Deficit Irrigation on Guar Genotypes (Cyamopsis tetragonoloba (L.) Taub.): Effects on Seed Yield and Water Use Efficiency
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description and Materials
2.2. Data Analysis
2.3. Weather Conditions
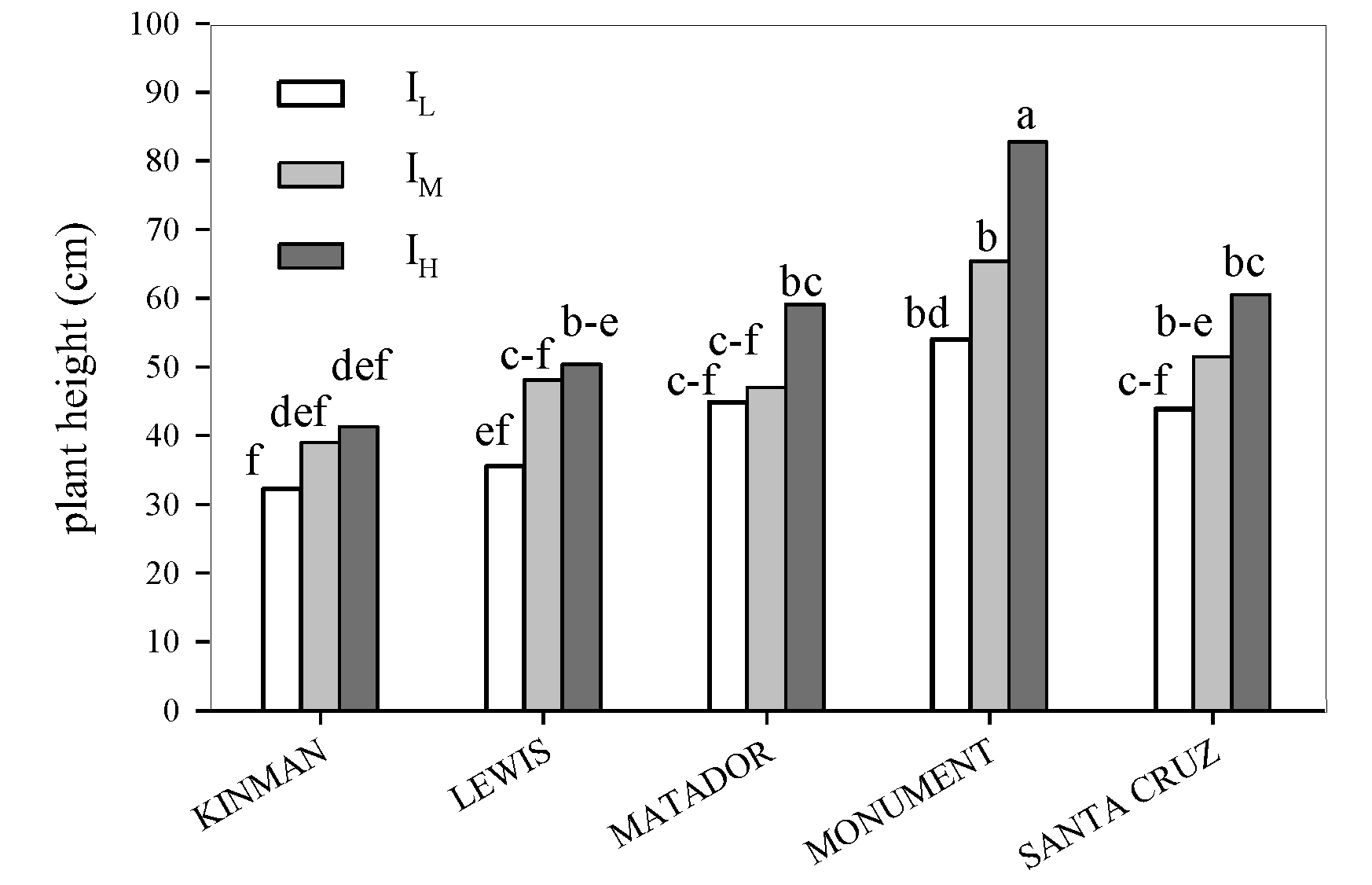
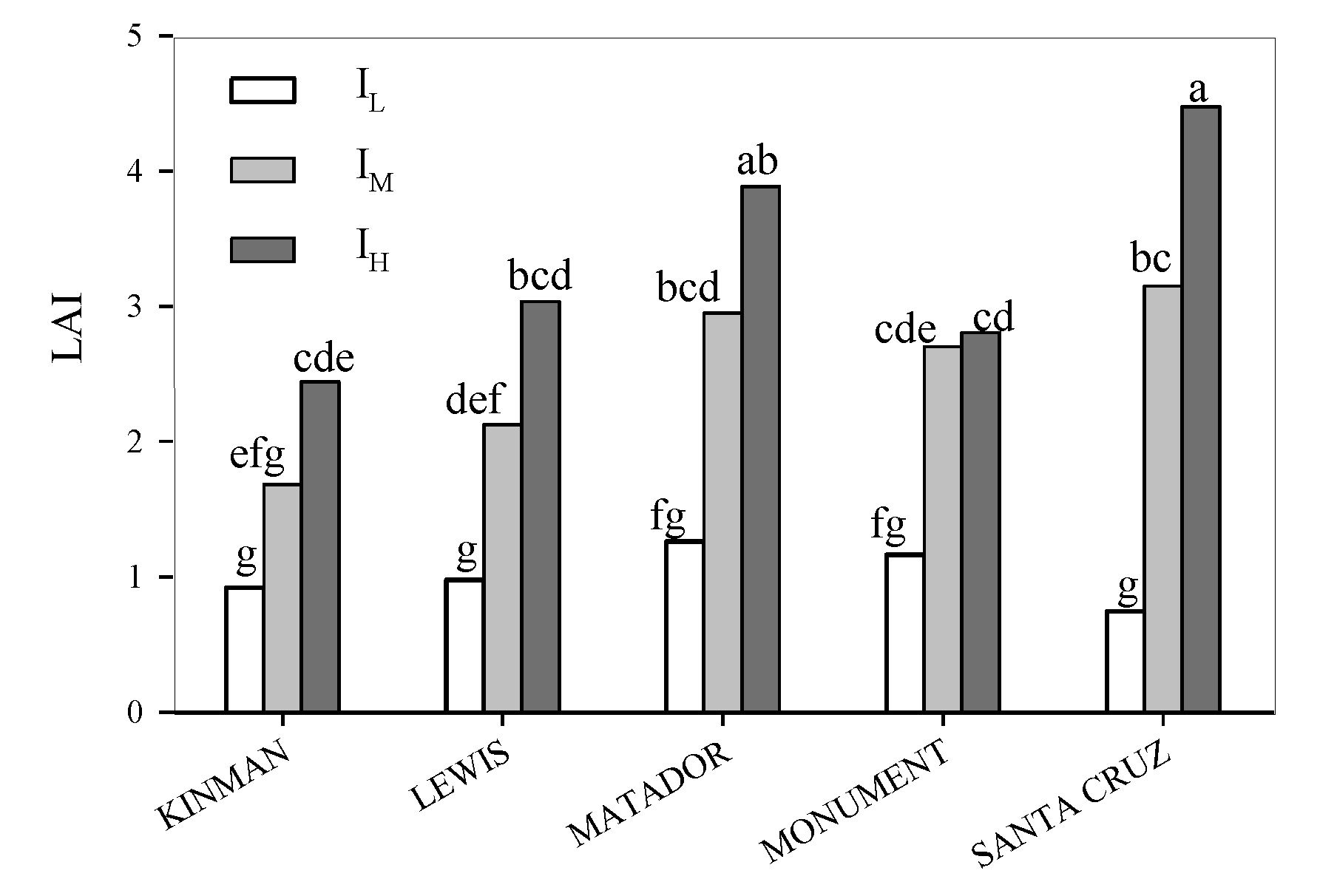
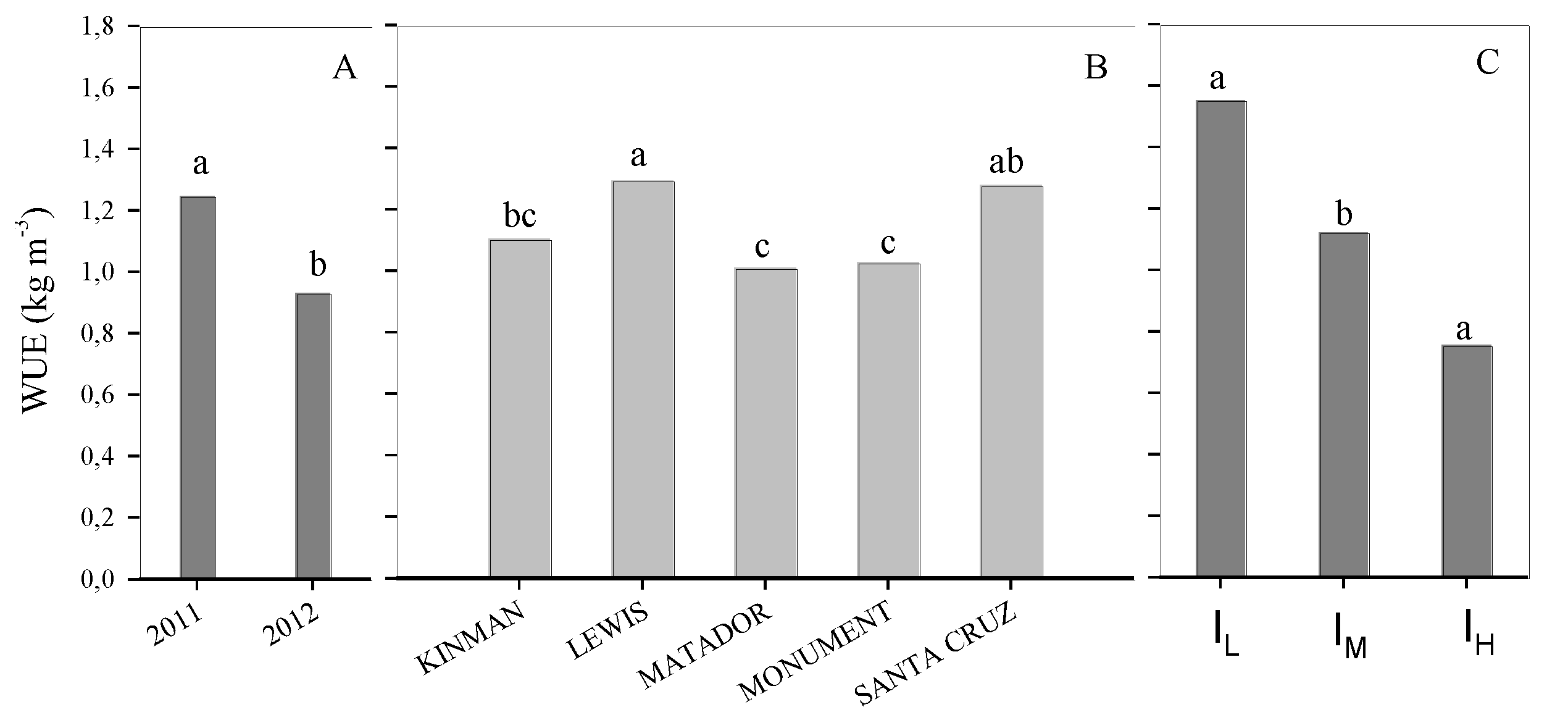
3. Results
3.1. Yield and Yield Components
3.2. Water Use Efficiency
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Trostle, C. Guar Production in Texas & SW Oklahoma. Available online: https://lubbock.tamu.edu/files/2020/04/Guar-2020-West-Texas-SW-Oklahoma-CTrostle.pdf (accessed on 2 April 2020).
- Sharma, P. Guar Industry Vision 2020: Single Vision Strategies; NIAM Research Report; CCS National Institute of Agricultural Marketing: Jaipur, India, 2010. [Google Scholar]
- Gresta, F.; De Luca, A.I.; Strano, A.; Falcone, G.; Santonoceto, C.; Anastasi, U.; Gulisano, G. Economic and environmental sustainability analysis of guar (Cyamopsis tetragonoloba L.) farming process in a Mediterranean area: Two case-study. Ital. J. Agron. 2014, 9, 20–24. [Google Scholar] [CrossRef]
- Ashraf, M.Y.; Akhtar, K.; Sarwar, G.; Ashraf, M. Evaluation of arid and semiarid ecotypes of guar (Cyamopsis tetragonoloba L.) for salinity (NaCl) tolerance. J. Arid Environ. 2002, 52, 473–482. [Google Scholar] [CrossRef]
