Breeding Alfalfa (Medicago sativa L.) Adapted to Subtropical Agroecosystems
Abstract
1. Introduction
2. Materials and Methods
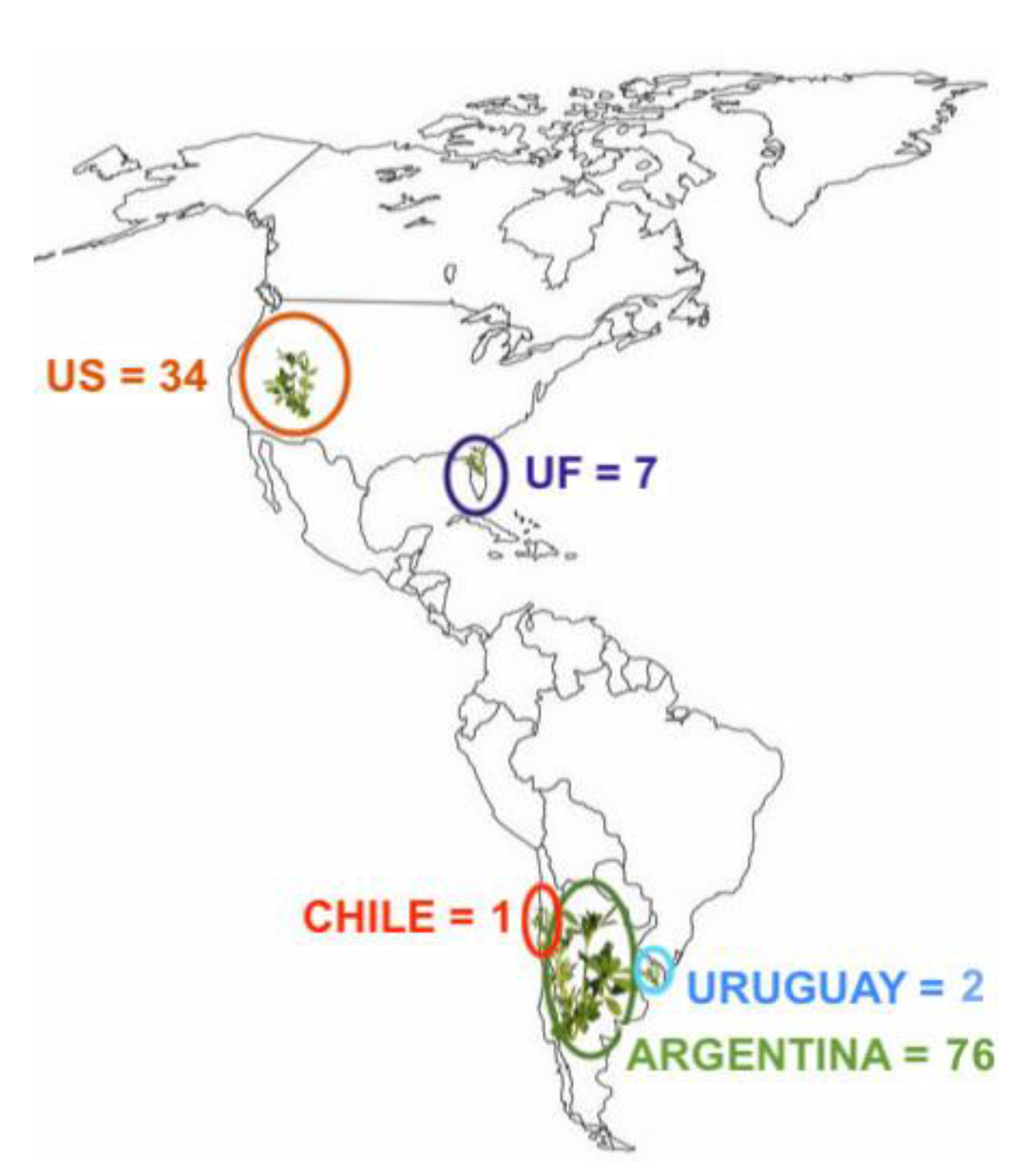
2.1. Initial Germplasm Screening
2.2. Development of the Reference Breeding Population
2.3. Experimental Design and Field Management
2.4. Data Collection
2.5. Statistical Analyses
3. Results
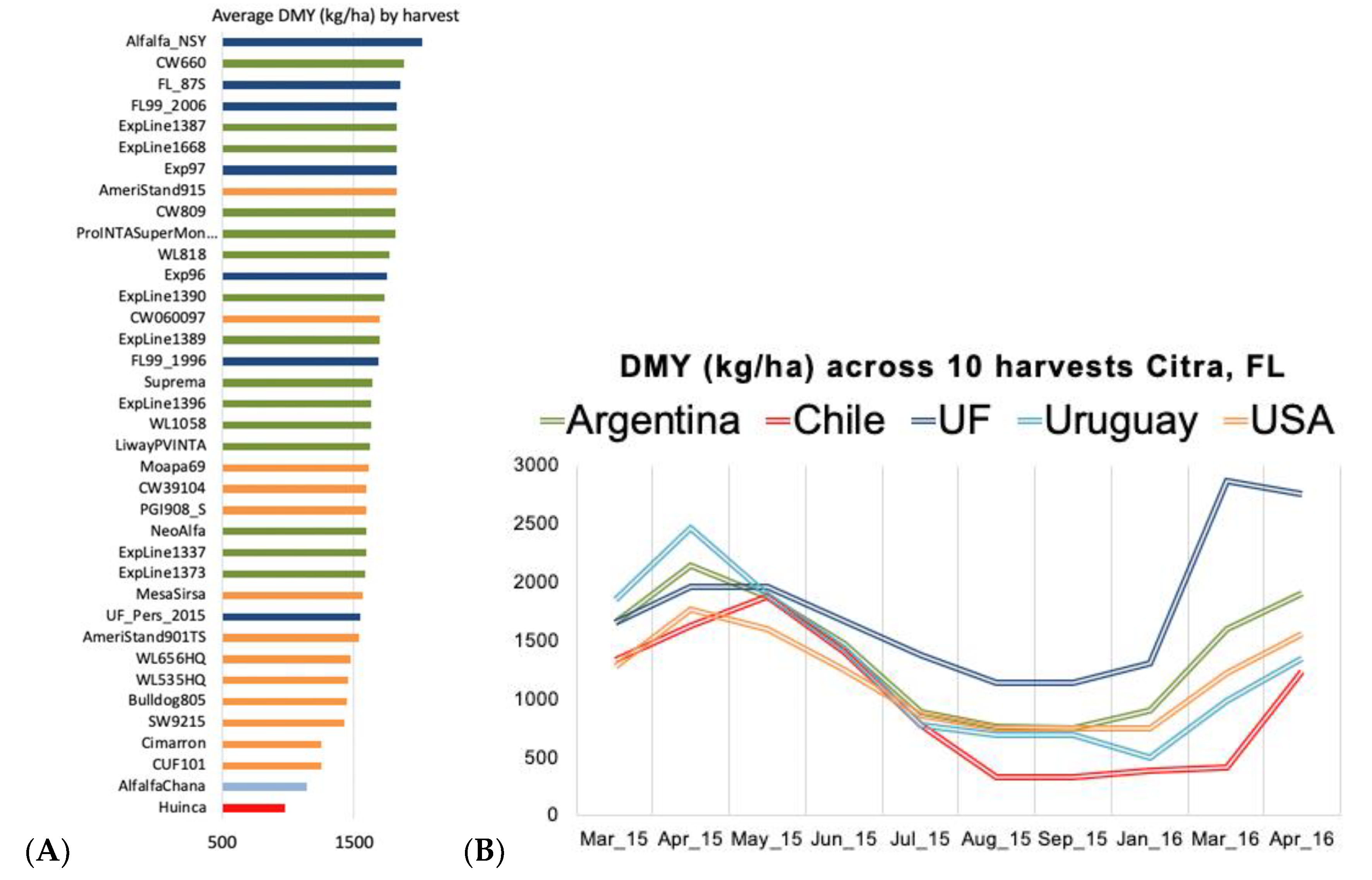
3.1. Initial Germplasm Screening and Mating Design
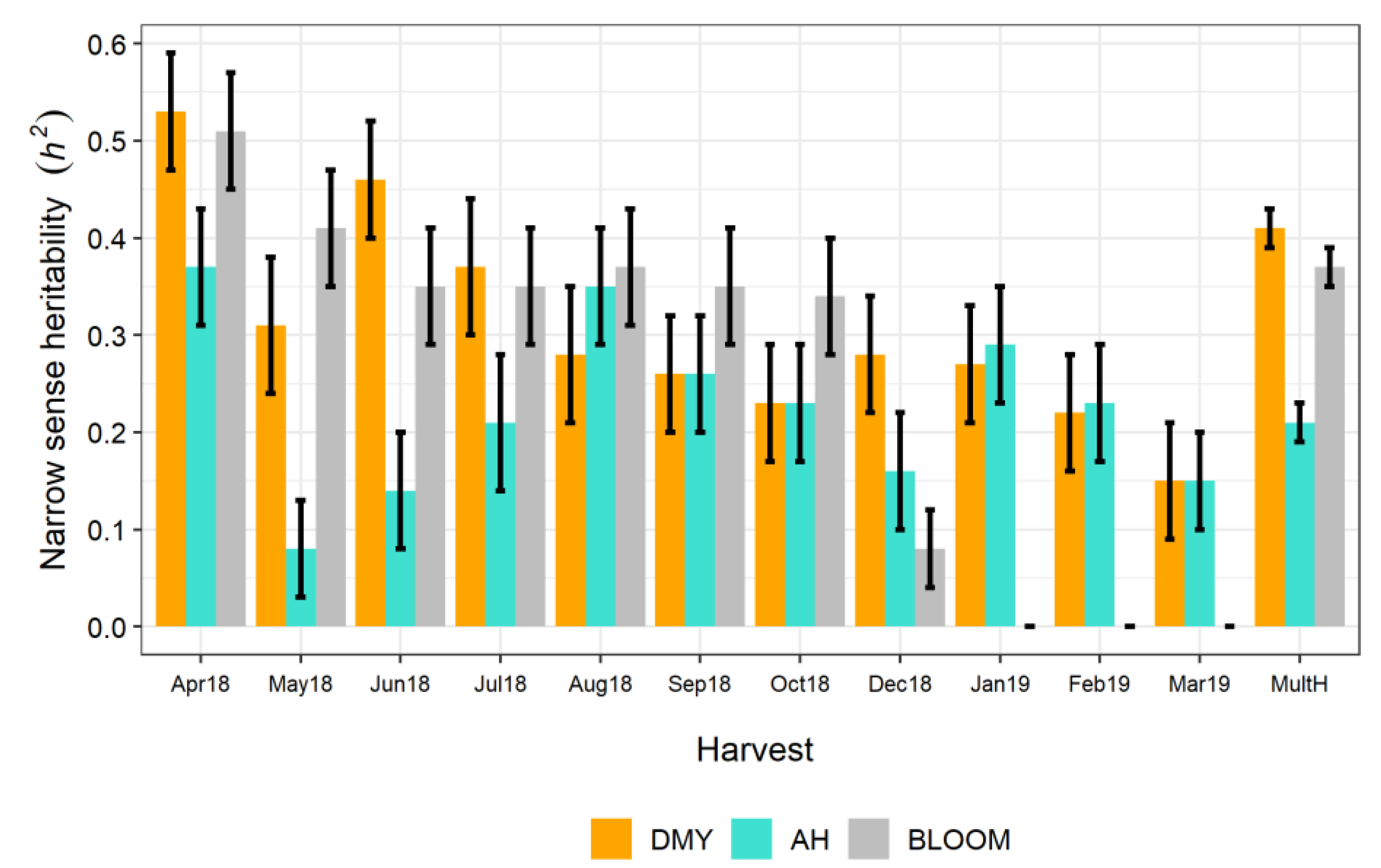
3.2. Variance Components and Genotypic Values
