Impact of Ionic Liquids on Induction of Wheat Microspore Embryogenesis and Plant Regeneration
Abstract
1. Introduction
2. Results
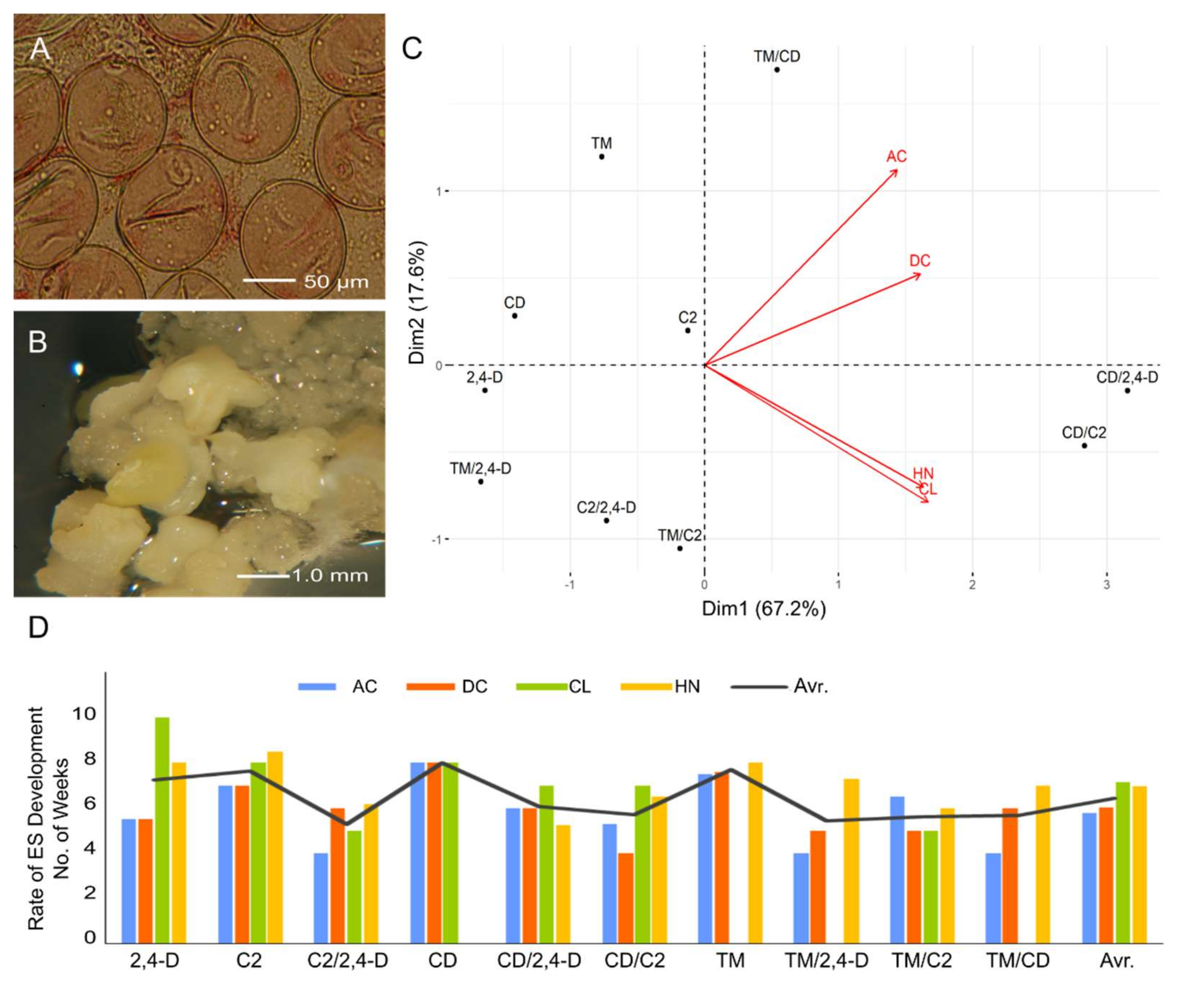
2.1. Influence of ILs and Genotypes on Microspore Embryogenesis Induction
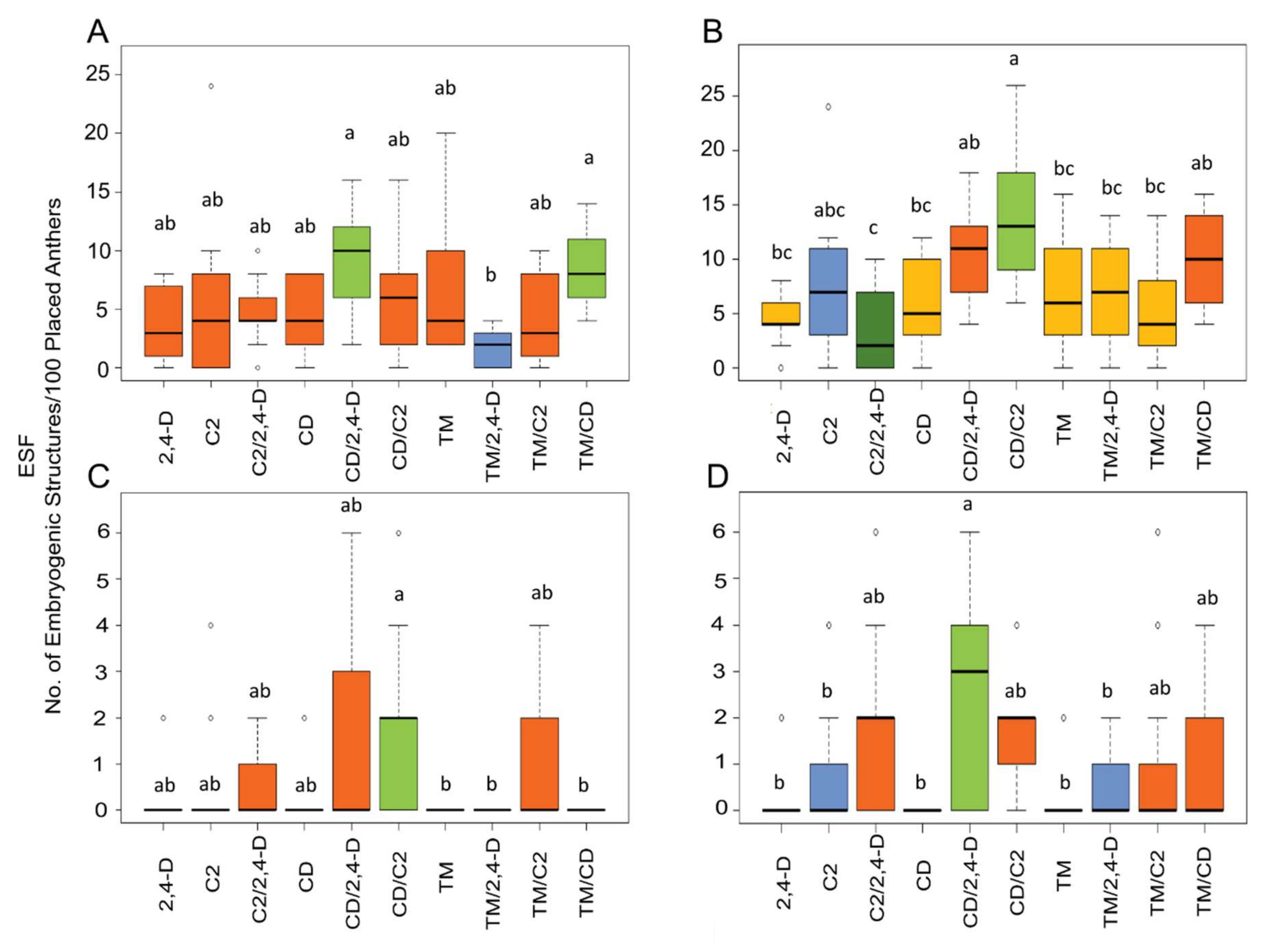
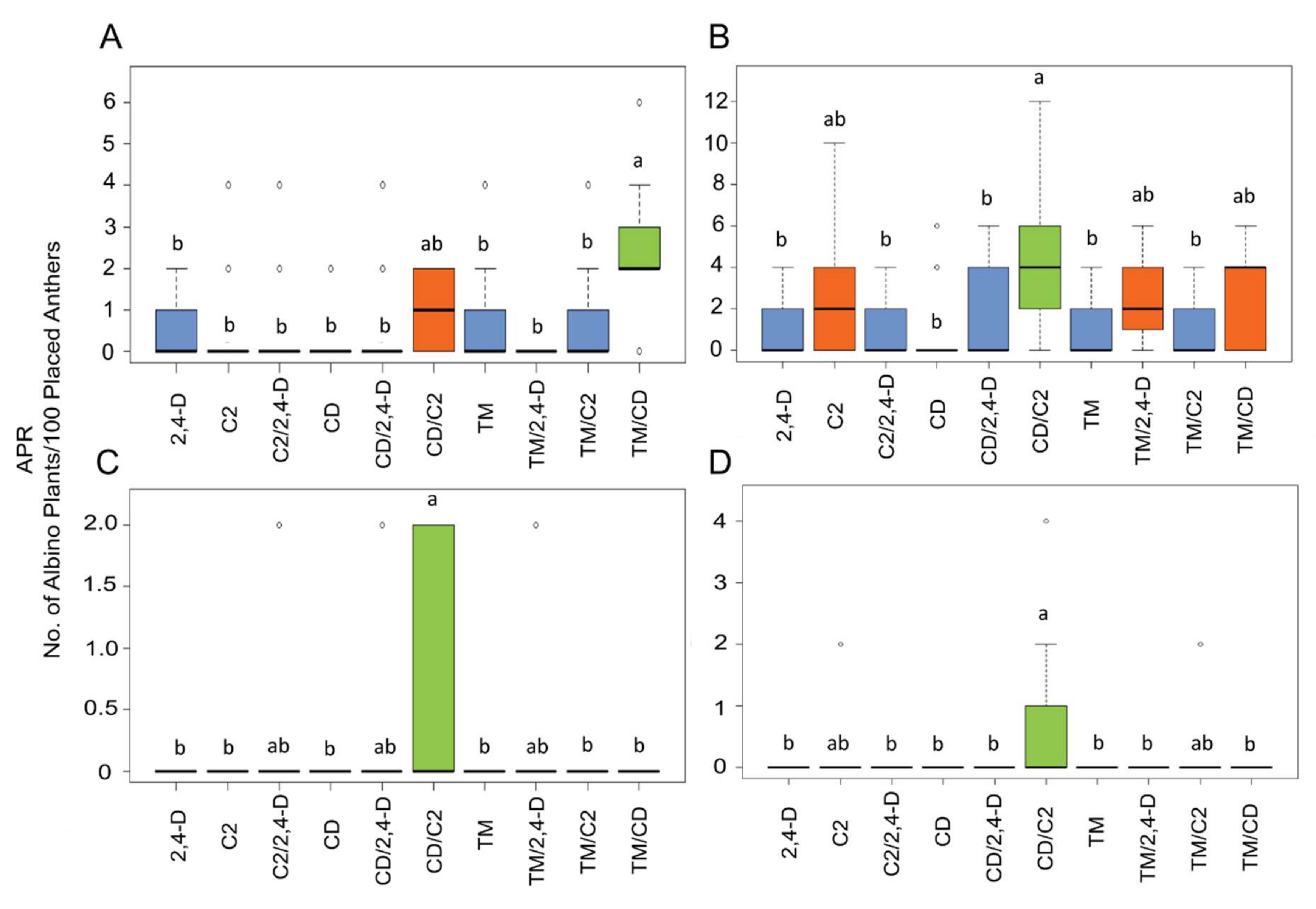
2.2. Influence of ILs and Genotypes on Plant Regeneration
3. Discussion
4. Material and Methods
4.1. Plant Material and Growing Conditions
4.2. Donor Tillers Pre-Treatment and Sterylization
4.3. Induction Medium Information
4.4. Course of Anther Culture
4.5. Data Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chauhan, H.; Khurana, P. Use of doubled haploid technology for development of stable drought tolerant bread wheat (Triticum aestivum L.) transgenics. Plant Biotechnol. J. 2010, 9, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Ravi, M.; Chan, S.W.L. Haploid plants produced by centromere-mediated genome elimination. Nature 2010, 464, 615–618. [Google Scholar] [CrossRef] [PubMed]
- Grauda, D.; Miķelsone, A.; Ļisina, N.; Žagata, K.; Ornicāns, R.; Fokina, O.; Lapiņa, L.; Rashal, I. Anther Culture Effectiveness in Producing Doubled Haploids of Cereals/ Putekðòu Kultûras Efektivitâte Graudaugu Dubultoto Haploîdu Izveidoðanâ. Proc. Latv. Acad. Sci. Sect. B Nat. Exact Appl. Sci. 2014, 68, 142–147. [Google Scholar] [CrossRef]
- Hennawy, M.A.; Abdalla, A.F.; Shafey, S.A.; Ashkar, I.M. Production of doubled haploid wheat lines (Triticum aestivum L.) using anther culture technique. Ann. Agric. Sci. 2011, 56, 63–72. [Google Scholar] [CrossRef]
- Datta, S.K. Androgenic Haploids: Factors controlling development and its application in crop improvement. Curr. Sci. 2005, 89, 1870–1878. [Google Scholar] [CrossRef]
- Sánchez, M.A.; Coronado, Y.M.; Morillo-Coronado, A.C. Androgenic studies in the production of haploids and doubled haploids in Capsicum spp. Rev. Fac. Nac. Agron. Medellín 2020, 73, 9047–9056. [Google Scholar] [CrossRef]
