Wild Miscanthus Germplasm in a Drought-Affected Area: Physiology and Agronomy Appraisals
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Trial Set-Up
2.2. Measurements
2.3. Statistical Analysis
3. Results
3.1. Environmental Conditions
3.2. Morphological and Productive Traits
3.3. Gas Exchange
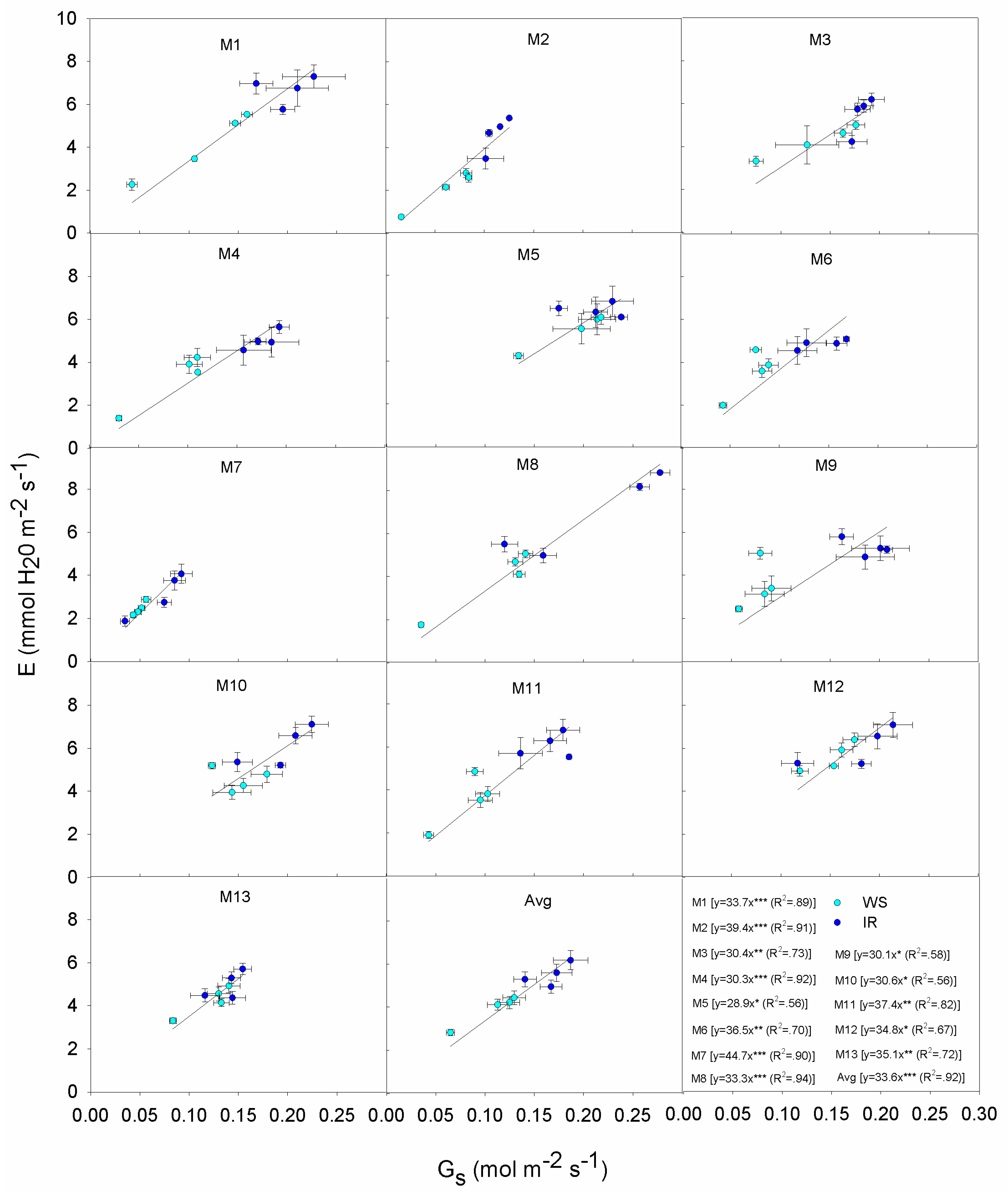
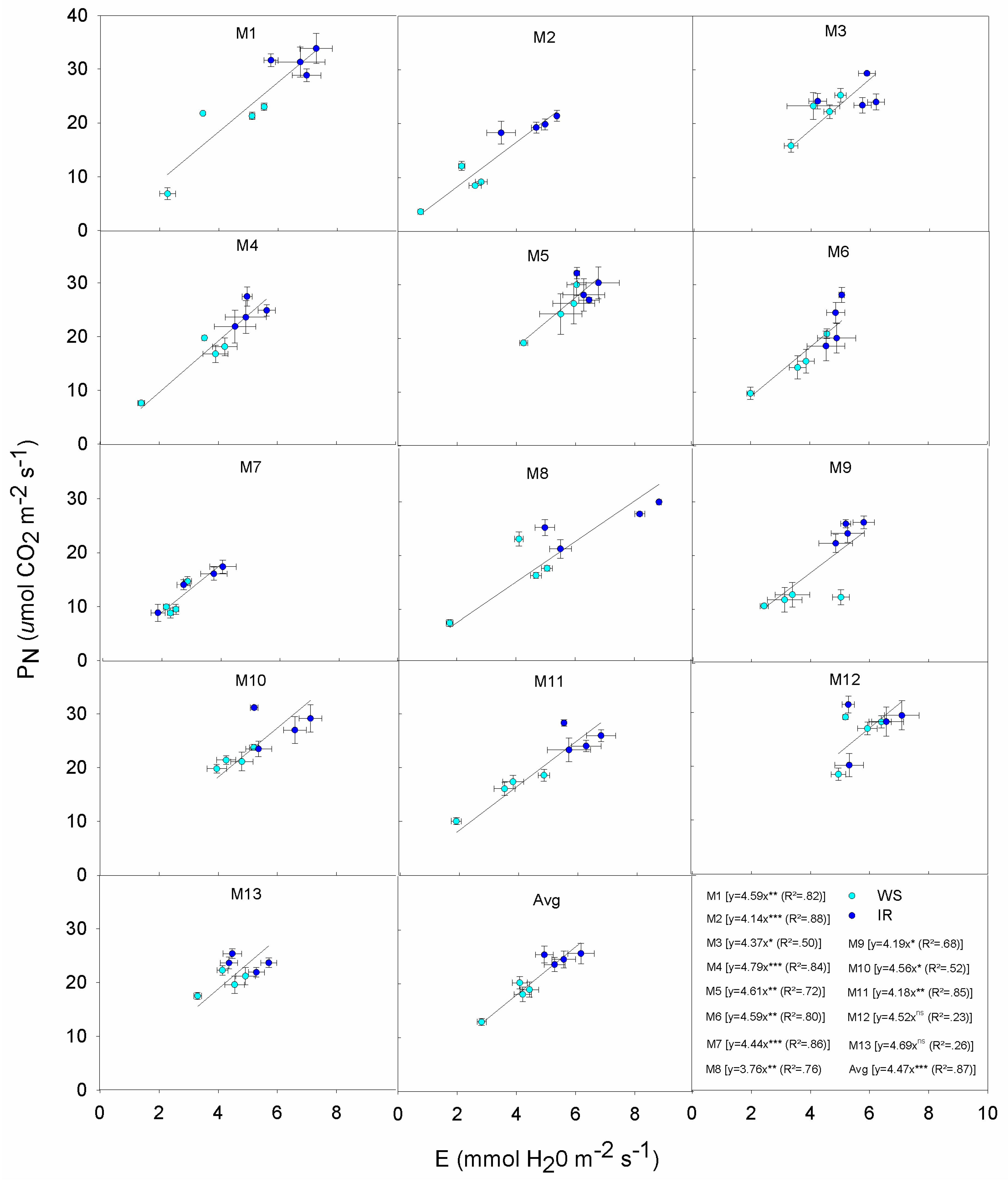
3.4. Correlations and Relationships
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Scarlat, N.; Dallemand, J.F.; Monforti-Ferrario, F.; Nita, V. The role of biomass and bioenergy in a future bioeconomy: Policies and facts. Environ. Dev. 2015, 15, 3–34. [Google Scholar] [CrossRef]
- Tilman, D.; Socolow, R.; Foley, J.A.; Hill, J.; Larson, E.; Lynd, L.; Pacala, S.; Reilly, J.; Searchinger, T.; Somerville, C.; et al. Beneficial biofuels—The food, energy, and environment trilemma. Science 2009, 325, 270–271. [Google Scholar] [CrossRef] [PubMed]
- Dauber, J.; Brown, C.; Fernando, A.L.; Finnan, J.; Krasuska, E.; Ponitka, J.; Styles, D.; Thrän, D.; Van Groenigen, K.J.; Weih, M.; et al. Bioenergy from “surplus” land: Environmental and socio-economic implications. BioRisk 2012, 7, 5–50. [Google Scholar] [CrossRef]
- Von Cossel, M.; Wagner, M.; Lask, J.; Magenau, E.; Bauerle, A.; Cossel, V.V.; Warrach-Sagi, K.; Elbersen, B.; Staritsky, I.; van Eupen, M.; et al. Prospects of bioenergy cropping systems for a more social-ecologically sound bioeconomy. Agronomy 2019, 9, 605. [Google Scholar] [CrossRef]
- Von Cossel, M.; Lewandowski, I.; Elbersen, B.; Staritsky, I.; Van Eupen, M.; Iqbal, Y.; Mantel, S.; Scordia, D.; Testa, G.; Cosentino, S.L.; et al. Marginal agricultural land low-input systems for biomass production. Energies 2019, 12, 3123. [Google Scholar] [CrossRef]
- Robson, P.R.H.; Hastings, A.; Clifton-Brown, J.C.; McAlmont, J.P. Sustainable use of Miscanthus for biofuel. In Achieving Carbon-Negative Bioenergy Systems from Plant Materials; Saffron, C., Ed.; Burleigh Dodds Science Publishing: Cambridge, UK, 2019; Chapter 7; ISBN 9781786762528. [Google Scholar]
- Lewandowski, I.; Scurlock, J.M.; Lindvall, E.; Christou, M. The development and current status of perennial rhizomatous grasses as energy crops in the US and Europe. Biomass Bioenergy 2003, 25, 335–361. [Google Scholar] [CrossRef]
