Abstract
Understanding the mechanisms of plant tolerance to osmotic and chemical stress is fundamental to maintaining high crop productivity. Soil drought often occurs in combination with physiological drought, which causes chemical stress due to high concentrations of ions. Hence, it is often assumed that the acclimatization of plants to salinity and drought follows the same mechanisms. Grass pea (Lathyrus sativus L.) is a legume plant with extraordinary tolerance to severe drought and moderate salinity. The aim of the presented study was to compare acclimatization strategies of grass pea seedlings to osmotic (PEG) and chemical (NaCl) stress on a physiological level. Concentrations of NaCl and PEG were adjusted to create an osmotic potential of a medium at the level of 0.0, −0.45 and −0.65 MPa. The seedlings on the media with PEG were much smaller than those growing in the presence of NaCl, but had a significantly higher content percentage of dry weight. Moreover, the stressors triggered different accumulation patterns of phenolic compounds, soluble and insoluble sugars, proline and β-N-oxalyl-L-α,β-diamino propionic acid, as well as peroxidase and catalase activity. Our results showed that drought stress induced a resistance mechanism consisting of growth rate limitation in favor of osmotic adjustment, while salinity stress induced primarily the mechanisms of efficient compartmentation of harmful ions in the roots and shoots. Furthermore, our results indicated that grass pea plants differed in their response to drought and salinity from the very beginning of stress occurrence.
1. Introduction
Adverse climatic and soil conditions force us to look for crop plants with innate resistance to abiotic stresses, in order to attain and maintain food security. The second most important food source, after cereals, is legume plants [1,2]. Legumes are appreciated not only for their nutritional (mainly high protein content) and health (bioactive compounds) values, but also economical (cheap meat replacement, especially in poorer regions) and ecological (nitrogen soil enrichment) ones [2]. This paper focuses on grass pea (Lathyrus sativus L.) [3], one of the oldest domesticated plants in Europe (i.e., since approximately 6000 BC) [4]. However, it is currently neglected by breeders and unused by farmers [3]. The nutritional composition of grass pea seeds surpasses those of chickpea, pea, broad bean or lupine. The average protein content in its seeds ranges from 25 to 30% of dry matter, and its amino acid pool is abundant in lysine but, as with other grain-legumes, it is deficient in methionine, cysteine and tryptophan [5,6]. Due to its very strong and deep-reaching root system, grass pea is tolerant to different soil pHs, and is capable of growing and developing on different soil types, which makes it unique among legumes [7]. Moreover, grass pea is extremely tolerant to adverse environmental factors, such as periodic flooding, low temperature, soil salinity and above all, prolonged drought. It grows in areas where the sum of precipitation is only 250 mm per year. It is basically the last crop to survive and fruit during periods of drought [8,9]. Its high degree of atmospheric nitrogen fixing, being conducive to soil fertility, and low grass pea production costs, make it an important component of sustainable agriculture [3,7,10].
It is widely believed that amongst abiotic stresses, drought and salinity limit plant productivity the most [11,12,13,14]. Drought generates water stress in plants due to limited or no water availability [15]. Soil salinity, in turn, generates both water stress resulting from physiological drought and chemical stress resulting from the accumulation of harmful ions (Na+ and Cl−) in toxic concentrations [16]. In both cases, the common denominator is the reduced ability of plants to absorb water; therefore, both stresses trigger the same initial response in plants [16,17]. This response is a loss of water from the cells and their shrinkage, which subsides after a few hours and the cells regain their original volume, but their ability to continue elongation growth is reduced, leading to the inhibition of root, stem and especially leaf growth [17]. Changing the soil–water relationship limits plant transpiration by reducing stomatal conductance, which, in turn, directly affects photosynthesis [18,19,20]. Disturbances in photosynthesis negatively affect primarily the production of assimilates (reduced plant growth), but also contribute to the formation of reactive oxygen species (ROS) which evoke secondary oxidative stress in cells, leading, among others, to lipid peroxidation, protein and DNA damage [19,20,21]. Disruptions in soil–water relationships also decrease nutrient availability to plants and disturb ion homeostasis [19]. In plants subjected to salinity stress, the symptoms of chemical stress become visible after a few days. The accumulation of toxic concentrations of Na+ and Cl−, mainly in the cytoplasm, but also in the vacuole, disturbs the water structure (kosmo and chaotropic effects), and thus, interferes with the biochemical activity of proteins and nucleic acids whose polar groups bind water. In addition, high concentrations of Na+ can inhibit enzyme activity (by replacing K+) and disrupt Na+/K+ balance, causing not only problems with the intake of K+ ions, but also their efflux [22].
Being sessile organisms, plants have developed two general mechanisms that allow them to survive environmental stresses: stress avoidance and stress tolerance [19]. The ability of plants to survive water and salt stress is manifested at different levels of plant organization: from cells (molecular, biochemical and physiological responses) to the entire organism (physiological, morphological and phenological responses) [12,19]. The mechanisms of tolerance to water (osmotic) stress are based on an increase in the root to shoot ratio (increase of absorbent root surface), reduction of water loss (stomata closure), accumulation of osmolytes (reduction of the cell water potential enabling water uptake from the soil solution) and removal of oxidative stress effects (activation of enzymatic and nonenzymatic antioxidants) [12,19]. The basic mechanisms of tolerance to salt stress include salt exclusion, consisting of limited the transport of harmful ions to the leaves. This is most often executed by selective ion uptake by the roots and retaining them in the lower part of the plant body, or their compartmentation in vacuoles if they reach the leaves [16,17,23].
Although plant growth is limited mainly by the effects of water stress, in the case of chemical stress, plants belonging to the ‘salt-sensitive’ category also experience an additional reduction in the production of assimilates, and further growth inhibition [17]. To be able to properly use the potential of plant resistance in breeding, it is necessary to understand which mechanisms underlie the response to a particular type of stress [16].
The aim of the present study was to evaluate the response of grass pea to PEG stress (drought imitating) and NaCl stress (salinity imitating), generating the same osmotic potential of the media. We hypothesized that grass pea acclimatization to drought and salinity stress would result from different physiological mechanisms. To our knowledge, this is the first research where a direct comparison of grass pea response to these two stressors was examined. To eliminate the influence of other adverse factors (extreme temperatures, pathogens, changing light intensities, etc.), experiments were conducted in vitro.
Our findings demonstrated that drought stress induced a whole-plant level response in the form of growth rate limitation and increased production of a secondary metabolite used in osmotic adjustment. In contrast, salinity stress induced acclimatization mechanisms involving mainly the compartmentation of harmful ions.
2. Materials and Methods
2.1. Plant Material, Growth Conditions and Stress Treatments
Plant material consisted of seeds of Polish grass pea (Lathyrus sativus L.) cultivar ‘Krab’. The seeds were multiplied from those obtained from a collection of legume plants of the Institute of Plant Genetics, Polish Academy of Sciences in Poznań in 2007. The seeds were surface disinfected according to Piwowarczyk et al. [24] and seeded on a medium solidified with 5 g l−1 Phytagel (Merck KGaA, Darmstadt, Germany). Basal medium comprised macro- and microelements of MS medium [25] and 20 g l−1 sucrose. Drought stress and salinity stress were induced by adding polyethylene glycol (PEG) and sodium chloride (NaCl) to the basal medium. PEG and NaCl were added at concentrations generating the same osmotic potential of the medium (Table 1), as calculated according to Kawasaki et al. [26]. For each treatment, ten disinfected seeds were placed in each of five vessels, and the experiments were repeated twice. The vessels with the seeds were kept at 24 ± 1 °C, 16 h day/8 h night photoperiod and fluorescent light of 50 (μmol (quantum) m−2 s−1) intensity.

Table 1.
Osmotic potential of the media and corresponding PEG and NaCl concentrations.
2.2. Evaluation of Seedling Response to Stress Factors
2.2.1. Determination of Germination, Seedling Emergence Rates and Biometric Parameters
Seven days after sowing the seeds, the rates of seed germination and seedling emergence were assessed and presented as percentages of all sowed seeds. Seeds with a visible root were treated as germinating seeds. Seeds with visible roots and shoots were counted as emerging seedlings. After 14 days of culture, the shoot and root lengths of the seedlings were measured, and their fresh and dry weight were weighed. Separated shoots and roots were weighed, and then dried at 120 °C for 48 h and weighed again. Additionally, the percentage of organ dry weight content was calculated according to the formula: x = a × 100% ÷ b, where x is the percentage dry matter content, a is dry weight (mg) and b is fresh weight (mg).
2.2.2. Determination of Na+ and K+ Content
Sodium and potassium content were determined using atomic absorption spectrometry (AAS). Freeze-dried root and shoot tissue samples (approx. 50 mg) treated with NaCl were placed in digestion vessels and predigested at room temperature in 5 mL of 65% HNO3. After one hour, 2 mL of 30% H2O2 was added to the suspension and digested for another 20 min. After preliminary digestion, the samples were mineralized for 40 min using a microwave digester (speedwave ENTRY, Berghof, Eningen unter Achalm, Germany), cooled to room temperature, transferred to flasks and adjusted to 25 mL with Milli-Q® water. The solutions were analyzed using atomic absorption spectrometer (Thermo iCE3000, Waltham, MA, USA). The cation concentrations were measured against the calibration curves (Na+ and K+ standards of trace metal basis purity).
2.2.3. Determination of Malonyldialdehyde Content
Malonyldialdehyde (MDA) content was determined according to the spectrophotometric method of Dhindsa et al. [27], with modifications. Freeze-dried shoot and root tissues were homogenized in 0.1% trichloroacetic acid (TCA) solution (1 mL) and centrifuged (15,000 rpm, 10 min, 4 °C). The extract (0.2 mL) was mixed with 20% TCA containing 0.5% thiobarbituric acid (TBA) (0.8 mL). The mixture was incubated for 30 min at 95 °C and centrifuged (15,000 rpm, 10 min, 4 °C). The absorbance of the samples was measured at 532 nm and 600 nm (correction value). The content of MDA (nM g−1 dw) was calculated using the absorbance coefficient for MDA (ε = 155 mM cm−1).
2.2.4. Determination of Photosynthetic Pigment Content
The content of photosynthetic pigments was determined using the spectrophotometric method described by Lichtenchtaler [28]. Freeze-dried shoot tissues were extracted twice in 1.0 and 0.5 mL of 80% acetone, and the extract was centrifuged (15,000 rpm, 15 min, 4 °C). The absorbance of the diluted extract was measured using a spectrophotometer (U-2900 Hitachi High Technologies Corporation, Tokyo, Japan) at 649 nm, 665 nm and 480 nm. The contents of chlorophyll a (Chl a), chlorophyll b (Chl b) and carotenoids (Car) were calculated according to Wellburn’s [29] equations. In addition, total chlorophyll and the ratio of chlorophyll a to chlorophyll b (Chl a/b) were calculated.
