What is the Difference between the Response of Grass Pea (Lathyrus sativus L.) to Salinity and Drought Stress?—A Physiological Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Growth Conditions and Stress Treatments
2.2. Evaluation of Seedling Response to Stress Factors
2.2.1. Determination of Germination, Seedling Emergence Rates and Biometric Parameters
2.2.2. Determination of Na+ and K+ Content
2.2.3. Determination of Malonyldialdehyde Content
2.2.4. Determination of Photosynthetic Pigment Content
2.2.5. Determination of Soluble and Insoluble Sugars
2.2.6. Determination of β-N-oxalyl-L-α,β-diamino propionic acid (ODAP) Content
2.2.7. Determination of Proline Content
2.2.8. Determination of Antioxidant Enzymes Activity
2.2.9. Determination of Phenolic Compounds Content
2.2.10. Determination of Antioxidant Capacity
2.3. Statistical Analysis
3. Results
3.1. Germination Rate and Seedling Performance under Salinity and Drought Stress
3.2. Na+ and K+ Content in NaCl Treated Seedlings
3.3. Content of MDA and Leaf Pigments under Drought and Salinity Stress
3.4. Osmolyte Accumulation under Salinity and Drought Stress
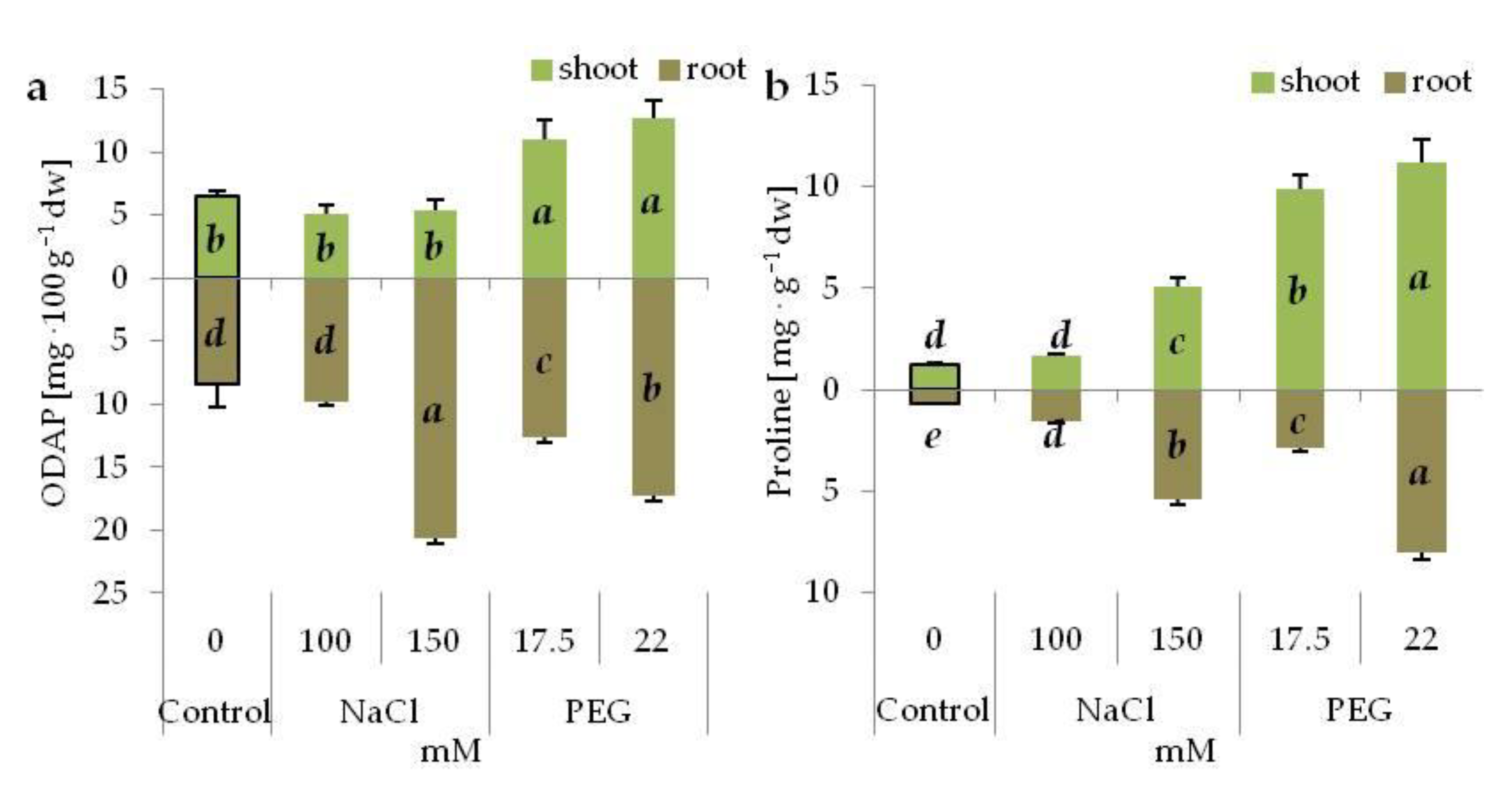
3.5. Neurotoxin Accumulation under Salinity and Drought Stress
3.6. Proline Content under Salinity and Drought Stress
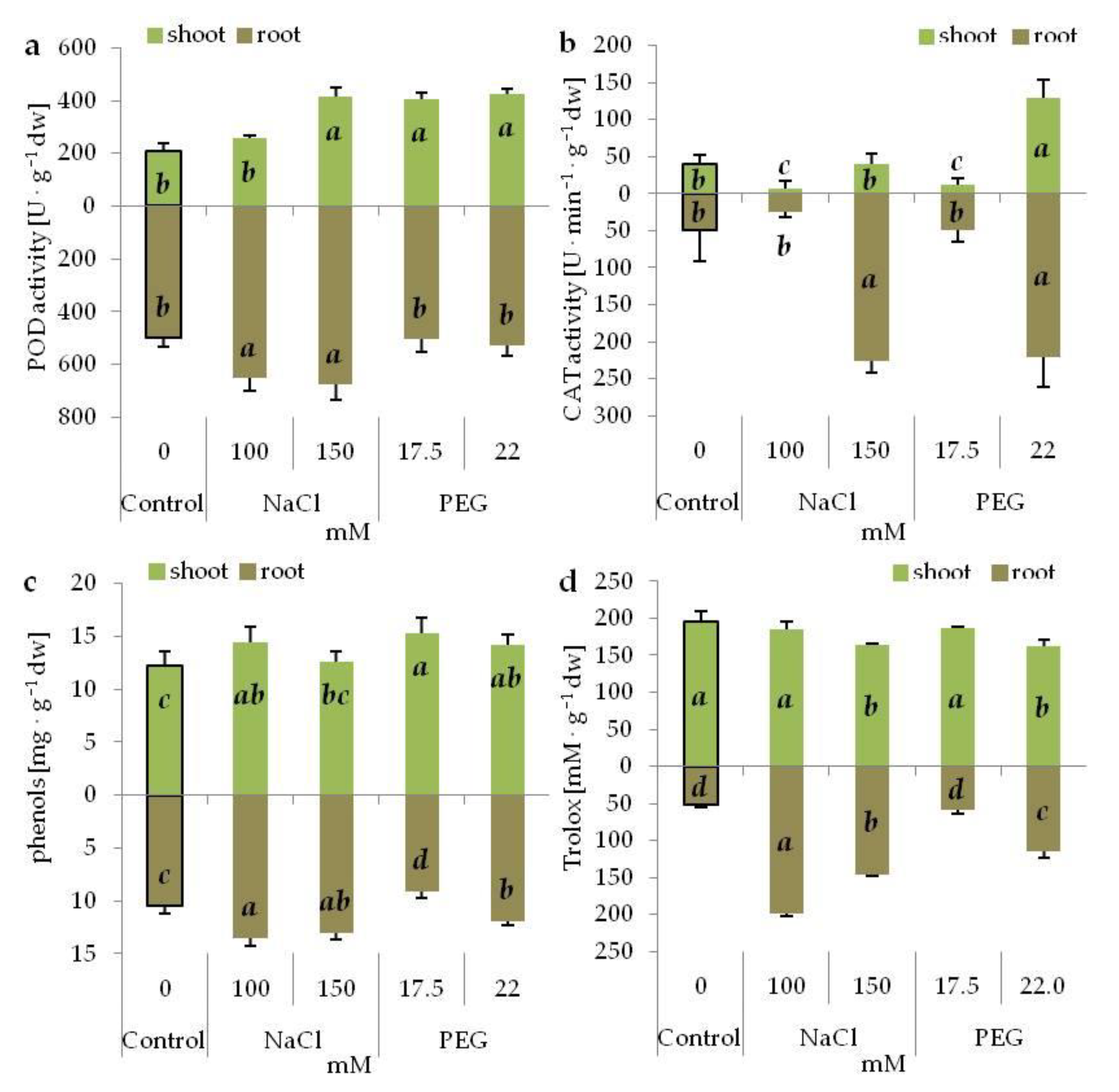
3.7. Antioxidant System under Salinity and Drought Stress
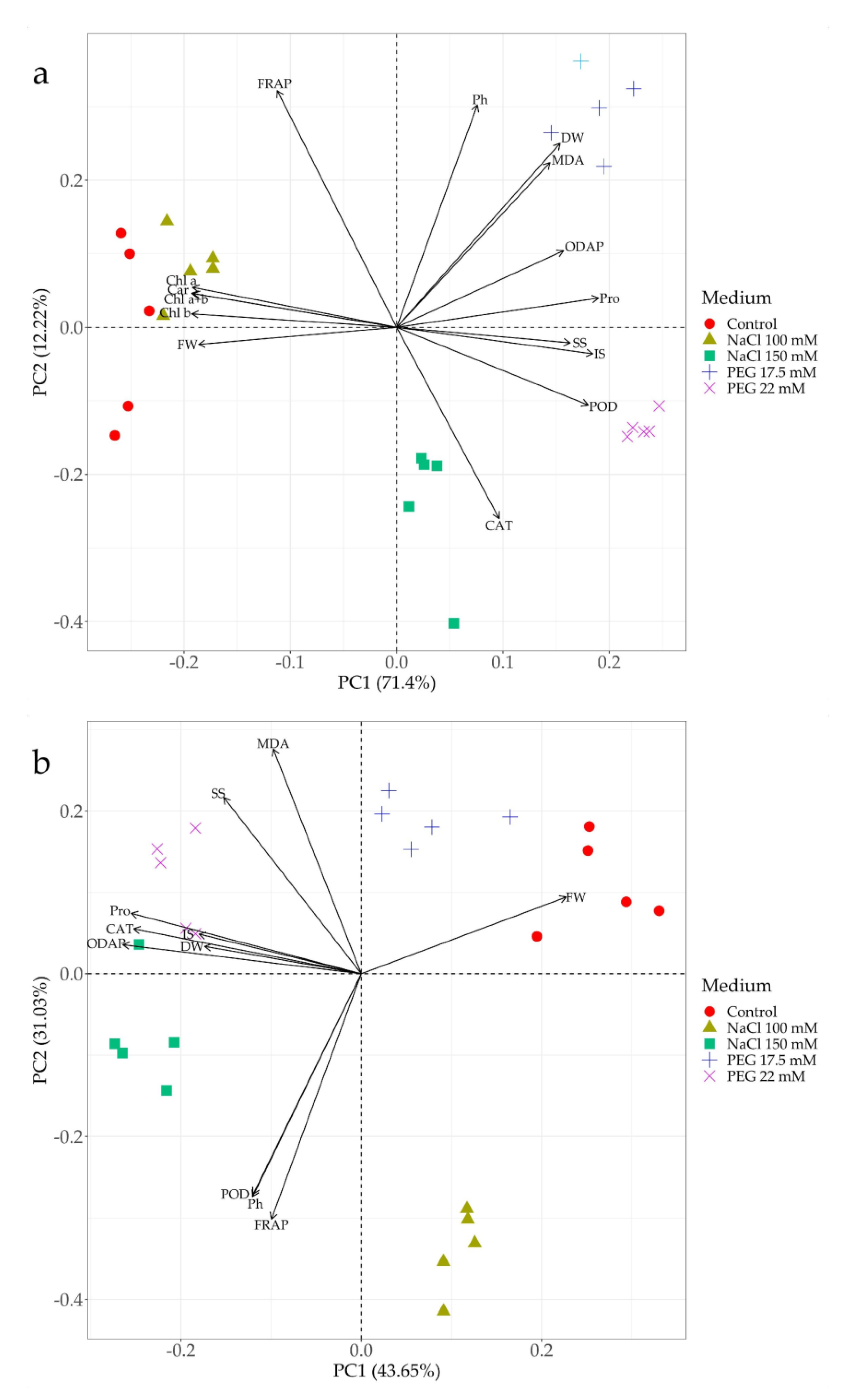
3.8. Principal Component Biplot Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Maphosa, Y.; Jideani, V.A. The role of legumes in human nutrition. In Functional Food-Improve Health through Adequate Food; Hueda, M.C., Ed.; In Tech d.o.o.: Rijeka, Croatia, 2017; Volume 1, pp. 103–121. [Google Scholar]
- Martín-Cabrejas, M.A. Legumes: An overview. In Legumes: Nutritional Quality, Processing and Potential Health Benefits; Martín-Cabrejas, M.A., Ed.; CPI Group (UK) Ltd.: Croydon, UK, 2019; pp. 1–18. [Google Scholar]
- Vaz Patto, M.C.; Rubiales, D. Lathyrus diversity: Available resources with relevance to crop improvement–L. sativus and L. cicera as case studies. Ann. Bot. 2014, 113, 895–908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kislev, M.E. Origins of the cultivation of Lathyrus sativus and L. cicera (Fabaceae). Econ. Bot. 1989, 43, 262–270. [Google Scholar] [CrossRef]
- Grela, E.R.; Kiczorowska, B.; Samolińska, W.; Matras, J.; Kiczorowski, P.; Rybiński, W.; Hanczakowska, E. Chemical composition of leguminous seeds: Part I—Content of basic nutrients, amino acids, phytochemical compounds, and antioxidant activity. Eur. Food Res. Technol. 2017, 243, 1385–1395. [Google Scholar] [CrossRef]
- Rybiński, W.; Karamać, M.; Sulewska, K.; Börner, A.; Amarowicz, R. Antioxidant potential of grass pea seeds from European Countries. Foods 2018, 7, 142. [Google Scholar] [CrossRef] [Green Version]
- Campbell, C.G.; Mehra, R.B.; Agrawal, S.K.; Chen, Y.Z.; Abd El Moneim, A.M.; Khawaja, H.I.T.; Yadov, C.R.; Tay, J.U.; Araya, W.A. Current status and future strategy in breeding grasspea (Lathyrus sativus). Euphytica 1994, 73, 167–175. [Google Scholar] [CrossRef]
- Singh, S.P.; Dhiraj, B.; Ajit, P.; Nirmal, V. An epidemiological study on incidence and determinants of Lathyrism. J. Community Health Manag. 2016, 3, 113–122. [Google Scholar] [CrossRef]
- Khandare, A.L.; Kumar, R.H.; Meshram, I.I.; Arlappa, N.; Laxmaiah, A.; Venkaiah, K.; Rao, P.A.; Validandi, V.; Toteja, G.S. Current scenario of consumption of Lathyrus sativus and lathyrism in three districts of Chhattisgarh State, India. Toxicon 2018, 150, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Vaz Patto, M.C.; Skiba, B.; Pang, E.C.K.; Ochatt, S.J.; Lambein, F.; Rubiales, D. Lathyrus Improvement for resistance against biotic and abiotic stresses: From classical breeding to marker assisted selection. Euphytica 2006, 147, 133–147. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Akram, N.A.; Ashraf, M.; Alyemeni, M.N.; Wijaya, L.; Ahmad, P. Plant responses to environmental stresses—From gene to biotechnology. AoB Plants 2017, 9, plx025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saxena, R.; Kumar, M.; Tomar, R.S. Plant responses and resilience towards drought and salinity stress. Plant Arch. 2019, 19, 50–58. [Google Scholar]