- Schmidt, T.; Fernando, A.L.; Monti, A.; Rettenmaier, N. Life Cycle Assessment of Bioenergy and Bio-Based Products from Perennial Grasses Cultivated on Marginal Land in the Mediterranean Region. Bioenergy Res. 2015, 8, 1548–1561. [Google Scholar] [CrossRef]
- Alexopoulou, E.; Zanetti, F.; Scordia, D.; Zegada-Lizarazu, W.; Christou, M.; Testa, G.; Cosentino, S.L.; Monti, A. Long-Term Yields of Switchgrass, Giant Reed, and Miscanthus in the Mediterranean Basin. Bioenergy Res. 2015, 8, 1492–1499. [Google Scholar] [CrossRef]
- Fernando, A.L.; Boléo, S.; Barbosa, B.; Costa, J.; Duarte, M.P.; Monti, A. Perennial Grass Production Opportunities on Marginal Mediterranean Land. Bioenergy Res. 2015, 8, 1523–1537. [Google Scholar] [CrossRef]
- Scordia, D.; Cosentino, S.L. Perennial Energy Grasses: Resilient Crops in a Changing European Agriculture. Agriculture 2019, 9, 169. [Google Scholar] [CrossRef]
- Zanetti, F.; Scordia, D.; Calcagno, S.; Acciai, M.; Grasso, A.; Cosentino, S.L.; Monti, A. Trade-off between harvest date and lignocellulosic crop choice for advanced biofuel production in the Mediterranean area. Ind. Crop. Prod. 2019, 138, 111439. [Google Scholar] [CrossRef]
- Clifton-Brown, J.C.; Lewandowski, I.; Andersson, B.; Basch, G.; Christian, D.G.; Bonderup Kjeldsene, J.; Jorgensen, U.; Mortensene, J.V.; Riched, A.B.; Schwarz, K.U.; et al. Performance of 15 Miscanthus genotypes at five sites in Europe. Agron. J. 2001, 93, 1013–1019. [Google Scholar] [CrossRef]
- Clifton-Brown, J.C.; Robson, P.R.H.; Allison, G.G.; Lister, S.; Sanderson, R.; Morris, C.; Hodgson, E.; Farrar, K.; Hawkins, S.; Jensen, E.; et al. Miscanthus: Breeding our way to a better future. Asp. Appl. Biol. 2008, 90, 199–206. [Google Scholar]
- Clifton-Brown, J.; Harfouche, A.; Casler, M.D.; Jones, H.D.; Macalpine, W.J.; Murphy-Bokern, D.; Smart, L.B.; Adler, A.; Ashman, C.; Awty-Carroll, D.; et al. Breeding progress and preparedness for mass-scale deployment of perennial lignocellulosic biomass crops switchgrass, miscanthus, willow and poplar. Glob. Chang. Biol. Bioenergy 2019, 11, 118–151. [Google Scholar] [CrossRef] [PubMed]
- Clifton-Brown, J.; Schwarz, K.U.; Awty-Carroll, D.; Iurato, A.; Meyer, H.; Greef, J.; Gwyn, J.; Mos, M.; Ashman, C.; Hayes, C.; et al. Breeding strategies to improve Miscanthus as a sustainable source of biomass for bioenergy and biorenewable products. Agronomy 2019, 9, 673. [Google Scholar] [CrossRef]