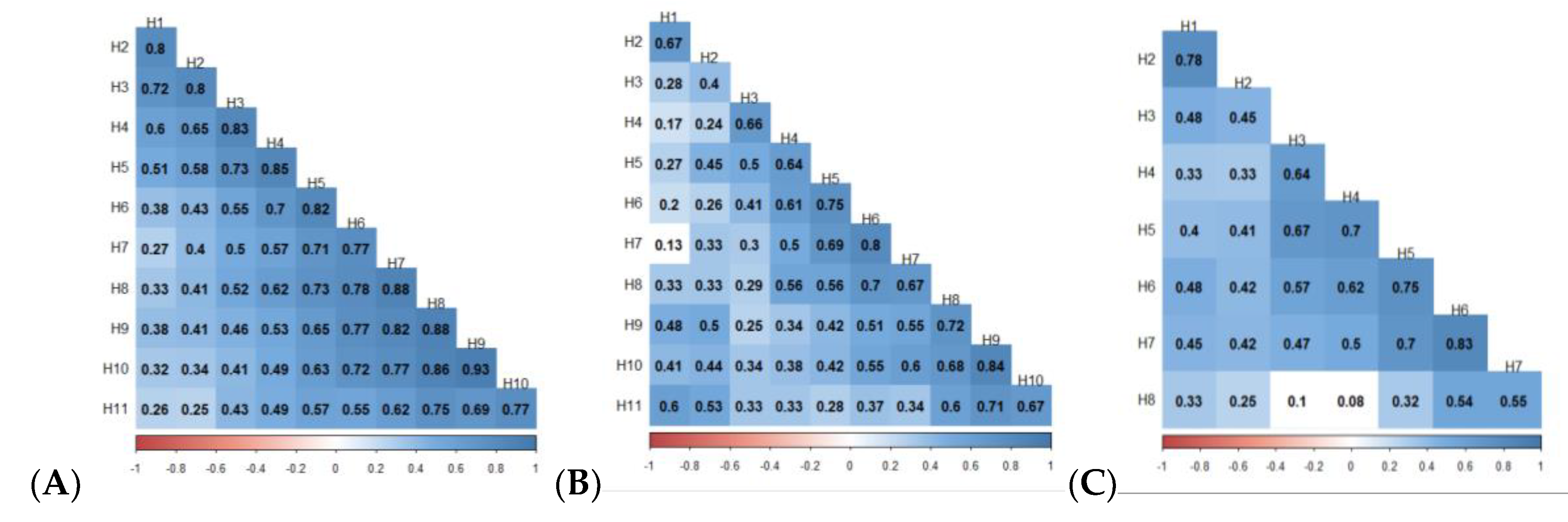
3.3. Spearman Correlation Between Harvests, and Type-A Genetic Correlation Between Traits
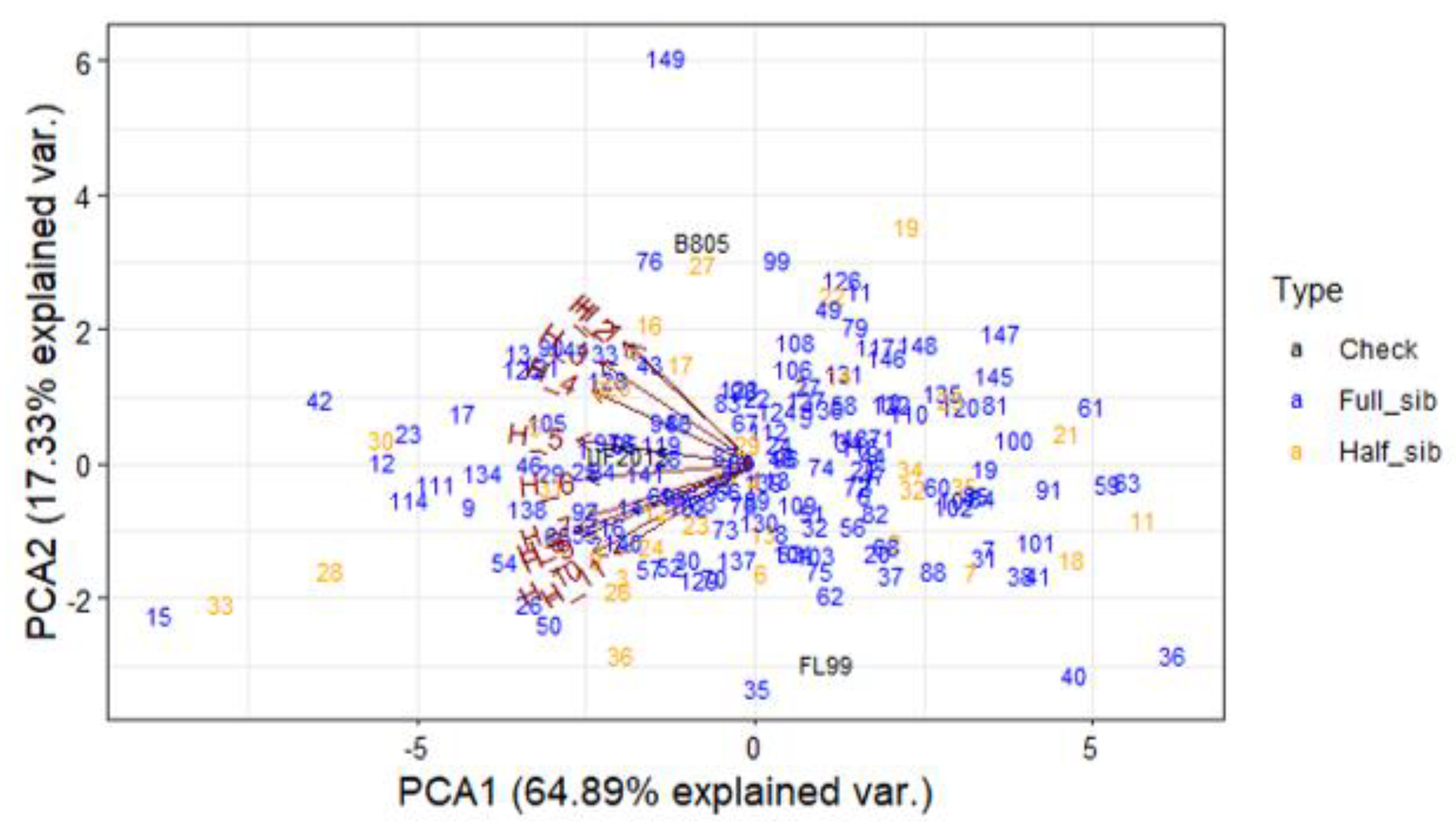
3.4. Principal Component Analysis for DMY
4. Discussion
4.1. Initial Germplasm Screening and Mating Design
4.2. Variance Components and Genotypic Values for the Reference Breeding Population
4.3. Type-B (rGxH) Genetic Correlation Between Families and Harvest
4.4. Type-A (rG) Genetic Correlation Between Traits