- Garg, R.; Shankar, R.; Thakkar, B.; Kudapa, H.; Krishnamurthy, L.; Mantri, N.; Varshney, R.K.; Bhatia, S.; Jain, M. Transcriptome analyses reveal genotype-and developmental stage-specific molecular responses to drought and salinity stresses in chickpea. Sci. Rep. 2016, 6, 19228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munns, R.; Gilliham, M. Salinity tolerance of crops–what is the cost? New Phytol. 2015, 208, 668–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taiz, L.; Zeiger, E. Plant Physiology, 5th ed.; Sinauer Associates Inc.: Sunderland, MA, USA, 2010; pp. 591–623. [Google Scholar]
- Munns, R. Plant adaptations to salt and water stress. In Plant Responses to Drought and Salinity Stress—Developments in a Post-Genomic Era; Turkan, I., Ed.; Academic Press: Boston, MA, USA, 2011; Volume 57, pp. 1–32. [Google Scholar]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; James, R.A.; Sirault, X.R.; Furbank, R.T.; Jones, H.G. New phenotyping methods for screening wheat and barley for beneficial responses to water deficit. J. Exp. Bot. 2010, 61, 3499–3507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salehi-Lisar, S.Y.; Bakhshayeshan-Agdam, H. Drought stress in plants: Causes, consequences, and tolerance. In Drought Stress Tolerance in Plants; Hossain, M.A., Wani, S.H., Bhattacharjee, S., Burritt, D.J., Tran, L.S.P., Eds.; Springer: Cham, Germany, 2016; Volume 1, pp. 1–16. [Google Scholar]
- Kaur, G.; Asthir, B. Molecular responses to drought stress in plants. Biol. Plant 2017, 61, 201–209. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isayenkov, S.V.; Maathuis, F.J. Plant salinity stress: Many unanswered questions remain. Front. Plant Sci. 2019, 10, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piwowarczyk, B.; Tokarz, K.; Kamińska, I. Responses of grass pea seedlings to salinity stress in in vitro culture conditions. Plant Cell Tissue Organ Cult. 2016, 124, 227–240. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Kawasaki, T.; Shimizu, G.; Moritsugu, M. Effects of high concentrations of sodium chloride and polyethylene glycol on the growth and ion absorption in plants. Plant Soil 1983, 75, 87–93. [Google Scholar] [CrossRef]
- Dhindsa, R.H.; Plumb-Dhindsa, R.; Thorpe, T.A. Leaf senescence correlated with increased level of membrane permeability, lipid peroxidation and decreased level of SOD and CAT. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Method Enzymol. 1987, 148, 350–356. [Google Scholar]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Yemm, E.W.; Willis, A. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Addis, G.; Narayan, R.K.J. Developmental Variation of the Neurotoxin, β-N-Oxalyl-l-α, β-diamino propionic acid (ODAP), in Lathyrus sativus. Ann. Bot. 1994, 74, 209–215. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldern, R.P.; Teare, I.D. Rapid determination of free proline from water stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Lűck, H. Peroxidase. In Methoden der Enzymatischen Analyse; Bergmeyer, H.U., Ed.; Verlag Chemie: Weinheim, Germany, 1962; pp. 895–897. [Google Scholar]
- Bartosz, G. Another Side of Oxygen. Free Radicals in Nature; PWN: Warsaw, Poland, 2006. (In Polish) [Google Scholar]
- Swain, T.; Hillis, W.E. Phenolic constituents of Prunus domestica. I. Quantitative analysis of phenolic constituents. J. Sci. Food Agric. 1959, 10, 63–68. [Google Scholar] [CrossRef]
- Benzie, I.; Strain, J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org (accessed on 6 April 2020).
- Tang, Y.; Horikoshi, M.; Li, W. Ggfortify: Unified interface to visualize statistical result of popular R packages. R J. 2016, 8, 474–485. [Google Scholar] [CrossRef] [Green Version]