- Clifton-Brown, J.C.; Lewandowski, I. Water use efficiency and biomass partitioning of three different Miscanthus genotypes with limited and unlimited water supply. Ann. Bot. 2000, 86, 191–200. [Google Scholar] [CrossRef]
- Cosentino, S.L.; Patane, C.; Sanzone, E.; Copani, V.; Foti, S. Effect of soil water content and nitrogen supply on the productivity of Miscanthus × giganteus Greef and Deu. in Mediterranean environment. Ind. Crop. Prod. 2007, 25, 75–88. [Google Scholar] [CrossRef]
- Robson, P.; Jensen, E.; Hawkins, S.; White, S.R.; Kenobi, K.; Clifton-Brown, J.; Donnison, I.; Farrar, K. Accelerating the domestication of a bioenergy crop: Identifying and modelling morphological targets for sustainable yield increase in Miscanthus. J. Exp. Bot. 2013, 64, 4143–4415. [Google Scholar] [CrossRef]
- Allison, G.G.; Morris, C.; Clifton-Brown, J.; Lister, S.J.; Donnison, I.S. Genotypic variation in cell wall composition in a diverse set of 244 accessions of Miscanthus. Biomass Bioenergy 2011, 35, 4740–4747. [Google Scholar] [CrossRef]
- Scordia, D.; van den Berg, D.; van Sleen, P.; Alexopoulou, E.; Cosentino, S.L. Are herbaceous perennial grasses suitable feedstock for thermochemical conversion pathways? Ind. Crop. Prod. 2016, 91, 350–357. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, B.; He, J.; Yang, J.; Pan, L.; Sun, D.; Peng, J. Genetic diversity and population structure of Miscanthus sinensis germplasm in China. PLoS ONE 2013, 8, e75672. [Google Scholar] [CrossRef]
- Clark, L.V.; Dzyubenko, E.; Dzyubenko, N.; Bagmet, L.; Sabitov, A.; Chebukin, P.; Johnson, D.A.; Kjeldsen, J.B.; Petersen, K.K.; Jørgensen, U.; et al. Ecological characteristics and in situ genetic associations for yield-component traits of wild Miscanthus from eastern Russia. Ann. Bot. 2016, 118, 941–955. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, S.L.; Testa, G.; Scordia, D.; Alexopoulou, E. Future yields assessment of bioenergy crops in relation to climate change and technological development in Europe. Ital. J. Agron. 2012, 7, 22. [Google Scholar] [CrossRef]
- Cramer, W.; Guiot, J.; Fader, M.; Garrabou, J.; Gattuso, J.P.; Iglesias, A.; Lange, M.A.; Llasat, M.C.; Paz, S.; Penuelas, J.; et al. Climate change and interconnected risks to sustainable development in the Mediterranean. Nat. Clim. Chang. 2018, 8, 972–980. [Google Scholar] [CrossRef]