- Ponitka, A.; Ślusarkiewicz-Jarzina, A. Regeneration of oat androgenic plants in relation to induction media and culture conditions of embryo-like structures. Acta Soc. Bot. Pol. 2011, 78, 209–213. [Google Scholar] [CrossRef]
- Ikeuchi, M.; Favero, D.S.; Sakamoto, Y.; Iwase, A.; Coleman, D.; Rymen, B.; Sugimoto, K. Molecular Mechanisms of Plant Regeneration. Annu. Rev. Plant Boil. 2019, 70, 377–406. [Google Scholar] [CrossRef]
- Gorbunova, V.Y.; Kruglova, N.N.; Abramov, S.N. The Induction of Androgenesis in vitro in Spring Soft Wheat. Balance of Exogenous and Endogenous Phytohormones. Boil. Bull. 2001, 28, 25–30. [Google Scholar] [CrossRef]
- Ghandi, K. A Review of Ionic Liquids, Their Limits and Applications. Green Sustain. Chem. 2014, 4, 44–53. [Google Scholar] [CrossRef]
- Jastorff, B.; Mölter, K.; Behrend, P.; Bottin-Weber, U.; Filser, J.; Heimers, A.; Ondruschka, B.; Ranke, J.; Schaefer, M.; Schröder, H.; et al. Progress in evaluation of risk potential of ionic liquids-basis for an eco-design of sustainable products. Green Chem. R. Soc. Chem. 2005, 7, 362–372. [Google Scholar] [CrossRef]
- Biczak, R.; Pawłowska, B.; Telesiński, A.; Kapusniak, J. Role of cation structure in the phytotoxicity of ionic liquids: Growth inhibition and oxidative stress in spring barley and common radish. Environ. Sci. Pollut. Res. 2017, 24, 18444–18457. [Google Scholar] [CrossRef] [PubMed]
- Egorova, K.S.; Gordeev, E.G.; Ananikov, V.P. Biological Activity of Ionic Liquids and Their Application in Pharmaceutics and Medicine. Chem. Rev. 2017, 117, 7132–7189. [Google Scholar] [CrossRef] [PubMed]
- Zajac, A.; Kukawka, R.; Pawlowska-Zygarowicz, A.; Stolarska, O.; Smiglak, M. Ionic liquids as bioactive chemical tools for use in agriculture and the preservation of agricultural products. Green Chem. 2018, 20, 4764–4789. [Google Scholar] [CrossRef]