- Rajjou, L.; Duval, M.; Gallardo, K.; Catusse, J.; Bally, J.; Job, C.; Job, D. Seed germination and vigor. Annu. Rev. Plant Biol. 2012, 63, 507–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nonogaki, H.; Bassel, G.W.; Bewley, J.D. Germination—Still a mystery. Plant Sci. 2010, 179, 574–581. [Google Scholar] [CrossRef]
- Verslues, P.E.; Agarwal, M.; Katiyar-Agarwal, S.; Zhu, J.; Zhu, J.K. Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status. Plant J. 2006, 45, 523–539. [Google Scholar] [CrossRef] [PubMed]
- Patanè, C.; Saita, A.; Sortino, O. Comparative effects of salt and water stress on seed germination and early embryo growth in two cultivars of sweet sorghum. J. Agron. Crop Sci. 2013, 199, 30–37. [Google Scholar] [CrossRef]
- Zhang, H.; Irving, J.; McGill, C.; Mathhew, C.; Zhou, D.; Kemp, P. The effects of salinity and osmotic stress on barley germination rate: Sodium as an osmotic regulator. Ann. Bot. 2010, 106, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Petrović, G.; Jovičić, D.; Nikolić, Z.; Tamindžić, G.; Ignjatov, M.; Milošević, D.; Milošević, B. Comparative study of drought and salt stress effects on germination and seedling growth of pea. Genetika 2016, 48, 373–381. [Google Scholar] [CrossRef]
- Kaya, M.; Kaya, G.; Kaya, M.D.; Atak, M. Interaction between seed size and NaCl on germination and early seedling growth of some Turkish cultivars of chickpea (Cicer arietinum L.). J. Zhejiang Univ. Sci. B 2008, 9, 371–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okcu, G.; Kaya, M.D.; Atak, M. Effects of salt and drought stresses on germination and seedling growth of pea (Pisum sativum L.). Turk. J. Agric. For. 2005, 29, 237–242. [Google Scholar]
- Piwowarczyk, B.; Pindel, A. Determination of an optimal isolation and culture conditions of grass pea protoplasts. Biotechnologia 2015, 96, 192–202. [Google Scholar] [CrossRef] [Green Version]
- Wiszniewska, A.; Piwowarczyk, B. Studies on cell wall regeneration in protoplast culture of legumes-the effect of organic medium additives on cell wall components. Czech J. Genet. Plant Breed. 2014, 50, 84–91. [Google Scholar] [CrossRef] [Green Version]
- Piwowarczyk, B.; Kaminska, I.; Rybinski, W. Influence of PEG generated osmotic stress on shoot regeneration and some biochemical parameters in Lathyrus culture. Czech J. Genet. Plant Breed. 2014, 50, 77–83. [Google Scholar] [CrossRef] [Green Version]
- Piwowarczyk, B.; Tokarz, K.; Makowski, W.; Łukasiewicz, A. Different acclimatization mechanisms of two grass pea cultivars to osmotic stress in in vitro culture. Acta Physiol. Plant. 2017, 39, 96. [Google Scholar] [CrossRef] [Green Version]
- Alam, M.Z.; Stuchbury, T.; Naylor, R.E.L. Effect of NaCl and PEG induced osmotic potentials on germination and early seedling growth of rice cultivars differing in salt tolerance. Pak. J. Biol. Sci. 2002, 5, 1207–1210. [Google Scholar]
- Mokhberdoran, F.; Kalat, S.N.; Haghighi, R.S. Effect of temperature, iso-osmotic concentrations of NaCl and PEG agents on germination and some seedling growth yield components in rice (Oryza sativa L). Asian J. Plant Sci. 2009, 8, 409–416. [Google Scholar] [CrossRef] [Green Version]
- Jaleel, C.A.; Manivannan, P.; Wahid, A.; Farooq, M.; Al-Jubur, H.J.; Somasundaram, R.; Panneerselvam, R. Drought stress in plants: A review on morphological characteristics and pigments composition. Int. J. Agric. Biol. 2009, 11, 100–105. [Google Scholar]
- Shitole, S.M.; Dhumal, K.N. Effect of water stress by polyethylene glycol 6000 and sodium chloride on seed germination and seedling growth of Cassia angustifolia. Int. J. Pharmaceut. Sci. Res. 2012, 3, 528–531. [Google Scholar]
- Kaydan, D.; Yagmur, M. Germination, seedling growth and relative water content of shoot in different seed sizes of triticale under osmotic stress of water and NaCl. Afr. J. Biotechnol. 2008, 7, 2862–2868. [Google Scholar]