- Vicente-Serrano, S.M.; Lopez-Moreno, J.I.; Beguería, S.; Lorenzo-Lacruz, J.; Sanchez-Lorenzo, A.; García-Ruiz, J.M.; Azorin-Molina, C.; Morán-Tejeda, E.; Revuelto, J.; Trigo, R.; et al. Evidence of increasing drought severity caused by temperature rise in southern Europe. Environ. Res. Lett. 2014, 9, 044001. [Google Scholar] [CrossRef]
- Flexas, J.; Diaz-Espejo, A.; Galmés, J.; Kaldenhoff, R.; Medrano, H.; Ribas-Carbo, M. Rapid variations of mesophyll conductance in response to changes in CO2 concentration around leaves. Plant Cell Environ. 2007, 30, 1284–1298. [Google Scholar] [CrossRef] [PubMed]
- Gulías, J.; Melis, R.; Scordia, D.; Cifre, J.; Testa, G.; Cosentino, S.L.; Porqueddu, C. Exploring the potential of wild perennial grasses as a biomass source in semi-arid Mediterranean environments. Ital. J. Agron. 2018, 13, 937. [Google Scholar] [CrossRef]
- Fader, M.; Shi, S.; Von Bloh, W.; Bondeau, A.; Cramer, W. Mediterranean irrigation under climate change: More efficient irrigation needed to compensate increases in irrigation water requirements. Hydrol. Earth Syst. Sci. 2016, 20, 953–973. [Google Scholar] [CrossRef]
- Stavridou, E.; Webster, R.J.; Robson, P.R.H. Novel Miscanthus genotypes selected for different drought tolerance phenotypes show enhanced tolerance across combinations of salinity and drought treatments. Ann. Bot. 2019, 124, 653–674. [Google Scholar] [CrossRef]
- Chaves, M.M.; Maroco, J.P.; Pereira, J.S. Understanding plant responses to drought—From genes to the whole plant. Func. Plant Biol. 2003, 30, 239–264. [Google Scholar] [CrossRef]
- Huang, L.S.; Flavell, R.; Donnison, I.S.; Chiang, Y.C.; Hastings, A.; Hayes, C.; Heidt, C.; Hong, H.; Hsu, T.W.; Humphreys, M.; et al. Collecting wild Miscanthus germplasm in Asia for crop improvement and conservation in Europe whilst adhering to the guidelines of the United Nations’ Convention on Biological Diversity. Ann. Bot. 2019, 124, 591–604. [Google Scholar] [CrossRef] [PubMed]
- Nunn, C.; Hastings, A.F.S.J.; Kalinina, O.; Özgüven, M.; Schüle, H.; Tarakanov, I.; van Der Weijde, T.; Anisimov, A.A.; Iqbal, Y.; Kiesel, A.; et al. Environmental influences on the growing season duration and ripening of diverse Miscanthus germplasm grown in six countries. Front. Plant Sci. 2017, 8, 907. [Google Scholar] [CrossRef] [PubMed]
