Inoculation with Different Nitrogen-Fixing Bacteria and Arbuscular Mycorrhiza Affects Grain Protein Content and Nodule Bacterial Communities of a Fava Bean Crop
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cultivation Conditions and Experimental Design
2.2. Soil Sampling and Analyses
2.3. Inoculum Preparation
2.4. Plant Sampling
2.5. Plant Analyses
2.6. Efficiency of Biological Nitrogen Fixation
2.7. DNA Extraction, PCR Amplification and Processing of Sequencing Data
2.8. Statistical Analyses
3. Results
3.1. Soil Fertility
3.2. Biological Nitrogen Fixation
3.3. Plant Nutritional Characteristics
3.4. Crop Yield and Quality
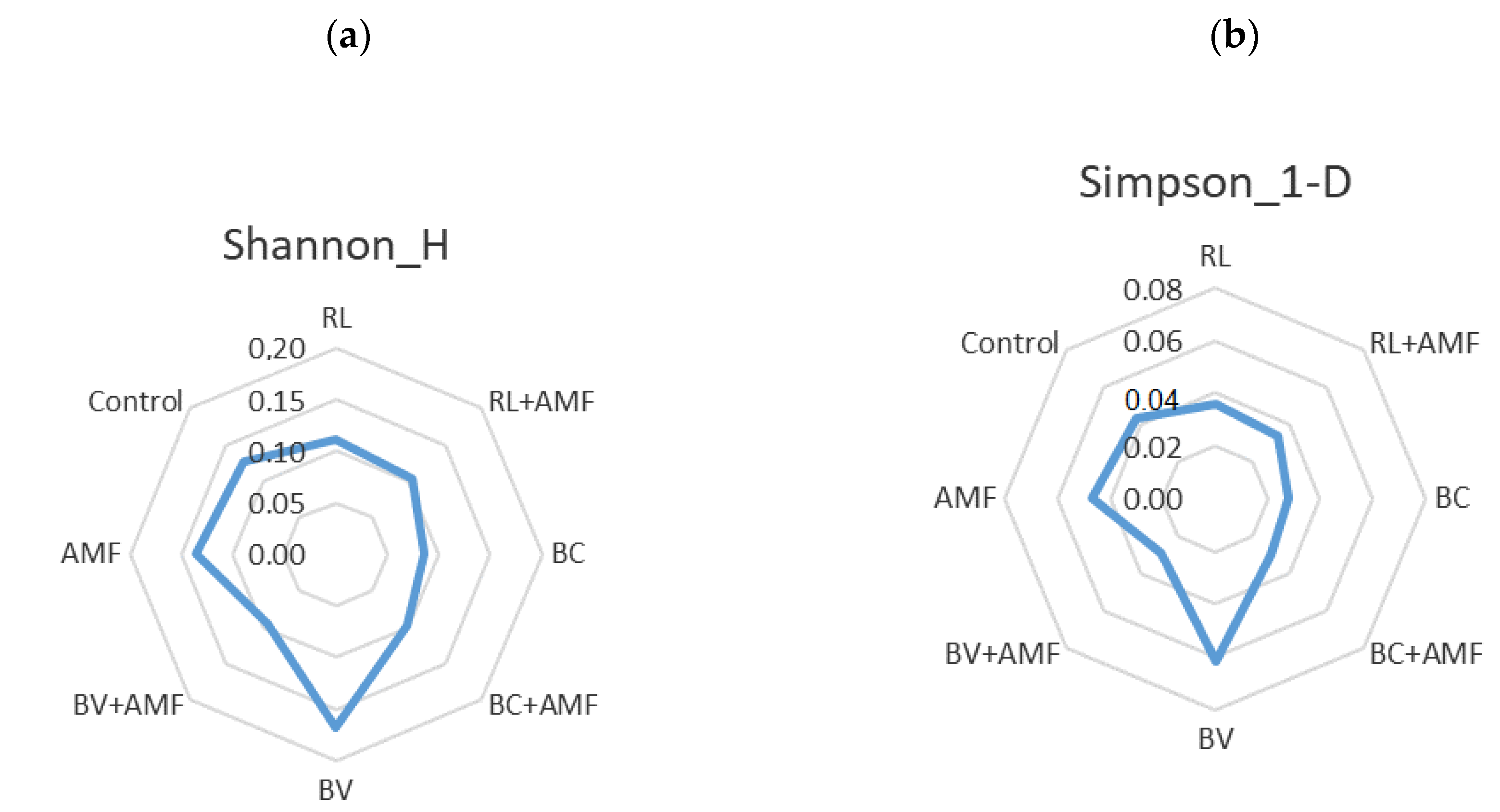
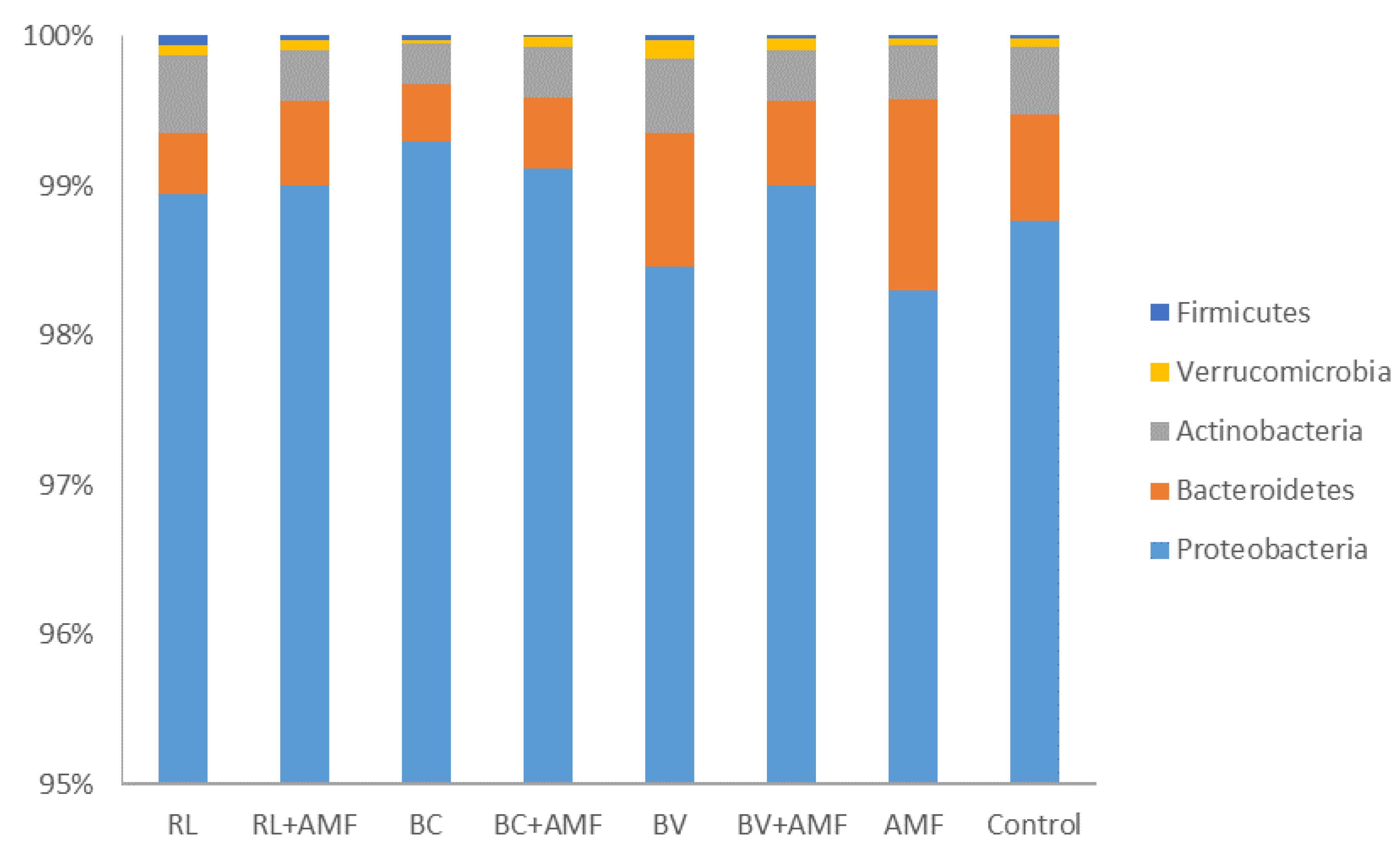
3.5. Bacterial Diversity and Community Structure
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lugtenberg, B.J.J.; Chin-A-Woeng, T.F.C.; Bloemberg, G.V. Microbee plant interactions: Principles and mechanisms. Antonie Van Leeuwenhoek 2002, 81, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Peoples, M.B.; Herridge, D.E.; Ladha, J.K. Biological nitrogen fixation: An efficient source of nitrogen for sustainable agricultural production? Plant Soil 1995, 174, 3–28. [Google Scholar] [CrossRef]
- Giller, K.E. Nitrogen Fixation in Tropical Cropping Systems, 2nd ed.; CABI Publishing: Wallingford, UK, 2001. [Google Scholar]
- Lu, J.; Yang, F.; Wang, S.; Ma, H.; Liang, J.; Chen, Y. Co-existence of rhizobia and diverse non-rhizobial bacteria in the rhizosphere and nodules of Dalbergia odorifera seedlings inoculated with Bradyrhizobium elkani, Rhizobium multihospitium like and Burkholderia pyrrocinia like strains. Front. Microbiol. 2017, 8, 2255. [Google Scholar] [CrossRef] [PubMed]
- Laranjo, M.; Alexandre, A.; Oliveira, S. Legume growth-promoting rhizobia: An overview on the Mesorhizobium genus. Microbiol. Res. 2014, 169, 2–17. [Google Scholar] [CrossRef]
- Vandamme, P.; Goris, J.; Chen, W.M.; De Vos, P.; Willems, A. Burkholderia tuberum sp. nov. and Burkholderia phymatum sp. nov., nodulate the roots of tropical legumes. Syst. Appl. Microbiol. 2002, 25, 507–512. [Google Scholar] [CrossRef]
- Benhizia, Y.; Benhizia, H.; Benguedouar, A.; Muresu, R.; Giacomini, A.; Squartini, A. Gamma proteobacteria can nodulate legumes of the genus Hedysarum. Syst. Appl. Microbiol. 2004, 27, 462–468. [Google Scholar] [CrossRef]
- Glick, B.R. Plant Growth-Promoting Bacteria: Mechanisms and Applications; Hindawi Publishing Corporation Scientific: Waterloo, ON, Canada, 2012. [Google Scholar]
- Zaidi, A.; Ahmad, E.; Khan, M.S.; Saif, S.; Rizvi, A. Role of plant growth promoting rhizobacteria in sustainable production of vegetables: Current perspective. Sci. Hortic. 2015, 193, 231–239. [Google Scholar] [CrossRef]
- Nadeem, S.M.; Ahmad, M.; Zahir, Z.A.; Javaid, A.; Ashral, M. The role of mycorrhizae and plant growth promoting rhizobacteria (PGPR) in improving crop productivity under stressful environments. Biotechnol. Adv. 2014, 32, 429–448. [Google Scholar] [CrossRef]
- Busby, R.R.; Rodriguez, G.; Gebhart, D.L.; Yannarell, A.C. Native Lespedeza species harbour greater non-rhizobial bacterial diversity in root nodules compared to the coexisting invader, L. cuneata. Plant Soil 2016, 401, 427–436. [Google Scholar] [CrossRef]
