Discovery of Four Novel ORFs Responsible for Cytoplasmic Male Sterility (CMS) in Cotton (Gossypium hirsutum L.) through Comparative Analysis of the Mitochondrial Genomes of Four Isoplasmic Lines
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Sequencing and Assembly of Mitochondrial (mt) Genomes
2.3. Analysis and Annotations of the mt Genomes
2.4. Detection and Annotation of Single Nucleotide Polymorphisms (SNPs)
3. Results
3.1. Sequencing Data Statistics and Assembly
3.2. Structures and Contents of the mt Genomes
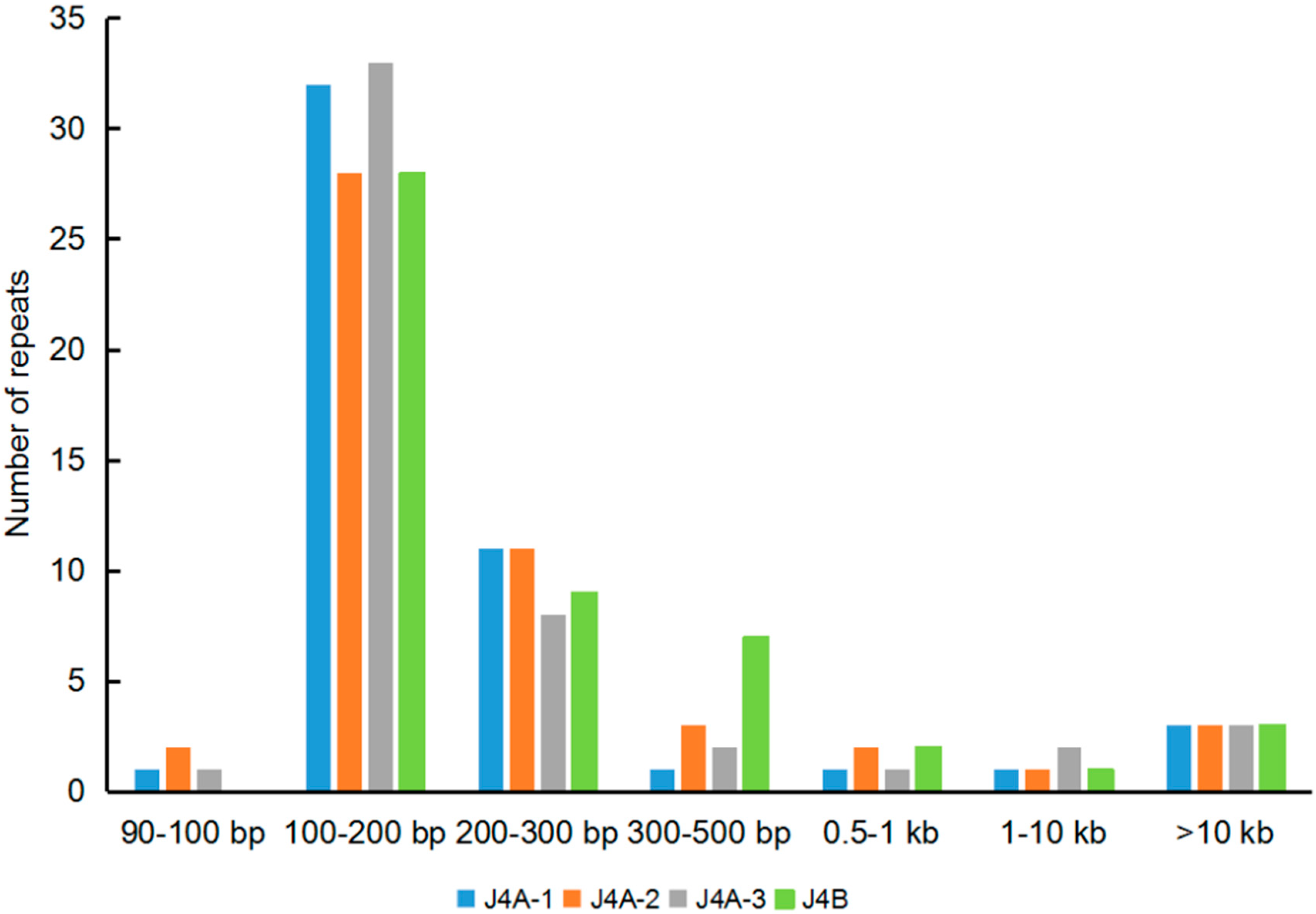
3.3. Repeat Sequences in the mt Genome
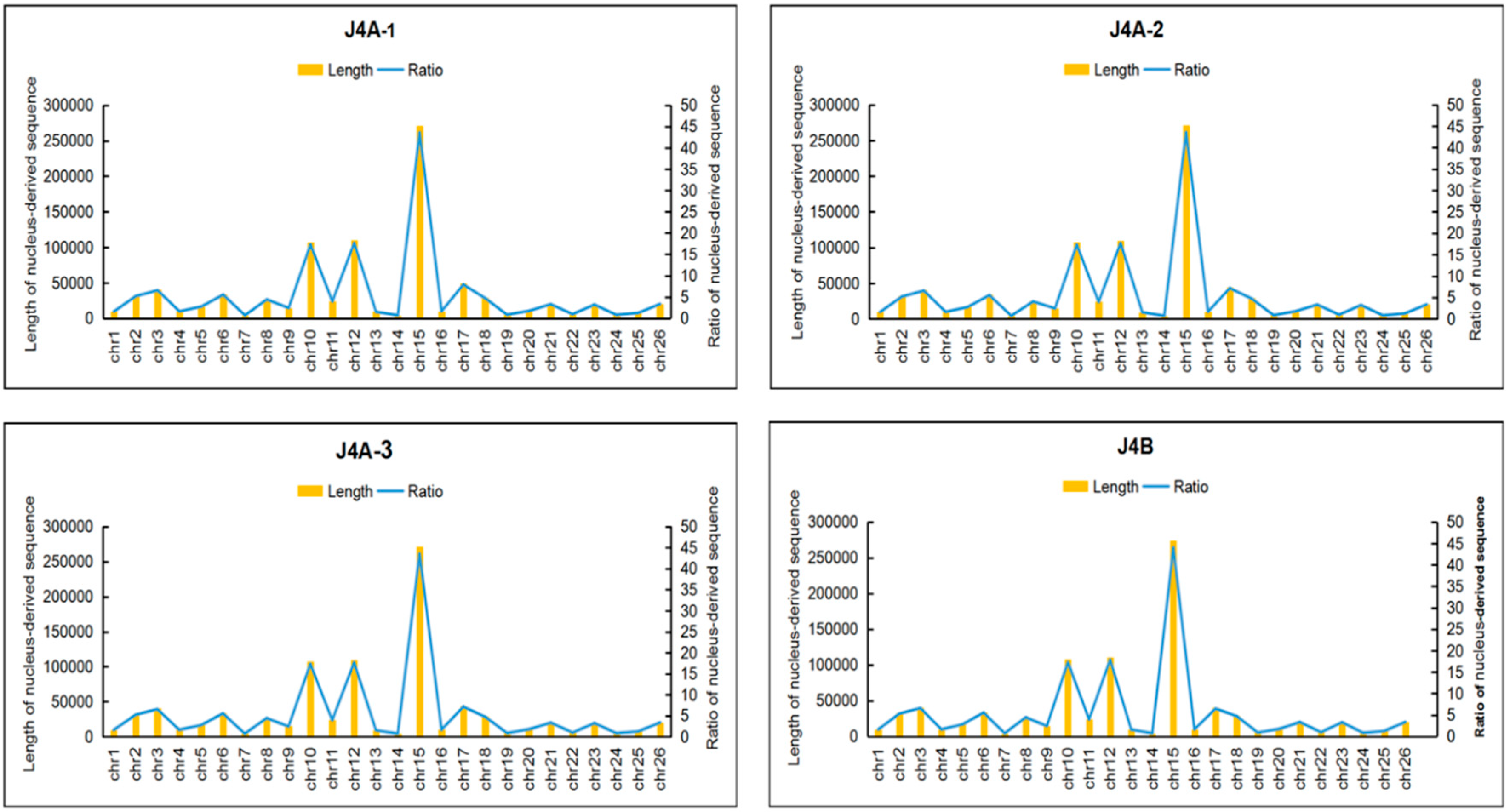
3.4. Similarity of mtDNA with the Cotton Chloroplast and Nuclear Genomes
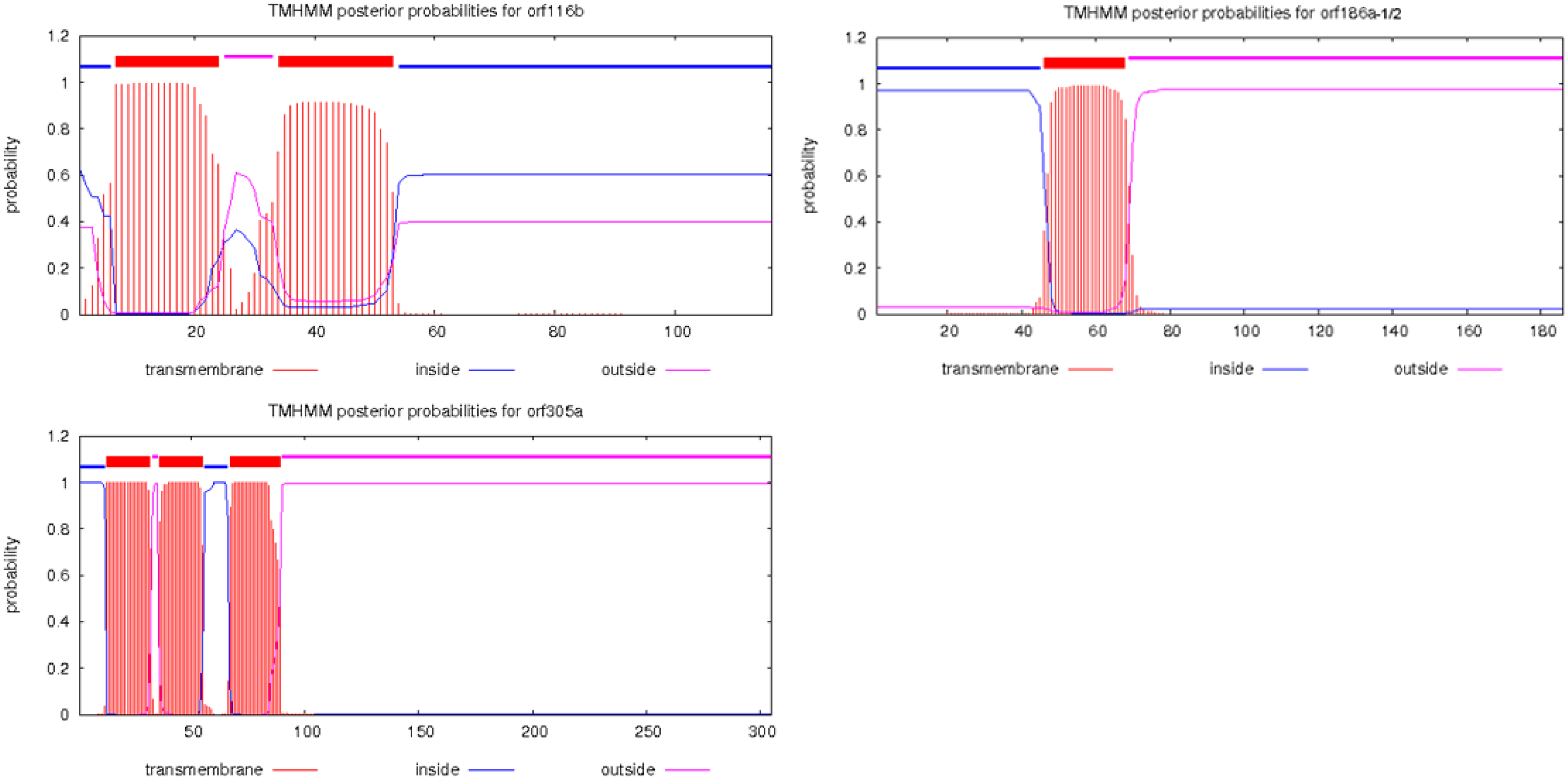
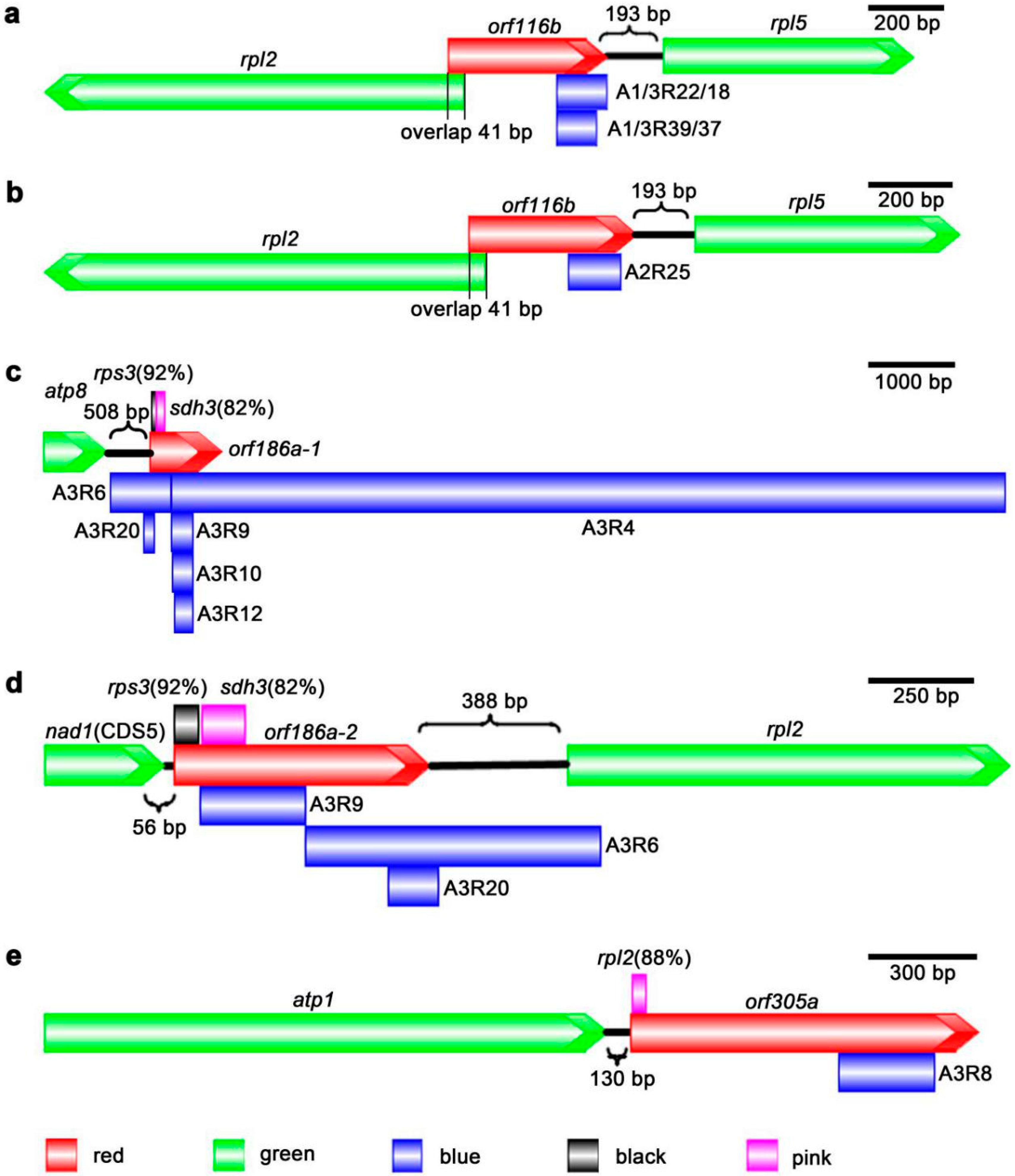
3.5. Unique Open Reading Frames (ORFs) of J4A mtDNA
3.6. Statistical Analysis and Annotation of SNPs
4. Discussion
4.1. Characteristics of Plant Mitochondrial Genes
4.2. ORFs Associated with Cytoplasmic Male Sterility (CMS)
4.3. SNPs and Plant Mitochondrial DNA Evolve
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Clifton, S.W.; Minx, P.; Fauron, C.M.; Gibson, M.; Allen, J.O.; Sun, H.; Thompson, M.; Barbazuk, W.B.; Kanuganti, S.; Tayloe, C.; et al. Sequence and comparative analysis of the maize NB mitochondrial genome. Plant Physiol. 2004, 136, 3486–3503. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Cui, P.; Zhan, K.; Lin, Q.; Zhuo, G.; Guo, X.; Ding, F.; Yang, W.; Liu, D.; Hu, S.; et al. Comparative analysis of mitochondrial genomes between a wheat K-type cytoplasmic male sterility (CMS) line and its maintainer line. BMC Genom. 2011, 12, 163. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Xu, H.; Liu, Z.; Guo, J.; Li, H.; Chen, L.; Fang, C.; Zhang, Q.; Bai, M.; Yao, N.; et al. A detrimental mitochondrial-nuclear interaction causes cytoplasmic male sterility in rice. Nat. Genet. 2013, 45, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, Y.G. Male sterility and fertility restoration in crops. Annu. Rev. Plant Biol. 2014, 65, 579–606. [Google Scholar] [CrossRef]
- Sloan, D.B.; Alverson, A.J.; Storchova, H.; Palmer, J.D.; Taylor, D.R. Extensive loss of translational genes in the structurally dynamic mitochondrial genome of the angiosperm Silene latifolia. BMC Evol. Biol. 2010, 10, 274. [Google Scholar] [CrossRef]
- Archibald, J.M. Endosymbiosis and eukaryotic cell evolution. Curr. Biol. 2015, 25, R911–R921. [Google Scholar] [CrossRef]
- Liao, X.; Zhao, Y.; Kong, X.; Khan, A.; Zhou, B.; Liu, D.; Kashif, M.H.; Chen, P.; Wang, H.; Zhou, R. Complete sequence of kenaf (Hibiscus cannabinus) mitochondrial genome and comparative analysis with the mitochondrial genomes of other plants. Sci. Rep. 2018, 8, 12714. [Google Scholar] [CrossRef]