4.5. Principal Component Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Quesenberry, K.; Munoz, P.; Blount, A.; Kenworthy, K.; Crow, W. Breeding forages in Florida for resistance to nematodes. Crop Pasture Sci. 2014, 65, 1192–1198. [Google Scholar] [CrossRef]
- Jank, L.; Quesenberry, K.H.; Sollenberger, L.E.; Wofford, D.S.; Lyrene, P.M. Selection of morphological traits to improve forage characteristics of Setaria sphacelata grown in Florida. N. Z. J. Agric. Rses. 2007, 50, 73–83. [Google Scholar] [CrossRef]
- Acuña, C.A.; Blount, A.R.; Quesenberry, K.H.; Kenworthy, K.E.; Hanna, W.W. Tetraploid bahiagrass hybrids: Breeding technique, genetic variability and proportion of heterotic hybrids. Euphytica 2011, 179, 227–235. [Google Scholar] [CrossRef]
- Wallau, M.O.; Sollenberger, L.E.; Vendramini, J.M.B.; Gomide, C.A.M.; Mullenix, M.K.; Quesenberry, K.H. Performance of limpograss breeding lines under various grazing management strategies. Crop Sci. 2016, 56, 3345–3353. [Google Scholar] [CrossRef]
- Bouton, J.H. Breeding lucerne for persistence. Crop Pasture Sci. 2012, 63, 95–106. [Google Scholar] [CrossRef]
- Michaud, R.; Lehman, W.F.; Rumbaugh, M.D. World distribution and historical development. Alfalfa Alfalfa Improv. 1988, 29, 25–91. [Google Scholar]
- Annicchiarico, P. Alfalfa forage yield and leaf/stem ratio: Narrow-sense heritability, genetic correlation, and parent selection procedures. Euphytica 2015, 205, 409–420. [Google Scholar] [CrossRef]
- North American Alfalfa Improvement Conference. Available online: https://www.naaic.org/resource/importance.php (accessed on 8 May 2020).