- Ashraf, M.Y.; Akhtar, K.; Sarwar, G.; Ashraf, M. Role of the rooting system in salt tolerance potential of different guar accessions. Agron. Sustain. Dev. 2005, 25, 243–249. [Google Scholar] [CrossRef]
- Mathur, N.K. Industrial Galactomannan Polysaccharides; CRC Press; Taylor & Francis Group: Boca Raton, FL, USA, 2012; p. 187. [Google Scholar]
- Abidi, N.; Liyanage, S.; Auld, D.; Imel, R.K.; Norman, L.; Grover, K.; Angadi, S.; Singla, S.; Trostle, C. Chapter 12: Challenges and opportunities for increasing guar production in the United States to support unconventional oil and gas production. In Hydraulic Fracturing Impacts and Technologies; CRC Press: Boca Raton, FL, USA, 2015; pp. 207–226. [Google Scholar]
- Mudgil, D.; Barak, S.; Khatkar, B.S. Guar gum: Processing, properties and food applications—A review. J. Food Sci. Technol. 2011, 51, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, S.F.; Berhow, M.A.; Winkler-Moser, J.K.; Lee, E. Formulation of a biodegradable: Odor-reducing cat litter from solvent-extracted corn dried distillers grains. Ind. Crop Prod. 2011, 34, 999–1002. [Google Scholar] [CrossRef]
- Lubbe, A.; Verpoorte, R. Cultivation of medicinal and aromatic plants for specialty industrial materials. Ind. Crop Prod. 2011, 34, 785–801. [Google Scholar] [CrossRef]