- Scordia, D.; Testa, G.; Copani, V.; Patanè, C.; Cosentino, S.L. Lignocellulosic biomass production of Mediterranean wild accessions (Oryzopsis miliacea, Cymbopogon hirtus, Sorghum halepense and Saccharum spontaneum) in a semi-arid environment. Field Crop. Res. 2017, 214, 56–65. [Google Scholar] [CrossRef]
- Hastings, A.; Tallis, M.; Casella, E.; Matthews, R.W.; Henshall, P.A.; Milner, S.; Smith, P.; Taylor, G. The technical potential of Great Britain to produce lignocellulosic biomass for bioenergy in current and future climates. Glob. Chang. Biol. Bioenergy 2014, 6, 108–122. [Google Scholar] [CrossRef]
- Cosentino, S.L.; Scordia, D.; Sanzone, E.; Testa, G.; Copani, V. Response of giant reed (Arundo donax L.) to nitrogen fertilization and soil water availability in semi-arid Mediterranean environment. Eur. J. Agron. 2014, 60, 22–32. [Google Scholar] [CrossRef]
- Confalonieri, R.; Jones, B.; Van Diepen, K.; Van Orshoven, J. Scientific contribution on combining biophysical criteria underpinning the delineation of agricultural areas affected by specific constraints. In RC Science and Policy Reports; Terres, J.M., Hagyo, A., Wania, A., Eds.; European Commission, Joint Research Centre, Institute for Environment and Sustainability: Brussels, Belgium, 2014; ISBN 978-92-79-44340-4. [Google Scholar]
- Cosentino, S.L.; Copani, V.; Testa, G.; Scordia, D. Saccharum spontaneum L. ssp. aegyptiacum (Willd.) Hack. A potential perennial grass for biomass production in marginal land in semi-arid Mediterranean environment. Ind. Crops Prod. 2015, 75, 93–102. [Google Scholar]
- Scordia, D.; Testa, G.; Cosentino, S.L.; Copani, V.; Patanè, C. Soil water effect on crop growth, leaf gas exchange, water and radiation use efficiency of Saccharum spontaneum L. ssp. aegyptiacum (Willd.) Hackel in semi-arid Mediterranean environment. Ital. J. Agron. 2015, 10, 185. [Google Scholar]
- Cosentino, S.L.; Patanè, C.; Sanzone, E.; Testa, G.; Scordia, D. Leaf gas exchange, water status and radiation use efficiency of giant reed (Arundo donax L.) in a changing soil nitrogen fertilization and soil water availability in a semi-arid Mediterranean area. Eur. J. Agron. 2016, 72, 56–69. [Google Scholar] [CrossRef]