- Crop Production Summary Report, USDA, NASS. 2018. Available online: https://www.nass.usda.gov/Publications/Todays_Reports/reports/cropan19.pdf (accessed on 19 May 2020).
- Belda, M.; Holtanová, E.; Halenka, T.; Kalvová, J. Climate classification revisited: From Köppen to Trewartha. Clim. Res. 2014, 59, 1–13. [Google Scholar] [CrossRef]
- Peters, G.P.; Andrew, R.M.; Boden, T.; Canadell, J.G.; Ciais, P.; Le Quéré, C.; Marland, G.; Raupach, M.R.; Wilson, C. The challenge to keep global warming below 2C. Nat. Clim. Chang. 2013, 3, 4–6. [Google Scholar] [CrossRef]
- Hanberry, B.B.; Fraser, J.S. Visualizing current and future climate boundaries of the conterminous United States: Implications for forests. Forests 2019, 10, 280. [Google Scholar] [CrossRef]
- Blount, A.R. Forage Production in the Southern Coastal Plain; Agronomy Department, IFAS Extension, Univ. of Florida: Gainesville, FL, USA, 2017; pp. 1–2. Available online: https://edis.ifas.ufl.edu/aa265 (accessed on 8 May 2020).
- Horner, E.S. Registration of Florida 66 Alfalfa (Reg. No. 48). Crop Sci. 1970, 10, 456. [Google Scholar] [CrossRef]
- Horner, E.S.; Ruelke, O.C. Registraton of Florida 77 Alfalfa (Reg. No. 99). Crop Sci. 1981, 21, 797. [Google Scholar] [CrossRef]
- Vilela, D.; Basigalup, D.H.; Juntolli, F.V.; Ferreira, R.P. Research priorities and future of alfalfa in Latin America. In Proceedings of the Second World Alfalfa Congress, Cordoba, Agentina, 11–14 November 2018; pp. 140–143. Available online: https://repositorio.inta.gob.ar/handle/20.500.12123/4031 (accessed on 8 May 2020).