- Chiofalo, B.; Lo Presti, V.; D’Agata, A.; Raso, R.; Ceravolo, G.; Gresta, F. Qualitative profile of degummed guar (Cyamopsis tetragonoloba L.) seeds grown in a Mediterranean area for use as animal feed. J. Anim. Physiol. Anim. Nutr. 2018, 102, 260–267. [Google Scholar] [CrossRef]
- Gresta, F.; Ceravolo, G.; Lo Presti, V.; D’Agata, A.; Rao, R.; Chiofalo, B. Seed yield: Galactomannans content and quality traits of different guar (Cyamopsis tetragonoloba L.) genotypes. Ind. Crop Prod. 2017, 107, 122–129. [Google Scholar] [CrossRef]
- Sortino, O.; Gresta, F. Growth and yield performance of five guar cultivars in a Mediterranean environment. Ital. J. Agron. 2007, 4, 359–364. [Google Scholar] [CrossRef]
- Gresta, F.; Mercati, F.; Santonoceto, C.; Abenavoli, M.R.; Ceravolo, G.; Araniti, F.; Anastasi, U.; Sunseri, F. Morpho-agronomic and AFLP characterization to explore guar (Cyamopsis tetragonoloba L.) genotypes for the Mediterranean environment. Ind. Crop Prod. 2016, 86, 23–30. [Google Scholar] [CrossRef]
- Santonoceto, C.; Mauceri, A.; Lupini, A.; Anastasi, U.; Chiera, E.; Sunseri, F.; Mercati, F.; Gresta, F. Morpho-agronomic characterization and genetic variability assessment of a guar germplasm collection by a novel SSR panel. Ind. Crop Prod. 2019, 138, 111568. [Google Scholar] [CrossRef]