- Peix, A.; Ramirez-Bahena, M.H.; Velazquez, E.; Bedmar, E.J. Bacterial associations with legumes. Crit. Rev. Plant Sci. 2015, 34, 17–42. [Google Scholar] [CrossRef]
- Sharda, J.N.; Koide, R.T. Exploring the role of root anatomy in P-mediated control of colonization by arbuscular mycorrhizal fungi. Botany 2010, 88, 165–173. [Google Scholar] [CrossRef]
- Al-Niemi, T.S.; Kahn, M.L.; Mc Dermott, T.R. P metabolism in the bean-Rhizobium tropic symbiosis. Plant Physiol. 1997, 113, 1233–1242. [Google Scholar] [CrossRef] [Green Version]
- Shen, J.; Yuan, L.; Zhang, J.; Li, H.; Bai, Z.; Chen, X.; Zhang, W.; Zhang, F. Phosphorus dynamics: From soil to plant. Plant Physiol. 2011, 156, 997–1005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brockwell, J.; Bottomley, P.J. Recent advances in inoculant technology and prospects for the future. Soil Biol. Biochem. 1995, 27, 683–697. [Google Scholar] [CrossRef]
- Catroux, G.; Hartmann, A.; Revellin, C. Trends in rhizobial inoculant production and use. Plant Soil 2001, 230, 21–30. [Google Scholar] [CrossRef]
- Meghvansi, M.K.; Prasad, K.; Mahna, S.K. Symbiotic potential, competitiveness and compatibility of indigenous Bradyrhizobium japonicum isolates to three soybean genotypes of two distinct agro-climatic regions of Rajasthan. India Saudi J. Biol. Sci. 2010, 17, 303–310. [Google Scholar] [CrossRef] [Green Version]
- Pastor-Bueis, R.; Sánchez-Cañizares, C.; James, E.K.; González-Andrés, F. Formulation of a highly effective inoculant for common bean based on an autochthonous elite strain of Rhizobium leguminosarum bv. phaseoli, and genomic-based insights into its agronomic performance. Front. Microbiol. 2019, 10, 2724. [Google Scholar] [CrossRef]
- Pacovsky, R.S.; Fuller, G.; Stafford, A.E. Nutrient and growth interactions in soybeans colonized with Glomus fasciculatum and Rhizobium japonicum. Plant Soil 1986, 92, 37–45. [Google Scholar] [CrossRef]
- Azcon, R.; Rubio, R.; Barea, J.M. Selective interactions between different species of mycorrhizal fungi and Rhizobium meliloti strains and their effects on growth, N2-fixation (15N) and nutrition of Medicago sativa L. New Phytol. 1991, 117, 399–404. [Google Scholar] [CrossRef]
- Tajini, F.; Trabelsi, M.; Drevon, J.J. Combined inoculation with Glomus intraradices and Rhizobium tropici CIAT899 increases phosphorus use efficiency for symbiotic nitrogen fixation in common bean (Phaseolus vulgaris L.). Saudi J. Biol. Sci. 2012, 19, 157–163. [Google Scholar] [CrossRef]
- Abd-Alla, M.H.; El-Enany, A.W.E.; Nafady, N.A.; Khalaf, D.M.; Morsy, F.M. Synergistic interaction of Rhizobium leguminosarum bv. viciae and arbuscular mycorrhizal fungi as a plant growth promoting biofertilizers for faba bean (Vicia faba L.) in alkaline soil. Microbiol. Res. 2014, 169, 49–58. [Google Scholar] [PubMed]
- Hoeger, R. Training Papers Nitrogen Determination According to Kjeldahl; BÜCHI Labortechnik AG: Flawil, Switzerland, 1998; pp. 4–10. [Google Scholar]
- Roig, A.; Romero, M.; Lax, A.; Fernández, F.G. Estudio comparativo de métodos de determinación de capacidad de cambio catiónica en suelos calizos. Anal. Edaf. Agrobiol. 1980, 39, 2021–2032. [Google Scholar]