- Pernak, J.; Niemczak, M.; Materna, K.; Marcinkowska, K.; Praczyk, T. Ionic liquids as herbicides and plant growth regulators. Tetrahedron 2013, 69, 4665–4669. [Google Scholar] [CrossRef]
- Lewandowski, P.; Kukawka, R.; Pospieszny, H.; Smiglak, M. Bifunctional quaternary ammonium salts based on benzo[1,2,3]thiadiazole-7-carboxylate as plant systemic acquired resistance inducers. New J. Chem. 2014, 38, 1372. [Google Scholar] [CrossRef]
- Smiglak, M.; Pringle, J.M.; Lu, X.; Han, L.; Zhang, S.; Gao, H.; Macfarlane, D.R.; Rogers, R.D. Ionic liquids for energy, materials, and medicine. Chem. Commun. 2014, 50, 9228–9250. [Google Scholar] [CrossRef]
- Smiglak, M.; Lewandowski, P.; Kukawka, R.; Budziszewska, M.; Krawczyk, K.; Obrępalska-Stęplowska, A.; Pospieszny, H. Dual Functional Salts of Benzo[1.2.3]thiadiazole-7-carboxylates as a Highly Efficient Weapon Against Viral Plant Diseases. ACS Sustain. Chem. Eng. 2017, 5, 4197–4204. [Google Scholar] [CrossRef]
- Pernak, J.; Markiewicz, B.; Zgoła-Grześkowiak, A.; Chrzanowski, Ł.; Gwiazdowski, R.; Marcinkowska, K.; Praczyk, T. Ionic liquids with dual pesticidal function. RSC Adv. 2014, 4, 39751–39754. [Google Scholar] [CrossRef]
- Biczak, R.; Pawłowska, B.; Feder-Kubis, J. The Effect of Ionic Liquids With (–)-Menthol Derivative Containing a Chloride Anion to Weed. Ecol. Chem. Eng. S 2017, 24, 637–651. [Google Scholar] [CrossRef][Green Version]
- Soriano, M.; Li, H.; Boutilier, K. Microspore embryogenesis: Establishment of embryo identity and pattern in culture. Plant Reprod. 2013, 26, 181–196. [Google Scholar] [CrossRef] [PubMed]
- Shariatpanahi, M.E.; Bal, U.; Heberle-Bors, E.; Touraev, A. Stresses applied for the re-programming of plant microspores towards in vitro embryogenesis. Physiol. Plant. 2006, 127, 519–534. [Google Scholar] [CrossRef]