- Blum, A. Osmotic adjustment is a prime drought stress adaptive engine in support of plant production. Plant Cell Environ. 2017, 40, 4–10. [Google Scholar] [CrossRef]
- Turner, N.C. Turgor maintenance by osmotic adjustment: 40 years of progress. J. Exp. Bot. 2018, 69, 3223–3233. [Google Scholar] [CrossRef] [Green Version]
- Zivcak, M.; Brestic, M.; Sytar, O. Osmotic adjustment and plant adaptation to drought stress. In Drought Stress Tolerance in Plants; Hossain, M.A., Wani, S.H., Bhattacharjee, S., Burritt, D.J., Tran, L.S.P., Eds.; Springer: Cham, Germany, 2016; Volume 1, pp. 105–143. [Google Scholar]
- Munns, R.; Passioura, J.B.; Colmer, T.D.; Byrt, C.S. Osmotic adjustment and energy limitations to plant growth in saline soil. New Phytol. 2020, 225, 1091–1096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munns, R. Genes and salt tolerance: Bringing them together. New Phytol. 2005, 167, 645–663. [Google Scholar] [CrossRef]
- Eziz, A.; Yan, Z.; Tian, D.; Han, W.; Tang, Z.; Fang, J. Drought effect on plant biomass allocation: A meta-analysis. Ecol. Evol. 2017, 7, 11002–11010. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Hamid, A.; Khaliq, M.A. Evaluation of mungbean (Vigna radiata (L.) Wilczek) genotypes on the basis of photosynthesis and dry matter accumulation. J. Agric. Rural. Dev. 2009, 7, 1–8. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.B.S.M.A.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. In Sustainable Agriculture; Lichtfouse, E., Navarrete, M., Debaeke, P., Souchere, V., Alberola, C., Eds.; Springer: Dordrecht, Germany, 2009; pp. 153–188. [Google Scholar]
- Gill, P.K.; Sharma, A.D.; Singh, P.; Bhullar, S.S. Changes in germination, growth and soluble sugar contents of Sorghum bicolor (L.) Moench seeds under various abiotic stresses. Plant Growth Regul. 2003, 40, 157–162. [Google Scholar] [CrossRef]
- Gapińska, M.; Skłodowska, M.; Gabara, B. Effect of short-and long-term salinity on the activities of antioxidative enzymes and lipid peroxidation in tomato roots. Acta Physiol. Plant. 2008, 30, 11. [Google Scholar] [CrossRef]
- Nxele, X.; Klein, A.; Ndimba, B.K. Drought and salinity stress alters ROS accumulation, water retention, and osmolyte content in sorghum plants. S. Afr. J. Bot. 2017, 108, 261–266. [Google Scholar] [CrossRef]
- Davey, M.W.; Stals, E.; Panis, B.; Keulemans, J.; Swennen, R.L. High-throughput determination of malondialdehyde in plant tissues. Anal. Biochem. 2005, 347, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Ekinci, M.; Ors, S.; Turan, M.; Yildiz, S.; Yildirim, E. Effects of individual and combined effects of salinity and drought on physiological, nutritional and biochemical properties of cabbage (Brassica oleracea var. capitata). Sci. Hortic. 2018, 240, 196–204. [Google Scholar] [CrossRef]
- Dugasa, M.T.; Cao, F.; Ibrahim, W.; Wu, F. Differences in physiological and biochemical characteristics in response to single and combined drought and salinity stresses between wheat genotypes differing in salt tolerance. Physiol. Plant. 2019, 165, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Tokarz, K.M.; Makowski, W.; Tokarz, B.; Hanula, M.; Sitek, E.; Muszyńska, E.; Jędrzejczyk, R.; Banasiuk, R.; Chajec, Ł.; Mazur, S. Can Ceylon Leadwort (Plumbago zeylanica L.) Acclimate to Lead Toxicity?—Studies of Photosynthetic Apparatus Efficiency. Int. J. Mol. Sci. 2020, 21, 1866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, C.V. Regulation of chlorophyll biosynthesis and degradation by salt stress in sunflower leaves. Sci. Hortic. 2004, 103, 93–99. [Google Scholar] [CrossRef]
- Dalal, V.K.; Tripathy, B.C. Modulation of chlorophyll biosynthesis by water stress in rice seedlings during chloroplast biogenesis. Plant Cell Environ. 2012, 35, 1685–1703. [Google Scholar] [CrossRef] [PubMed]