- Undersander, D.J.; Putnam, D.H.; Kaminski, A.R.; Kelling, K.A.; Doll, J.D.; Oplinger, E.S.; Gunsolus, J.L. Alternative Field Crops Manual; Guar. University of Wisconsin and University of Minnesota: Madison, WI, USA, 1991; Available online: http://www.hort.purdue.edu/newcrop/afcm/guar.html (accessed on 2 April 2020).
- Gresta, F.; Cristaudo, A.; Trostle, C.; Anastasi, U.; Guarnaccia, P.; Catara, S.; Onofri, A. Germination of guar (Cyamopsis tetragonoloba (L.) Taub.) genotypes with reduced temperature requirements. Aust. J. Crop Sci. 2018, 12, 954–960. [Google Scholar] [CrossRef]
- Alexander, W.L.; Bucks, D.A.; Backhaus, R.A. Irrigation water management for guar seed production. Agron. J. 1988, 80, 447–453. [Google Scholar] [CrossRef]
- Venkateswarlu, B.; Rao, A.V.; Lahiri, A.N. Effect of water stress on nodulation and N2-ase activity in guar (Cyamopsis tetragonoloba (L.) Taub.). Proc. Ind. Acad. Sci. 1983, 92, 297–301. [Google Scholar]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration—Guidelines for Computing Crop Water Requirements—FAO Irrigation and Drainage Paper No. 56; FAO: Rome, Italy, 1998. [Google Scholar]
- Doorenbos, J.; Kassam, A.H. Yield Response to Water Irrigation and Drainage, Paper No. 33; FAO: Rome, Italy, 1979. [Google Scholar]
- Onofri, A. Routine statistical analyses of field experiments by using an Excel extension. In Proceedings of the 6th National Conference Italian Biometric Society: La statistica nelle scienze della vita e dell’ambiente, Pisa, Italy, 20–22 June 2007; pp. 93–99. [Google Scholar]