- Weigt, D.; Nawracała, J.; Kurasiak-Popowska, D.; Nijak, K. RESEARCH PAPER Examination of ability to androgenesis of spring wheat genotypes resistant to Fusarium. Biotechnologia 2012, 2, 116–122. [Google Scholar] [CrossRef]
- Redha, A.; Talaat, A. Improvement of green plant regeneration by manipulation of anther culture induction medium of hexaploid wheat. Plant Cell Tissue Organ Cult. 2007, 92, 141–146. [Google Scholar] [CrossRef]
- Chaudhary, H.; Dhaliwal, I.; Singh, S.; Sethi, G. Genetics of androgenesis in winter and spring wheat genotypes. Euphytica 2003, 132, 311–319. [Google Scholar] [CrossRef]
- Diyachuk, T.I.; Khomyakova, O.V.; Akinina, V.N.; Kibkalo, I.A.; Pominov, A.V. Microspore embryogenesis in vitro: The role of stresses. Vavilov J. Genet. Breed. 2019, 23, 86–94. [Google Scholar] [CrossRef]
- Krzewska, M.; Gołębiowska-Pikania, G.; Dubas, E.; Gawin, M.; Żur, I. Identification of proteins related to microspore embryogenesis responsiveness in anther cultures of winter triticale (×Triticosecale Wittm.). Euphytica 2017, 213, 192. [Google Scholar] [CrossRef]
- Szechyńska-Hebda, M.; Skrzypek, E.; Dąbrowska, G.; Biesaga-Kościelniak, J.; Filek, M.; Wędzony, M. The role of oxidative stress induced by growth regulators in the regeneration process of wheat. Acta Physiol. Plant. 2007, 29, 327–337. [Google Scholar] [CrossRef]
- Pérez-Pérez, Y.; El-Tantawy, A.-A.; Solís, M.T.; Risueño, M.C.; Testillano, P.S. Stress-Induced Microspore Embryogenesis Requires Endogenous Auxin Synthesis and Polar Transport in Barley. Front. Plant Sci. 2019, 10, 1200. [Google Scholar] [CrossRef]
- Prem, D.; Solís, M.-T.; Bárány, I.; Rodríguez-Sanz, H.; Risueño, M.C.; Testillano, P.S. A new microspore embryogenesis system under low temperature which mimics zygotic embryogenesis initials, expresses auxin and efficiently regenerates doubled-haploid plants in Brassica napus. BMC Plant Boil. 2012, 12, 127. [Google Scholar] [CrossRef]