- Haworth, M.; Cosentino, S.L.; Marino, G.; Brunetti, C.; Scordia, D.; Testa, G.; Riggi, E.; Avola, G.; Loreto, F.; Centritto, M. Physiological responses of Arundo donax ecotypes to drought: A common garden study. GCB Bioenergy 2017, 9, 132–143. [Google Scholar] [CrossRef]
- Jones, M.B.; Finnan, J.; Hodkinson, T.R. Morphological and physiological traits for higher biomass production in perennial rhizomatous grasses grown on marginal land. Glob. Chang. Biol. Bioenergy 2015, 7, 375–385. [Google Scholar] [CrossRef]
- Haworth, M.; Marino, G.; Riggi, E.; Avola, G.; Brunetti, C.; Scordia, D.; Testa, G.; Gomes, M.T.G.; Loreto, F.; Cosentino, S.L.; et al. The effect of summer drought on the yield of Arundo donax is reduced by the retention of photosynthetic capacity and leaf growth later in the growing season. Ann. Bot. 2019, 124, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Foti, S.; Cosentino, S.L.; Patanè, C.; Copani, V.; Sanzone, E. Plant indicators of available soil water in Miscanthus × giganteus Greef and Deu. Agronomie 2003, 23, 29–36. [Google Scholar] [CrossRef]
- El-Sharkawy, A.M.; Cock, J.H.; Del Pilar Hernandez, A. Stomatal response to air humidity and its relation to stomatal density in a wide range of warm climate species. Photosyn. Res. 1985, 7, 137–149. [Google Scholar] [CrossRef] [PubMed]
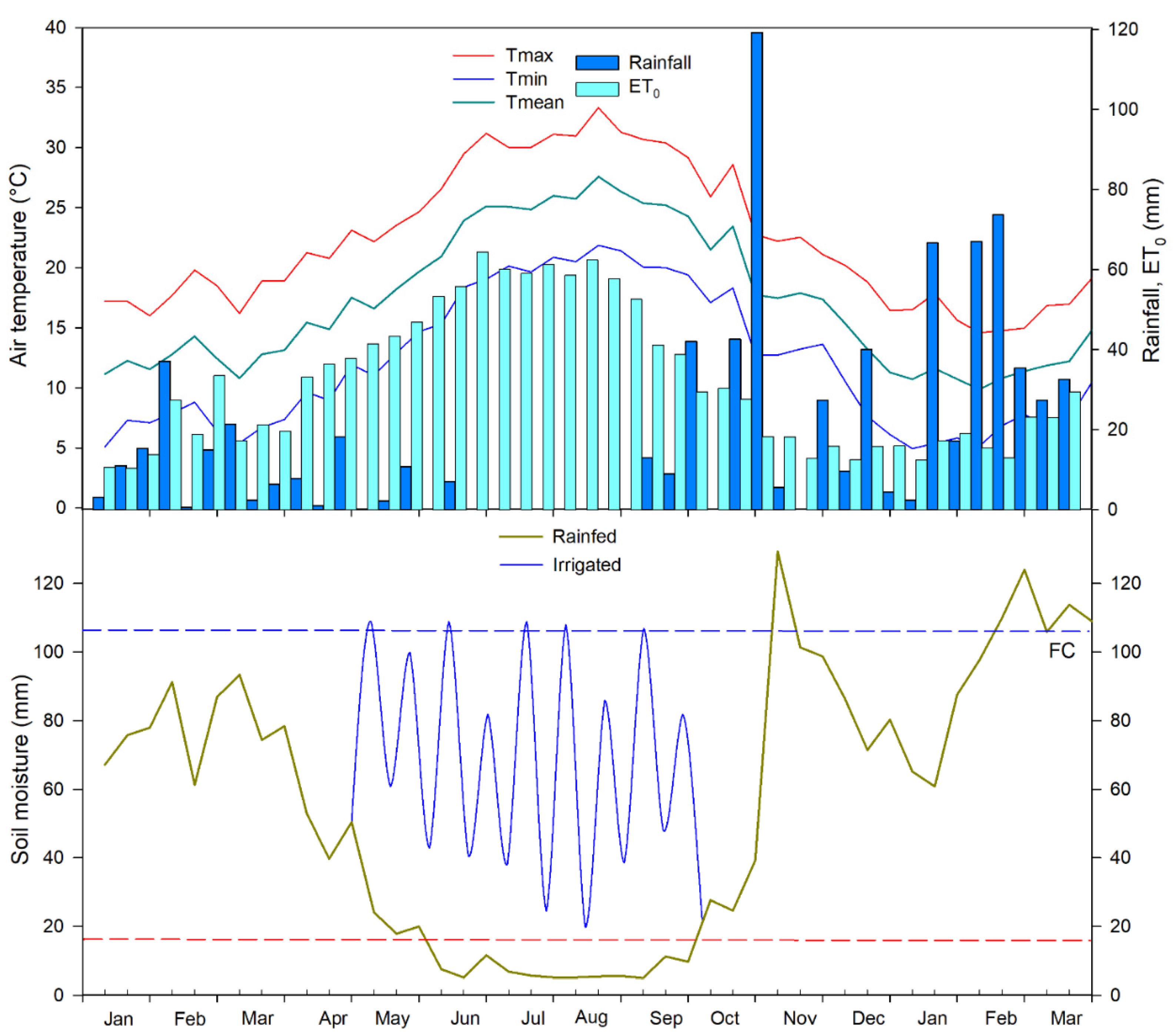
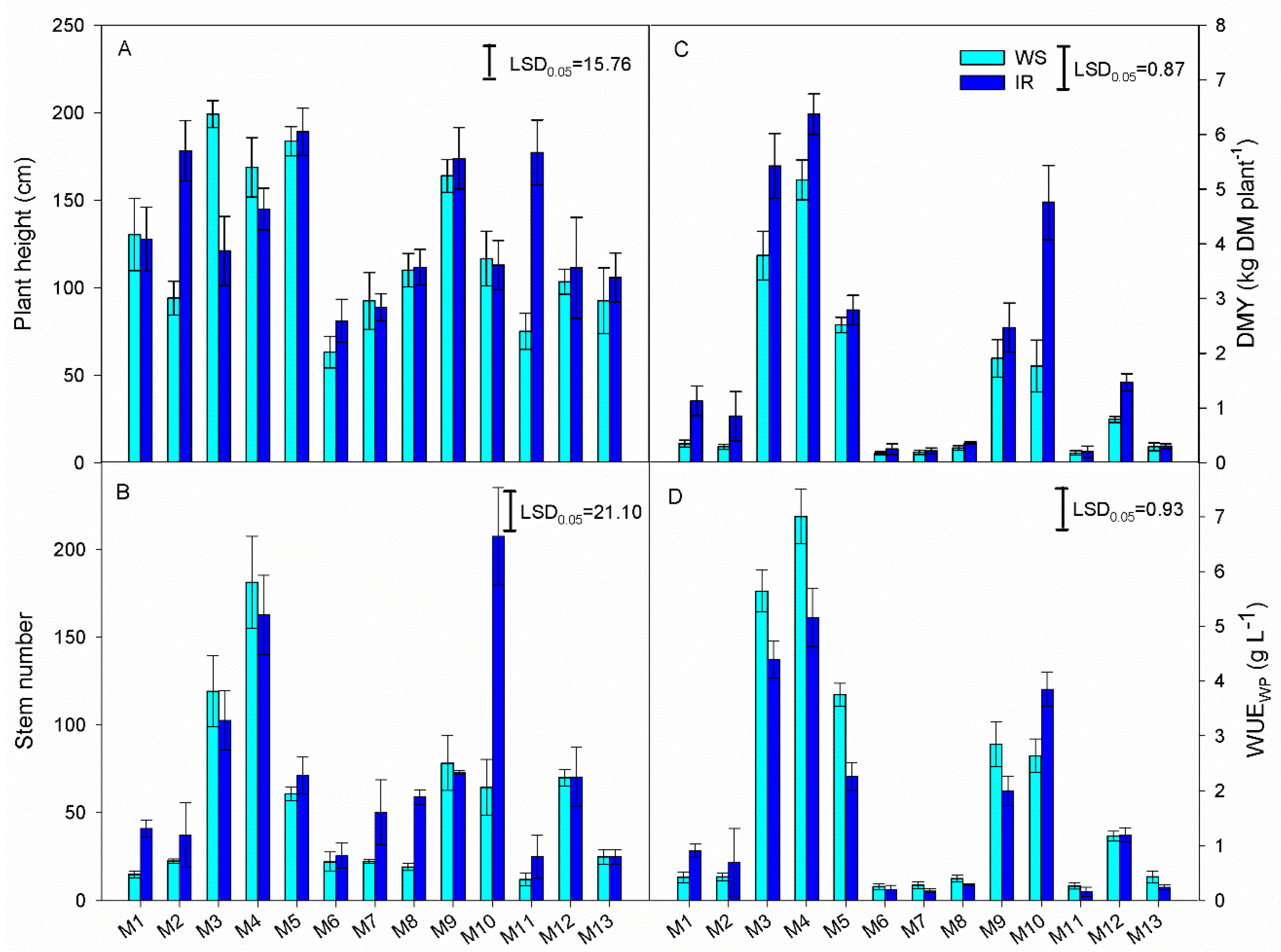
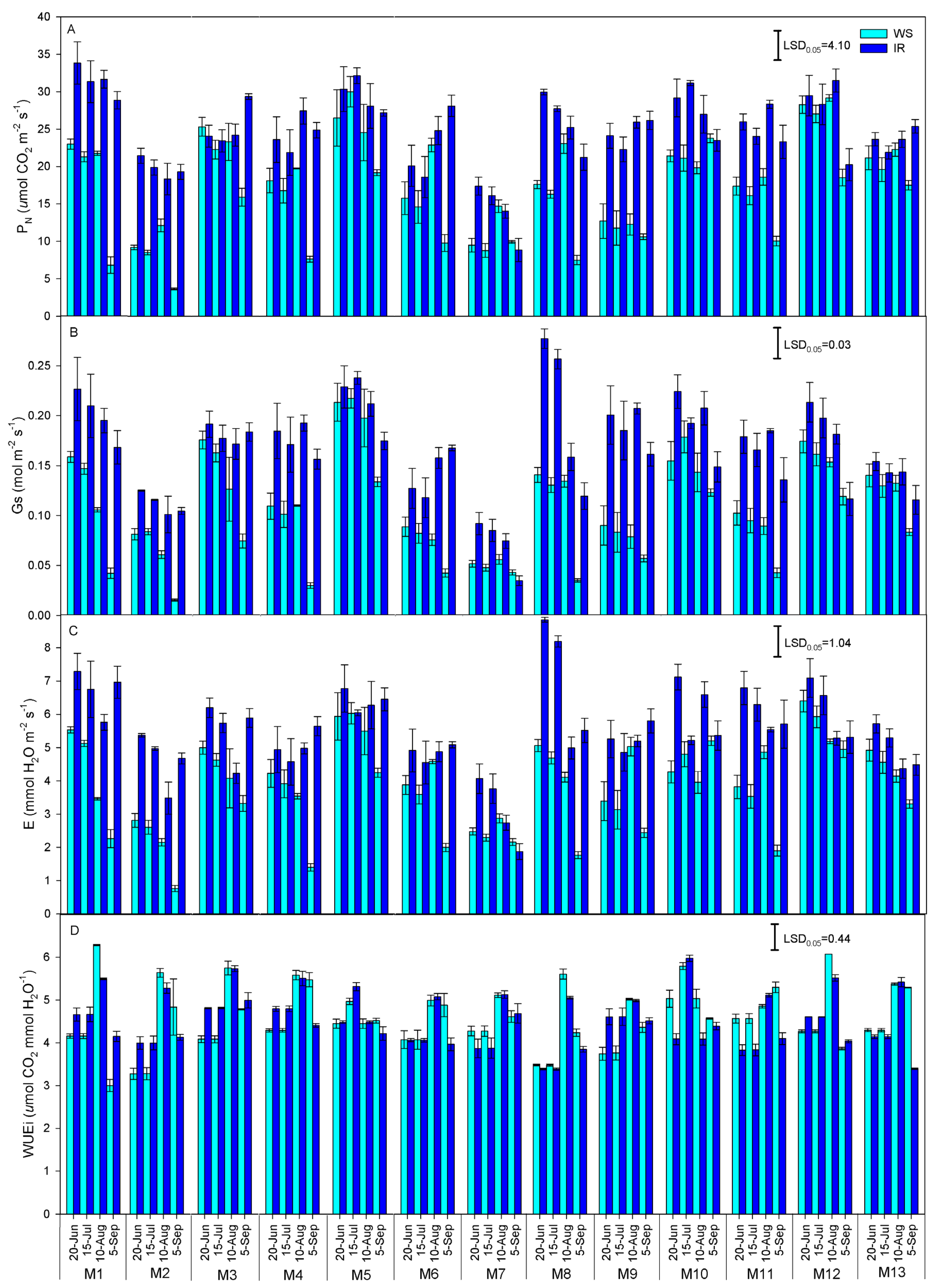
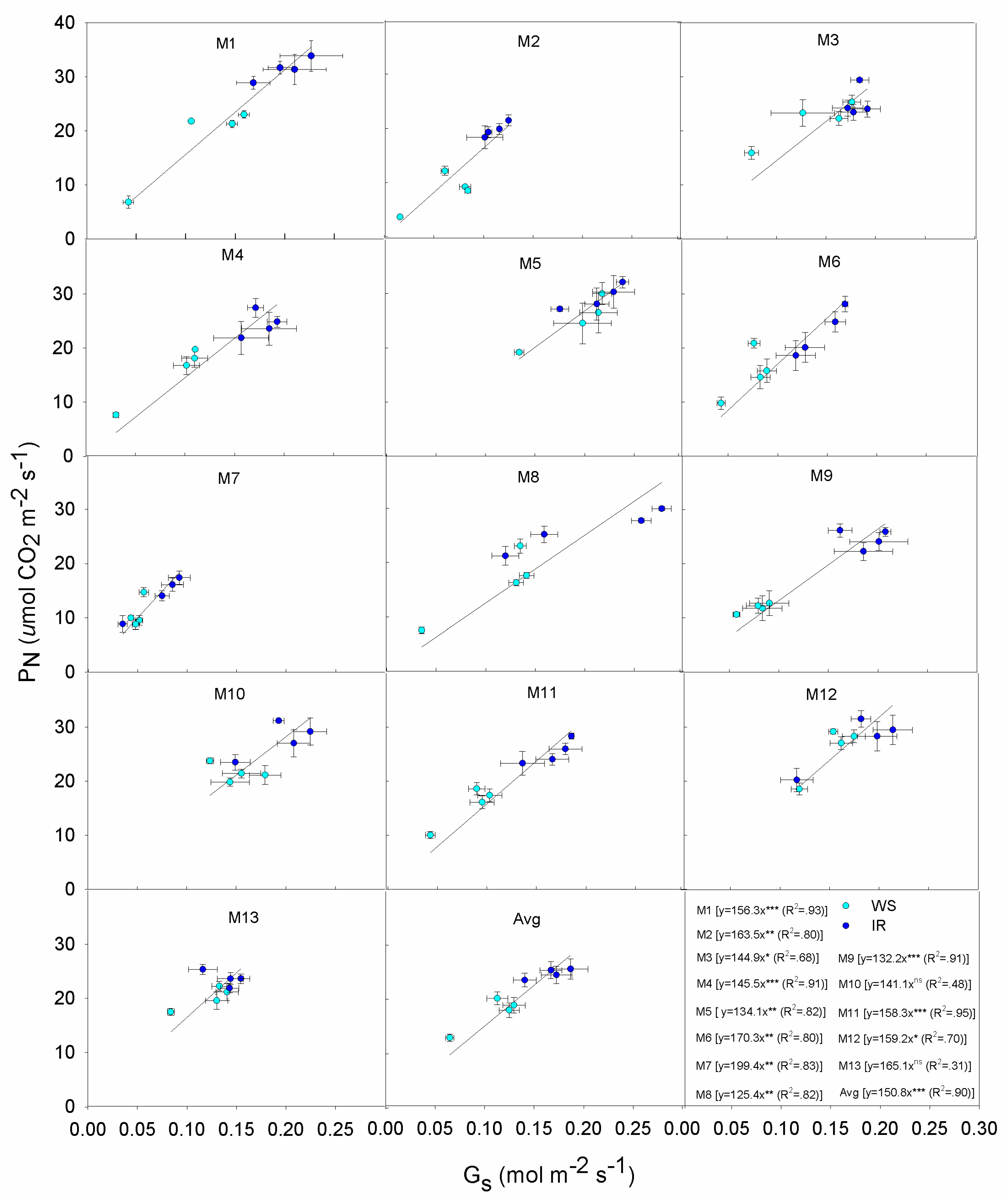
| Species | Acronym | Origin (Latitude) | Summer Rain (mm) |
|---|---|---|---|
| M. x giganteus | M1 | ||
| M. sacchariflorus | M2 | China (29°03′ N) | 922.5 |
| M. floridulus | M3 | Taiwan (23°56′ N) | 1509.5 |
| M4 | Taiwan (23°56′ N) | 1509.5 | |
| M5 | Taiwan (24°60′ N | 1461.5 | |
| M6 | Taiwan (24°03′ N) | 1468.7 | |
| M. sinensis | M7 | Japan (35°47′ N) | 1203.9 |
| M8 | Japan (37°85′ N) | 1166.2 | |
| M9 | Taiwan (24°62′ N) | 1461.6 | |
| M10 | China (18°50′ N) | 1260.5 | |
| M11 | Japan (35°41′ N) | 1295.6 | |
| M12 | Taiwan (22°01′ N) | 1495.6 | |
| M13 | Japan (37°85′ N) | 1166.2 |
| Source | DF | Stem Number | Plant Height | DMY | WUEWP |
|---|---|---|---|---|---|
| Adj MS | |||||
| Genotype (G) | 12 | 14,162.79 * | 6629.21 * | 35.82 * | 44.72 * |
| Irrigation (I) | 1 | 6587.21 * | 1968.04 * | 8.69 * | 6.36 * |
| G × I | 12 | 2538.06 * | 2955.89 * | 1.05 * | 2.76 * |