- Garg, B.K.; Kathju, S.; Vyas, S.P.; Lahiri, A.N. Influence of irrigation and nitrogen levels on Indian mustard. Indian J. Plant. Physiol. 2001, 6, 289–294. [Google Scholar]
- Gresta, F.; Sortino, O.; Santonoceto, C.; Issi, L.; Formantici, C.; Galante, Y.M. Effects of sowing times on seed yield: Protein and galactomannans content of four varieties of guar (Cyamopsis tetragonoloba L) in a Mediterranean environment. Ind. Crop Prod. 2013, 41, 46–52. [Google Scholar] [CrossRef]
- Pathak, R.; Roy, M.M. Climatic responses; environmental indices and interrelationships between qualitative and quantitative traits in clusterbean under arid conditions. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2015, 85, 147–154. [Google Scholar] [CrossRef]
- Gresta, F.; Trostle, C.; Sortino, O.; Santonoceto, C.; Avola, G. Rhizobium inoculation and phosphate fertilization effects on the productive and qualitative traits of guar (Cyamopsis tetragonoloba (L.) Taub.). Ind. Crop Prod. 2019, 139, 111513. [Google Scholar] [CrossRef]
- Meftahizade, H.; Ghorbanpourb, M.; Asarehc, M.H. Changes in phenological attributes; yield and phytochemical compositions of guar (Cyamopsis tetragonoloba L.) landaraces under various irrigation regimes and planting dates. Sci. Hortic. 2019, 256, 108577. [Google Scholar] [CrossRef]