- Vincent, J.M. A Manual for the Practical Study of Root Nodule Bacteria; Blackwell: Oxford-Edinburgh, UK, 1970. [Google Scholar]
- Cunniff, P. Official Methods of Analysis of AOAC International, 16th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1995. [Google Scholar]
- Keeney, D.R.; Nelson, D.W. Nitrogen-inorganic forms. In Methods of Soil Analysis, Part 2 Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy, Inc.: Madison, WI, USA, 1982; pp. 643–693. [Google Scholar]
- Bedard-Haughn, A.; Van Groenigen, J.W.; Van Kessel, C. Tracing 15N through landscapes: Potential uses and precautions. J. Hydrol. 2003, 272, 175–190. [Google Scholar] [CrossRef]
- Unkovich, M.; Herridge, D.; Peoples, M.; Cadish, G.; Boddey, R.; Giller, K.; Alves, B.; Chalk, P. Measuring Plant Associated Nitrogen Fixation in Agricultural Systems; Clarus Design Pty Ltd.: Canberra, Australia, 2008. [Google Scholar]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M.; et al. Ultra-high-throughput microbial community analysis on the Illimina HiSeq and MiSeq platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef] [Green Version]
- Žifčáková, L.; Vetrovsky, T.; Howe, A.; Baldrian, P. Microbial activity in forest soil reflects the changes in ecosystem properties between summer and winter. Environ. Microbiol. 2016, 18, 288–301. [Google Scholar] [CrossRef]
- Větrovský, T.; Baldrian, P.; Morais, D. SEED 2: A user-friendly platform for amplicon high-throughput sequencing data analyses. Bioinformatics 2018, 34, 2292–2294. [Google Scholar] [CrossRef]
- Aronesty, E. Comparison of sequencing utility programs. Open Bioinform. J. 2013, 7, 1–8. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 1, 2460–2461. [Google Scholar] [CrossRef] [Green Version]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Hammer, O.; Harper, D.; Ryan, P. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Zahir, Z.A.; Arshad, M.; Frankenberger, W.T., Jr. Plant growth promoting rhizobacteria application and perspectives in agriculture. Adv. Agron. 2004, 81, 96–168. [Google Scholar]
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; van der Putten, W.H. Going back to the roots: The microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 2013, 11, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Nardi, S.; Concheri, G.; Pizzeghello, D.; Sturano, A.; Rella, R.; Parvoli, G. Soil organic matter mobilization by root exudates. Chemosphere 2000, 5, 653–658. [Google Scholar] [CrossRef]
- Walker, T.S.; Bais, H.P.; Groteworld, E.; Vivanco, J.M. Root exudation and rhizosphere biology. Plant Physiol. 2003, 132, 44–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alsina, I.; Dubova, L.; Karlovska, A.; Steinberga, V.; Strauta, L. Evaluation of effectiveness of Rhizobium leguminosarum strains on broad beans. Acta Hortic. 2016, 1142, 417–422. [Google Scholar] [CrossRef]