2.2.5. Determination of Soluble and Insoluble Sugars
The sugar content was determined by a spectrophotometric method with anthrone reagent [30], with modifications. Freeze-dried tissues (shoots and roots, separately) were extracted overnight in 1 mL of Milli-Q-ultrapure water (Millipore Direct system Q3). After centrifugation (15,000 rpm, 10 min), the supernatant was collected, and the pellet was resuspended in 0.5 mL of 0.1 M H2SO4 and incubated for 60 min at 80 °C. Aqueous or acid extracts (0.2 mL) were mixed with 1 mL of anthrone reagent (1 g anthrone in 500 mL 72% H2SO4) and incubated for 15 min at 95 °C. The reaction was stopped on ice and the samples were left to cool to room temperature. The absorbance of the samples was measured at 630 nm. The content of sugars (mg g−1 dw) was calculated from the calibration curve of glucose.
2.2.6. Determination of β-N-oxalyl-L-α,β-diamino propionic acid (ODAP) Content
ODAP content estimation was done according to the spectrophotometric method of Addis and Narayan [31]. Freeze-dried tissues (shoots and roots, separately) were extracted on a shaker overnight in 1 mL of 60% methanol. The extracts were treated with activated charcoal to remove pigments, mixed and centrifuged (15,000 rpm, 10 min). The supernatant was divided, and one part was hydrolyzed with 3N KOH for 30 min in 95 °C. Unhydrolyzed and hydrolyzed extracts were mixed with O-phtalaldehyde reagent (OPT) or borate buffer. The OPT reagent (pH 9.9) consisted of 50 mg OPT (Merck KGaA, Darmstadt, Germany), 100 µM β-mercaptoethanol, 0.5 mL of 95% ethanol and 49.5 mL of borate buffer. All the samples were incubated at 38 °C for 2 h. The absorbance of the samples was measured at 425 nm. The absorbance of the hydrolyzed sample with OPT reagent was reduced by the absorbance of the hydrolyzed sample with borate buffer, and by one third of the difference between the absorbance of the unhydrolyzed sample with OPT and the unhydrolyzed sample with the buffer. The content of ODAP (mg g−1 dw) was calculated from the calibration curve made for DL-2,3-diaminopropionic acid (DAP) (Merck KGaA, Darmstadt, Germany).
2.2.7. Determination of Proline Content
The proline content was determined by the spectrophotometric method of Bates et al. [32]. Freeze-dried shoots and roots were homogenized in 1 mL of 3% C7H6O6S×2H2O and centrifuged (15,000 rpm, 10 min, at 4 °C). The reaction mixture consisting of the extract (0.5 mL), acid-ninhydrin (0.5 mL) and glacial acetic acid (0.5 mL) was incubated for 1 h at 100 °C. The reaction was terminated on ice. Then, the mixture was extracted with 1 mL of toluene, and the absorbance of the samples was measured at 520 nm. Proline concentration (mg g−1 dw) was determined from a calibration curve made for L-proline (Sigma-Aldrich Chemie, GmBH, Steinheim, Germany).
2.2.8. Determination of Antioxidant Enzymes Activity
Freeze-dried tissues (shoots and roots, separately) were homogenized in 1 mL of phosphate buffer (pH 6.2) and centrifuged (15,000 rpm, 10 min, at 4 °C). The reaction mixture, consisting of the diluted extract (1 mL), phosphate buffer (1 mL), p-phenyldiamine (0.1 mL) and 0.1 mL of H2O2 solution, was kept at 30 °C for 10 min. Absorbance was measured at 485 nm after 1 and 2 min [33]. An increase in absorbance of 0.1 equals one unit of peroxidase (POD) activity.
Freeze-dried tissues (shoots and roots, separately) were homogenized in 1 mL of phosphate buffer (pH 7.0) and centrifuged (15,000 rpm, 10 min, at 4 °C). The absorbance (240 nm) of the reaction mixture consisting of 0.2 mL of the extract, 1.8 mL of phosphate buffer and 1 mL of 0.3% H2O2 solution in phosphate buffer was measured for 4 min at 1-min intervals [34]. One unit of catalase (CAT) activity is the amount of enzyme that decomposed 1 μmol H2O2 in 1 min.
2.2.9. Determination of Phenolic Compounds Content
The content of phenolic compounds was determined according to the Folin-Ciocalteu reagent method [35]. Freeze-dried shoot and root tissues were separately homogenized in 1 mL of 80% methanol. The extract was centrifuged (15,000 rpm, 10 min, 4 °C). The diluted supernatant (1 mL) was mixed with 0.2 mL of Folin-Ciocalteu reagent and 1.6 mL of 5% sodium carbonate (Na2CO3). The samples were incubated for 20 min at 40 °C. The absorbance of the mixture was measured at 740 nm. The content of phenolic compounds (mg g−1 dw) was calculated based on the standard curve made for chlorogenic acid.
2.2.10. Determination of Antioxidant Capacity
Total antioxidant capacity was determined using the FRAP (ferric reducing antioxidant power) assay [36]. The method is based on the reduction of ferric–tripyridyl-s-triazine (Fe3+-TPTZ) complex to its ferrous derivative (Fe2+). Freeze-dried shoot and root tissues were separately homogenized in 0.5 mL of 80% methanol. The extract was centrifuged (13,000 rpm, 3 min, room temperature), and then 0.1 mL of the extract was mixed with 3 mL of FRAP solution and 0.3 mL of H2O. The FRAP solution (300 mM acetate buffer (pH 3.6), 20 mM FeCl3 and 10 mM TPTZ in ethanol (10:1:1, v:v:v) was prepared fresh and warmed to 37 °C before preparing the mixtures. After 30 min, the absorbance of the samples was measured at 595 nm (UV/Vis Spectrophotometer JASCO V-530). The antioxidant capacity was presented as Trolox equivalents calculated from standard curves using Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) at 0.1 to 1.0 mM.
2.3. Statistical Analysis
All determinations were made in five replications. Statistical analyses were done using STATISTICA 12.0 (StatSoft Inc., Tulsa, OK, USA). The results, within the cultivar, organ and parameter, were subjected to a one-way analysis of variance (ANOVA), and the significance of differences between the arithmetical means was determined by Duncan post hoc test at p ≤ 0.05. The data were subjected to principal component analysis (PCA) performed for all analyzed traits in the shoots and roots in R v 3.6.1 [37] with the prcomp() function. The results were visualized for the first and second principal component with the autoplot() function from the ggfortify package v 0.4.9 [38].
3. Results
3.1. Germination Rate and Seedling Performance under Salinity and Drought Stress
Generally, seedling emergence rate was more negatively affected by the applied stresses than seed germination rate. Seed germination was reduced by both stress treatments at their higher concentration (150 mM NaCl and 22 mM PEG) (Table 2), while the seedling emergence rate was reduced by both stress factors, regardless of the treatment type and concentration (Table 2). Both parameters showed no differences in intensity for the corresponding concentrations of PEG and NaCl, generating the same osmotic potential of the medium (Table 2). Seedling growth was also negatively influenced by the applied stresses. Shoot growth was more severely inhibited by drought-imitating conditions than by salinity (Figure 1a–c). Shoot length was reduced on the media with both PEG concentrations, and on the media with higher NaCl concentration (150 mM) (Figure 1a). However, the fresh weight of the shoots was reduced also on the medium with a lower NaCl concentration (Figure 1b). The root length and fresh weight decreased both on the media with NaCl and PEG; however, no differences were observed between the corresponding NaCl and PEG concentrations (Figure 1a,b). Both stressors reduced the average dry weight of shoots and roots (Figure 1d). However, an evaluation of dry matter content with reference to fresh weight of organs revealed the opposite direction of changes (Figure 1e); the salinity generated by NaCl did not change the percentage of dry weight content in the shoots (Figure 1e), but it increased the percentage of root dry matter (Figure 1e). In contrast, stress generated by PEG increased dry weight content [%] in both the shoots and roots (Figure 1e). In addition, a significantly higher content of dry weight was found in both the shoots and roots of the seedlings subjected to PEG-induced stress compared to NaCl-induced stress (Figure 1e).

Table 2.
Percentage of grass pea seed germination and seedling emergence under NaCl and PEG-induced stress after 7 days of culture.
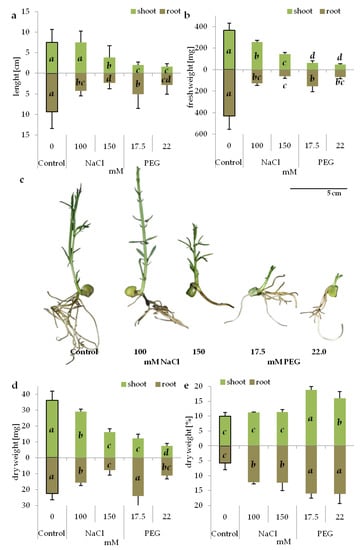
Figure 1.
Biometric parameters of 14-day old grass pea seedlings under NaCl and PEG-induced stress; (a) length of shoots and roots, (b) fresh weight of single shoot and root, (c) morphology of seedlings, (d) dry weight of single shoot and root, (e) percentage of shoot and root dry weight content. Different letters in italic denote significant differences at p ≤ 0.05 within one organ.
3.2. Na+ and K+ Content in NaCl Treated Seedlings
In 14-day old grass pea seedlings treated with NaCl, the content of K+ ions did not change in the shoots, but decreased significantly in the roots (Figure 2a). In turn, sodium ion accumulation increased gradually with increasing NaCl concentration in the medium, both in the shoots and roots (Figure 2b). The Na+/K+ ratio changed in the same way (Figure 2c).
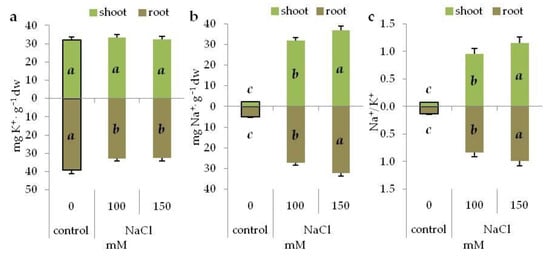
Figure 2.
Content of K+ (a) and Na+ (b) ions and Na+/ K+ ratio (c) in the shoots and roots of 14-day old grass pea seedlings under NaCl and PEG-induced stress. Different letters in italic denote significant differences at p ≤ 0.05 within one organ.