- Phung, T.H.; Jung, H.I.; Park, J.H.; Kim, J.G.; Back, K.; Jung, S. Porphyrin biosynthesis control under water stress: Sustained porphyrin status correlates with drought tolerance in transgenic rice. Plant Physiol. 2011, 157, 1746–1764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beale, S.I. Enzymes of chlorophyll biosynthesis. Photosynth. Res. 1999, 60, 43–73. [Google Scholar] [CrossRef]
- Nouet, C.; Motte, P.; Hanikenne, M. Chloroplastic and mitochondrial metal homeostasis. Trends Plant Sci. 2011, 16, 395–404. [Google Scholar] [CrossRef]
- Hanikenne, M.; Bernal, M.; Urzica, E.I. Ion homeostasis in the Chloroplast. In Plastid Biology. Advances in Plant Biology; Theg, S., Wollman, F.A., Eds.; Springer: New York, NY, USA, 2014; Volume 5, pp. 465–514. [Google Scholar]
- Piwowarczyk, B.; Tokarz, K.; Muszyńska, E.; Makowski, W.; Jędrzejczyk, R.; Gajewski, Z.; Hanus-Fajerska, E. The acclimatization strategies of kidney vetch (Anthyllis vulneraria L.) to Pb toxicity. Environ. Sci. Pollut. Res. 2018, 25, 19739–19752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wani, S.H.; Singh, N.B.; Haribhushan, A.; Mir, J.I. Compatible solute engineering in plants for abiotic stress tolerance-role of glycine betaine. Curr. Genom. 2013, 14, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Couée, I.; Sulmon, C.; Gouesbet, G.; El Amrani, A. Involvement of soluble sugars in reactive oxygen species balance and responses to oxidative stress in plants. J. Exp. Bot. 2006, 57, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Patakas, A.; Nikolaou, N.; Zioziou, E.; Radoglou, K.; Noitsakis, B. The role of organic solute and ion accumulation in osmotic adjustment in drought-stressed grapevines. Plant Sci. 2002, 163, 361–367. [Google Scholar] [CrossRef]
- Byrt, C.S.; Munns, R.; Burton, R.A.; Gilliham, M.; Wege, S. Root cell wall solutions for crop plants in saline soils. Plant Sci. 2018, 269, 47–55. [Google Scholar] [CrossRef]
- An, P.; Li, X.; Zheng, Y.; Matsuura, A.; Abe, J.; Eneji, A.E.; Tanimoto, E.; Inanaga, S. Effects of NaCl on root growth and cell wall composition of two soya bean cultivars with contrasting salt tolerance. J. Agron. Crop Sci. 2014, 200, 212–218. [Google Scholar] [CrossRef]
- Matros, A.; Peshev, D.; Peukert, M.; Mock, H.P.; Van den Ende, W. Sugars as hydroxyl radical scavengers: Proof-of-concept by studying the fate of sucralose in Arabidopsis. Plant J. 2015, 82, 822–839. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.Y.; Spencer, P.S.; Li, Z.X.; Liang, Y.M.; Wang, Y.F.; Wang, C.Y.; Li, F.M. Lathyrus sativus (grass pea) and its neurotoxin ODAP. Phytochemistry 2006, 67, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Lambein, F.; Travella, S.; Kuo, Y.H.; Van Montagu, M.; Heijde, M. Grass pea (Lathyrus sativus L.): Orphan crop, nutraceutical or just plain food? Planta 2019, 250, 821–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haque, R.M.; Kuo, Y.H.; Lambein, F.; Hussain, M. Effect of environmental factors on the biosynthesis of the neuro-excitatory amino acid β-ODAP (β-N-oxalyl-l-α, β-diaminopropionic acid) in callus tissue of Lathyrus sativus. Food Chem. Toxicol. 2011, 49, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Jiao, C.J.; Jiang, J.L.; Ke, L.M.; Cheng, W.; Li, F.M.; Li, Z.X.; Wang, C.Y. Factors affecting β-ODAP content in Lathyrus sativus and their possible physiological mechanisms. Food Chem. Toxicol. 2011, 49, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.W.; Xing, G.M.; Xu, H.; Yan, Z.Y.; Wang, C.Y.; Wang, Y.F.; Li, Z.X. Relationship between oxalic acid and the metabolism of ß-N-oxalyl-α, ß-diaminopropionic acid (ODAP) in grass pea (Lathyrus sativus L). Isr. J. Plant Sci. 2005, 53, 89–96. [Google Scholar]