- Wang, M.; Tu, L.; Yuan, D.; Zhu, D.; Shen, C.; Li, J.; Liu, F.; Pei, L.; Wang, P.; Zhao, G.; et al. Reference genome sequences of two cultivated allotetraploid cottons, Gossypium hirsutum and Gossypium barbadense. Nat. Genet. 2019, 51, 224–229. [Google Scholar] [CrossRef]
- Tang, H.; Zheng, X.; Li, C.; Xie, X.; Chen, Y.; Chen, L.; Zhao, X.; Zheng, H.; Zhou, J.; Ye, S.; et al. Multi-step formation, evolution, and functionalization of new cytoplasmic male sterility genes in the plant mitochondrial genomes. Cell Res. 2017, 27, 130–146. [Google Scholar] [CrossRef]
- Horn, R. Molecular diversity of male sterility inducing and male-fertile cytoplasms in the genus Helianthus. Theor. Appl. Genet. 2002, 104, 562–570. [Google Scholar] [CrossRef]
- Touzet, P.; Meyer, E.H. Cytoplasmic male sterility and mitochondrial metabolism in plants. Mitochondrion 2014, 19 Pt B, 166–171. [Google Scholar] [CrossRef]
- Small, I.; Suffolk, R.; Leaver, C.J. Evolution of plant mitochondrial genomes via substoichiometric intermediates. Cell 1989, 58, 69–76. [Google Scholar] [CrossRef]
- Albert, B.; Godelle, B.; Gouyon, P. Evolution of the plant mitochondrial genome: Dynamics of duplication and deletion of sequences. J. Mol. Evol. 1998, 46, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Kmiec, B.; Woloszynska, M.; Janska, H. Heteroplasmy as a common state of mitochondrial genetic information in plants and animals. Curr. Genet. 2006, 50, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Woloszynska, M.; Trojanowski, D. Counting mtDNA molecules in Phaseolus vulgaris: Sublimons are constantly produced by recombination via short repeats and undergo rigorous selection during substoichiometric shifting. Plant Mol. Biol. 2009, 70, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Gabay-Laughnan, S.; Newton, K. Plant mitochondrial mutations. In Genomics of Chloroplasts and Mitochondria. Advances in Photosynthesis and Respiration (Including Bioenergy and Related Processes); Bock, R., Knoop, V., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 267–292. [Google Scholar]
- Arrieta-Montiel, M.P.; Mackenzie, S.A. Plant mitochondrial genomes and recombination. In Plant Mitochondria; Kempken, F., Ed.; Springer: New York, NY, USA, 2011; pp. 65–82. [Google Scholar]
- Dewey, R.E.; Timothy, D.H.; Levings, C.S. A mitochondrial protein associated with cytoplasmic male sterility in the T cytoplasm of maize. Proc. Natl. Acad. Sci. USA 1987, 84, 5374–5378. [Google Scholar] [CrossRef] [PubMed]
- Yi, P. Discovery of mitochondrial chimeric-gene associated with cytoplasmic male sterility of HL-rice. Chin. Sci. Bull. 2002, 47, 744. [Google Scholar] [CrossRef]
- Satoh, M.; Kubo, T.; Nishizawa, S.; Estiati, A.; Itchoda, N.; Mikami, T. The cytoplasmic male-sterile type and normal type mitochondrial genomes of sugar beet share the same complement of genes of known function but differ in the content of expressed ORFs. Mol. Genet. Genom. 2004, 272, 247–256. [Google Scholar] [CrossRef]