3.3. Content of MDA and Leaf Pigments under Drought and Salinity Stress
In 14-day old grass pea seedlings, MDA accumulation increased in the shoots at lower PEG content, and decreased in the roots at lower NaCl content in comparison with the control and the corresponding concentration of the other stressor (Figure 3).

Figure 3.
Malonyldialdehyde (MDA) content in the shoots and roots of 14-day old grass pea seedlings under NaCl and PEG-induced stress. Different letters in italic denote significant differences at p ≤ 0.05 within one organ.
Stress generated by both NaCl and PEG reduced photosynthetic pigment (Chl a, Chl b and Car) content in grass pea shoots, with the exception of shoots at lower NaCl concentration (Table 3). The decrease in pigment content was more pronounced in the seedlings cultivated under PEG-induced stress than NaCl-induced stress. There were no significant differences in the pigment content ratio (Chl a/b) between the control and the corresponding stress factors (Table 3).

Table 3.
Chlorophyll a (Chl a), chlorophyll b (Chl b) and carotenoid (Car) content (mg g−1 dw) and pigment ratio of 14 day-old grass pea shoots under NaCl and PEG-induced stress.
3.4. Osmolyte Accumulation under Salinity and Drought Stress
The soluble sugar content increased in the shoots grown on the media with PEG and with higher NaCl concentration, compared with the control. In the shoots at a lower level of NaCl, it did not change in comparison to the control, and was lower than in the shoots from the corresponding PEG treatment (Figure 4a). In the roots, the accumulation of soluble sugars increased only at a higher PEG concentration and decreased at lower NaCl concentration (Figure 4a). In turn, the accumulation of insoluble sugar in the shoots intensified under both stresses, and was greater in PEG-stressed than NaCl-stressed shoots (Figure 4b). Increased accumulation of insoluble sugars in the roots occurred only for higher NaCl concentration (Figure 4b). The ratio of soluble to insoluble sugars decreased in the shoots under both stress conditions (Table 4), while in the roots, it increased at higher PEG concentration and decreased at lower NaCl concentration. Furthermore, the ratio was higher under drought-imitating conditions than in the presence of NaCl (Table 4).
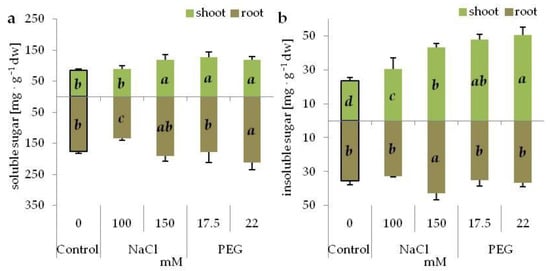
Figure 4.
Soluble (a) and insoluble (b) sugar content in the shoots and roots of 14-day old grass pea seedlings under NaCl and PEG-induced stress. Different letters denote significant differences at p ≤ 0.05 within one cultivar and organ.

Table 4.
Ratio of soluble to insoluble sugar content in 14-day old grass pea shoots and roots under NaCl and PEG-induced stress.
3.5. Neurotoxin Accumulation under Salinity and Drought Stress
ODAP accumulation increased both in the shoots and roots under PEG stress (Figure 5a). Moreover, its significantly higher content was noted in the shoots grown in the media with PEG than that with NaCl. In the roots, accumulation of ODAP was also noted with a higher concentration of NaCl, and it was greater than for the corresponding PEG concentration (Figure 5a).
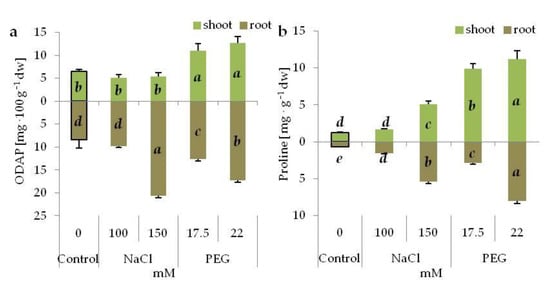
Figure 5.
β-N-oxalyl-L-α,β-diamino propionic acid (ODAP) (a) and proline (b) content in the shoots and roots of 14-day old grass pea seedlings under NaCl and PEG-induced stress. Different letters in italic denote significant differences at p ≤ 0.05 within one organ.
3.6. Proline Content under Salinity and Drought Stress
Generally, both stress treatments increased proline accumulation in the shoots and roots of ‘Krab’ grass pea seedlings (Figure 5b). However, the increase was significantly higher in the shoots exposed to PEG-induced stress than to salinity (Figure 5b). Similarly, proline accumulation in the roots was higher in the seedlings treated with PEG than with corresponding NaCl concentrations (Figure 5b).
3.7. Antioxidant System under Salinity and Drought Stress
POD activity was higher, compared with the control, in the shoots subjected to both PEG and higher NaCl concentration, and in the roots growing on the media containing NaCl (Figure 6a). Moreover, higher POD activity was noted in the roots cultivated on NaCl media than in those cultivated on PEG media (Figure 6a). CAT activity, as compared with the control, increased in the shoots of the seedlings cultivated at a higher PEG concentration, and decreased at lower salinity and drought stress (Figure 6b). In the roots, CAT activity increased following exposure to higher PEG and NaCl concentrations, and did not change at the lower ones (Figure 6b). The accumulation of phenolic compounds, in relation to the control seedlings, enhanced in the shoots cultivated on the media with PEG and lower NaCl concentration (Figure 6c). No differences between the stressors and concentrations were noted. In the roots, phenol content increased in the seedlings cultivated on the media with NaCl and higher PEG concentration (Figure 6c). Greater accumulation of phenols was observed in the roots of the seedlings growing on the media with lower NaCl concentration vs. roots from the media with PEG generating the same osmotic potential (Figure 6c). The total antioxidant capacity dropped in the shoots of seedlings growing at higher concentration of NaCl and PEG (Figure 6d). No significant differences were noted between the corresponding treatments. In contrast to this, the antioxidant capacity was significantly higher in the roots under NaCl-induced stress than under the corresponding PEG-induced stress (Figure 6d).
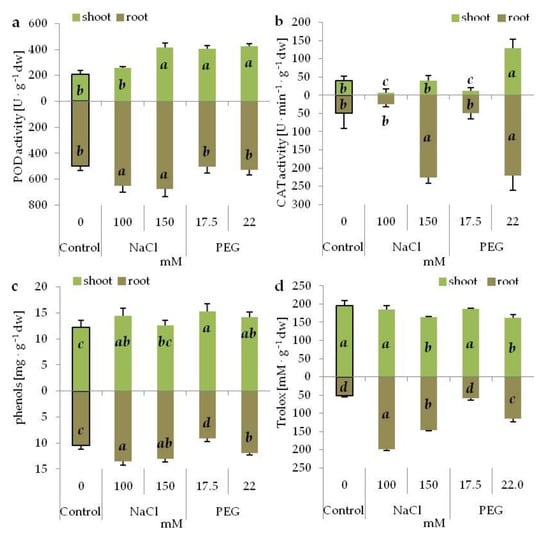
Figure 6.
Peroxidase (POD) (a) and catalase (CAT) (b) activity, phenolic compound content (c) and total antioxidant capacity (as Trolox equivalent) (d) in the shoots and roots of 14-day old grass pea seedlings under NaCl and PEG-induced stress. Different letters in italic denote significant differences at p ≤ 0.05 within one organ.
3.8. Principal Component Biplot Analysis
PCA biplots were generated for shoots and roots separately. They indicated that both principal components explained almost 84% of the total variance in the shoots, with the first principal component (PC1) explaining 71.4%, and the second (PC2) only 12.22% (Figure 7a). The analysis revealed clear differences in grass pea shoot responses depending on the medium used, as distinguished by PC1. Under drought stress induced by PEG, shoot response was rather similar, regardless of the stressor concentration. Under salt stress, a lower NaCl concentration resulted in a similar plant response as the control, while a higher sodium chloride concentration triggered a different reaction, as distinguished by PC1 and PC2 (Figure 7a). The PCA biplots explained also almost 75% (PC1: 43.65%, PC2: 31.03%) of the total variance in the roots (Figure 7b). Plant roots reacted similarly, regardless of the stressor concentration, with a clear difference in reaction between PEG and NaCl, as explained by the second principal component (PC2) (Figure 7b). Furthermore, the analysis revealed that the mechanism of response to drought stress in shoot cells was associated with an increase in the content of proline, ODAP, soluble and insoluble sugars and POD activity. Additionally, these parameters correlated negatively with the content of photosynthetic pigments and fresh weight (Figure 7a). The response to the higher NaCl level involved CAT activity. The analysis also revealed positive correlations between ODAP, proline and sugars, and negative correlations between CAT activity and total antioxidant capacity (Figure 7a). In the roots, the response to salinity stress manifested in increased POD activity, phenol accumulation and total antioxidant capacity, which were also positively correlated (Figure 7b). In turn, root response to drought stress manifested in increased soluble sugar accumulation and MDA content (Figure 7b). Furthermore, in the case of more intense stress (both drought and salinity), the roots responded similarly by increasing accumulation of proline, ODAP, insoluble sugar, dry weight and activity of CAT (Figure 7b).
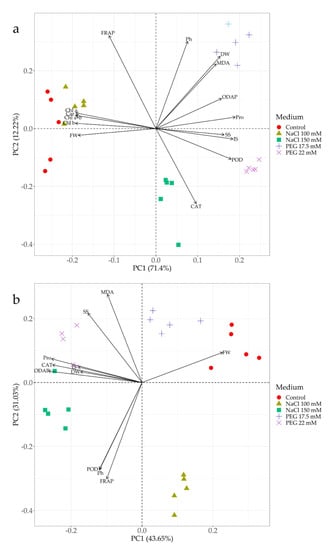
Figure 7.
Principal component analysis biplots showing relationships between the medium and biochemical parameters in the shoots (a) and roots (b) of 14-day old grass pea seedlings. Chl a: chlorophyll a, Chl b: chlorophyll b, Chl a+b: total chlorophylls, Car: carotenoids, MDA: malonyldialdehyde, SS: soluble sugar, IS: insoluble sugar, ODAP: β-N-oxalyl-L-α,β-diamino propionic acid, CAT: catalase, POD: peroxidase, Ph: phenolic compounds., Pro: proline, FW: fresh weight, DW: percentage of dry weight content., FRAP: total antioxidant capacity.