- de Assis, G.M.L.; Ruggieri, A.C.; Mercadante, M.E.Z.; De Camargo, G.M.F.; Carneiro Júnior, J.M. Selection of alfalfa cultivars adapted for tropical environments with repeated measures using PROC MIXED of SAS® System. Plant Genet. Resour. 2010, 8, 55–62. [Google Scholar] [CrossRef]
- Annicchiarico, P.; Scotti, C.; Carelli, M.; Pecetti, L. Questions and avenues for lucerne improvement. Czech J. Genet. Plant Breed. 2010, 46, 1–13. [Google Scholar] [CrossRef]
- Bingham, E.T.; Groose, R.W.; Woodfield, D.R.; Kidwell, K.K. Complementary gene interactions in alfalfa are greater in autotetraploids than diploids. Crop Sci. 1994, 34, 823–829. [Google Scholar] [CrossRef]
- Brummer, E.C. Capturing heterosis in forage crop cultivar development. Crop Sci. 1999, 39, 943–954. [Google Scholar] [CrossRef]
- Li, X.; Brummer, E.C. Applied genetics and genomics in alfalfa breeding. Agronomy 2012, 2, 40–61. [Google Scholar] [CrossRef]
- Annicchiarico, P.; Barrett, B.; Brummer, E.C.; Julier, B.; Marshall, A.H. Achievements and challenges in improving temperate perennial forage legumes. CRC Crit. Rev. Plant Sci. 2015, 34, 327–380. [Google Scholar] [CrossRef]
- Biazzi, E.; Nazzicari, N.; Pecetti, L.; Brummer, E.C.; Palmonari, A.; Tava, A.; Annicchiarico, P. Genome-wide association mapping and genomic selection for alfalfa (Medicago sativa) forage quality traits. PLoS ONE 2017, 12, e0169234. [Google Scholar] [CrossRef]
- Li, X.; Wei, Y.; Acharya, A.; Hansen, J.L.; Crawford, J.L.; Viands, D.R.; Michaud, R.; Claessens, A.; Brummer, C.E. Genomic prediction of biomass yield in two selection cycles of a tetraploid alfalfa breeding population. Plant Genome 2015, 8, 1–10. [Google Scholar] [CrossRef]
- Brummer, E.C.; Casler, M.D. Cool-Season Forages. Yield Gains Major US Field Crop 2014, 1108, 33–52. [Google Scholar]
- Bowley, S.R.; Christie, B.R. Inheritance of Dry Matter yield in a Heterozygous Population of Alfalfa. Can. J. Plant Sci. 1981, 61, 313–318. [Google Scholar] [CrossRef]
- Riday, H.; Brummer, E.C. Crop breeding, genetics & cytology: Heterosis in a broad range of alfalfa germplasm. Crop Sci. 2005, 45, 8–17. [Google Scholar]
- Casler, M.D.; Brummer, E.C. Theoretical expected genetic gains for among-and-within-family selection methods in perennial forage crops. Crop Sci. 2008, 48, 890–902. [Google Scholar] [CrossRef]
- Piepho, H.P.; Möhring, J.; Melchinger, A.E.; Büchse, A. BLUP for phenotypic selection in plant breeding and variety testing. Euphytica 2008, 161, 209–228. [Google Scholar] [CrossRef]
- John, J.A.; Williams, E.R. The Construction of Efficient Two-Replicate Row-Column Designs for Use in Field. Appl. Statist. 1997, 46, 207–214. [Google Scholar] [CrossRef]
- Gilmour, A.R. Average information residual maximum likelihood in practice. J. Anim. Breed. Genet. 2019, 136, 262–272. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Stat. Comput.: Vienna, Austria, 2018; Available online: https://www.R-project.org/ (accessed on 8 May 2020).
- Amadeu, R.R.; Cellon, C.; Olmstead, J.W.; Garcia, A.A.F.; Resende, M.F.R.; Muñoz, P.R. AGHmatrix: R package to construct relationship matrices for autotetraploid and diploid species: A blueberry example. Plant Genome 2016, 9, 1–10. [Google Scholar] [CrossRef]
- Smith, B.A.; Stringer, J.K.; Wei, X.; Cullis, B.R. Varietal selection for perennial crops where data relate to multiple harvests from a series of field trials. Euphytica 2007, 157, 253–266. [Google Scholar] [CrossRef]
- Andrade, V.T.; Gonçalves, F.M.; Nunes, J.A.R.; Botelho, C.E. Statistical modeling implications for coffee progenies selection. Euphytica 2016, 207, 177–189. [Google Scholar] [CrossRef]
- Schwarz, G. Estimating The Dimension of a Model. Ann. Stat. 1978, 6, 461–464. [Google Scholar] [CrossRef]
- de Mendiburu, F. Agricolae: Statistical procedures for agricultural research. R Packag. Version 2014, 1. [Google Scholar]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 3319242776. [Google Scholar]
- FAO. The State of the World’s Land and Water Resources for Food and Agriculture; Food and Agriculture Organization of the United Nations: London, UK, 2011; Available online: http://www.fao.org/docrep/017/i1688e/i1688e.pdf (accessed on 11 May 2020).