- Balzan, S.; Johal, G.S.; Carraro, N. The role of auxin transporters in monocots development. Front. Plant Sci. 2014, 5, 393. [Google Scholar] [CrossRef] [PubMed]
- Rubtsova, M.; Gnad, H.; Melzer, M.; Weyen, J.; Gils, M. The auxins centrophenoxine and 2,4-D differ in their effects on non-directly induced chromosome doubling in anther culture of wheat (T. aestivum L.). Plant Biotechnol. Rep. 2012, 7, 247–255. [Google Scholar] [CrossRef]
- Malik, S.i.; Rashid, H.; Yasmin, T.; Minhas, N.M. Effect of 2,4-dichlorophenoxyacetic acid on callus induction from mature wheat (Triticum aestivum L.) seeds. Int. J. Agric. Biol. 2003, 6, 156. [Google Scholar]
- Żur, I.; Dubas, E.; Krzewska, M.; Janowiak, F. Current insights into hormonal regulation of microspore embryogenesis. Front. Plant Sci. 2015, 6, 424. [Google Scholar] [CrossRef] [PubMed]
- Weigt, D.; Kiel, A.; Siatkowski, I.; Zyprych-Walczak, J.; Tomkowiak, A.; Kwiatek, M.T. Comparison of the Androgenic Response of Spring and Winter Wheat (Triticum aestivum L.). Plants 2019, 9, 49. [Google Scholar] [CrossRef]
- Weigt, D.; Kiel, A.; Nawracała, J.; Pluta, M.; Łacka, A. Solid-stemmed spring wheat cultivars give better androgenic response than hollow-stemmed cultivars in anther culture. Vitr. Cell. Dev. Boil. Anim. 2016, 52, 619–625. [Google Scholar] [CrossRef][Green Version]
- Li, S.-B.; Xie, Z.-Z.; Hu, C.-G.; Hu, Z.C.-G. A Review of Auxin Response Factors (ARFs) in Plants. Front. Plant Sci. 2016, 7, 137. [Google Scholar] [CrossRef]
- Liscum, E.; Reed, J. Genetics of Aux/IAA and ARF action in plant growth and development. Plant Mol. Boil. 2002, 49, 387–400. [Google Scholar] [CrossRef]
- Guilfoyle, T.J. The PB1 Domain in Auxin Response Factor and Aux/IAA Proteins: A Versatile Protein Interaction Module in the Auxin Response [OPEN]. Plant Cell 2015, 27, 33–43. [Google Scholar] [CrossRef]