4. Discussion
Germination is considered the most critical stage in a plant life cycle; the process is highly sensitive to stresses, mainly abiotic ones. It starts with the uptake of water (imbibition), and completes with the appearance of a radicle [39]. Germination is a very complex process during which seeds need to quickly switch from dormancy to accelerated metabolic activity to enable the appearance of the radicle, followed by a plumule [40]. Imbibition consists of rapid water uptake until the complete hydration of all cell components and the reactivation of metabolism have occurred, allowing for further stages of germination. Thus, environmental conditions limiting water availability, such as drought, salinity or freezing, negatively affect this key process [41]. Many authors, who compared germination efficiency under stress induced by NaCl and PEG, observed a stronger reduction of germination rates in the presence of PEG, compared to NaCl [42,43,44,45,46]. In our study, no significant differences were observed in grass pea seed germination on media with the same osmotic potential generated by various stressors (NaCl and PEG). Our results do not confirm the observation of Zhang et al. [43], i.e., that sodium ions can act as an osmotic regulator that increases water uptake by germinating seeds. Moreover, Na+ ions may have a toxic effect on seed germination. Desiccation of seeds upon entering dormancy, and subsequent changes related to seed rehydration during imbibition, generate free radicals and damage cellular components. After the seed rehydration phase, the seed cells synthesize enzymes associated with the repair and reduction of cellular damage that can be adversely affected by sodium ions [40]. The different outcomes of previous studies may also have resulted from different experimental techniques. The use of in vitro cultures requires seed sterilization by soaking in aqueous solutions for several to a few dozen minutes. Grass pea seeds have to be sterilized for over 30 min [47,48,49,50]; in this way, preliminary imbibition already occurs during sterilization. Under stressful conditions, this may facilitate the transition to further germination stages, but may also delay them. The delay would be due to the increased water potential of the seeds impeding the further uptake of water from an environment with lower water potential. This was observed in our study at higher concentrations of NaCl and PEG. Moreover, the mobilization of storage material from the cells and the reactivation of cell energy metabolism control the seed transition to the next (final) stage of germination. This germination stage is often inhibited by harsh environmental factors. The final stage of germination (sensu stricto) is the elongation of a radicle and its breaking through the seed coat; this depends on the growth of the embryo cells (radicle and surrounding tissues), which is executed by water absorption [40,51]. That is why seed germination decline is so often observed in the conditions preventing water uptake from the environment [24].
Some authors report that the germination capacity of seeds under stress may better reflect seedling growth, followed by better plant yield [44]. In our research, however, the percentage of seedling emergence was lower (between 10 to 50%), relative to the percentage of germinating seeds. Other authors also noted that both salinity and drought had a more negative impact on the growth of a hypocotyl than of a radicle [45,46,52]. The postgermination stage of shoot growth commences with intensive cell divisions that are very sensitive to water deficit [40]. Interestingly, also for this parameter, no significant differences were spotted between NaCl and PEG-induced stress, which may suggest that the observed plant response is a reaction to osmotic, rather than chemical, stress. Still, dividing cells should be more sensitive to adverse ion ratios, due to a lack of a well-developed vacuole into which harmful ions could be discharged [51].
The physiological process most sensitive to water deficit is cell growth. Water stress is a highly limiting factor in the initial phase of growth, because low turgor pressure inhibits cell growth and development [53]. The stressors applied in our study significantly affected shoot and root length and weight after two weeks of culture. In this case, our observations were consistent with those of other authors, who reported a stronger inhibition of organ growth under stress imitating drought than salinity [42,44,46,54,55]. According to Munns [17], the harmful effects of salinity stress involve primarily water (osmotic) stress, and chemical stress only after a few days, resulting from the accumulation of harmful ions. One of the most important adaptations of plants to water shortage is osmotic adjustment [56,57,58,59]. Osmotic adjustment, consisting of the accumulation of organic and inorganic water-soluble compounds, has two main roles to fulfil: osmoregulation and osmoprotection [56,58]. Osmoregulation involves the accumulation of osmotically active compounds to reduce the osmotic potential and to maintain the cell turgor necessary for the proper course of many physiological processes [58]. In addition, under salinity stress, this process may be associated with either complete exclusion of toxic sodium and chlorine ions, or their accumulation, but only to a level that is nontoxic to cells [59]. Our results indicate that salinity stress is less harmful to grass pea seedling growth than drought stress. One possible explanation is the reduction of root osmotic potential, due to the accumulation of Na+ ions, which allows plants to absorb water more efficiently and continue uninhibited growth [51]. High content of Na+ ions, both in the roots and shoots of the examined grass pea seedlings, indicated that this parameter controlled the effective seedling growth under salinity. Such an effect was not observed in the case of PEG 6000, because its high molecular weight does not allow its transport via cell wall pores and into the protoplast [41]. As a result, water is withdrawn not only from the protoplast, but also from the cell wall causing cytorrhysis, not plasmolysis, which is observed in the case of drought in natural conditions [41]. On the other hand, the accumulation of Na+ ions, and to a lesser extent, also Cl-, has a disastrous effect on cell growth and function over time [16,23,59]. Consequently, an effective mechanism of ion exclusion and the compartmentation of harmful ions in the vacuole, increasing with growing cells, and/or preclusion of ion transport to seedling shoots, has to occur [16,17]. A high content of Na+ ions in the shoots of cv. ‘Krab’ grass pea seedlings, together with milder growth inhibition effects, suggest the occurrence of an effective mechanism of ion compartmentation. Our results also revealed no limitations in ion transport to the shoots, despite reduced transpiration in the tissue cultures [41].
Crops respond to salinity or drought stress by changes in dry matter content [24,46,50,60,61], which is considered one of the key indicators in assessments of plant tolerance to abiotic stress [60]. Dry matter production in plants is a result of a complex physiological process whose main substrates are carbohydrates. Their synthesis occurs primarily during photosynthesis, but also in alternative cycles such as gluconeogenesis [62,63]. Disorders affecting photosynthesis and resulting from water (osmotic) and chemical stress significantly impair the production of assimilates and their distribution in the plant. Often, the main organs using sugars from primary and secondary production are the roots, in which osmotically active compounds are produced to change the water potential of cells in order to maintain water uptake from the environment [61,63]. Thus, an increase in dry matter content is often treated as a feature typical of plants tolerant to water and chemical stress [24,50]. Disturbances in carbohydrate metabolism under stress conditions may lead to a negative carbon balance, unless at the same time there is a reduction of growth [63]. In the presented research, dry matter content increase was observed in the shoots and roots of grass pea in conditions imitating drought (PEG-induced stress), as compared with control conditions. Moreover, the dry matter content in these seedlings was significantly higher than in the seedlings growing at corresponding NaCl concentrations. Such a significant increase in dry matter content, in both the above- and underground parts of the seedlings growing in drought-imitating conditions, most likely resulted from the production of osmoregulatory compounds (e.g., sugars, proline, glycine betaine), as well as osmoprotective and antioxidant compounds (e.g., phenolic compounds) [64]. At the same time, as mentioned earlier, these seedlings showed a stronger inhibition of shoot and root growth in relation to the control, and shoot growth in relation to the corresponding NaCl concentrations. To synthesize 1 mole of an osmolyte, a plant requires much more ATP molecules than to accumulate 1 mole of NaCl, and ATP synthesis this takes place at the expense of plant growth [16,59]. Moreover, a lower increase of dry matter content in the seedlings growing under salinity additionally confirmed the role of Na+ ions as osmoregulators.
To determine plant sensitivity to stress conditions, cell membrane integrity is often assessed by evaluating lipid peroxidation [65,66]. The unsaturated membrane lipids of plant cells are attacked by ROS overproduced during stress and leading to lipid oxidation (peroxidation) [67]. Peroxidation breaks down the unsaturated lipids resulting in, among others, the formation of malonyldialdehyde (MDA) [65,67]. The increased accumulation of MDA indicates intense lipid peroxidation, and is often interpreted as a symptom of impaired antioxidant system activity [65,68,69]. In the presented research, the content of MDA remained fairly stable in both organs (shoots and roots) and under both stresses (salinity and drought). This indicates either effective scavenging of free radicals by the antioxidant system, or the prevention of free radical production. However, it seems that the mechanisms that protect membranes from excessive lipid peroxidation are different, depending on the type of stress and organ, and can be associated with increased phenol accumulation or peroxidase activity.
The sensitivity of plants to stress conditions is very often evaluated by photosynthetic pigment content analysis, which allows for an initial assessment of the photosynthetic apparatus condition [16,70]. A decrease in the photosynthetic pigment content under conditions of water scarcity, both due to drought and salinity, results primarily from disorders in their synthesis [71,72,73]. A number of enzymes are involved in the synthesis of plant pigments [74]. Studies indicate that both drought and salinity reduce the content of 5-aminolevulinic acid (ALA), a precursor of porphyrins and, consequently, chlorophylls, by interfering with ALA dehydrogenase [71,72,73]. Dalal and Tripathy [72] reported that under stress, plants reduce the expression of genes associated with porphyrin biosynthesis, preventing the production of intermediates that could generate free radicals. Pigment synthesis disturbances may also result from impaired enzyme activity as a result of disturbances in the ionic homeostasis of cells, affecting the availability of enzyme cofactors [75,76]. In the shoots of grass pea, the content of photosynthetic pigments decreased under both NaCl- and PEG-induced stress. A significantly greater decrease was observed under drought-imitating conditions than under salinity. Under NaCl-induced stress, cells are most likely capable of uptaking water for longer periods, due to the better osmotic adjustment resulting from (Na+) ion accumulation [59]. This way, they can protect the enzymes involved in pigment biosynthesis from dehydration and/or ensure ion homeostasis. The drop in pigment content as a result of chlorophyll synthesis disorders, not degradation, was also confirmed by no changes in Chl a/b ratio. During the process of chlorophyll degradation, Chl b is transformed into Chl a, and this changes the ratio of these pigments [71].