- Ugo, P.C.; Baker, D.; Morgan, N.; Ly, C.; Nouala, S. Investing in African Livestock: Business opportunities in 2030–2050. 2013. Available online: http://www.fao.org/3/al757e/al757e.pdf (accessed on 11 May 2020).
- Basigalup, D.H.; Rossanigo, R.O.; Rodríguez, N.E.; Spada, M.; Del, C.; Collino, D.J.; Dardanelli, J.L.; De Luca, M.J.; Racca, R.W.; González, N.S.; et al. El Cultivo De La Alfalfa En La Argentina; Ediciones INTA: Buenos Aires, Argentina, 2007; 476p, ISBN 9875212428. [Google Scholar]
- Smith, S.R.; Bouton, J.H. Selection within Alfalfa Cultivars for Persistence under Continous Stocking. Crop Sci. 1993, 33, 1321–1328. [Google Scholar] [CrossRef]
- Falconer, D.S.; Mackay, T.F.C. Introduction to Quantitative Genetics, 4th ed.; Longman: Essex, UK, 1996; p. 464. [Google Scholar]
- Brummer, E.C.; Shah, M.M.; Luth, D. Reexamining the relationship between fall dormancy and winter hardiness in alfalfa. Crop Sci. 2000, 40, 971–977. [Google Scholar] [CrossRef]
- Riday, H.; Brummer, E.C. Narrow Sense Heritability and Additive Genetic Correlations in Alfalfa subsp. falcata. J. Iowa Acad. Sci. JIAS 2007, 114, 28–34. [Google Scholar]
- Rios, E.F.; Kenworthy, K.E.; Gezan, S.A.; Munoz, P.R. Genetic Parameters for Phenotypic Traits in Annual Ryegrass. Crop Sci. 2019, 59, 2128–2140. [Google Scholar] [CrossRef]
- Anthony, D.; Elbaum, S.; Lorenz, A.; Detweiler, C. On crop height estimation with UAVs. In Proceedings of the 2014 IEEE/RSJ International Conference on Intelligent Robots and Systems, Chicago, IL, USA, 14–18 September 2014; 2014; pp. 4805–4812. [Google Scholar] [CrossRef]
- Berhongaray, G.; Basanta, M.; Jauregui, J.M. Water table depth affects persistence and productivity of alfalfa in Central Argentina. Field Crops Res. 2019, 235, 54–58. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acharya, J.P.; Lopez, Y.; Gouveia, B.T.; de Bem Oliveira, I.; Resende, M.F.R., Jr.; Muñoz, P.R.; Rios, E.F. Breeding Alfalfa (Medicago sativa L.) Adapted to Subtropical Agroecosystems. Agronomy 2020, 10, 742. https://doi.org/10.3390/agronomy10050742
Acharya JP, Lopez Y, Gouveia BT, de Bem Oliveira I, Resende MFR Jr., Muñoz PR, Rios EF. Breeding Alfalfa (Medicago sativa L.) Adapted to Subtropical Agroecosystems. Agronomy. 2020; 10(5):742. https://doi.org/10.3390/agronomy10050742
Chicago/Turabian StyleAcharya, Janam P., Yolanda Lopez, Beatriz Tome Gouveia, Ivone de Bem Oliveira, Marcio F. R. Resende, Jr., Patricio R. Muñoz, and Esteban F. Rios. 2020. "Breeding Alfalfa (Medicago sativa L.) Adapted to Subtropical Agroecosystems" Agronomy 10, no. 5: 742. https://doi.org/10.3390/agronomy10050742
APA StyleAcharya, J. P., Lopez, Y., Gouveia, B. T., de Bem Oliveira, I., Resende, M. F. R., Jr., Muñoz, P. R., & Rios, E. F. (2020). Breeding Alfalfa (Medicago sativa L.) Adapted to Subtropical Agroecosystems. Agronomy, 10(5), 742. https://doi.org/10.3390/agronomy10050742