- Singla, S.; Grover, K.; Angad, S.V.; Begna, S.H.; Schutte, B.; Van Leeuwen, D. Growth and yield of guar (Cyamopsis tetragonoloba L.) genotypes under different planting dates in the semi-arid Southern High Plains. Am. J. Plant. Sci. 2016, 7, 1246–1258. [Google Scholar] [CrossRef]
- Gresta, F.; Santonoceto, C.; Ceravolo, G.; Formantici, C.; Grillo, O.; Ravalli, C.; Venora, G. Productive, qualitative and seed image analysis traits of guar (Cyamopsis tetragonoloba (L.) Taub). Aust. J. Crop Sci. 2016, 10, 1052–1060. [Google Scholar] [CrossRef]
- Pathak, R. Clusterbean: Physiology, Genetics and Cultivation; Springer: Berlin/Heidelberg, Germany, 2015; p. 151. [Google Scholar]
- Stafford, R.E.; McMichael, B.L. Effect of Water Stress on Yield Components in Guar. J. Agron. Crop Sci. 1991, 166, 63–68. [Google Scholar] [CrossRef]
- Gresta, F.; Avola, G.; Cannavò, S.; Santonoceto, C. Morphological, biological, productive and qualitative characterization of 68 guar (Cyamopsis tetragonoloba (L.) Taub.) genotypes. Ind. Crop Prod. 2018, 114, 98–107. [Google Scholar] [CrossRef]
- Meftahizade, H.; Hamidoghli, Y.; Assareh, M.H.; Dakheli, M.J. Effect of sowing date and irrigation regimes on yield components; protein and galactomannan content of guar (Cyamopsis Tetragonoloba L.) in Iran climate. Aust. J. Crop Sci. 2017, 11, 1481–1487. [Google Scholar] [CrossRef]