| Error | 50 | 235.53 | 131.51 | 0.42 | 0.45 |
| Source | DF | PN | Gs | E | WUEi |
|---|---|---|---|---|---|
| Adj MS | |||||
| Genotype (G) | 12 | 514.32 * | 0.032 * | 21.166 * | 1.388 * |
| Irrigation (I) | 1 | 1650.09 * | 0.128 * | 89.401 * | 0.041 ns |
| Time (T) | 3 | 535.84 * | 0.046 * | 16.501 * | 25.768 * |
| G × T | 36 | 26.00 * | 0.002 * | 1.844 * | 0.532 * |
| I × T | 3 | 302.53 * | 0.014 * | 25.930 * | 2.034 * |
| G × I | 12 | 219.57 * | 0.012 * | 12.853 * | 0.816 * |
| G × I × T | 36 | 21.38 * | 0.002 * | 1.637 * | 0.365 * |
| Error(T) | 156 | 0.900 | 0.0005 | 0.054 | 0.020 |
| Error | 52 | 13.31 | 0.01 | 0.698 | 0.042 |
| PN | Gs | E | WUEi | Plant Height | Stem Number | DMY | |
|---|---|---|---|---|---|---|---|
| Gs | 0.95 *** | ||||||
| E | 0.96 *** | 0.94 *** | |||||
| WUEi | 0.26 * | 0.15 ns | −0.00 ns | ||||
| Plant Height | 0.24 * | 0.28 * | 0.22 ns | 0.07 ns | |||
| Stem Number | 0.16 ns | 0.20 ns | 0.04 ns | 0.54 *** | 0.38 ** | ||
| DMY | 0.25 ns | 0.30 * | 0.10 ns | 0.62 *** | 0.45 ** | 0.88 *** | |
| WUEWP | 0.15 ns | 0.17 ns | 0.02 ns | 0.56 *** | 0.53 *** | 0.84 *** | 0.92 *** |
| Genotype | Response |
|---|---|
| M1 | Exhibits a wide variation in PN from 36 down to 7 μmol m−2 s−1: high PN is associated with high Gs even under WS. This displays the typical Mxg ‘optimistic’ strategy, with profligate water use even during the onset of mild to severe drought. Overall M1 produced lower DMY relative to other genotypes both IR and WS treatments. This indicates that more assimilates are being partitioned to the below ground roots and rhizomes. |
| M2 | Maintains PN under WS and IR, low Gs also under IR; contains E under WS and IR; low E but low increase of PN per unit of E; low DMY in IR and lower in WS. |
| M3 | Medium-high PN under moderate Gs, increase slightly PN under IR; moderate E due to moderate Gs under both WS and IR; moderate PN due to moderate E under both conditions; high DMY in IR, significantly reduces in WS. |
| M4 | High PN under IR, reduces Gs under WS but PN is still at high levels; moderate E due to limited Gs, even under IR; high PN under little increases of E; high DMY in both IR and WS. |
| M5 | High PN but high Gs in both IR and WS; high E due to low Gs control; high PN but at high water expenses; medium-high DMY in both IR and WS. |