- Sistani, N.R.; Kaul, H.P.; Desalegn, G.; Wienkoop, S. Rhizobium Impacts on Seed Productivity, Quality, and Protection of Pisum sativum upon Disease Stress Caused by Didymellapinodes: Phenotypic, Proteomic, and Metabolomic Traits. Front. Plant Sci. 2017, 8, 1961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elsheikh, E.A.; Elzidany, A.A. Effects of Rhizobium inoculation, organic and chemical fertilizers on yield and physical properties of faba bean seeds. Plant Foods Hum. Nutr. 1997, 51, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Massa, N.; Cesaro, P.; Todeschini, V.; Capraro, J.; Scarafoni, A.; Cantamessa, S.; Copetta, A.; Anastasia, F.; Gamalero, E.; Lingua, G.; et al. Selected autochthonous rhizobia, applied in combination with AM fungi, improve seed quality of common bean cultivated in reduced fertilization condition. Appl. Soil Ecol. 2020, 148, 103507. [Google Scholar] [CrossRef]
- Zhang, B.; Chang, S.X.; Angya, A.O. Mycorrhizal inoculation and nitrogen fertilization affect the physiology and growth of spring wheat under two contrasting water regimes. Plant Soil 2016, 398, 47–57. [Google Scholar] [CrossRef]
- Requena, N.; Perez-Solis, E.; Azcon-Aguilar, C.; Jeffries, P.; Barea, J. Management of indigenous plant-microbe symbioses aids restoration of desertified ecosystems. Appl. Environ. Microbiol. 2001, 67, 495–498. [Google Scholar] [CrossRef] [Green Version]
- Lodwig, E.M.; Hosie, A.H.F.; Bourdes, A.; Findlay, K.; Allaway, D.; Karunakaran, R.; Dowie, J.A.; Poole, P.S. Amino-acid cycling drives nitrogen fixation in the Legume-Rhizobium symbiosis. Nature 2003, 422, 722–726. [Google Scholar] [CrossRef]
- Scheublin, T.R.; Ridgway, P.; Van Der Heijden, M.G. Nonlegumes, legumes, and root nodules harbor different arbuscular mycorhizal fungal communities. Appl. Environ. Microbiol. 2004, 70, 6240–6246. [Google Scholar] [CrossRef] [Green Version]
- Pennanen, T.; Liski, J.; Baath, E.; Kitunea, V.V.; Uotila, J.; Westman, C.J. Structure of the microbial communities in coniferous forest soils in relation to site fertility and stand development stage. Microb. Ecol. 1999, 38, 168–179. [Google Scholar] [CrossRef]
- Menge, D.; Wolf, A.; Funk, J. Diversity of nitrogen fixation strategies in Mediterranean legumes. Nat. Plants 2015, 1, 15064. [Google Scholar] [CrossRef] [PubMed]
- Bottomley, D.J. Ecology of Bradyrhizobium and Rhizobium. In Biological Nitrogen Fixation; Stacey, G., Burris, R.H., Evans, H.J., Eds.; Chapman and Hall: New York, NY, USA, 1992; pp. 293–348. [Google Scholar]
- Zgadzaj, R.; James, E.K.; Kelly, S.; Kawaharada, Y.; de Jonge, N.; Jensen, D.B.; Madsen, L.H.; Radutoiu, S. A legume genetic framework controls infection of nodules by symbiotic and endophytic bacteria. PLoS Genet. 2015, 11, e1005280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez, J.; Negrete-Yankelevich, S.; Godinez, L.G.; Reyes, J.; Esposti, M.D.; Martínez Romero, E. Short-Term evolution of rhizobial strains toward sustainability in agriculture. In Microbial Models: From Environmental to Industrial Sustainability; Springer: Singapore, 2016. [Google Scholar]
- Dludlu, M.N.; Chimphango, S.B.M.; Stirton, C.H.; Muasya, A.M. Differential Preference of Burkholderia and Mesorhizobium to pH and Soil Types in the Core Cape Subregion, South Africa. Genes 2018, 9, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gano-Cohen, K.A.; Stokes, P.J.; Blanton, M.A.; Wendlandt, C.E.; Hallowell, A.C.; Regus, J.U.; Kim, D.; Patel, S.; Pahua, V.J.; Sachs, J.L. Nonnodulating Bradyrhizobium spp. modulate the benefits of legume Rhizobium mutualism. Appl. Environ. Microb. 2016, 82, 5259–5268. [Google Scholar] [CrossRef] [Green Version]