- Xing, G.; Cui, K.; Li, J.; Wang, Y.; Li, Z. Water stress and accumulation of beta- N-oxalyl-L-alpha,beta-diaminopropionic acid in grass pea (Lathyrus sativus). J. Agric. Food Chem. 2001, 49, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.C.; Xing, G.M.; Li, F.M.; Wang, S.M.; Fan, X.W.; Li, Z.X.; Wang, Y.F. Abscisic acid promotes accumulation of toxin ODAP in relation to free spermine level in grass pea seedlings (Lathyrus sativus L). Plant Physiol. Biochem. 2006, 44, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.M.; Zhang, X.Y.; Wang, G.X. Relationships between stomatal character, photosynthetic character and seed chemical composition in grass pea at different water availabilities. J. Agric. Sci. 2004, 142, 675–681. [Google Scholar] [CrossRef]
- Xiong, J.L.; Kong, H.Y.; Akram, N.A.; Bai, X.; Ashraf, M.; Tan, R.Y.; Zhy, H.; Siddique, K.H.M.; Xiong, Y.C.; Turner, N.C. 24-epibrassinolide increases growth, grain yield and β-ODAP production in seeds of well-watered and moderately water-stressed grass pea. Plant Growth Regul. 2016, 78, 217–231. [Google Scholar] [CrossRef]
- Jiao, C.J.; Xu, Q.L.; Wang, C.Y.; Li, F.M.; Li, Z.X.; Wang, Y.F. Accumulation pattern of toxin β-ODAP during lifespan and effect of nutrient elements on β-ODAP content in Lathyrus sativus seedlings. J. Agric. Sci. 2006, 144, 369–375. [Google Scholar] [CrossRef]
- Zhou, G.K.; Kong, Y.Z.; Cui, K.R.; Li, Z.X.; Wang, Y.F. Hydroxyl radical scavenging activity of β-N-oxalyl-l-α, β-diaminopropionic acid. Phytochemistry 2001, 58, 759–762. [Google Scholar]
- Suzuki, N.; Koussevitzky, S.H.A.I.; Mittler, R.O.N.; Miller, G.A.D. ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ. 2012, 35, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Sofo, A.; Scopa, A.; Nuzzaci, M.; Vitti, A. Ascorbate peroxidase and catalase activities and their genetic regulation in plants subjected to drought and salinity stresses. Int. J. Mol. Sci. 2015, 16, 13561–13578. [Google Scholar] [CrossRef] [Green Version]
- Demidchik, V. Mechanisms of oxidative stress in plants: From classical chemistry to cell biology. Environ. Exp. Bot. 2015, 109, 212–228. [Google Scholar] [CrossRef]
- Noctor, G.; Mhamdi, A.; Foyer, C.H. The roles of reactive oxygen metabolism in drought: Not so cut and dried. Plant Physiol. 2014, 164, 1636–1648. [Google Scholar] [CrossRef] [Green Version]
- Golemiec, E.; Tokarz, K.; Wielanek, M.; Niewiadomska, E. A dissection of the effects of ethylene, H2O2 and high irradiance on antioxidants and several genes associated with stress and senescence in tobacco leaves. J. Plant Physiol. 2014, 171, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Willekens, H.; Langebartels, C.; Tire, C.; Van Montagu, M.; Inze, D.; Van Camp, W. Differential expression of catalase genes in Nicotiana plumbaginifolia (L). Proc. Nat. Acad. Sci. USA 1994, 91, 10450–10454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonifacio, A.; Martins, M.O.; Ribeiro, C.W.; Fontenele, A.V.; Carvalho, F.E.; Margis-Pinheiro, M.Á.R.C.I.A.; Silveira, J.A. Role of peroxidases in the compensation of cytosolic ascorbate peroxidase knockdown in rice plants under abiotic stress. Plant Cell Environ. 2011, 34, 1705–1722. [Google Scholar] [CrossRef] [PubMed]
- van Doorn, W.G.; Ketsa, S. Cross reactivity between ascorbate peroxidase and phenol (guaiacol) peroxidase. Postharvest Biol. Technol. 2014, 95, 64–69. [Google Scholar] [CrossRef]
- Świętek, M.; Lu, Y.C.; Konefał, R.; Ferreira, L.P.; Cruz, M.M.; Ma, Y.H.; Horák, D. Scavenging of reactive oxygen species by phenolic compound-modified maghemite nanoparticles. Beilstein J. Nanotech. 2019, 10, 1073–1088. [Google Scholar] [CrossRef] [PubMed]
- Makowski, W.; Tokarz, K.M.; Tokarz, B.; Banasiuk, R.; Witek, K.; Królicka, A. Elicitation-based method for increasing the production of antioxidant and bactericidal phenolic compounds in Dionaea muscipula J. Ellis Tissue. Molecules 2020, 25, 1794. [Google Scholar] [CrossRef] [PubMed]
- Ahanger, M.A.; Tomar, N.S.; Tittal, M.; Argal, S.; Agarwal, R.M. Plant growth under water/salt stress: ROS production; antioxidants and significance of added potassium under such conditions. Physiol. Mol. Biol. Plants 2017, 23, 731–744. [Google Scholar] [CrossRef] [PubMed]
