ± 0.2 c | 23.5 ± 4.6 a–c | 6856.8 ± 164.6 e |
| Basic Type | N20P20 | N18P46 | ||
|---|---|---|---|---|
| RDA Axis | RDA 1 | RDA 2 | RDA 1 | RDA 2 |
| Variance explained (%) | 73.50 | 8.13 | 74.27 | 15.72 |
| Interaction | F | p-value | F | p-value |
| year | 130.86 | 0.005 | 54.77 | 0.005 |
| phase | 6.61 | 0.005 | 13.79 | 0.005 |
| split | 6.66 | 0.015 | 6.41 | 0.015 |
| year × phase | 3.61 | 0.005 | 39.13 | 0.005 |
| year × split | 6.22 | 0.005 | 1.81 | 0.205 |
| phase × split | 2.42 | 0.095 | 64.10 | 0.005 |
| year × phase × split | 5.08 | 0.010 | 20.48 | 0.005 |
| Mycorrhizas (%) | Inter | Na | Pa | Nv | ||||
| Freq (%) | 93.51 | 0.19 | −0.28 | 0.12 | ||||
| Int (%) | 31.55 | 0.27 | −0.37 | 0.09 | ||||
| Arb (%) | 0.68 | −0.005 | 0.003 | 0.003 | ||||
| Cdeg (%) | 29.76 | 0.29 | −0.39 | 0.11 | ||||
| Yield (kg ha−1) | Inter | Na | Pa | Nv | Freq | Int | Arb | Cdeg |
| Model 1 | 6439.88 | 6.80 | −4.72 | 10.26 | ||||
| Model 2 | 5858.45 | 6.81 | −4.53 | 9.83 | 8.20 | −5.76 | −4.85 | |
| Model 3 | 6500.10 | 7.11 | −5.25 | 10.48 | −22.96 | −1.50 | ||
| Model 4 | 6024.77 | 15.08 | −9.34 | 32.01 | ||||
| Model 5 | 7214.41 | 6.18 | −1.82 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vidican, R.; Păcurar, F.; Vâtcă, S.D.; Pleșa, A.; Stoian, V. Arbuscular Mycorrhizas Traits and Yield of Winter Wheat Profiled by Mineral Fertilization. Agronomy 2020, 10, 846. https://doi.org/10.3390/agronomy10060846
Vidican R, Păcurar F, Vâtcă SD, Pleșa A, Stoian V. Arbuscular Mycorrhizas Traits and Yield of Winter Wheat Profiled by Mineral Fertilization. Agronomy. 2020; 10(6):846. https://doi.org/10.3390/agronomy10060846
Chicago/Turabian StyleVidican, Roxana, Florin Păcurar, Sorin Daniel Vâtcă, Anca Pleșa, and Vlad Stoian. 2020. "Arbuscular Mycorrhizas Traits and Yield of Winter Wheat Profiled by Mineral Fertilization" Agronomy 10, no. 6: 846. https://doi.org/10.3390/agronomy10060846
APA StyleVidican, R., Păcurar, F., Vâtcă, S. D., Pleșa, A., & Stoian, V. (2020). Arbuscular Mycorrhizas Traits and Yield of Winter Wheat Profiled by Mineral Fertilization. Agronomy, 10(6), 846. https://doi.org/10.3390/agronomy10060846