- Ibañez, F.; Angelini, J.; Taurin, T.; Tonelli, M.L.; Fabra, A. Endophytic occupation of peanut root nodules by opportunistic gammaproteobacteria. Syst. Appl. Microbiol. 2009, 32, 49–55. [Google Scholar] [CrossRef]
- Ampomah, O.; Huss-Danell, K. Genetic diversity of root nodule bacteria nodulating Lotus corniculatus and Anthyllis vulneraria Sweden. Syst. Appl. Microbiol. 2011, 34, 267–275. [Google Scholar] [CrossRef]
- Youseif, S.H.; El-Megeed, F.; Ageez, A.; Mohamed, Z.K.; Shamseldin, A.; Saleh, S.A. Phenotypic characteristics and genetic diversity of rhizobia nodulating soybean in Egyptian soils. Eur. J. Soil Biol. 2014, 60, 34–43. [Google Scholar] [CrossRef]
- Hoque, M.S.; Broadhurst, L.M.; Thrall, P.H. Genetic characterization of root-nodule bacteria associated with Acacia salicina and A. stenophylla (Mimosaceae) across south-eastern Australia. Int. J. Syst. Evol. Microbiol. 2011, 61, 299–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnawal, D.; Bharti, N.; Maji, D.; Chanotiya, C.S.; Kalra, A. ACC deaminase-containing Arthrobacter protophormiae induces NaCl stress tolerance through reduced ACC oxidase activity and ethylene production resulting in improved nodulation and mycorrhization in Pisum sativum. J. Plant Physiol. 2014, 171, 884–894. [Google Scholar] [CrossRef] [PubMed]
- Sreevidya, M.; Gopalakrishnan, S.; Kudapa, H.; Varshney, R.K. Exploring plant growth-promotion actinomycetes from vermicompost and rhizosphere soil for yield enhancement in chickpea. Braz. J. Microbiol. 2016, 47, 85–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, L.; Zhang, Y.; Wang, L.; Chen, W.; Wei, G. Diversity of endophytic bacteria associated with nodules of two indigenous legumes at different altitudes of the Qilian Mountains in China. Syst. Appl. Microbiol. 2014, 37, 457–465. [Google Scholar] [CrossRef]
- Leite, J.; Fischer, D.; Rouws, L.F.; Fernandes-J´unior, P.I.; Hofmann, A.; Kublik, S.; Schloter, M.; Xavier, G.R.; Radl, V. Cowpea nodules harbor non-rhizobial bacterial communities that are shaped by soil type rather than plant genotype. Front. Plant Sci. 2017, 7, 2064. [Google Scholar] [CrossRef]
- Samac, D.A.; Willert, A.M.; McBride, M.J.; Kinkel, L.L. Effects of antibiotic producing Streptomyces on nodulation and leaf spot in Alfalfa. Appl. Soil Ecol. 2003, 22, 55–66. [Google Scholar] [CrossRef]
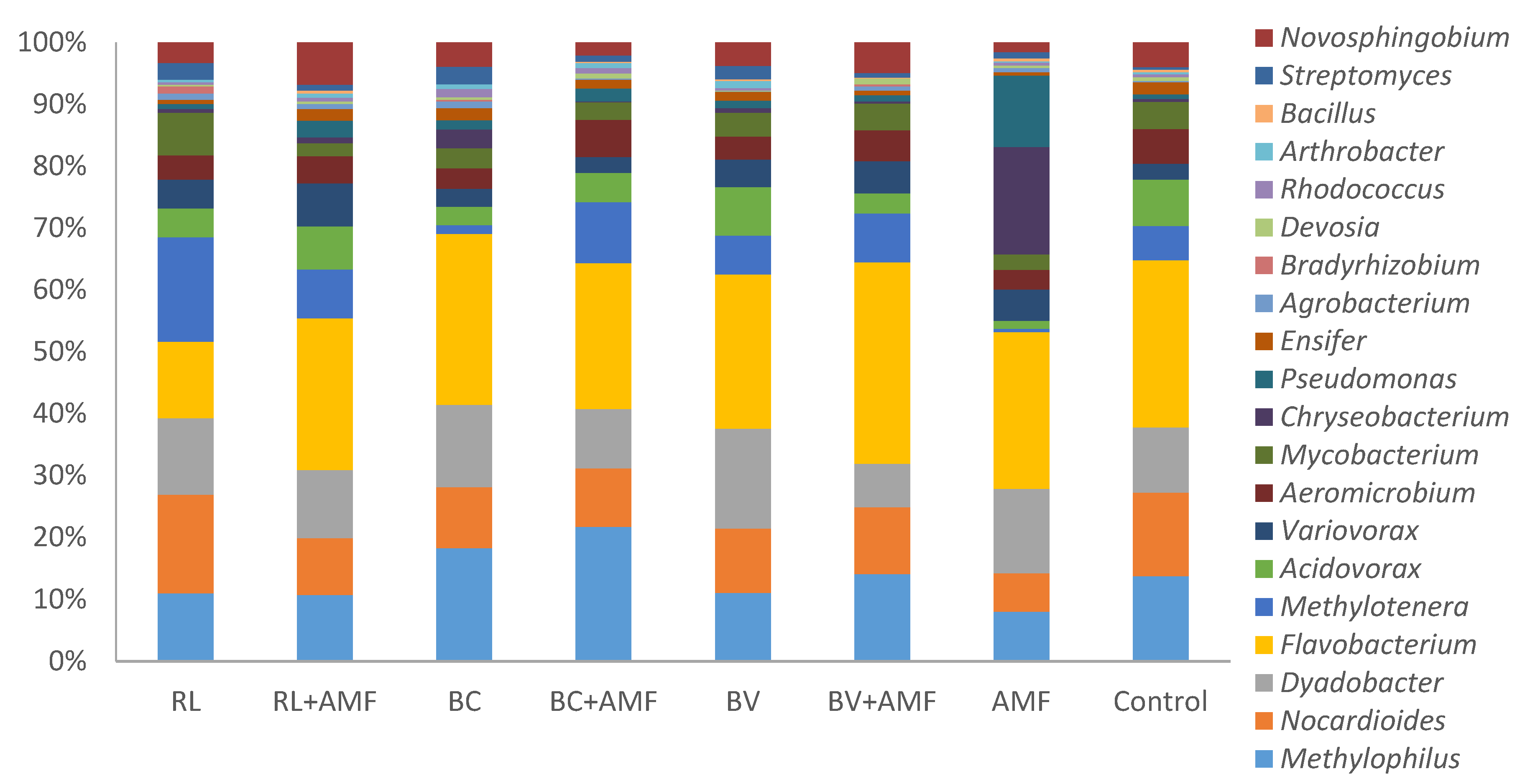
| Treatment (T) a | Season 1 | Season 2 | |
|---|---|---|---|
| RL | 62.8 ± 6.2 | 53.3 ± 5.9 | |
| RL + AMF | 61.2 ± 6.5 | 63.8 ± 6.7 | |
| BC | 63.0 ± 2.5 | 56.7 ± 10.7 | |
| BC + AMF | 61.3 ± 7.8 | 48.3 ± 2.9 | |
| BV | 57.8 ± 6.2 | 55.0 ± 12.4 | |
| BV + AMF | 59.6 ± 2.7 | 55.1 ± 10.1 | |
| AMF | 59.1 ± 0.8 | 58.3 ± 1.2 | |
| CONTROL | 61.8 ± 10.3 | 56.6 ± 8.2 | |
| F values b | |||
| Between subjects | |||
| Inoculation (IT) | 0.81 ns | ||
| Within subjects | |||
| Season (S) | 7.37 * | ||
| S × T | 1.56 ns | ||
| Treatment (T) a | Mg (mg kg−1) | K (mg kg−1) | P (mg kg−1) | Treatment (T) a | N (g kg−1) | Ca (mg kg−1) | Na (mg kg−1) | ||