- Yamamoto, M.P.; Kubo, T.; Mikami, T. The 5’-leader sequence of sugar beet mitochondrial atp6 encodes a novel polypeptide that is characteristic of Owen cytoplasmic male sterility. Mol. Genet. Genom. 2005, 273, 342–349. [Google Scholar] [CrossRef]
- Meyer, V.G. Male sterility from gossypium harknessii. J. Hered. 1975, 66, 23–27. [Google Scholar] [CrossRef]
- Stewart, J.M. A new male sterility from G. trilobum. In Proceedings of the Beltwide Cotton Conference (National Cotton Council), Memphis, TN, USA; 1992; p. 610. [Google Scholar]
- Zhang, X.; Meng, Z.; Zhou, T.; Sun, G.; Shi, J.; Yu, Y.; Zhang, R.; Guo, S. Mitochondrial SCAR and SSR Markers for distinguishing cytoplasmic male sterile lines from their isogenic maintainer lines in cotton. Plant Breed. 2012, 131, 563–570. [Google Scholar] [CrossRef]
- Chen, Z.; Nie, H.; Wang, Y.; Pei, H.; Li, S.; Zhang, L.; Hua, J. Rapid evolutionary divergence of diploid and allotetraploid Gossypium mitochondrial genomes. BMC Genom. 2017, 18, 876. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, Z.; Zhao, N.; Wang, Y.; Nie, H.; Hua, J. The comparison of four mitochondrial genomes reveals cytoplasmic male sterility candidate genes in cotton. BMC Genom. 2018, 19, 775. [Google Scholar] [CrossRef] [PubMed]
- Ling, X.; Zhou, P.; Guan, H.; Jing, R.; Zhu, Y. Isolation of the mtDNA bands associated with CMS by AFLP technique. Hereditas 1999, 21, 33–36. [Google Scholar]
- Nurk, S.; Anton, B.; Dmitry, A.; Alexey, G.; Anton, K.; Alla, L.; Andrey, P.; Alexey, P.; Alexander, S.; Yakov, S.; et al. Assembling Genomes and Mini-metagenomes from Highly Chimeric Reads. In Research in Computational Molecular Biology; Deng, M., Jiang, R., Sun, F., Zhang, X., Eds.; RECOMB, 2013, Lecture Notes in Computer Science; Springer: Berlin/Heidelberg, Germany, 2013; Volume 7821, pp. 158–170. [Google Scholar] [CrossRef]
- Liu, G.Z.; Cao, D.; Li, S.S.; Su, A.G.; Geng, J.n.; Grover, C.E.; Hu, S.N.; Hua, J.P. The complete mitochondrial genome of Gossypium hirsutum and evolutionary analysis of higher plant mitochondrial genomes. PLoS ONE 2013, 8, e69476. [Google Scholar] [CrossRef]
- Luo, R.B.; Liu, B.H.; Xie, Y.L.; Li, Z.Y.; Huang, W.H.; Yuan, J.Y.; He, G.Z.; Chen, Y.X.; Pan, Q.; Liu, Y.J.; et al. Soapdenovo2: An empirically improved memory-efficient short-read de novo assembler. Gigascience 2012, 1, 1–18. [Google Scholar] [CrossRef]
- Lohse, M.; Drechsel, O.; Bock, R. OrganellarGenomeDRAW (OGDRAW): A tool for the easy generation of high-quality custom graphical maps of plastid and mitochondrial genomes. Curr. Genet. 2007, 52, 267–274. [Google Scholar] [CrossRef]
- Stanke, M.; Steinkamp, R.; Waack, S.; Morgenstern, B. AUGUSTUS: A web server for gene finding in eukaryotes. Nucleic Acids Res. 2004, 32, W309–W312. [Google Scholar] [CrossRef]