- Weigt, D.; Kiel, A.; Nawracała, J.; Tomkowiak, A.; Kurasiak-Popowska, D.; Siatkowski, I.; Ługowska, B. Obtaining doubled haploid lines of the Lr19 gene using anther cultures of winter wheat genotypes. Biotechnologia 2016, 4, 285–293. [Google Scholar] [CrossRef]
- Weigt, D.; Niemann, J.; Siatkowski, I.; Zyprych-Walczak, J.; Olejnik, P.; Kurasiak-Popowska, D. Effect of Zearalenone and Hormone Regulators on Microspore Embryogenesis in Anther Culture of Wheat. Plants 2019, 8, 487. [Google Scholar] [CrossRef] [PubMed]
- Coelho, M.B.; Scagliusi, S.M.M.; Lima, M.I.P.M.; Consoli, L.; Grando, M.F. Androgenic response of wheat genotypes resistant to fusariosis. Pesqui. Agropecuária Bras. 2018, 53, 575–582. [Google Scholar] [CrossRef]
- Kunz, C.; Islam, S.; Berberat, J.; Peter, S.; Büter, B.; Stamp, P.; Schmid, J. Assessment and Improvement of Wheat Microspore derived Embryo Induction and Regeneration. J. Plant Physiol. 2000, 156, 190–196. [Google Scholar] [CrossRef]
- Poersch-Bortolon, L.B.; Scagliusi, S.M.M.; Yamazaki-Lau, E.; Bodanese-Zanettini, M.H.; Trigo, B.E. Androgenic response of Brazilian wheat genotypes to different pretreatments of spikes and to a gelling agent. Pesqui. Agropecuária Bras. 2016, 51, 1839–1847. [Google Scholar] [CrossRef]
- Zamani, I.; Roupakias, D.G.; Gouli-Vavdinoudi, E.; Kovács, G.; Barnabás, B. Regeneration of fertile doubled haploid plants from colchicine-supplemented media in wheat anther culture. Plant Breed. 2000, 119, 461–465. [Google Scholar] [CrossRef]
- Makowska, K.; Oleszczuk, S. Albinism in barley androgenesis. Plant Cell Rep. 2013, 33, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Sibikeeva, Y.E.; Sibikeev, S.N. The influence of combinations of alien translocations on in vitro androgenesis in near-isogenic lines of spring bread wheat. Russ. J. Genet. 2014, 50, 728–735. [Google Scholar] [CrossRef]
- Wang, P.; Chen, Y. Preliminary study on production of height of pollen H2 generation in winter wheat grown in the field. Acta Agron. Sin. 1983, 9, 283–284. [Google Scholar]