|---|---|---|---|---|---|---|---|---|---|
| Season 1 | RL | 295 ± 146 | 2919 ± 1283 | 1090 ± 474 | Season 1 | RL | 48.2 ± 0.6 | 457 ± 320 | 91.7 ± 52.6 |
| RL + AMF | 195 ± 75 | 2333 ± 838 | 762 ± 249 | RL + AMF | 42.4 ± 1.0 | 172 ± 46 | 81.6 ± 27.2 | ||
| BC | 184 ± 65 | 1992 ± 687 | 760 ± 263 | BC | 41.6 ± 1.3 | 166 ± 75 | 52.2 ± 6.4 | ||
| BC + AMF | 236 ± 59 | 2899 ± 598 | 988 ± 232 | BC + AMF | 44.4 ± 5.1 | 217 ± 58 | 84.5 ± 5.7 | ||
| BV | 208 ± 167 | 2120 ± 1726 | 825 ± 658 | BV | 45.8 ± 1.8 | 186 ± 151 | 53.6 ± 28.6 | ||
| BV + AMF | 114 ± 45 | 1441 ± 544 | 501 ± 148 | BV + AMF | 44.6 ± 1.9 | 117 ± 45 | 49.8 ± 9.6 | ||
| AMF | 316 ± 96 | 2910 ± 727 | 454 ± 283 | AMF | 46.0 ± 0.8 | 237 ± 56 | 79.0 ± 23.4 | ||
| CONTROL | 217 ± 44 | 2482 ± 530 | 883 ± 181 | CONTROL | 47.1 ± 1.0 | 176 ± 55 | 72.5 ± 9.0 | ||
| Season 2 | RL | 780 ± 296 | 13,820 ± 3735 | 3836 ± 1060 | Season 2 | RL | 44.3 ± 1.5 | 443 ± 88 | 194 ± 100 |
| RL + AMF | 873 ± 251 | 14,764 ± 2411 | 3976 ± 668 | RL + AMF | 41.5 ± 2.8 | 543 ± 124 | 193 ± 15 | ||
| BC | 511 ± 96 | 10,954 ± 1090 | 2906 ± 391 | BC | 40.8 ± 2.5 | 343 ± 60 | 105 ± 43 | ||
| BC + AMF | 396 ± 62 | 10,101 ± 931 | 2369 ± 344 | BC + AMF | 44.0 ± 5.7 | 275 ± 26 | 117 ± 11 | ||
| BV | 567 ± 153 | 11,609 ± 1961 | 3206 ± 672 | BV | 46.1 ± 7.7 | 372 ± 97 | 134 ± 27 | ||
| BV + AMF | 757 ± 130 | 14,656 ± 2942 | 3834 ± 55 | BV + AMF | 44.6 ± 5.4 | 518 ± 101 | 147 ± 54 | ||
| AMF | 516 ± 118 | 10,777 ± 1249 | 2916 ± 329 | AMF | 43.2 ± 3.2 | 347 ± 73 | 155 ± 13 | ||
| CONTROL | 660 ± 340 | 12,510 ± 3505 | 3247 ± 1299 | CONTROL | 36.5 ± 2.9 | 415 ± 241 | 213 ± 87 | ||
| F values b | χ2 values b | ||||||||
| Between subjects | Inoculation (IT) | 4.81 * | 3.0 ns | 3.2 ns | |||||
| Inoculation (IT) | 4.26 * | 2.64 ns | 2.80 ns | ||||||
| Within subjects | |||||||||
| Season (S) | 65.65 *** | 327.31 *** | 224.17 *** | Season (S) | 2.66 ns | 16.66 *** | 20.16 *** | ||
| S × T | 2.28 ns | 2.55 ns | 3.40 * | ||||||
| Treatment (T) a | Na (mg kg−1) | Treatment (T) a | N (g kg−1) | Ca (mg kg−1) | Mg (mg kg−1) | K (mg kg−1) | P (mg kg−1) | ||
|---|---|---|---|---|---|---|---|---|---|
| Season 1 | RL | 1032 ± 487 | Season 1 | RL | 30.5 ± 6.4 | 2158 ± 1242 | 584 ± 290 | 9055 ± 3097 | 726 ± 192 |
| RL + AMF | 986 ± 139 | RL + AMF | 36.7 ± 4.3 | 3270 ± 779 | 939 ± 164 | 10,229 ± 1548 | 851 ± 145 | ||
| BC | 935 ± 195 | BC | 27.5 ± 2.3 | 2457 ± 1144 | 732 ± 242 | 9197 ± 2141 | 961 ± 233 | ||
| BC + AMF | 1250 ± 176 | BC + AMF | 29.7 ± 5.8 | 2308 ± 1159 | 670 ± 267 | 8254 ± 2192 | 599 ± 303 | ||
| BV | 1094 ± 561 | BV | 33.3 ± 3.3 | 2308 ± 720 | 688 ± 256 | 9028 ± 4288 | 842 ± 251 | ||
| BV + AMF | 1372 ± 147 | BV + AMF | 20.7 ± 7.4 | 3143 ± 429 | 961 ± 146 | 6536 ± 1176 | 1057 ± 136 | ||
| AMF | 795 ± 33 | AMF | 30.1 ± 2.3 | 1347 ± 239 | 403 ± 83 | 4375 ± 1085 | 556 ± 130 | ||
| CONTROL | 2182 ± 287 | CONTROL | 27.4 ± 1.8 | 4283 ± 743 | 1407 ± 91 | 16,445 ± 2527 | 1442 ± 166 | ||
| Season 2 | RL | 1550 ± 159 | Season 2 | RL | 25.3 ± 3.2 | 6633 ± 1373 | 1250 ± 291 | 20,623 ± 3343 | 1538 ± 369 |
| RL + AMF | 1680 ± 180 | RL + AMF | 35.1 ± 4.2 | 4552 ± 1389 | 931 ± 219 | 19,849 ± 177 | 1479 ± 631 | ||