- Haas, B.J.; Salzberg, S.L.; Zhu, W.; Pertea, M.; Allen, J.E.; Orvis, J.; White, O.; Buell, C.R.; Wortman, J.R. Automated eukaryotic gene structure annotation using EVidenceModeler and the program to assemble spliced alignments. Genome Biol. 2008, 9, R7. [Google Scholar] [CrossRef]
- Lowe, T.M.; Eddy, S.R. tRNAscan-SE: A program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 1997, 25, 955–964. [Google Scholar] [CrossRef]
- Ibba, M.; Soll, D. Aminoacyl-tRNA synthesis. Annu. Rev. Biochem. 2000, 69, 617–650. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.R.; Wang, Q.; Cardenas, E.; Fish, J.; Chai, B.; Farris, R.J.; Kulam-Syed-Mohideen, A.S.; McGarrell, D.M.; Marsh, T.; Garrity, G.M.; et al. The ribosomal database project: Improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 2009, 37, D141–D145. [Google Scholar] [CrossRef]
- Lowe, T.M.; Chan, P.P. tRNAscan-SE On-line: Integrating search and context for analysis of transfer RNA genes. Nucleic Acids Res. 2016, 44, W54–W57. [Google Scholar] [CrossRef] [PubMed]
- Olson, S.A. EMBOSS opens up sequence analysis. European molecular biology open software suite. Brief. Bioinform. 2002, 3, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Rombel, I.T.; Sykes, K.F.; Rayner, S.; Johnston, S.A. ORF-FINDER: A vector for high-throughput gene identification. Gene 2002, 282, 33–41. [Google Scholar] [CrossRef]
- Song, J.; He, Q.-F. Bioinformatics analysis of the structure and linear B–cell epitopes of aquaporin–3 from Schistosoma japonicum. Asian Pac. J. Trop. Med. 2012, 5, 107–109. [Google Scholar] [CrossRef]
- Kurtz, S.; Phillippy, A.; Delcher, A.L.; Smoot, M.; Shumway, M.; Antonescu, C.; Salzberg, S.L. Versatile and open software for comparing large genomes. Genome Biol. 2004, 5, R12. [Google Scholar] [CrossRef]
- Hulse-Kemp, A.M.; Ashrafi, H.; Zheng, X.; Wang, F.; Hoegenauer, K.A.; Maeda, A.B.; Yang, S.S.; Stoffel, K.; Matvienko, M.; Clemons, K.; et al. Development and bin mapping of gene-associated interspecific SNPs for cotton (Gossypium hirsutum L.) introgression breeding efforts. BMC Genom. 2014, 15, 945. [Google Scholar] [CrossRef]
- Birney, E.; Clamp, M.; Durbin, R. GeneWise and genomewise. Genome Res. 2004, 14, 988–995. [Google Scholar] [CrossRef]
- Yang, Z. Likelihood ratio tests for detecting positive selection and application to primate lysozyme evolution. Mol. Biol. Evol. 1998, 15, 568–573. [Google Scholar] [CrossRef]
- Allen, J.O.; Fauron, C.M.; Minx, P.; Roark, L.; Oddiraju, S.; Lin, G.N.; Meyer, L.; Sun, H.; Kim, K.; Wang, C.; et al. Comparisons among two fertile and three male-sterile mitochondrial genomes of maize. Genetics 2007, 177, 1173–1192. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Lee, Y.P.; Lee, J.; Choi, B.S.; Kim, S.; Yang, T.J. Complete mitochondrial genome sequence and identification of a candidate gene responsible for cytoplasmic male sterility in radish (Raphanus sativus L.) containing DCGMS cytoplasm. Theor. Appl. Genet. 2013, 126, 1763–1774. [Google Scholar] [CrossRef] [PubMed]