- Ahmed, M.; Mac, D.; Bashir, A. Effect of water stress at different periods on seed yield and water use efficiency of guar under Shambat conditions. Agric. Sci. 2011, 2, 262–266. [Google Scholar] [CrossRef]
- Baath, G.S.; Kakani, V.G.; Gowda, P.H.; Rocateli, A.C.; Northup, B.K.; Singh, H.; Katta, J.R. Guar responses to temperature: Estimation of cardinal temperatures and photosynthetic parameters. Ind. Crop Prod. 2020, 145, 111940. [Google Scholar] [CrossRef]
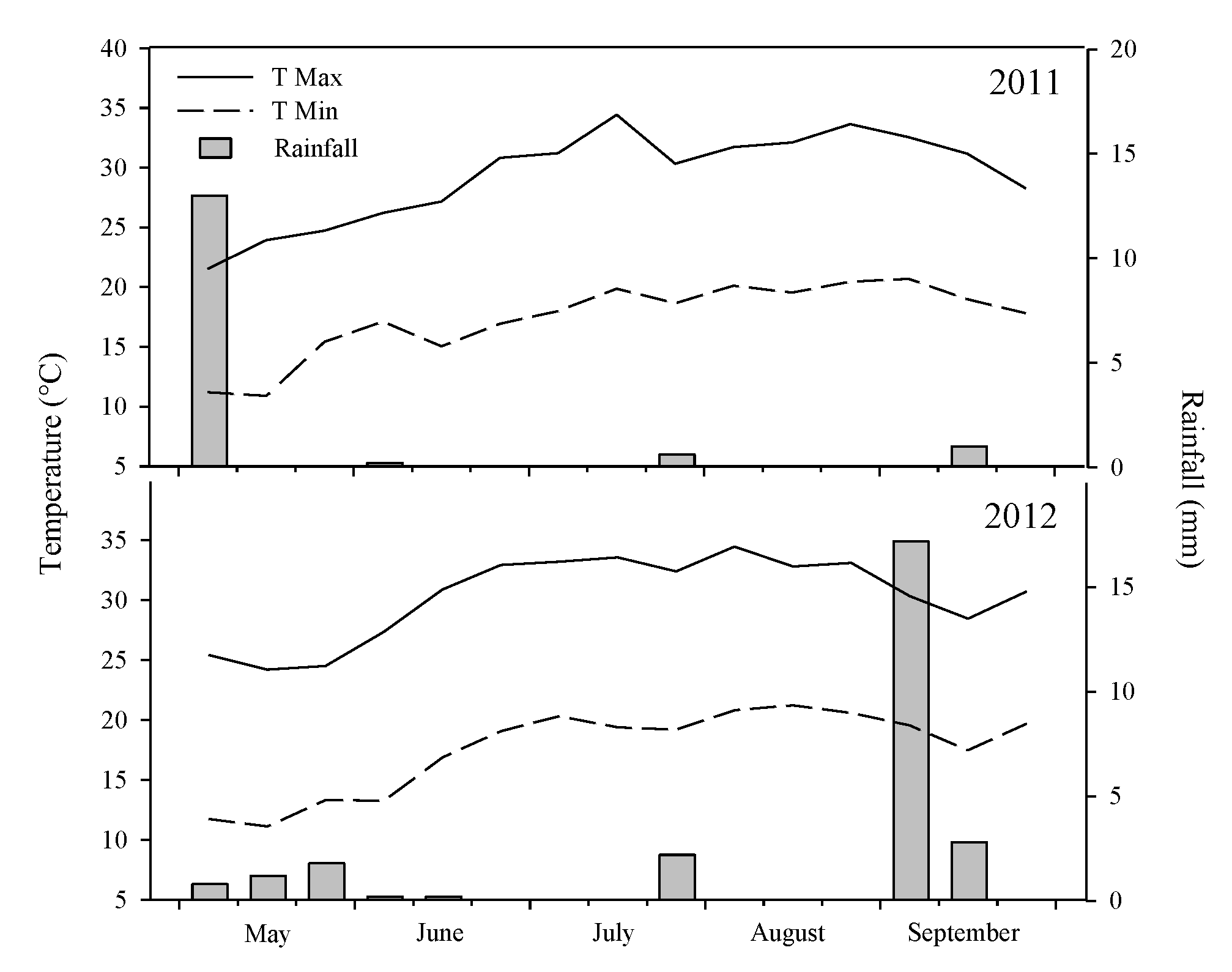
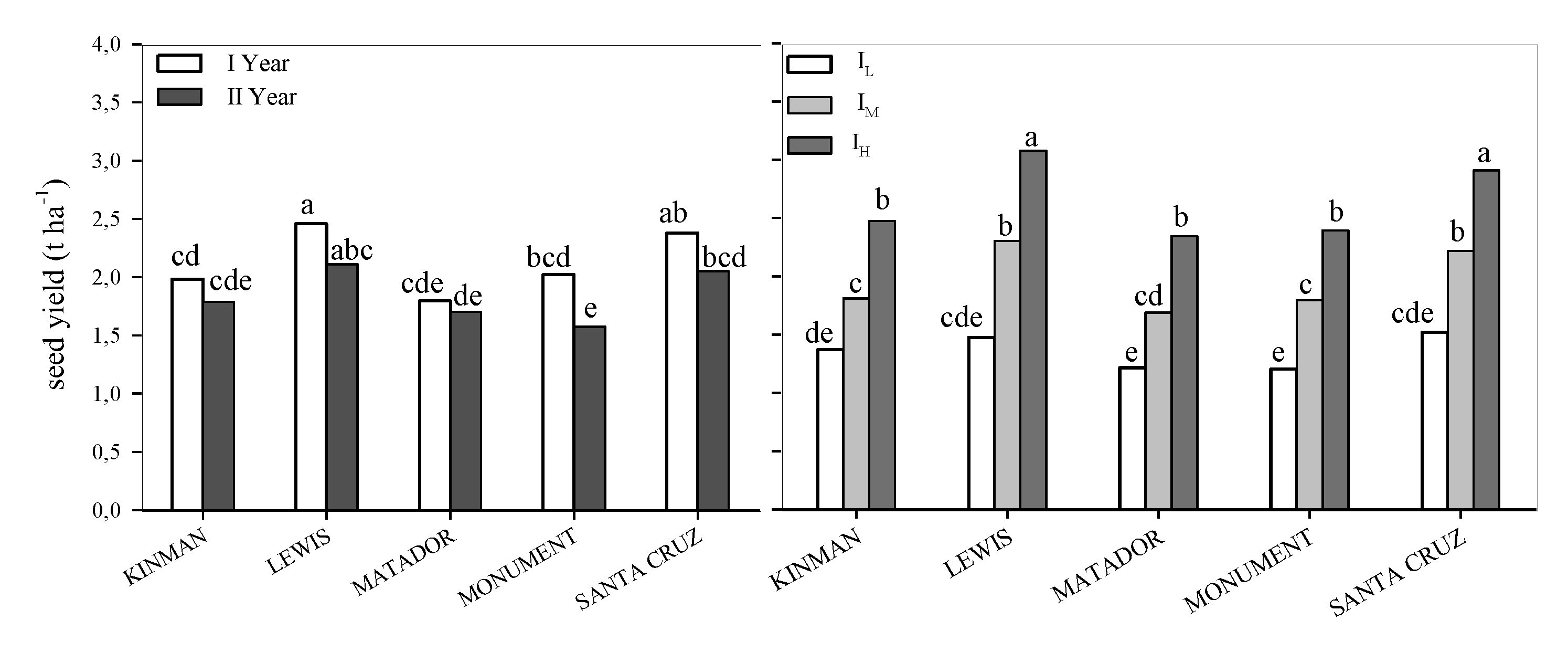
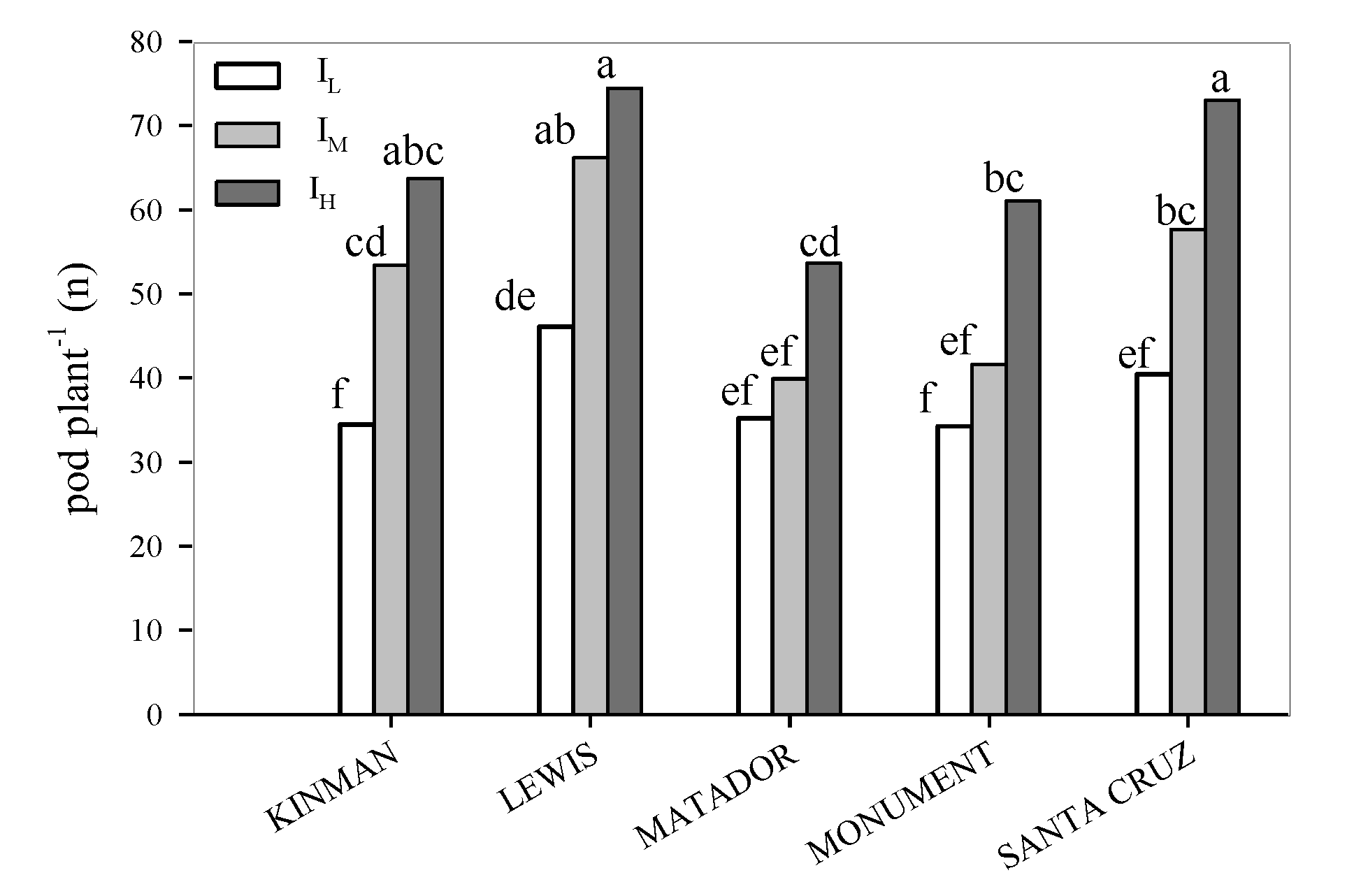
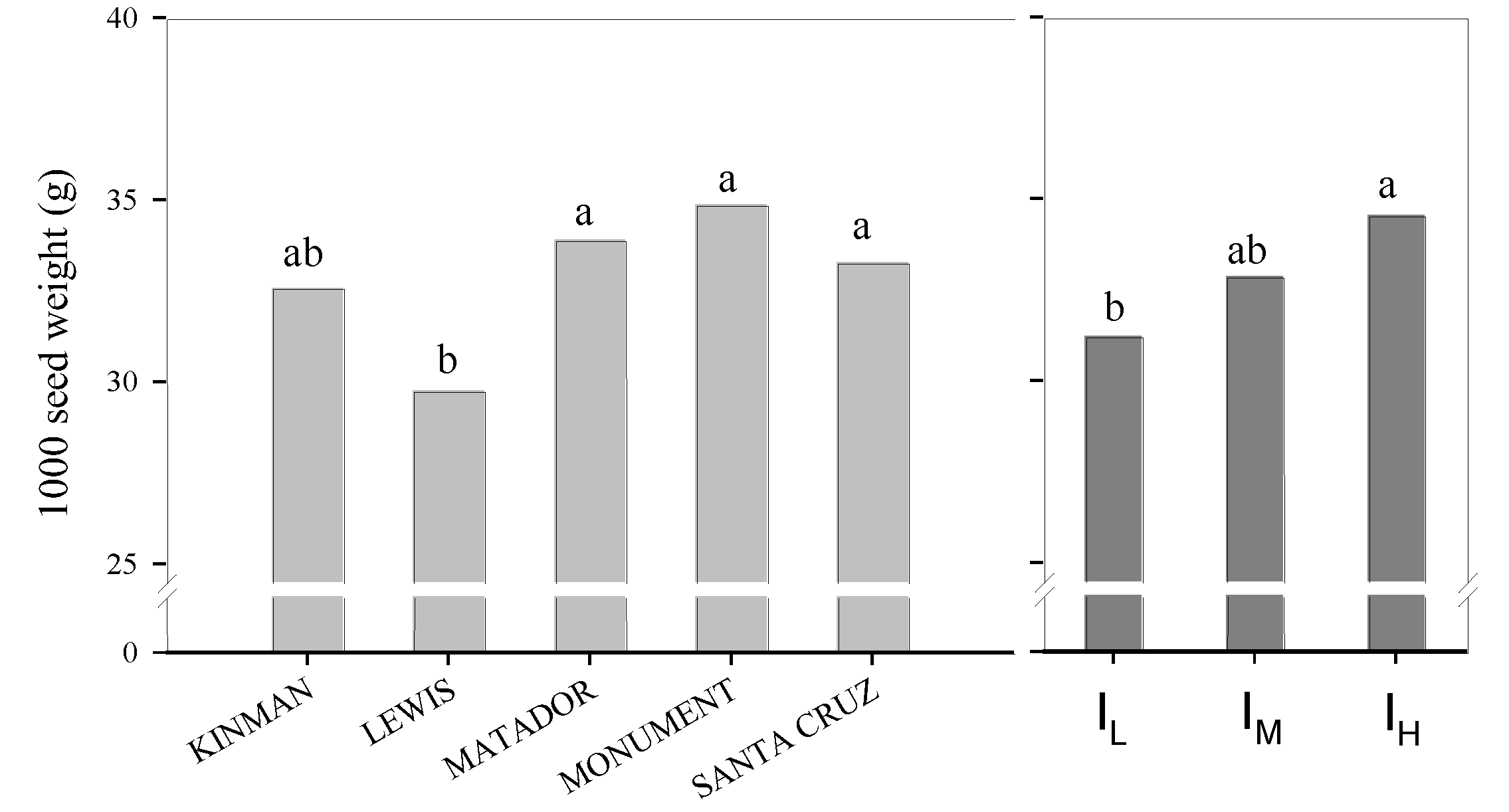

| EFFECT | DF | Grain Yield | Pods Plant | 1000 Seed Weight | Seed Pods | Plant Height | LAI | Harvest Index | WUE |
|---|---|---|---|---|---|---|---|---|---|
| Year (Y) | 1 | 0.011 | 0.150 | 0.385 | 0.556 | 0.672 | 0.994 | 0.159 | 0.013 |
| Water Regimes (I) | 2 | <0.001 | <0.001 | <0.001 | 0.109 | <0.001 | <0.001 | 0.132 | <0.001 |
| Cultivar (C) | 4 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.002 | <0.001 |
| Y × I | 2 | 0.777 | 0.200 | 0.180 | 0.468 | 0.868 | 0.496 | 0.323 | 0.052 |
| Y × C | 4 | 0.005 | 0.719 | 0.161 | 0.767 | 0.748 | 0.282 | 0.074 | 0.114 |
| I × C | 8 | 0.002 | <0.001 | 0.188 | 0.929 | 0.004 | <0.001 | 0.008 | 0.469 |
| Y × I × C | 8 | 0.172 | 0.061 | 0.271 | 0.715 | 0.745 | 0.111 | 0.455 | 0.720 |
| r | F | p | |
|---|---|---|---|