| BC | 2670 ± 674 | BC | 29.8 ± 0.7 | 23,068 ± 6704 | 3576 ± 288 | 34,786 ± 7097 | 3877 ± 730 | ||
| BC + AMF | 2410 ± 780 | BC + AMF | 34.0 ± 1.7 | 42,355 ± 3941 | 3588 ± 852 | 20,314 ± 4004 | 4046 ± 377 | ||
| BV | 1422 ± 293 | BV | 26.7 ± 2.0 | 7630 ± 822 | 1520 ± 130 | 23,080 ± 5230 | 2247 ± 1065 | ||
| BV + AMF | 2018 ± 756 | BV + AMF | 37.1 ± 7.2 | 8728 ± 4136 | 1810 ± 899 | 29,378 ± 12020 | 2348 ± 143 | ||
| AMF | 2436 ± 319 | AMF | 28.4 ± 1.7 | 46,638 ± 7428 | 4413 ± 860 | 26,915 ± 4053 | 4327 ± 133 | ||
| CONTROL | 2326 ± 340 | CONTROL | 25.9 ± 1.7 | 47,820 ± 2898 | 3810 ± 484 | 21,441 ± 3150 | 3666 ± 122 | ||
| F values b | χ2 values b | ||||||||
| Between subjects | Inoculation (IT) | 11.60 *** | 0.96 ns | 1.33 ns | 0.03 ns | 0.85 ns | |||
| Inoculation (IT) | 4.14 * | ||||||||
| Within subjects | |||||||||
| Season (S) | 39.75 *** | Season (S) | 4.16 * | 20.16 *** | 16.66 *** | 24.00 *** | 24.00 *** | ||
| S × T | 2.47 ns | ||||||||
| Treatment (T) a | N (g kg−1) | Na (mg kg−1) | Treatment (T) a | Ca (mg kg−1) | Mg (mg kg−1) | K (mg kg−1) | P (mg kg−1) | ||
|---|---|---|---|---|---|---|---|---|---|
| Season 1 | RL | 12.8 ± 0.9 | 1035 ± 366 | Season 1 | RL | 12,338 ± 4247 | 803 ± 103 | 3967 ± 1838 | 421 ± 112 |
| RL + AMF | 14.5 ± 1.6 | 1278 ± 773 | RL + AMF | 3079 ± 1151 | 614 ± 314 | 5138 ± 2249 | 410 ± 197 | ||
| BC | 15.5 ± 0.3 | 1932 ± 557 | BC | 13,544 ± 6189 | 1141 ± 272 | 7419 ± 3562 | 740 ± 193 | ||
| BC + AMF | 14.6 ± 3.7 | 967 ± 536 | BC + AMF | 2570 ± 532 | 433 ± 134 | 3622 ± 1503 | 280 ± 62 | ||
| BV | 11.8 ± 0.9 | 903 ± 118 | BV | 8198 ± 790 | 610 ± 57 | 4096 ± 1218 | 380 ± 67 | ||
| BV + AMF | 14.3 ± 2.7 | 1054 ± 391 | BV + AMF | 2585 ± 448 | 517 ± 139 | 4411 ± 2084 | 374 ± 130 | ||
| AMF | 12.6 ± 2.5 | 1364 ± 972 | AMF | 14,207 ± 9458 | 1012 ± 618 | 4678 ± 2389 | 557 ± 343 | ||
| CONTROL | 14.0 ± 0.5 | 814 ± 636 | CONTROL | 2665 ± 1409 | 396 ± 250 | 3755 ± 2614 | 283 ± 186 | ||
| Season 2 | RL | 11.0 ± 0.7 | 2427 ± 780 | Season 2 | RL | 11,223 ± 3896 | 1166 ± 310 | 16,856 ± 4059 | 1112 ± 326 |
| RL + AMF | 10.1 ± 1.2 | 2506 ± 600 | RL + AMF | 23,672 ± 15915 | 1795 ± 518 | 13,787 ± 1829 | 999 ± 259 | ||
| BC | 13.2 ± 0.8 | 3047 ± 1296 | BC | 9566 ± 2019 | 1077 ± 753 | 15,491 ± 1806 | 1101 ± 77 | ||
| BC + AMF | 13.4 ± 0.3 | 2192 ± 319 | BC + AMF | 17,601 ± 8106 | 1357 ± 194 | 12,722 ± 897 | 834 ± 119 | ||
| BV | 9.8 ± 0.7 | 2649 ± 784 | BV | 13,769 ± 3957 | 1294 ± 404 | 18,360 ± 8763 | 1209 ± 509 | ||
| BV + AMF | 11.5 ± 1.3 | 3244 ± 1136 | BV + AMF | 19,758 ± 12867 | 1832 ± 248 | 18,628 ± 5196 | 1266 ± 494 | ||
| AMF | 13.0 ± 1.3 | 2050 ± 449 | AMF | 10,705 ± 3476 | 969 ± 1044 | 14,013 ± 1607 | 918 ± 142 | ||
| CONTROL | 11.8 ± 1.3 | 2153 ± 522 | CONTROL | 13,251 ± 4610 | 1227 ± 317 | 11,744 ± 1361 | 708 ± 99 | ||
| F values b | χ2 values b | ||||||||
| Between subjects | Inoculation (IT) | 0.08 ns | 0.97 ns | 0.003 ns | 0.003 ns | ||||
| Inoculation (IT) | 4.58 * | 0.81 ns | |||||||
| Within subjects | |||||||||
| Season (S) | 20.70 *** | 52.79 *** | Season (S) | 8.16 ** | 10.66 ** | 24.00 *** | 24.00 *** | ||
| S × T | 1.11 ns | 1.25 ns | |||||||
| Treatment (T) a | Crop Yield (kg ha−1) | Nodule Dry Weight (g/Plant) | Weight of 100 Seeds (g) | Number of Pods Per Plant | Treatment (T) a | Protein Content of Grain (%) | ||