- Alverson, A.J.; Wei, X.; Rice, D.W.; Stern, D.B.; Barry, K.; Palmer, J.D. Insights into the evolution of mitochondrial genome size from complete sequences of Citrullus lanatus and Cucurbita pepo (Cucurbitaceae). Mol. Biol. Evol. 2010, 27, 1436–1448. [Google Scholar] [CrossRef] [PubMed]
- Goremykin, V.V.; Salamini, F.; Velasco, R.; Viola, R. Mitochondrial DNA of Vitis vinifera and the issue of rampant horizontal gene transfer. Mol. Biol. Evol. 2009, 26, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Bergthorsson, U.; Adams, K.L.; Thomason, B.; Palmer, J.D. Widespread horizontal transfer of mitochondrial genes in flowering plants. Nature 2003, 424, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Rice, D.W.; Alverson, A.J.; Richardson, A.O.; Young, G.J.; Sanchez-Puerta, M.V.; Munzinger, J.; Barry, K.; Boore, J.L.; Zhang, Y.; de Pamphilis, C.W.; et al. Horizontal transfer of entire genomes via mitochondrial fusion in the angiosperm Amborella. Science 2013, 342, 1468–1473. [Google Scholar] [CrossRef]
- Straub, S.C.; Cronn, R.C.; Edwards, C.; Fishbein, M.; Liston, A. Horizontal transfer of DNA from the mitochondrial to the plastid genome and its subsequent evolution in milkweeds (apocynaceae). Genome Biol. Evol. 2013, 5, 1872–1885. [Google Scholar] [CrossRef]
- Koizuka, N.; Imai, R.; Iwabuchi, M.; Sakai, T.; Imamura, J. Genetic analysis of fertility restoration and accumulation of ORF125 mitochondrial protein in the kosena radish (Raphanus sativus cv. Kosena) and a Brassica napus restorer line. Theor. Appl. Genet. 2000, 100, 949–955. [Google Scholar] [CrossRef]
- Kubo, T.; Newton, K.J. Angiosperm mitochondrial genomes and mutations. Mitochondrion 2008, 8, 5–14. [Google Scholar] [CrossRef]
- Yang, J.H.; Huai, Y.; Zhang, M.F. Mitochondrial atpA gene is altered in a new orf220-type cytoplasmic male-sterile line of stem mustard (Brassica juncea). Mol. Biol. Rep. 2009, 36, 273–280. [Google Scholar] [CrossRef]
- Yang, J.H.; Zhang, M.F.; Yu, J.Q. Mitochondrial nad2 gene is co-transcripted with CMS-associated orfB gene in cytoplasmic male-sterile stem mustard (Brassica juncea). Mol. Biol. Rep. 2009, 36, 345–351. [Google Scholar] [CrossRef]
- Heng, S.; Wei, C.; Jing, B.; Wan, Z.; Wen, J.; Yi, B.; Ma, C.; Tu, J.; Fu, T.; Shen, J. Comparative analysis of mitochondrial genomes between the hau cytoplasmic male sterility (CMS) line and its iso-nuclear maintainer line in Brassica juncea to reveal the origin of the CMS-associated gene orf288. BMC Genom. 2014, 15, 322. [Google Scholar] [CrossRef] [PubMed]