One of the most important functions of the photosynthetic apparatus is the production of carbohydrates that are indispensable for plant primary and secondary metabolism [77]. Under stress conditions, carbohydrates are accumulated and used, e.g., for the osmotic adjustment [56,57]. In addition to the aforementioned osmoregulation, osmotic adjustment also plays a protective (osmoprotective) role by accumulating compounds that stabilize proteins, especially enzymes, or participating in scavenging of newly formed free radicals, thus protecting the plasma membrane integrity [78,79]. In the present study, a significant increase in soluble sugar content was observed in the shoots of grass pea growing under conditions of PEG-induced stress. In the case of lower salinity, other compounds or ions participated in the osmotic adjustment of shoot cells, as evidenced by the shoot growth rate and lower content of soluble sugars. Generally, the production of osmotically active compounds depends on the efficiency of photosynthesis and/or gluconeogenesis activity under stress conditions, and their accumulation in the roots from the partitioning of assimilates [80]. The increase in soluble sugar content in the roots was slightly higher on the medium with PEG than NaCl. No changes in the content of insoluble sugars, with one exception, were noted. A significant increase in insoluble sugar content and no change in the ratio of soluble to insoluble sugars was observed in the roots on the medium with higher NaCl concentration, which may suggest effective osmotic adjustment mediated by Na+. In addition, the increment in insoluble sugar content indicated an increase of other polysaccharides content, e.g., the ones building the cell wall, which may act as free radical scavengers [81] or regulate cell growth [82]. In addition to their osmoregulatory function, carbohydrates can also serve as osmoprotectants [58]. Simple sugars, together with plant hormones, create a signal network in plants participating not only in the cell cycle and cell division, but also enabling plant response to emerging stress. Moreover, sugars are believed to play an important role in scavenging hydroxyl radicals (OH•) through the Fenton reaction in the presence of Fe2+ ions [83]. Regardless of the strategy chosen, effective protection against the negative effects of both ionic and osmotic stress was observed in the roots.
The neurotoxin ODAP is a characteristic compound for grass pea [84]. Grass pea breeding has sought to produce cultivars and lines with the lowest content of ODAP [7,10,85]. Meanwhile, some research studies have suggested changes in the content of this compound under the influence of environmental stresses, indicating that it can play a significant role in plant response to abiotic stress [86,87,88,89,90,91,92,93]. Drought stress, most often induced by PEG, increased the ODAP content in various organs and tissues [86,88,89,90,91]. Under salinity stress, greater variability was observed in ODAP accumulation patterns. The content of ODAP decreased at lower concentrations of NaCl (below 0.4%), but it increased at higher ones (above 0.4%) [86]. In our study, a higher accumulation of ODAP was also observed in both the shoots and roots of grass pea seedlings under PEG-induced stress. Furthermore, salinity stress did not reduce ODAP content either in shoots or roots, and did not change or increased it in the roots under higher NaCl concentration. It is believed that ODAP, as a soluble nonprotein amino acid, can act as an osmoregulator [81,88], or even as free hydroxyl radical (OH•) scavenger [94].
Under abiotic stress, plant cells struggle to maintain a balance between energy production and consumption. Imbalances, occurring mainly in chloroplasts and mitochondria, result in excessive production of ROS and free radicals [95,96]. Plant cells cope with the excess by activating and synthesizing de novo antioxidants, both enzymatic and nonenzymatic [97].
Under stress conditions, the efficiency of the dark reactions (Calvin cycle) of photosynthesis is reduced, leading to the photo-oxidative generation of huge amounts of ROS. To protect cells against the uncontrolled generation of oxygen free radicals, plants increase the rate of photorespiration, preventing limitation on the PSII acceptor side. However, as a result of intensive photorespiration in peroxisomes, H2O2 is intensively produced. CAT is an enzyme that very effectively and efficiently scavenges hydrogen peroxide molecules, and its high activity was observed in the leaves of many plant species as a response to osmotic stress [96,98]. At the same time, catalase activity may also be associated with lignification [99,100]. Grass pea shoots showed intense catalase activity only at higher PEG concentrations, indicating massive production of H2O2 during drought stress, which was not observed in the case of severe salinity stress. At the same time, higher concentrations of NaCl and PEG induced high CAT activity in the roots. In the case of seedlings growing under NaCl-induced stress, increased CAT activity, together with increased accumulation of phenolic compounds and POD activity, may indicate a progressive process of root lignification.
Peroxidases (ascorbate, glutathione, guaiacol) belong to the most common enzymatic antioxidants [101]. The method used in this study to determine the enzyme activity using p-phenylenediamine as a substrate is useful in the determination of guaiacol peroxidase (POD) activity [24,102]. Guaiacol peroxidase, also called phenolic peroxidase, is involved in H2O2 scavenging, as well as in lignin biosynthesis, and therefore, in cell growth and development [101]. Different POD isoforms occur in the cytosol, vacuoles, and primarily in the cell wall. It is believed that the latter take part in plant defence responses to various stresses [102]. In our research, POD activity increased in both shoots and roots under stress conditions. The increase was greater in shoots exposed to PEG-induced stress, and in the roots under NaCl-induced stress. Enhanced POD activity in the shoots most likely results from stronger stress, as evidenced by stronger growth reduction. Increased POD activity in the roots may result from the intensification of lignin biosynthesis in the cell walls that can permanently bind Na+ ions [81]. This was also confirmed by the observation of increased accumulation of phenolic compounds in these roots.
Nonenzymatic components of the antioxidant system comprise glutathione, ascorbate, tocopherol, carotenoids, and phenolic compounds [103]. Phenolic compounds belong to a very wide family of secondary metabolites synthesized in the shikimic acid pathway [104]. It is estimated that about 20% of carbon assimilated in photosynthesis is directed to the phenylpropanoid pathway [103]. Studies indicate the importance of phenolic compounds in alleviating the negative effects of abiotic stresses [105,106,107]. An increase in drought and salinity tolerance was observed, along with an increase in phenolic compound content in both wild and crop plants [108,109,110]. The antioxidant properties of phenolic compounds result from their structure, which makes them excellent reducing agents, as well as from the possibility of chelating metal ions, which prevents uncontrolled redox reactions [106]. Phenolic compounds can also react with free radicals, becoming phenoxyl radicals, which, thanks to their stable structure, stop radical propagation and terminate the radical chain reaction [106]. Phenolic compounds also participate in the synthesis of components building the secondary cell wall, increasing its thickness and, at the same time, resistance to stress [105]. The increase in the content of phenolic compounds that we witnessed in plant roots exposed to NaCl, especially at lower concentration, and in comparison with PEG-induced stress, indicated that these compounds play important roles in the mechanism of root resistance to salinity stress. Most likely, they participated in the chelation of Na+ ions mainly in the cell wall. A similar increase in phenols in plants treated with both stressors, as compared with the control, indicated that shoot phenolic compounds acted as osmoprotectants during salinity and drought stress.
Apart from phenols, other low molecular compounds may also play an important role in scavenging free radicals or repairing their harmful effects. Therefore, a FRAP assay was used to estimate the total antioxidant capacity. This method allows for quick assessments of the total antioxidant activity of low molecular weight antioxidants including phenols, but also ascorbic acid and simple sugars [111,112]. Generally, our results indicated that the activity of low molecular weight antioxidants was of greater importance in the roots than in the shoots of stressed seedlings. Furthermore, the observed alterations point to a greater contribution of low molecular weight antioxidants to the acclimatization of grass pea roots to salinity than to drought stress. However, strong correlation between FRAP and phenol accumulation, as revealed by PCA analyses, indicated a crucial antioxidant role of phenols.
Different stresses imply disturbances of anabolic process efficiency, leading to a reduction in carbon skeleton synthesis during photosynthesis. Moreover, stress imposes the allocation of these carbon skeletons from primary metabolism to secondary metabolite production, and leads to the synthesis of secondary metabolites involved in plant resistance and/or tolerance mechanisms [113]; one such secondary metabolite is proline [114]. The role of proline and the mechanism of its action are not clear. Over the years, proline has been treated as an osmotically active substance, responsible for the rapid and effective reduction of the cell water potential [114]. Many studies pointed to its marginal role in the cell osmotic adjustment [115,116,117]. It seems that under stress conditions, the mechanism of proline action is associated with the protection of enzymes and cellular membranes. Due to its kosmotropic properties, proline protects key enzymes against conformational changes induced by dehydration under osmotic and/or salt stress. Additionally, it indirectly reduces the effects of oxidative stress by protecting antioxidant enzymes against denaturation [118]. The higher accumulation of proline in the shoots and roots of grass pea seedlings under PEG-induced stress indicated its greater importance in the acclimatization of grass pea to osmotic than to chemical stress. Proline, in addition to its role in stabilizing tertiary and quaternary structures of enzymatic and structural proteins, is also an important element of osmotin and late embryogenesis abundant (LEA) proteins. These proteins are part of a large protein system involved in plant response to abiotic stresses, including drought and salinity [119,120]. Under stress conditions, the action of these proteins consists of the regulation of the osmotic adjustment by intracellular compartmentation and the redistribution of osmolytes, changes in the metabolic rate, as well as increasing cell wall elasticity and membrane permeability [121]. Moreover, under salinity stress, these proteins are responsible for the redistribution and complementation of Na+ in vacuoles and/or in the cytosol, as well as the distribution of sodium ions to the apoplast and its sequestration in the cell wall [122,123,124]. Increasing knowledge of the importance of these proteins in the mechanisms of plant acclimatization to abiotic stress underlines the importance of analyses of osmotin and LEA proteins in the future research of the response mechanisms of grass pea to stresses.
5. Conclusions
Our experiment on NaCl and PEG stressors at concentrations generating the same osmotic potential of the medium revealed differences in the mechanisms of grass pea response to salinity and drought stress. Grass pea cv. ‘Krab’ was characterized by greater tolerance to salinity (NaCl induced) than to drought. The mechanism of grass pea response to salinity stress seems to be primarily associated with an effective distribution of Na+ ions, which can be used as osmotically active compounds, ensuring adequate hydration of cells, and reducing the need for excessive production of osmoprotectants (lower proline accumulation), thus not limiting the growth and development of the seedlings. Moreover, increasing phenol and insoluble sugar accumulation, as well as the lack of severe stress symptoms (low MDA accumulation), may indicate the sequestration of excessive Na+ ions in cell walls. In contrast, grass pea acclimatization to drought depends mainly on the mechanism of osmotic adjustment, using soluble sugars as osmoregulators, and proline as well as probably ODAP as osmoprotectants, at the expense of plant growth and development. It is believed that in the initial stages, salinity stress is also water (osmotic) stress, and that plant responses follow the same mechanisms in both cases. However, our findings indicate that grass pea exhibits a clearly differentiated response to salinity and drought stress from the very beginning of its vegetation.
Author Contributions
Conceptualization, B.T. and K.M.T.; validation, B.T. and T.W.; formal analysis, B.T. and T.W.; investigation, B.T., W.M. and R.J.J.; methodology, R.J.J.; writing—original draft preparation, B.T., R.J.J. and K.M.T.; writing—review and editing, B.T., T.W. and K.M.T.; visualization, B.T. and T.W.; supervision, B.T.; project administration, B.T. All authors have read and agreed to the published version of the manuscript.