| M6 | Moderate PN due to limited Gs, increases PN quickly but it remains still low per unit of Gs; moderate E but reaches its maximum even under low Gs; low PN under moderate E; low DMY in IR and lower in WS. |
| M7 | Low PN at low Gs, little differences between IR and WS; low E due to low Gs; low E but low PN; low DMY in IR and lower in WS. |
| M8 | High PN under IR due to high Gs, but moderate PN in WS; high E under IR due to high Gs, reduces Gs and E under WS; moderate PN under quite high E; low DMY in IR and lower in WS. |
| M9 | Low PN and E under WS due to very low Gs; low PN under moderate E, but increases PN in small ranges of E; medium-high DMY in both IR and WS. |
| M10 | High PN and E under moderate Gs, increase slightly PN and E under IR; high PN but at high water expenses; high DMY in IR, significantly reduces in WS. |
| M11 | Medium-high PN and E under moderate Gs, increase PN and E under IR; quite high E under moderate Gs, and high PN at high E; low DMY and similar in both conditions. |
| M12 | High PN but high Gs in both IR and WS; high E due to low Gs regulation; high PN but high water losses; medium-high DMY in IR, reduces in WS. |
| M13 | Moderate Gs to support moderate PN and E in both IR and WS; low DMY and similar in both IR and WS conditions. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scordia, D.; Scalici, G.; Clifton-Brown, J.; Robson, P.; Patanè, C.; Cosentino, S.L. Wild Miscanthus Germplasm in a Drought-Affected Area: Physiology and Agronomy Appraisals. Agronomy 2020, 10, 679. https://doi.org/10.3390/agronomy10050679
Scordia D, Scalici G, Clifton-Brown J, Robson P, Patanè C, Cosentino SL. Wild Miscanthus Germplasm in a Drought-Affected Area: Physiology and Agronomy Appraisals. Agronomy. 2020; 10(5):679. https://doi.org/10.3390/agronomy10050679
Chicago/Turabian StyleScordia, Danilo, Giovanni Scalici, John Clifton-Brown, Paul Robson, Cristina Patanè, and Salvatore Luciano Cosentino. 2020. "Wild Miscanthus Germplasm in a Drought-Affected Area: Physiology and Agronomy Appraisals" Agronomy 10, no. 5: 679. https://doi.org/10.3390/agronomy10050679
APA StyleScordia, D., Scalici, G., Clifton-Brown, J., Robson, P., Patanè, C., & Cosentino, S. L. (2020). Wild Miscanthus Germplasm in a Drought-Affected Area: Physiology and Agronomy Appraisals. Agronomy, 10(5), 679. https://doi.org/10.3390/agronomy10050679