| Pods Plant−1 | 0.924 | 511.050 | 0.000 |
| 1000 seed weight | 0.247 | 5.723 | 0.019 |
| Seeds Pod−1 | 0.010 | 0.009 | 0.924 |
| Plant height | 0.378 | 14.712 | 0.000 |
| LAI | 0.713 | 90.741 | 0.000 |
| WUE | 0.482 | 26.667 | 0.000 |
| HI | 0.029 | 0.072 | 0.790 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avola, G.; Riggi, E.; Trostle, C.; Sortino, O.; Gresta, F. Deficit Irrigation on Guar Genotypes (Cyamopsis tetragonoloba (L.) Taub.): Effects on Seed Yield and Water Use Efficiency. Agronomy 2020, 10, 789. https://doi.org/10.3390/agronomy10060789
Avola G, Riggi E, Trostle C, Sortino O, Gresta F. Deficit Irrigation on Guar Genotypes (Cyamopsis tetragonoloba (L.) Taub.): Effects on Seed Yield and Water Use Efficiency. Agronomy. 2020; 10(6):789. https://doi.org/10.3390/agronomy10060789
Chicago/Turabian StyleAvola, Giovanni, Ezio Riggi, Calvin Trostle, Orazio Sortino, and Fabio Gresta. 2020. "Deficit Irrigation on Guar Genotypes (Cyamopsis tetragonoloba (L.) Taub.): Effects on Seed Yield and Water Use Efficiency" Agronomy 10, no. 6: 789. https://doi.org/10.3390/agronomy10060789
APA StyleAvola, G., Riggi, E., Trostle, C., Sortino, O., & Gresta, F. (2020). Deficit Irrigation on Guar Genotypes (Cyamopsis tetragonoloba (L.) Taub.): Effects on Seed Yield and Water Use Efficiency. Agronomy, 10(6), 789. https://doi.org/10.3390/agronomy10060789