Funding
The research was supported by a subsidy (SUB/2019-0511000000-D507) of the Ministry of Science and Higher Education of the Republic of Poland aimed at maintenance and development of research potential of the Department of Botany, Physiology and Plant Protection.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Maphosa, Y.; Jideani, V.A. The role of legumes in human nutrition. In Functional Food-Improve Health through Adequate Food; Hueda, M.C., Ed.; In Tech d.o.o.: Rijeka, Croatia, 2017; Volume 1, pp. 103–121. [Google Scholar]
- Martín-Cabrejas, M.A. Legumes: An overview. In Legumes: Nutritional Quality, Processing and Potential Health Benefits; Martín-Cabrejas, M.A., Ed.; CPI Group (UK) Ltd.: Croydon, UK, 2019; pp. 1–18. [Google Scholar]
- Vaz Patto, M.C.; Rubiales, D. Lathyrus diversity: Available resources with relevance to crop improvement–L. sativus and L. cicera as case studies. Ann. Bot. 2014, 113, 895–908. [Google Scholar] [CrossRef] [PubMed]
- Kislev, M.E. Origins of the cultivation of Lathyrus sativus and L. cicera (Fabaceae). Econ. Bot. 1989, 43, 262–270. [Google Scholar] [CrossRef]
- Grela, E.R.; Kiczorowska, B.; Samolińska, W.; Matras, J.; Kiczorowski, P.; Rybiński, W.; Hanczakowska, E. Chemical composition of leguminous seeds: Part I—Content of basic nutrients, amino acids, phytochemical compounds, and antioxidant activity. Eur. Food Res. Technol. 2017, 243, 1385–1395. [Google Scholar] [CrossRef]
- Rybiński, W.; Karamać, M.; Sulewska, K.; Börner, A.; Amarowicz, R. Antioxidant potential of grass pea seeds from European Countries. Foods 2018, 7, 142. [Google Scholar] [CrossRef]
- Campbell, C.G.; Mehra, R.B.; Agrawal, S.K.; Chen, Y.Z.; Abd El Moneim, A.M.; Khawaja, H.I.T.; Yadov, C.R.; Tay, J.U.; Araya, W.A. Current status and future strategy in breeding grasspea (Lathyrus sativus). Euphytica 1994, 73, 167–175. [Google Scholar] [CrossRef]
- Singh, S.P.; Dhiraj, B.; Ajit, P.; Nirmal, V. An epidemiological study on incidence and determinants of Lathyrism. J. Community Health Manag. 2016, 3, 113–122. [Google Scholar] [CrossRef]
- Khandare, A.L.; Kumar, R.H.; Meshram, I.I.; Arlappa, N.; Laxmaiah, A.; Venkaiah, K.; Rao, P.A.; Validandi, V.; Toteja, G.S. Current scenario of consumption of Lathyrus sativus and lathyrism in three districts of Chhattisgarh State, India. Toxicon 2018, 150, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Vaz Patto, M.C.; Skiba, B.; Pang, E.C.K.; Ochatt, S.J.; Lambein, F.; Rubiales, D. Lathyrus Improvement for resistance against biotic and abiotic stresses: From classical breeding to marker assisted selection. Euphytica 2006, 147, 133–147. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Akram, N.A.; Ashraf, M.; Alyemeni, M.N.; Wijaya, L.; Ahmad, P. Plant responses to environmental stresses—From gene to biotechnology. AoB Plants 2017, 9, plx025. [Google Scholar] [CrossRef] [PubMed]
- Saxena, R.; Kumar, M.; Tomar, R.S. Plant responses and resilience towards drought and salinity stress. Plant Arch. 2019, 19, 50–58. [Google Scholar]
- Garg, R.; Shankar, R.; Thakkar, B.; Kudapa, H.; Krishnamurthy, L.; Mantri, N.; Varshney, R.K.; Bhatia, S.; Jain, M. Transcriptome analyses reveal genotype-and developmental stage-specific molecular responses to drought and salinity stresses in chickpea. Sci. Rep. 2016, 6, 19228. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Gilliham, M. Salinity tolerance of crops–what is the cost? New Phytol. 2015, 208, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Taiz, L.; Zeiger, E. Plant Physiology, 5th ed.; Sinauer Associates Inc.: Sunderland, MA, USA, 2010; pp. 591–623. [Google Scholar]
- Munns, R. Plant adaptations to salt and water stress. In Plant Responses to Drought and Salinity Stress—Developments in a Post-Genomic Era; Turkan, I., Ed.; Academic Press: Boston, MA, USA, 2011; Volume 57, pp. 1–32. [Google Scholar]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; James, R.A.; Sirault, X.R.; Furbank, R.T.; Jones, H.G. New phenotyping methods for screening wheat and barley for beneficial responses to water deficit. J. Exp. Bot. 2010, 61, 3499–3507. [Google Scholar] [CrossRef] [PubMed]
- Salehi-Lisar, S.Y.; Bakhshayeshan-Agdam, H. Drought stress in plants: Causes, consequences, and tolerance. In Drought Stress Tolerance in Plants; Hossain, M.A., Wani, S.H., Bhattacharjee, S., Burritt, D.J., Tran, L.S.P., Eds.; Springer: Cham, Germany, 2016; Volume 1, pp. 1–16. [Google Scholar]
- Kaur, G.; Asthir, B. Molecular responses to drought stress in plants. Biol. Plant 2017, 61, 201–209. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Isayenkov, S.V.; Maathuis, F.J. Plant salinity stress: Many unanswered questions remain. Front. Plant Sci. 2019, 10, 80. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Piwowarczyk, B.; Tokarz, K.; Kamińska, I. Responses of grass pea seedlings to salinity stress in in vitro culture conditions. Plant Cell Tissue Organ Cult. 2016, 124, 227–240. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Kawasaki, T.; Shimizu, G.; Moritsugu, M. Effects of high concentrations of sodium chloride and polyethylene glycol on the growth and ion absorption in plants. Plant Soil 1983, 75, 87–93. [Google Scholar] [CrossRef]
- Dhindsa, R.H.; Plumb-Dhindsa, R.; Thorpe, T.A. Leaf senescence correlated with increased level of membrane permeability, lipid peroxidation and decreased level of SOD and CAT. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Method Enzymol. 1987, 148, 350–356. [Google Scholar]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Yemm, E.W.; Willis, A. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Addis, G.; Narayan, R.K.J. Developmental Variation of the Neurotoxin, β-N-Oxalyl-l-α, β-diamino propionic acid (ODAP), in Lathyrus sativus. Ann. Bot. 1994, 74, 209–215. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldern, R.P.; Teare, I.D. Rapid determination of free proline from water stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Lűck, H. Peroxidase. In Methoden der Enzymatischen Analyse; Bergmeyer, H.U., Ed.; Verlag Chemie: Weinheim, Germany, 1962; pp. 895–897. [Google Scholar]
- Bartosz, G. Another Side of Oxygen. Free Radicals in Nature; PWN: Warsaw, Poland, 2006. (In Polish) [Google Scholar]
- Swain, T.; Hillis, W.E. Phenolic constituents of Prunus domestica. I. Quantitative analysis of phenolic constituents. J. Sci. Food Agric. 1959, 10, 63–68. [Google Scholar] [CrossRef]
- Benzie, I.; Strain, J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org (accessed on 6 April 2020).
- Tang, Y.; Horikoshi, M.; Li, W. Ggfortify: Unified interface to visualize statistical result of popular R packages. R J. 2016, 8, 474–485. [Google Scholar] [CrossRef]
- Rajjou, L.; Duval, M.; Gallardo, K.; Catusse, J.; Bally, J.; Job, C.; Job, D. Seed germination and vigor. Annu. Rev. Plant Biol. 2012, 63, 507–533. [Google Scholar] [CrossRef] [PubMed]
- Nonogaki, H.; Bassel, G.W.; Bewley, J.D. Germination—Still a mystery. Plant Sci. 2010, 179, 574–581. [Google Scholar] [CrossRef]
- Verslues, P.E.; Agarwal, M.; Katiyar-Agarwal, S.; Zhu, J.; Zhu, J.K. Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status. Plant J. 2006, 45, 523–539. [Google Scholar] [CrossRef] [PubMed]
- Patanè, C.; Saita, A.; Sortino, O. Comparative effects of salt and water stress on seed germination and early embryo growth in two cultivars of sweet sorghum. J. Agron. Crop Sci. 2013, 199, 30–37. [Google Scholar] [CrossRef]
- Zhang, H.; Irving, J.; McGill, C.; Mathhew, C.; Zhou, D.; Kemp, P. The effects of salinity and osmotic stress on barley germination rate: Sodium as an osmotic regulator. Ann. Bot. 2010, 106, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Petrović, G.; Jovičić, D.; Nikolić, Z.; Tamindžić, G.; Ignjatov, M.; Milošević, D.; Milošević, B. Comparative study of drought and salt stress effects on germination and seedling growth of pea. Genetika 2016, 48, 373–381. [Google Scholar] [CrossRef]
- Kaya, M.; Kaya, G.; Kaya, M.D.; Atak, M. Interaction between seed size and NaCl on germination and early seedling growth of some Turkish cultivars of chickpea (Cicer arietinum L.). J. Zhejiang Univ. Sci. B 2008, 9, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Okcu, G.; Kaya, M.D.; Atak, M. Effects of salt and drought stresses on germination and seedling growth of pea (Pisum sativum L.). Turk. J. Agric. For. 2005, 29, 237–242. [Google Scholar]
- Piwowarczyk, B.; Pindel, A. Determination of an optimal isolation and culture conditions of grass pea protoplasts. Biotechnologia 2015, 96, 192–202. [Google Scholar] [CrossRef]
- Wiszniewska, A.; Piwowarczyk, B. Studies on cell wall regeneration in protoplast culture of legumes-the effect of organic medium additives on cell wall components. Czech J. Genet. Plant Breed. 2014, 50, 84–91. [Google Scholar] [CrossRef]
- Piwowarczyk, B.; Kaminska, I.; Rybinski, W. Influence of PEG generated osmotic stress on shoot regeneration and some biochemical parameters in Lathyrus culture. Czech J. Genet. Plant Breed. 2014, 50, 77–83. [Google Scholar] [CrossRef]