- L’Homme, Y.; Stahl, R.J.; Li, X.Q.; Hameed, A.; Brown, G.G. Brassica nap cytoplasmic male sterility is associated with expression of a mtDNA region containing a chimeric gene similar to the pol CMS-associated orf224 gene. Curr. Genet. 1997, 31, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Menassa, R.; L’Homme, Y.; Brown, G.G. Post-transcriptional and developmental regulation of a CMS-associated mitochondrial gene region by a nuclear restorer gene. Plant J. 1999, 17, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, L.J.; Betz, S.K.; Chase, C.D. Mitochondrial RNA editing truncates a chimeric open reading frame associated with S male-sterility in maize. Curr. Genet. 2002, 42, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Hedgcoth, C. A chimeric gene (orf256) is expressed as protein only in cytoplasmic male-sterile lines of wheat. Plant Mol. Biol. 1994, 26, 535–539. [Google Scholar] [CrossRef]
- Palmer, J.D.; Herbon, L.A. Plant mitochondrial DNA evolves rapidly in structure, but slowly in sequence. J. Mol. Evol. 1988, 28, 87–97. [Google Scholar] [CrossRef]
- Kubo, T.; Mikami, T. Organization and variation of angiosperm mitochondrial genome. Physiol. Plant. 2007, 129, 6–13. [Google Scholar] [CrossRef]

| Genome Characteristic | J4A-1 | J4A-2 | J4A-3 | J4B |
|---|---|---|---|---|
| Genome size (bp) | 623,067 | 623,343 | 622,848 | 621,841 |
| Gene number (#) | 191 | 189 | 191 | 186 |
| Gene total length (bp) | 102,957 | 101,838 | 103,416 | 101,091 |
| Gene length/Genome (%) | 16.52 | 16.34 | 16.6 | 16.3 |
| Intergenic region length | 520,110 | 521,505 | 519,432 | 520,750 |
| Product Group | Gene | J4A-1 | J4A-2 | J4A-3 | J4B | |
|---|---|---|---|---|---|---|
| Complex I | nad1 | +4 a | +4 a | +4 a | +4 a | |
| nad2 | +2 a | +2 a | +2 a | +2 a | ||
| nad3 | + | + | + | + | ||
| nad4 | + | + | + | + | ||
| nad4L | + | + | + | + | ||
| nad5 | +3 a | +3 a | +3 a | +3 a | ||
| nad6 | + | + | + | + | ||
| nad7 | + | + | + | + | ||
| nad9 | + | + | + | + | ||
| Complex II | sdh3 | + | + | + | + | |
| sdh4 | + | + | + | + | ||
| Complex III | cob | + | + | + | + | |
| Complex IV | cox1 | + | + | + | + | |
| cox2 | + | + | + | + | ||
| cox3 | + | + | + | + | ||
| Complex V | atp1 | + | + | + | + | |
| atp4 | + | + | + | + | ||
| atp6 | + | + | + | + | ||
| atp8 | + | + | + | + | ||
| atp9 | + | + | + | + | ||
| Cytochrome C | ccmB | + | + | + | + | |
| ccmC | + | + | + | + | ||
| ccmFN | + | + | + | + | ||
| ccmFC | + | + | + | + | ||
| Other gene | mttB | + | + | + | + | |
| matR | + | + | + | + | ||
| Ribosome | rps3 | + | + | + | + | |
| rps4 | + | + | + | + | ||
| rps7 | + | + | + | + | ||
| rps10 | + | + | + | + | ||
| rps12 | + | + | + | + | ||
| rps14 | + | + | + | + | ||
| rpl2 | + | + | + | + | ||
| rpl5 | + | + | + | + | ||
| rpl10 | + | + | + | + | ||
| rpl16 | + | + | + | + | ||
| Total protein-coding genes | 36 | 36 | 36 | 36 | ||