- Pernak, J.; Syguda, A.; Materna, K.; Janus, E.; Kardasz, P.; Praczyk, T. 2,4-D based herbicidal ionic liquids. Tetrahedron 2012, 68, 4267–4273. [Google Scholar] [CrossRef]
- Cojocaru, O.A.; Shamshina, J.L.; Gurau, G.; Syguda, A.; Praczyk, T.; Pernak, J.; Rogers, R.D. Ionic liquid forms of the herbicide dicamba with increased efficacy and reduced volatility. Green Chem. 2013, 15, 2110. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 24 September 2019).


| No. of ES | No. of GP | No. of AP | |||||
|---|---|---|---|---|---|---|---|
| Source | Df | MSq | p-Value | MSq | p-Value | MSq | p-Value |
| Year | 1 | 0.3 | 0.921 | 3.20 | 0.43 | 0.622 | 0.432 |
| Treatment | 9 | 156.7 | <0.001 *** | 25.99 | <0.001 *** | 15.3 | <0.001 *** |
| Genotype | 3 | 2169.2 | <0.001 *** | 187.78 | <0.001 *** | 121.42 | <0.001 *** |
| Residuals | 66 | 31.8 | 5.08 | 3.94 | |||
| Treatment | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2,4-D | C2 | C2/2,4-D | CD | CD/2,4-D | CD/C2 | TM | TM/2,4-D | TM/C2 | TM/CD | |
| Genotype | ESF | |||||||||
| AC b | 3.8 | 5.5 | 4.5 | 4.5 | 9.2 | 6.3 | 6.8 | 1.7 | 4.3 | 8.5 |
| DC a | 4.7 | 8.0 | 3.3 | 5.8 | 10.7 | 14.2 | 7.0 | 6.8 | 5.2 | 10.2 |
| CL c | 0.2 | 0.5 | 0.2 | 0.2 | 1.5 | 1.7 | 0.0 | 0.0 | 1.0 | 0.0 |
| HN c | 0.2 | 0.7 | 1.7 | 0 | 2.5 | 1.8 | 0.2 | 0.5 | 1.0 | 0.8 |
| Avr. | 2.2 | 3.7 | 2.4 | 2.6 | 6.0 | 6.0 | 3.5 | 2.3 | 2.9 | 4.9 |
| Treatment | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2,4-D | C2 | C2/2,4-D | CD | CD/2,4-D | CD/C2 | TM | TM/2,4-D | TM/C2 | TM/CD | |
| Genotype | GPR | |||||||||
| AC a | 1.2 | 1.7 | 3.0 | 1.8 | 6.0 | 2.7 | 2.5 | 0.8 | 0.8 | 2.2 |
| DC b | 0.8 | 1.8 | 0.5 | 0.8 | 2.2 | 2.5 | 2.5 | 0.5 | 1.0 | 1.0 |
| CL c | 0.0 | 0.2 | 0.0 | 0.0 | 0.5 | 0.0 | 0.0 | 0.0 | 0.7 | 0.0 |
| HN c | 0.0 | 0.2 | 0.0 | 0.0 | 1.2 | 0.2 | 0.0 | 0.0 | 0.2 | 0.3 |