- Piwowarczyk, B.; Tokarz, K.; Makowski, W.; Łukasiewicz, A. Different acclimatization mechanisms of two grass pea cultivars to osmotic stress in in vitro culture. Acta Physiol. Plant. 2017, 39, 96. [Google Scholar] [CrossRef]
- Alam, M.Z.; Stuchbury, T.; Naylor, R.E.L. Effect of NaCl and PEG induced osmotic potentials on germination and early seedling growth of rice cultivars differing in salt tolerance. Pak. J. Biol. Sci. 2002, 5, 1207–1210. [Google Scholar]
- Mokhberdoran, F.; Kalat, S.N.; Haghighi, R.S. Effect of temperature, iso-osmotic concentrations of NaCl and PEG agents on germination and some seedling growth yield components in rice (Oryza sativa L). Asian J. Plant Sci. 2009, 8, 409–416. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Manivannan, P.; Wahid, A.; Farooq, M.; Al-Jubur, H.J.; Somasundaram, R.; Panneerselvam, R. Drought stress in plants: A review on morphological characteristics and pigments composition. Int. J. Agric. Biol. 2009, 11, 100–105. [Google Scholar]
- Shitole, S.M.; Dhumal, K.N. Effect of water stress by polyethylene glycol 6000 and sodium chloride on seed germination and seedling growth of Cassia angustifolia. Int. J. Pharmaceut. Sci. Res. 2012, 3, 528–531. [Google Scholar]
- Kaydan, D.; Yagmur, M. Germination, seedling growth and relative water content of shoot in different seed sizes of triticale under osmotic stress of water and NaCl. Afr. J. Biotechnol. 2008, 7, 2862–2868. [Google Scholar]
- Blum, A. Osmotic adjustment is a prime drought stress adaptive engine in support of plant production. Plant Cell Environ. 2017, 40, 4–10. [Google Scholar] [CrossRef]
- Turner, N.C. Turgor maintenance by osmotic adjustment: 40 years of progress. J. Exp. Bot. 2018, 69, 3223–3233. [Google Scholar] [CrossRef]
- Zivcak, M.; Brestic, M.; Sytar, O. Osmotic adjustment and plant adaptation to drought stress. In Drought Stress Tolerance in Plants; Hossain, M.A., Wani, S.H., Bhattacharjee, S., Burritt, D.J., Tran, L.S.P., Eds.; Springer: Cham, Germany, 2016; Volume 1, pp. 105–143. [Google Scholar]
- Munns, R.; Passioura, J.B.; Colmer, T.D.; Byrt, C.S. Osmotic adjustment and energy limitations to plant growth in saline soil. New Phytol. 2020, 225, 1091–1096. [Google Scholar] [CrossRef] [PubMed]
- Munns, R. Genes and salt tolerance: Bringing them together. New Phytol. 2005, 167, 645–663. [Google Scholar] [CrossRef]
- Eziz, A.; Yan, Z.; Tian, D.; Han, W.; Tang, Z.; Fang, J. Drought effect on plant biomass allocation: A meta-analysis. Ecol. Evol. 2017, 7, 11002–11010. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Hamid, A.; Khaliq, M.A. Evaluation of mungbean (Vigna radiata (L.) Wilczek) genotypes on the basis of photosynthesis and dry matter accumulation. J. Agric. Rural. Dev. 2009, 7, 1–8. [Google Scholar] [CrossRef][Green Version]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.B.S.M.A.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. In Sustainable Agriculture; Lichtfouse, E., Navarrete, M., Debaeke, P., Souchere, V., Alberola, C., Eds.; Springer: Dordrecht, Germany, 2009; pp. 153–188. [Google Scholar]
- Gill, P.K.; Sharma, A.D.; Singh, P.; Bhullar, S.S. Changes in germination, growth and soluble sugar contents of Sorghum bicolor (L.) Moench seeds under various abiotic stresses. Plant Growth Regul. 2003, 40, 157–162. [Google Scholar] [CrossRef]
- Gapińska, M.; Skłodowska, M.; Gabara, B. Effect of short-and long-term salinity on the activities of antioxidative enzymes and lipid peroxidation in tomato roots. Acta Physiol. Plant. 2008, 30, 11. [Google Scholar] [CrossRef]
- Nxele, X.; Klein, A.; Ndimba, B.K. Drought and salinity stress alters ROS accumulation, water retention, and osmolyte content in sorghum plants. S. Afr. J. Bot. 2017, 108, 261–266. [Google Scholar] [CrossRef]
- Davey, M.W.; Stals, E.; Panis, B.; Keulemans, J.; Swennen, R.L. High-throughput determination of malondialdehyde in plant tissues. Anal. Biochem. 2005, 347, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Ekinci, M.; Ors, S.; Turan, M.; Yildiz, S.; Yildirim, E. Effects of individual and combined effects of salinity and drought on physiological, nutritional and biochemical properties of cabbage (Brassica oleracea var. capitata). Sci. Hortic. 2018, 240, 196–204. [Google Scholar] [CrossRef]
- Dugasa, M.T.; Cao, F.; Ibrahim, W.; Wu, F. Differences in physiological and biochemical characteristics in response to single and combined drought and salinity stresses between wheat genotypes differing in salt tolerance. Physiol. Plant. 2019, 165, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Tokarz, K.M.; Makowski, W.; Tokarz, B.; Hanula, M.; Sitek, E.; Muszyńska, E.; Jędrzejczyk, R.; Banasiuk, R.; Chajec, Ł.; Mazur, S. Can Ceylon Leadwort (Plumbago zeylanica L.) Acclimate to Lead Toxicity?—Studies of Photosynthetic Apparatus Efficiency. Int. J. Mol. Sci. 2020, 21, 1866. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.V. Regulation of chlorophyll biosynthesis and degradation by salt stress in sunflower leaves. Sci. Hortic. 2004, 103, 93–99. [Google Scholar] [CrossRef]
- Dalal, V.K.; Tripathy, B.C. Modulation of chlorophyll biosynthesis by water stress in rice seedlings during chloroplast biogenesis. Plant Cell Environ. 2012, 35, 1685–1703. [Google Scholar] [CrossRef] [PubMed]
- Phung, T.H.; Jung, H.I.; Park, J.H.; Kim, J.G.; Back, K.; Jung, S. Porphyrin biosynthesis control under water stress: Sustained porphyrin status correlates with drought tolerance in transgenic rice. Plant Physiol. 2011, 157, 1746–1764. [Google Scholar] [CrossRef] [PubMed]
- Beale, S.I. Enzymes of chlorophyll biosynthesis. Photosynth. Res. 1999, 60, 43–73. [Google Scholar] [CrossRef]
- Nouet, C.; Motte, P.; Hanikenne, M. Chloroplastic and mitochondrial metal homeostasis. Trends Plant Sci. 2011, 16, 395–404. [Google Scholar] [CrossRef]
- Hanikenne, M.; Bernal, M.; Urzica, E.I. Ion homeostasis in the Chloroplast. In Plastid Biology. Advances in Plant Biology; Theg, S., Wollman, F.A., Eds.; Springer: New York, NY, USA, 2014; Volume 5, pp. 465–514. [Google Scholar]
- Piwowarczyk, B.; Tokarz, K.; Muszyńska, E.; Makowski, W.; Jędrzejczyk, R.; Gajewski, Z.; Hanus-Fajerska, E. The acclimatization strategies of kidney vetch (Anthyllis vulneraria L.) to Pb toxicity. Environ. Sci. Pollut. Res. 2018, 25, 19739–19752. [Google Scholar] [CrossRef] [PubMed]
- Wani, S.H.; Singh, N.B.; Haribhushan, A.; Mir, J.I. Compatible solute engineering in plants for abiotic stress tolerance-role of glycine betaine. Curr. Genom. 2013, 14, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Couée, I.; Sulmon, C.; Gouesbet, G.; El Amrani, A. Involvement of soluble sugars in reactive oxygen species balance and responses to oxidative stress in plants. J. Exp. Bot. 2006, 57, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Patakas, A.; Nikolaou, N.; Zioziou, E.; Radoglou, K.; Noitsakis, B. The role of organic solute and ion accumulation in osmotic adjustment in drought-stressed grapevines. Plant Sci. 2002, 163, 361–367. [Google Scholar] [CrossRef]
- Byrt, C.S.; Munns, R.; Burton, R.A.; Gilliham, M.; Wege, S. Root cell wall solutions for crop plants in saline soils. Plant Sci. 2018, 269, 47–55. [Google Scholar] [CrossRef]
- An, P.; Li, X.; Zheng, Y.; Matsuura, A.; Abe, J.; Eneji, A.E.; Tanimoto, E.; Inanaga, S. Effects of NaCl on root growth and cell wall composition of two soya bean cultivars with contrasting salt tolerance. J. Agron. Crop Sci. 2014, 200, 212–218. [Google Scholar] [CrossRef]
- Matros, A.; Peshev, D.; Peukert, M.; Mock, H.P.; Van den Ende, W. Sugars as hydroxyl radical scavengers: Proof-of-concept by studying the fate of sucralose in Arabidopsis. Plant J. 2015, 82, 822–839. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.Y.; Spencer, P.S.; Li, Z.X.; Liang, Y.M.; Wang, Y.F.; Wang, C.Y.; Li, F.M. Lathyrus sativus (grass pea) and its neurotoxin ODAP. Phytochemistry 2006, 67, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Lambein, F.; Travella, S.; Kuo, Y.H.; Van Montagu, M.; Heijde, M. Grass pea (Lathyrus sativus L.): Orphan crop, nutraceutical or just plain food? Planta 2019, 250, 821–838. [Google Scholar] [CrossRef] [PubMed]
- Haque, R.M.; Kuo, Y.H.; Lambein, F.; Hussain, M. Effect of environmental factors on the biosynthesis of the neuro-excitatory amino acid β-ODAP (β-N-oxalyl-l-α, β-diaminopropionic acid) in callus tissue of Lathyrus sativus. Food Chem. Toxicol. 2011, 49, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Jiao, C.J.; Jiang, J.L.; Ke, L.M.; Cheng, W.; Li, F.M.; Li, Z.X.; Wang, C.Y. Factors affecting β-ODAP content in Lathyrus sativus and their possible physiological mechanisms. Food Chem. Toxicol. 2011, 49, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.W.; Xing, G.M.; Xu, H.; Yan, Z.Y.; Wang, C.Y.; Wang, Y.F.; Li, Z.X. Relationship between oxalic acid and the metabolism of ß-N-oxalyl-α, ß-diaminopropionic acid (ODAP) in grass pea (Lathyrus sativus L). Isr. J. Plant Sci. 2005, 53, 89–96. [Google Scholar]