| tRNA | trnC(GCA)-cp | + | + | + | + | |
| trnD(GUC)-cp | +2 | +2 | +2 | +2 | ||
| trnE(UUC) | + | + | + | + | ||
| trnF(GAA) | +2 a | +2 a | +2 a | +2 a | ||
| trnfM(CAU)-cp | +4 a | +4 a | +4 a | +4 a | ||
| trnG(GCC) | + | + | + | + | ||
| trnH(GUG)-cp | + | + | + | + | ||
| trnK(UUU) | + | + | + | + | ||
| trnM(CAU) | + | + | + | +2 a | ||
| trnI(UAU) | + | + | + | + | ||
| trnN(GUU)-cp | + | + | + | + | ||
| trnP(UGG) | +3 a | +3 a | +3 a | +3 a | ||
| trnQ(UUG) | + | + | + | + | ||
| trnS(GGA)-cp | + | + | + | + | ||
| trnS(GCU) | +2 a | +2 a | +2 a | +2 a | ||
| trnS(UGA) | + | + | + | + | ||
| trnSup(UUA) | + | + | + | + | ||
| trnV(GAC) | + | + | + | + | ||
| trnW(CCA)-cp | +2 a | +2 a | +2 a | +2 a | ||
| trnY(GUA) | + | + | + | + | ||
| Total tRNA genes | 29 | 29 | 29 | 30 | - | |
| rRNA | rRNA_5S | + | + | + | + | - |
| rRNA_18S | + | + | + | + | - | |
| rRNA_26S | +2 a | +2 a | +2 a | +2 a | - | |
| Total rRNA genes | 4 | 4 | 4 | 4 | - | |
| Genome | Ge-Len (bp) a | Re-Len (bp) b | % of Genome | Min-Len (bp) c | Max-Len (bp) d | No. e | Ge-Len-without Dup f (bp) (%) |
|---|---|---|---|---|---|---|---|
| J4A-1 | 623,067 | 66,058 | 10.60 | 100 | 27,501 | 39 | 557,009 (89.40) |
| J4A-2 | 623,343 | 67,156 | 10.77 | 104 | 27,501 | 38 | 556,18 (89.23) |
| J4A-3 | 622,848 | 75,134 | 12.60 | 100 | 27,501 | 37 | 547,59 (87.92) |
| J4B | 621,841 | 67,514 | 10.90 | 105 | 27,362 | 37 | 554,19 (89.12) |
| ORF | Len a (bp) | Location | Tra-Dom b | ORF-Seq c | Homologous Sequence d |
|---|---|---|---|---|---|
| orf106e | 318 | 111193:111510 | 0 | - | - |
| orf111f | 333 | 188666:188998 | 0 | - | 181–288, 78%, psbE (chloroplast), Arabidopsis thaliana |
| orf116b | 348 | 534858:535205 | 2 | of rpl5; 41 bp overlap with rpl2; partial A1R22, A1R39 | - |
| orf118b | 354 | 429729:430082 | 0 | - | - |
| orf123e | 369 | 21393:21761 | 0 | - | - |
| orf123f | 369 | 403911:404279 | 0 | - | - |
| orf126b | 378 | 279972:280349 | 0 | A3R8 | - |
| orf129a | 378 | 566270:566656 | 0 | - | 76–366, 84%, orf174 (mitochondrion), Batis maritima |
| orf141a | 423 | 259437:259859 | 1 | - | 396 bp, sdh4; 69 bp overlap with cox3 |
| orf156a | 468 | 499754:500221 | 0 | - | - |
| orf186a-1 | 558 | 226860:227417 | 1 | 508 bp downstream of atp8; A3R9, A3R10, A3R12, partial A3R4, A3R6, A3R20 | 1–57 bp, 92%, rps3; 69–171 bp, 82%, sdh3 |
| orf186a-2 | 558 | 532727:533284 | 1 | 389 bp upstream of rpl2; 56 bp downstream of nad1 (CDS5), A3R9; partial A3R6, A3R20 | 1–57 bp, 92%, rps3; 69–171 bp, 82%, sdh3 |
| orf208a-1 | 624 | 613583:614206 | 3 | A1R7, A1R8, partial A1R1, A1R15 | - |
| orf208a-2 | 624 | 456201:456824 | 3 | A1R30 | - |
| orf228a | 684 | 261023:261706 | 0 | 304 bp downstream of cox3, 433 bp upstream of cox1, A1R27 | - |
| orf270b | 810 | 387038:387847 | 0 | - | - |