| Avr. | 0.5 | 1.0 | 0.8 | 0.7 | 2.5 | 1.3 | 1.3 | 0.3 | 0.7 | 0.9 |
| APR | ||||||||||
| AC b | 0.5 | 0.5 | 0.5 | 0.8 | 0.5 | 1.0 | 0.8 | 0.0 | 0.7 | 2.5 |
| DC a | 1.0 | 2.7 | 0.8 | 0.2 | 1.7 | 4.7 | 0.8 | 2.5 | 1.0 | 2.7 |
| CL c | 0.0 | 0.2 | 0.0 | 0.0 | 0.0 | 0.7 | 0.0 | 0.0 | 0.0 | 0.0 |
| HN c | 0.0 | 0.0 | 0.3 | 0.0 | 0.3 | 0.7 | 0.0 | 0.2 | 0.2 | 0.0 |
| Avr. | 0.4 | 0.8 | 0.4 | 0.3 | 0.6 | 1.8 | 0.4 | 0.7 | 0.5 | 1.3 |
| GPF | ||||||||||
| Avr. | 0.6 | 0.5 | 0.7 | 0.7 | 0.8 | 0.4 | 0.8 | 0.3 | 0.6 | 0.5 |
| Content of Ionic Liquids in the Induction Medium (mL/L) | ||||
|---|---|---|---|---|
| Treatment | 2,4-D | C2 | CD | TM |
| 2,4-D (Control) | 2 | - | - | - |
| C2 | - | 2 | - | - |
| C2/2,4-D | 1 | 1 | - | - |
| CD | - | - | 2 | - |
| CD/2,4-D | 1 | - | 1 | - |
| CD/C2 | - | 1 | 1 | - |
| TM | - | - | - | 2 |
| TM/2,4-D | 1 | - | - | 1 |
| TM/C2 | - | 1 | - | 1 |
| TM/CD | - | - | 1 | 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weigt, D.; Siatkowski, I.; Magaj, M.; Tomkowiak, A.; Nawracała, J. Impact of Ionic Liquids on Induction of Wheat Microspore Embryogenesis and Plant Regeneration. Agronomy 2020, 10, 839. https://doi.org/10.3390/agronomy10060839
Weigt D, Siatkowski I, Magaj M, Tomkowiak A, Nawracała J. Impact of Ionic Liquids on Induction of Wheat Microspore Embryogenesis and Plant Regeneration. Agronomy. 2020; 10(6):839. https://doi.org/10.3390/agronomy10060839
Chicago/Turabian StyleWeigt, Dorota, Idzi Siatkowski, Magdalena Magaj, Agnieszka Tomkowiak, and Jerzy Nawracała. 2020. "Impact of Ionic Liquids on Induction of Wheat Microspore Embryogenesis and Plant Regeneration" Agronomy 10, no. 6: 839. https://doi.org/10.3390/agronomy10060839
APA StyleWeigt, D., Siatkowski, I., Magaj, M., Tomkowiak, A., & Nawracała, J. (2020). Impact of Ionic Liquids on Induction of Wheat Microspore Embryogenesis and Plant Regeneration. Agronomy, 10(6), 839. https://doi.org/10.3390/agronomy10060839