- Naikoo, M.I.; Dar, M.I.; Raghib, F.; Jaleel, H.; Ahmad, B.; Raina, A.; Khan, F.A.; Naushin, F. Role and regulation of plants phenolics in abiotic stress tolerance: An overview. In Plant Signaling Molecules; Khan, M.I.R., Reddy, P.S., Ferrante, A., Khan, N.A., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 157–168. [Google Scholar]
- Makowski, W.; Tokarz, B.; Banasiuk, R.; Królicka, A.; Dziurka, M.; Wojciechowska, R.; Tokarz, K.M. Is a blue-red light a good elicitor of phenolic compounds in the family Droseraceae? A comparative study. J. Photochem. Photobio. B 2019, 201, 111679. [Google Scholar] [CrossRef] [PubMed]
- Nichols, S.N.; Hofmann, R.W.; Williams, W.M. Physiological drought resistance and accumulation of leaf phenolics in white clover interspecific hybrids. Environ. Exp. Bot. 2015, 119, 40–47. [Google Scholar] [CrossRef]
- Nakabayashi, R.; Yonekura-Sakakibara, K.; Urano, K.; Suzuki, M.; Yamada, Y.; Nishizawa, T.; Matsuda, F.; Kojima, M.; Sakakibara, H.; Shinozaki, K.; et al. Enhancement of oxidative and drought tolerance in Arabidopsis by overaccumulation of antioxidant flavonoids. Plant J. 2014, 77, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.A.; Ashraf, M.; Ali, Q. Response of two genetically diverse wheat cultivars to salt stress at different growth stages: Leaf lipid peroxidation and phenolic contents. Pak. J. Bot. 2010, 42, 559–565. [Google Scholar]
- Kostecka-Gugała, A.; Ledwożyw-Smoleń, I.; Augustynowicz, J.; Wyżgolik, G.; Kruczek, M.; Kaszycki, P. Antioxidant properties of fruits of raspberry and blackberry grown in central Europe. Open Chem. 2015, 13, 1313–1325. [Google Scholar] [CrossRef]
- Kostecka-Gugała, A.; Kruczek, M.; Ledwożyw-Smoleń, I.; Kaszycki, P. Antioxidants and health-beneficial nutrients in fruits of eighteen Cucurbita cultivars: Analysis of diversity and dietary implications. Molecules 2020, 25, 1792. [Google Scholar] [CrossRef] [Green Version]
- Lattanzio, V.; Caretto, S.; Linsalata, V.; Colella, G.; Mita, G. Signal transduction in artichoke [Cynara cardunculus L subsp. scolymus (L) Hayek] callus and cell suspension cultures under nutritional stress. Plant Physiol. Biochem. 2018, 127, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Verbruggen, N.; Hermans, C. Proline accumulation in plants: A review. Amino Acids 2008, 35, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Poustini, K.; Siosemardeh, A.; Ranjbar, M. Proline accumulation as a response to salt stress in 30 wheat (Triticum aestivum L.) cultivars differing in salt tolerance. Genet. Res. Crop Evol. 2007, 54, 925–934. [Google Scholar] [CrossRef]
- Aleksza, D.; Horváth, G.V.; Sándor, G.; Szabados, L. Proline accumulation is regulated by transcription factors associated with phosphate starvation. Plant Physiol. 2017, 175, 555–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertazzini, M.; Sacchi, G.A.; Forlani, G. A differential tolerance to mild salt stress conditions among six Italian rice genotypes does not rely on Na+ exclusion from shoots. J. Plant Physiol. 2018, 226, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Forlani, G.; Trovato, M.; Funck, D.; Signorelli, S. Regulation of proline accumulation and its molecular and physiological functions in stress. In Osmoprotectant-Mediated Abiotic Stress Tolerance in Plants: Recent Advances and Future Perspectives; Hossain, M.A., Kumar, V., Burritt, D.J., Fujita, M., Mäkelä, P.S., Eds.; Springer: Cham, Switzerland, 2019; pp. 73–97. [Google Scholar]
- Wan, Q.; Hongbo, S.; Zhaolong, X.; Jia, L.; Dayong, Z.; Yihong, H. Salinity tolerance mechanism of osmotin and osmotin-like proteins: A promising candidate for enhancing plant salt tolerance. Curr. Genom. 2017, 18, 553–556. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, C.; Zhang, B.; Yi, J.; Yang, Y.; Kong, C.; Lei, C.; Gong, M. The role of the late embryogenesis-abundant (LEA) protein family in development and the abiotic stress response: A comprehensive expression analysis of potato (Solanum tuberosum). Genes 2019, 10, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barthakur, S.B.V.B.; Babu, V.; Bansa, K.C. Over-expression of osmotin induces proline accumulation and confers tolerance to osmotic stress in transgenic tobacco. J. Plant Biochem. Biotech. 2001, 10, 31–37. [Google Scholar] [CrossRef]