|---|---|---|---|---|---|---|---|---|
| Season 1 | RL | 32,541 ± 3295 | 3.4 ± 0.5 | 152.4 ± 13.9 | 33 ± 2 | Season 1 | RL | 30 ± 0 |
| RL + AMF | 30,133 ± 6311 | 4.9 ± 2.4 | 178.3 ± 14.9 | 33 ± 5 | RL + AMF | 26 ± 1 | ||
| BC | 33,333 ± 2480 | 4.0 ± 1.4 | 178.7 ± 17.5 | 34 ± 2 | BC | 26 ± 1 | ||
| BC + AMF | 28,583 ± 4026 | 2.6 ± 1.1 | 165.0 ± 28.8 | 31 ± 1 | BC + AMF | 28 ± 3 | ||
| BV | 28,166 ± 7381 | 3.8 ± 0.9 | 179.9 ± 8.4 | 30 ± 4 | BV | 29 ± 1 | ||
| BV + AMF | 30,250 ± 4073 | 3.5 ± 0.8 | 158.5 ± 21.7 | 32 ± 7 | BV + AMF | 28 ± 1 | ||
| AMF | 27,100 ± 7258 | 4.9 ± 2.1 | 176.6 ± 28.8 | 29 ± 10 | AMF | 29 ± 0 | ||
| CONTROL | 27,016 ± 2759 | 4.8 ± 0.5 | 137.0 ± 7.3 | 29 ± 6 | CONTROL | 29 ± 1 | ||
| Season 2 | RL | 23,672 ± 2797 | 3.1 ± 0.5 | 170.7 ± 15.2 | 30 ± 1 | Season 2 | RL | 28 ± 1 |
| RL + AMF | 18,805 ± 6022 | 4.8 ± 1.6 | 183.5 ± 13.1 | 20 ± 7 | RL + AMF | 26 ± 2 | ||
| BC | 19,857 ± 4828 | 5.3 ± 2.7 | 199.6 ± 46.3 | 22 ± 6 | BC | 25 ± 2 | ||
| BC + AMF | 14,753 ± 2033 | 5.8 ± 3.4 | 169.8 ± 24.4 | 16 ± 2 | BC + AMF | 27 ± 4 | ||
| BV | 20,379 ± 7372 | 4.7 ± 1.7 | 158.8 ± 27.7 | 24 ± 10 | BV | 29 ± 5 | ||
| BV + AMF | 19,056 ± 2855 | 3.7 ± 0.7 | 154.0 ± 21.2 | 23 ± 2 | BV + AMF | 28 ± 3 | ||
| AMF | 24,137 ± 2599 | 6.2 ± 5.3 | 162.7 ± 15.8 | 26 ± 4 | AMF | 27 ± 2 | ||
| CONTROL | 19,506 ± 3791 | 4.3 ± 2.3 | 176.1 ± 8.0 | 22 ± 5 | CONTROL | 23 ± 2 | ||
| F values b | χ2 values b | |||||||
| Between subjects | 1.07 ns | Inoculation (IT) | 4.81 * | |||||
| Inoculation (IT) | 0.42 ns | 0.56 ns | 0.87 ns | |||||
| Within subjects | ||||||||
| Season (S) | 68.55 *** | 1.94 ns | 1.35 ns | 23.73 *** | Season (S) | 2.66 ns | ||
| S × T | 2.20 ns | 1.03 ns | 1.45 ns | 1.02 ns | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Navarro, V.; Zornoza, R.; Faz, Á.; Egea-Gilabert, C.; Ros, M.; Pascual, J.A.; Fernández, J.A. Inoculation with Different Nitrogen-Fixing Bacteria and Arbuscular Mycorrhiza Affects Grain Protein Content and Nodule Bacterial Communities of a Fava Bean Crop. Agronomy 2020, 10, 768. https://doi.org/10.3390/agronomy10060768
Sánchez-Navarro V, Zornoza R, Faz Á, Egea-Gilabert C, Ros M, Pascual JA, Fernández JA. Inoculation with Different Nitrogen-Fixing Bacteria and Arbuscular Mycorrhiza Affects Grain Protein Content and Nodule Bacterial Communities of a Fava Bean Crop. Agronomy. 2020; 10(6):768. https://doi.org/10.3390/agronomy10060768
Chicago/Turabian StyleSánchez-Navarro, Virginia, Raúl Zornoza, Ángel Faz, Catalina Egea-Gilabert, Margarita Ros, José A. Pascual, and Juan A. Fernández. 2020. "Inoculation with Different Nitrogen-Fixing Bacteria and Arbuscular Mycorrhiza Affects Grain Protein Content and Nodule Bacterial Communities of a Fava Bean Crop" Agronomy 10, no. 6: 768. https://doi.org/10.3390/agronomy10060768
APA StyleSánchez-Navarro, V., Zornoza, R., Faz, Á., Egea-Gilabert, C., Ros, M., Pascual, J. A., & Fernández, J. A. (2020). Inoculation with Different Nitrogen-Fixing Bacteria and Arbuscular Mycorrhiza Affects Grain Protein Content and Nodule Bacterial Communities of a Fava Bean Crop. Agronomy, 10(6), 768. https://doi.org/10.3390/agronomy10060768