| orf305a | 915 | 279431:280345 | 3 | 130 bp downstream of atp1, A3R8 | 4–45 bp, 88%, rpl2 |
| orf592a | 1776 | 508363:510138 | 4 | 1734 bp overlap with atp1 | 1734 bp, ccmFN |
| Gene | Len a | Var b | Loc c | J4A-1 | J4A-2 | J4A-3 | J4B | NSM d | SM e | aa-Var f | SNP Type |
|---|---|---|---|---|---|---|---|---|---|---|---|
| atp4 | 585 | 1 | 222 | ttT | ttT | ttT | ttC | 0 | 1 | transversion | |
| atp8 | 465 | 1 | 171 | agC | agA | agA | agA | 1 | 0 | Ser-Arg | |
| cox1 | 1593 | 2 | 960 | atA | atA | atA | atC | 0 | 2 | ||
| 1428 | atA | atA | atA | atC | |||||||
| cox3 | 798 | 1 | 157 | Atc | Atc | Atc | Ctc | 1 | 0 | Leu-Ile | |
| matR | 1967 | 1 | 1858 | Aaa | Aaa | Aaa | Caa | 1 | 0 | Gln-Lys | |
| nad2 | 1467 | 1 | 1227 | atC | atC | atC | atA | 0 | 1 | ||
| nad7 | 1185 | 1 | 24 | atC | atC | atC | atA | 0 | 1 | ||
| rpl2 | 1005 | 2 | 45 | ttG | ttG | ttG | ttT | 2 | 0 | Phe-Leu | |
| 292 | Ctc | Ctc | Ctc | Atc | Ile-Leu | ||||||
| rpl5 | 582 | 1 | 139 | Caa | Caa | Caa | Aaa | 1 | 0 | Lys-Gln | |
| rpl10 | 489 | 1 | 361 | Aaa | Aaa | Aaa | Gaa | 1 | 0 | Glu-Lys | |
| rpl16 | 435 | 1 | 270 | gtC | gtC | gtC | gtA | 0 | 1 | ||
| rps4 | 1098 | 1 | 535 | Caa | Caa | Caa | Aaa | 1 | 0 | Lys-Gln | |
| sdh3 | 435 | 1 | 33 | ttC | ttC | ttC | ttA | 1 | 0 | Leu-Phe | |
| Total number of non-synonymous mutations | 9 | ||||||||||
| Total number of synonymous mutations | 5 | ||||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Chen, L.; Tang, D.; Liao, X.; Kong, X.; Li, B.; You, J.; Zhou, R. Discovery of Four Novel ORFs Responsible for Cytoplasmic Male Sterility (CMS) in Cotton (Gossypium hirsutum L.) through Comparative Analysis of the Mitochondrial Genomes of Four Isoplasmic Lines. Agronomy 2020, 10, 765. https://doi.org/10.3390/agronomy10060765
Li M, Chen L, Tang D, Liao X, Kong X, Li B, You J, Zhou R. Discovery of Four Novel ORFs Responsible for Cytoplasmic Male Sterility (CMS) in Cotton (Gossypium hirsutum L.) through Comparative Analysis of the Mitochondrial Genomes of Four Isoplasmic Lines. Agronomy. 2020; 10(6):765. https://doi.org/10.3390/agronomy10060765
Chicago/Turabian StyleLi, Min, Li Chen, Danfeng Tang, Xiaofang Liao, Xiangjun Kong, Bin Li, Jingyi You, and Ruiyang Zhou. 2020. "Discovery of Four Novel ORFs Responsible for Cytoplasmic Male Sterility (CMS) in Cotton (Gossypium hirsutum L.) through Comparative Analysis of the Mitochondrial Genomes of Four Isoplasmic Lines" Agronomy 10, no. 6: 765. https://doi.org/10.3390/agronomy10060765
APA StyleLi, M., Chen, L., Tang, D., Liao, X., Kong, X., Li, B., You, J., & Zhou, R. (2020). Discovery of Four Novel ORFs Responsible for Cytoplasmic Male Sterility (CMS) in Cotton (Gossypium hirsutum L.) through Comparative Analysis of the Mitochondrial Genomes of Four Isoplasmic Lines. Agronomy, 10(6), 765. https://doi.org/10.3390/agronomy10060765