- Lan, T.; Gao, J.; Zeng, Q.Y. Genome-wide analysis of the LEA (late embryogenesis abundant) protein gene family in Populus trichocarpa. Tree Genet. Genomes 2013, 9, 253–264. [Google Scholar] [CrossRef]
- Huang, Z.; Zhong, X.J.; He, J.; Jin, S.H.; Guo, H.D.; Yu, X.F.; Zhou, Y.J.; Li, X.; Ma, M.D.; Chen, Q.B.; et al. Genome-wide identification, characterization, and stress-responsive expression profiling of genes encoding LEA (Late Embryogenesis Abundant) proteins in Moso bamboo (Phyllostachys edulis). PLoS ONE 2016, 11, e0165953. [Google Scholar] [CrossRef]
- Magwanga, R.O.; Lu, P.; Kirungu, J.N.; Lu, H.; Wang, X.; Cai, X.; Zhou, Z.; Zhang, Z.; Salih, S.; Wang, K.; et al. Characterization of the late embryogenesis abundant (LEA) proteins family and their role in drought stress tolerance in upland cotton. BMC Genet. 2018, 19, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
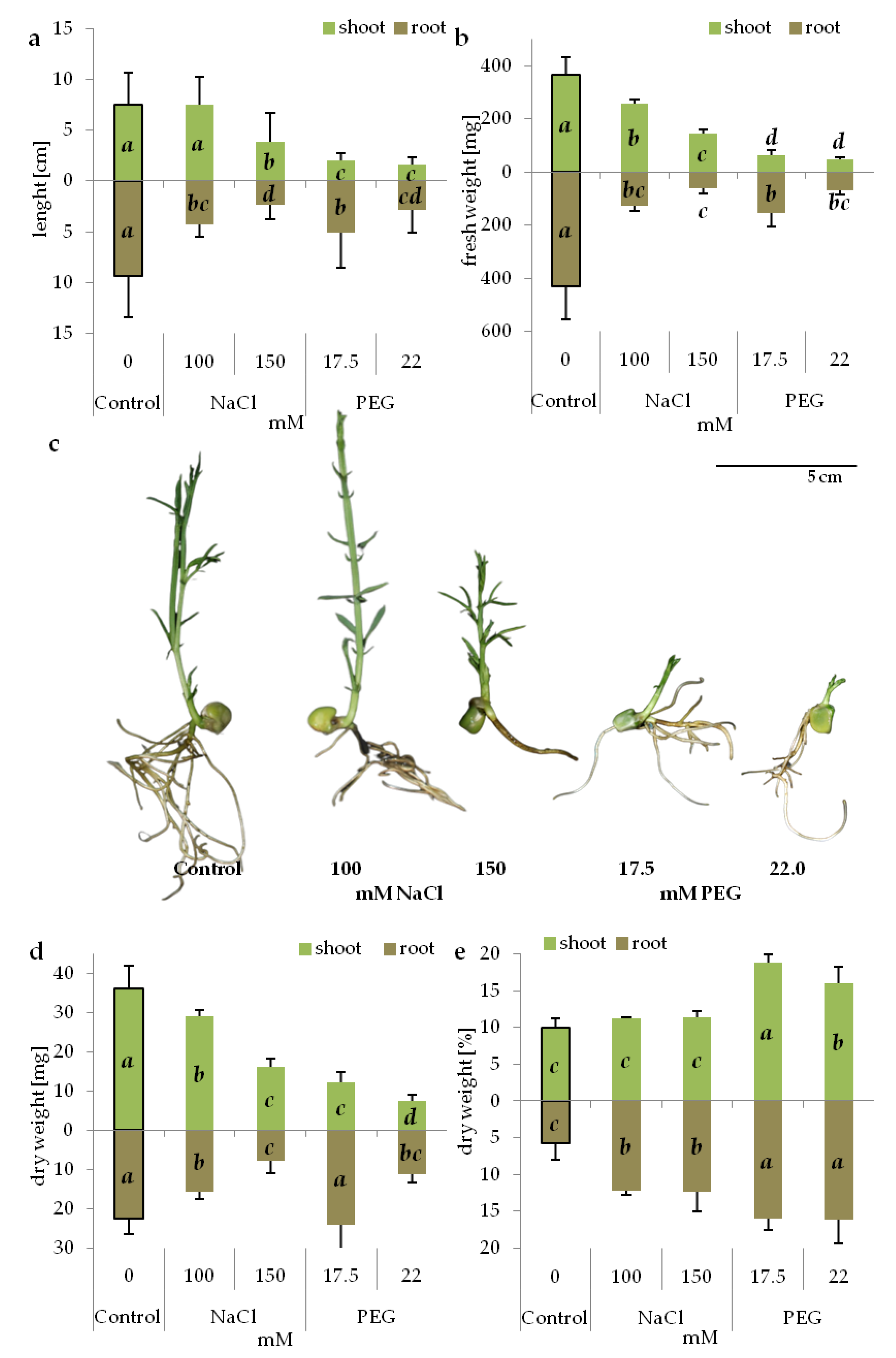
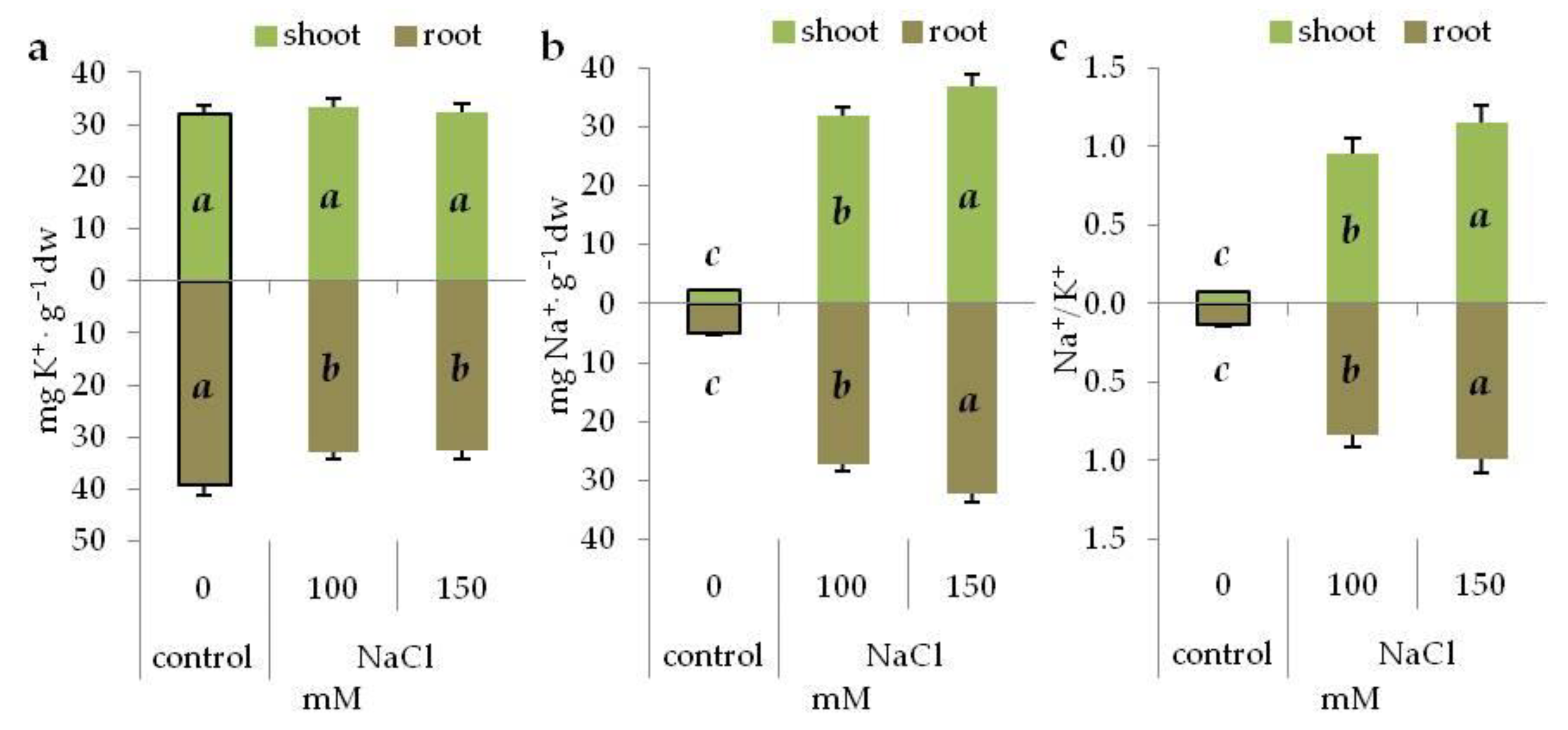
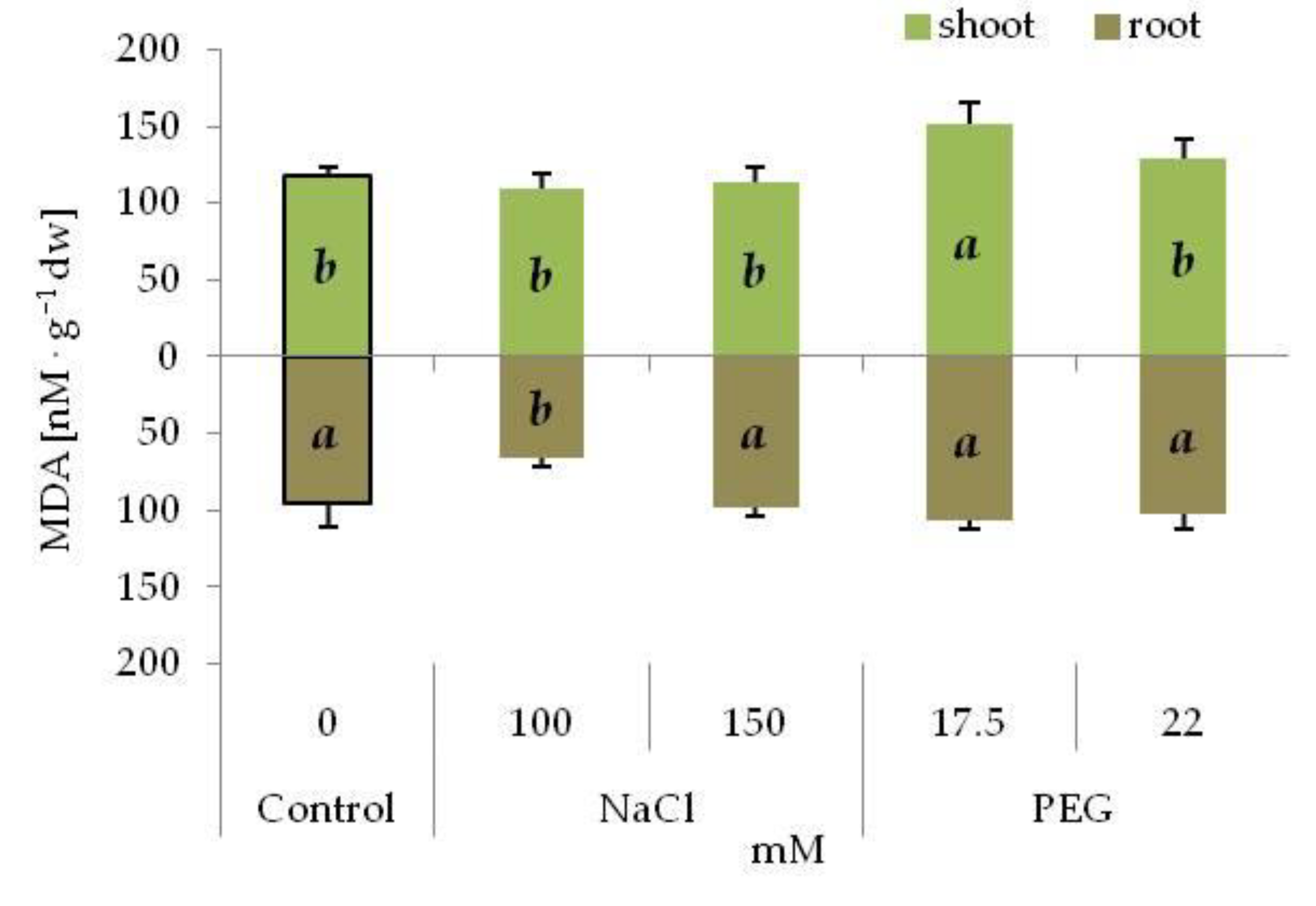
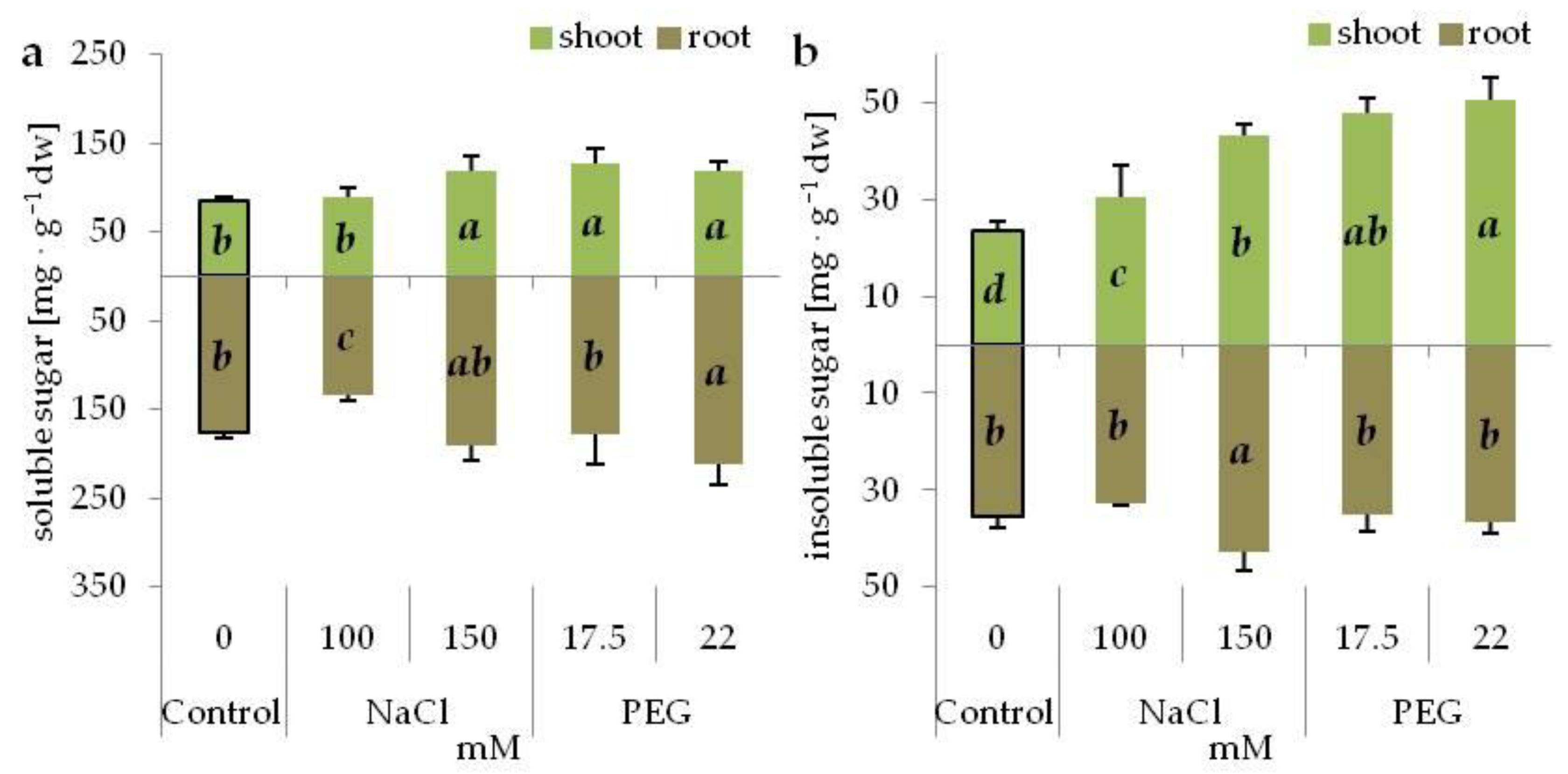
| Osmotic Potential (MPa) | Concentration (mM) | |
|---|---|---|
| PEG | NaCl | |
| 0.0 | 0.0 | 0.0 |
| −0.45 | 17.5 | 100.0 |
| −0.65 | 22.0 | 150.0 |
| Treatment | Concentration (mM) | Seed Germination (%) | Seedling Emergence (%) |
|---|---|---|---|
| Control | 0.0 | 100.0 a ± 0.0 | 96.7 a ± 5.8 |
| NaCl | 100.0 | 97.5 a ± 5.0 | 75.0 b ± 17.3 |
| 150.0 | 73.3 b ± 5.8 | 63.3 bc ± 5.8 | |
| PEG | 17.5 | 90.0 ab ± 17.3 | 68.0 b ± 11.0 |
| 22.0 | 80.0 b ± 8.2 | 47.5 c ± 12.6 |
| Pigment Content (mg∙g−1dw) Pigment Ratio | Treatment/Concentration (mM) | ||||
|---|---|---|---|---|---|
| Control | NaCl | PEG | |||
| 0.0 | 100.0 | 150.0 | 17.5 | 22.0 | |
| Chl a | 4.05 a ± 0.16 | 3.93 a ± 0.07 | 2.11 b ± 0.17 | 1.76 c ± 0.13 | 1.38 d ± 0.25 |
| Chl b | 1.12 a ± 0.04 | 1.08 a ± 0.01 | 0.71 b ± 0.16 | 0.52 c ± 0.07 | 0.40 c ± 0.06 |
| Chl a+b | 5.17 a ± 0.20 | 5.02 a ± 0.07 | 2.82 b ± 0.33 | 2.28 c ± 0.19 | 1.77 d ± 0.30 |
| Car | 0.93 a ± 0.04 | 0.91 a ± 0.03 | 0.49 b ± 0.01 | 0.40 c ± 0.02 | 0.34 c ± 0.06 |
| Chl a/b | 3.61 a ± 0.03 | 3.63 a ± 0.05 | 3.03 a ± 0.47 | 3.41 a ± 0.18 | 3.48 a ± 0.28 |
| Organ | Treatment/Concentration (mM) | ||||
|---|---|---|---|---|---|
| Control | NaCl | PEG | |||
| 0.0 | 100.0 | 150.0 | 17.5 | 22.0 | |
| shoot | 3.7 a ± 0.3 | 3.0 b ± 0.7 | 2.7 bc ± 0.4 | 2.7 bc ± 0.3 | 2.4 c ± 0.3 |
| root | 5.0 b ± 0.2 | 4.1 c ± 0.2 | 4.5 bc ± 0.3 | 5.0 b ± 0.8 | 5.8 a ± 0.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tokarz, B.; Wójtowicz, T.; Makowski, W.; Jędrzejczyk, R.J.; Tokarz, K.M. What is the Difference between the Response of Grass Pea (Lathyrus sativus L.) to Salinity and Drought Stress?—A Physiological Study. Agronomy 2020, 10, 833. https://doi.org/10.3390/agronomy10060833
Tokarz B, Wójtowicz T, Makowski W, Jędrzejczyk RJ, Tokarz KM. What is the Difference between the Response of Grass Pea (Lathyrus sativus L.) to Salinity and Drought Stress?—A Physiological Study. Agronomy. 2020; 10(6):833. https://doi.org/10.3390/agronomy10060833
Chicago/Turabian StyleTokarz, Barbara, Tomasz Wójtowicz, Wojciech Makowski, Roman J. Jędrzejczyk, and Krzysztof M. Tokarz. 2020. "What is the Difference between the Response of Grass Pea (Lathyrus sativus L.) to Salinity and Drought Stress?—A Physiological Study" Agronomy 10, no. 6: 833. https://doi.org/10.3390/agronomy10060833
APA StyleTokarz, B., Wójtowicz, T., Makowski, W., Jędrzejczyk, R. J., & Tokarz, K. M. (2020). What is the Difference between the Response of Grass Pea (Lathyrus sativus L.) to Salinity and Drought Stress?—A Physiological Study. Agronomy, 10(6), 833. https://doi.org/10.3390/agronomy10060833