- Xing, G.; Cui, K.; Li, J.; Wang, Y.; Li, Z. Water stress and accumulation of beta- N-oxalyl-L-alpha,beta-diaminopropionic acid in grass pea (Lathyrus sativus). J. Agric. Food Chem. 2001, 49, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.C.; Xing, G.M.; Li, F.M.; Wang, S.M.; Fan, X.W.; Li, Z.X.; Wang, Y.F. Abscisic acid promotes accumulation of toxin ODAP in relation to free spermine level in grass pea seedlings (Lathyrus sativus L). Plant Physiol. Biochem. 2006, 44, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.M.; Zhang, X.Y.; Wang, G.X. Relationships between stomatal character, photosynthetic character and seed chemical composition in grass pea at different water availabilities. J. Agric. Sci. 2004, 142, 675–681. [Google Scholar] [CrossRef]
- Xiong, J.L.; Kong, H.Y.; Akram, N.A.; Bai, X.; Ashraf, M.; Tan, R.Y.; Zhy, H.; Siddique, K.H.M.; Xiong, Y.C.; Turner, N.C. 24-epibrassinolide increases growth, grain yield and β-ODAP production in seeds of well-watered and moderately water-stressed grass pea. Plant Growth Regul. 2016, 78, 217–231. [Google Scholar] [CrossRef]
- Jiao, C.J.; Xu, Q.L.; Wang, C.Y.; Li, F.M.; Li, Z.X.; Wang, Y.F. Accumulation pattern of toxin β-ODAP during lifespan and effect of nutrient elements on β-ODAP content in Lathyrus sativus seedlings. J. Agric. Sci. 2006, 144, 369–375. [Google Scholar] [CrossRef]
- Zhou, G.K.; Kong, Y.Z.; Cui, K.R.; Li, Z.X.; Wang, Y.F. Hydroxyl radical scavenging activity of β-N-oxalyl-l-α, β-diaminopropionic acid. Phytochemistry 2001, 58, 759–762. [Google Scholar]
- Suzuki, N.; Koussevitzky, S.H.A.I.; Mittler, R.O.N.; Miller, G.A.D. ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ. 2012, 35, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Sofo, A.; Scopa, A.; Nuzzaci, M.; Vitti, A. Ascorbate peroxidase and catalase activities and their genetic regulation in plants subjected to drought and salinity stresses. Int. J. Mol. Sci. 2015, 16, 13561–13578. [Google Scholar] [CrossRef]
- Demidchik, V. Mechanisms of oxidative stress in plants: From classical chemistry to cell biology. Environ. Exp. Bot. 2015, 109, 212–228. [Google Scholar] [CrossRef]
- Noctor, G.; Mhamdi, A.; Foyer, C.H. The roles of reactive oxygen metabolism in drought: Not so cut and dried. Plant Physiol. 2014, 164, 1636–1648. [Google Scholar] [CrossRef]
- Golemiec, E.; Tokarz, K.; Wielanek, M.; Niewiadomska, E. A dissection of the effects of ethylene, H2O2 and high irradiance on antioxidants and several genes associated with stress and senescence in tobacco leaves. J. Plant Physiol. 2014, 171, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Willekens, H.; Langebartels, C.; Tire, C.; Van Montagu, M.; Inze, D.; Van Camp, W. Differential expression of catalase genes in Nicotiana plumbaginifolia (L). Proc. Nat. Acad. Sci. USA 1994, 91, 10450–10454. [Google Scholar] [CrossRef] [PubMed]
- Bonifacio, A.; Martins, M.O.; Ribeiro, C.W.; Fontenele, A.V.; Carvalho, F.E.; Margis-Pinheiro, M.Á.R.C.I.A.; Silveira, J.A. Role of peroxidases in the compensation of cytosolic ascorbate peroxidase knockdown in rice plants under abiotic stress. Plant Cell Environ. 2011, 34, 1705–1722. [Google Scholar] [CrossRef] [PubMed]
- van Doorn, W.G.; Ketsa, S. Cross reactivity between ascorbate peroxidase and phenol (guaiacol) peroxidase. Postharvest Biol. Technol. 2014, 95, 64–69. [Google Scholar] [CrossRef]
- Świętek, M.; Lu, Y.C.; Konefał, R.; Ferreira, L.P.; Cruz, M.M.; Ma, Y.H.; Horák, D. Scavenging of reactive oxygen species by phenolic compound-modified maghemite nanoparticles. Beilstein J. Nanotech. 2019, 10, 1073–1088. [Google Scholar] [CrossRef] [PubMed]
- Makowski, W.; Tokarz, K.M.; Tokarz, B.; Banasiuk, R.; Witek, K.; Królicka, A. Elicitation-based method for increasing the production of antioxidant and bactericidal phenolic compounds in Dionaea muscipula J. Ellis Tissue. Molecules 2020, 25, 1794. [Google Scholar] [CrossRef] [PubMed]
- Ahanger, M.A.; Tomar, N.S.; Tittal, M.; Argal, S.; Agarwal, R.M. Plant growth under water/salt stress: ROS production; antioxidants and significance of added potassium under such conditions. Physiol. Mol. Biol. Plants 2017, 23, 731–744. [Google Scholar] [CrossRef] [PubMed]
- Naikoo, M.I.; Dar, M.I.; Raghib, F.; Jaleel, H.; Ahmad, B.; Raina, A.; Khan, F.A.; Naushin, F. Role and regulation of plants phenolics in abiotic stress tolerance: An overview. In Plant Signaling Molecules; Khan, M.I.R., Reddy, P.S., Ferrante, A., Khan, N.A., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 157–168. [Google Scholar]
- Makowski, W.; Tokarz, B.; Banasiuk, R.; Królicka, A.; Dziurka, M.; Wojciechowska, R.; Tokarz, K.M. Is a blue-red light a good elicitor of phenolic compounds in the family Droseraceae? A comparative study. J. Photochem. Photobio. B 2019, 201, 111679. [Google Scholar] [CrossRef] [PubMed]
- Nichols, S.N.; Hofmann, R.W.; Williams, W.M. Physiological drought resistance and accumulation of leaf phenolics in white clover interspecific hybrids. Environ. Exp. Bot. 2015, 119, 40–47. [Google Scholar] [CrossRef]
- Nakabayashi, R.; Yonekura-Sakakibara, K.; Urano, K.; Suzuki, M.; Yamada, Y.; Nishizawa, T.; Matsuda, F.; Kojima, M.; Sakakibara, H.; Shinozaki, K.; et al. Enhancement of oxidative and drought tolerance in Arabidopsis by overaccumulation of antioxidant flavonoids. Plant J. 2014, 77, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.A.; Ashraf, M.; Ali, Q. Response of two genetically diverse wheat cultivars to salt stress at different growth stages: Leaf lipid peroxidation and phenolic contents. Pak. J. Bot. 2010, 42, 559–565. [Google Scholar]
- Kostecka-Gugała, A.; Ledwożyw-Smoleń, I.; Augustynowicz, J.; Wyżgolik, G.; Kruczek, M.; Kaszycki, P. Antioxidant properties of fruits of raspberry and blackberry grown in central Europe. Open Chem. 2015, 13, 1313–1325. [Google Scholar] [CrossRef]
- Kostecka-Gugała, A.; Kruczek, M.; Ledwożyw-Smoleń, I.; Kaszycki, P. Antioxidants and health-beneficial nutrients in fruits of eighteen Cucurbita cultivars: Analysis of diversity and dietary implications. Molecules 2020, 25, 1792. [Google Scholar] [CrossRef]
- Lattanzio, V.; Caretto, S.; Linsalata, V.; Colella, G.; Mita, G. Signal transduction in artichoke [Cynara cardunculus L subsp. scolymus (L) Hayek] callus and cell suspension cultures under nutritional stress. Plant Physiol. Biochem. 2018, 127, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Verbruggen, N.; Hermans, C. Proline accumulation in plants: A review. Amino Acids 2008, 35, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Poustini, K.; Siosemardeh, A.; Ranjbar, M. Proline accumulation as a response to salt stress in 30 wheat (Triticum aestivum L.) cultivars differing in salt tolerance. Genet. Res. Crop Evol. 2007, 54, 925–934. [Google Scholar] [CrossRef]
- Aleksza, D.; Horváth, G.V.; Sándor, G.; Szabados, L. Proline accumulation is regulated by transcription factors associated with phosphate starvation. Plant Physiol. 2017, 175, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Bertazzini, M.; Sacchi, G.A.; Forlani, G. A differential tolerance to mild salt stress conditions among six Italian rice genotypes does not rely on Na+ exclusion from shoots. J. Plant Physiol. 2018, 226, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Forlani, G.; Trovato, M.; Funck, D.; Signorelli, S. Regulation of proline accumulation and its molecular and physiological functions in stress. In Osmoprotectant-Mediated Abiotic Stress Tolerance in Plants: Recent Advances and Future Perspectives; Hossain, M.A., Kumar, V., Burritt, D.J., Fujita, M., Mäkelä, P.S., Eds.; Springer: Cham, Switzerland, 2019; pp. 73–97. [Google Scholar]
- Wan, Q.; Hongbo, S.; Zhaolong, X.; Jia, L.; Dayong, Z.; Yihong, H. Salinity tolerance mechanism of osmotin and osmotin-like proteins: A promising candidate for enhancing plant salt tolerance. Curr. Genom. 2017, 18, 553–556. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, C.; Zhang, B.; Yi, J.; Yang, Y.; Kong, C.; Lei, C.; Gong, M. The role of the late embryogenesis-abundant (LEA) protein family in development and the abiotic stress response: A comprehensive expression analysis of potato (Solanum tuberosum). Genes 2019, 10, 148. [Google Scholar] [CrossRef] [PubMed]
- Barthakur, S.B.V.B.; Babu, V.; Bansa, K.C. Over-expression of osmotin induces proline accumulation and confers tolerance to osmotic stress in transgenic tobacco. J. Plant Biochem. Biotech. 2001, 10, 31–37. [Google Scholar] [CrossRef]
- Lan, T.; Gao, J.; Zeng, Q.Y. Genome-wide analysis of the LEA (late embryogenesis abundant) protein gene family in Populus trichocarpa. Tree Genet. Genomes 2013, 9, 253–264. [Google Scholar] [CrossRef]
- Huang, Z.; Zhong, X.J.; He, J.; Jin, S.H.; Guo, H.D.; Yu, X.F.; Zhou, Y.J.; Li, X.; Ma, M.D.; Chen, Q.B.; et al. Genome-wide identification, characterization, and stress-responsive expression profiling of genes encoding LEA (Late Embryogenesis Abundant) proteins in Moso bamboo (Phyllostachys edulis). PLoS ONE 2016, 11, e0165953. [Google Scholar] [CrossRef]
- Magwanga, R.O.; Lu, P.; Kirungu, J.N.; Lu, H.; Wang, X.; Cai, X.; Zhou, Z.; Zhang, Z.; Salih, S.; Wang, K.; et al. Characterization of the late embryogenesis abundant (LEA) proteins family and their role in drought stress tolerance in upland cotton. BMC Genet. 2018, 19, 6. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).