Sewage Sludge as a Soil Amendment for Growing Biomass Plant Arundo donax L.
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
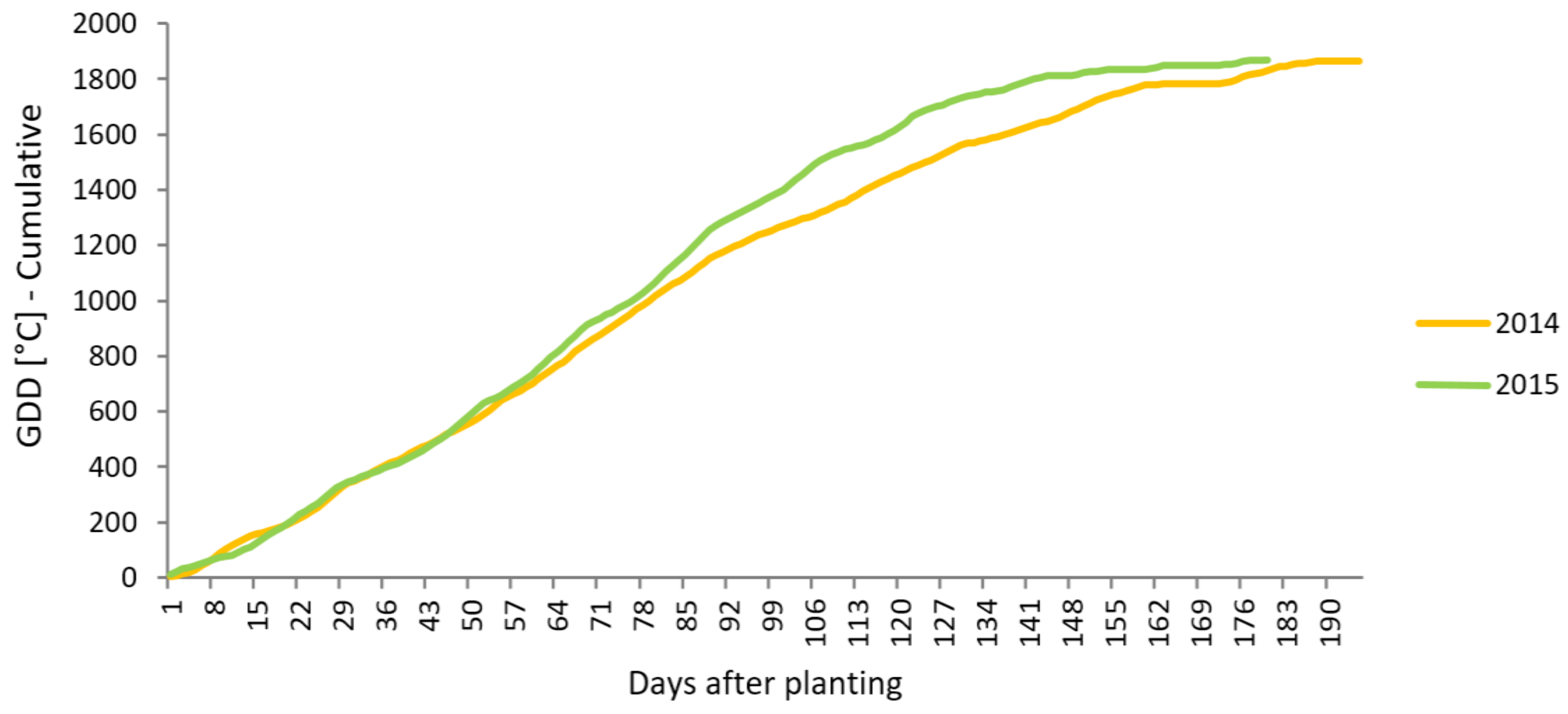
3.1. Plant Growth During the Vegetation Period
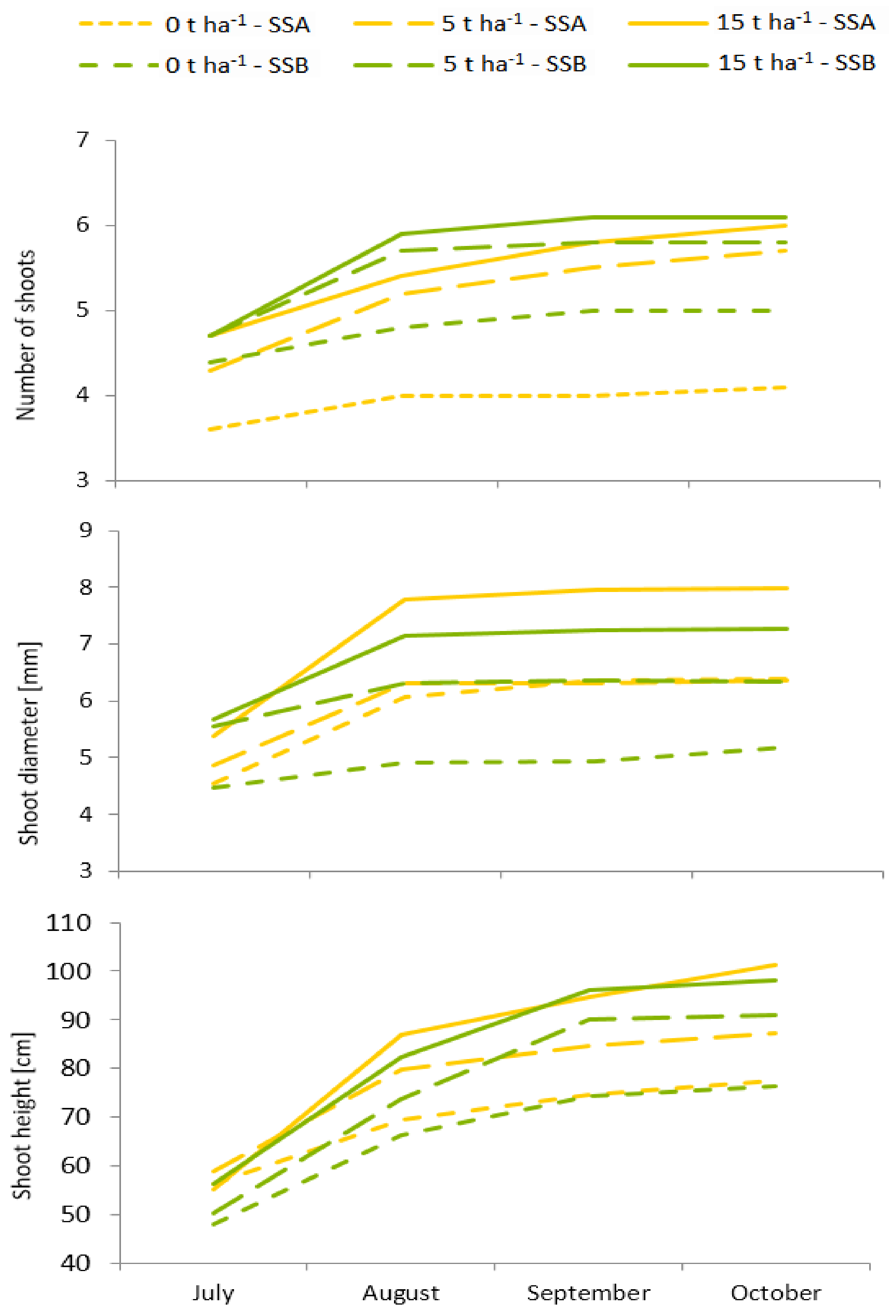
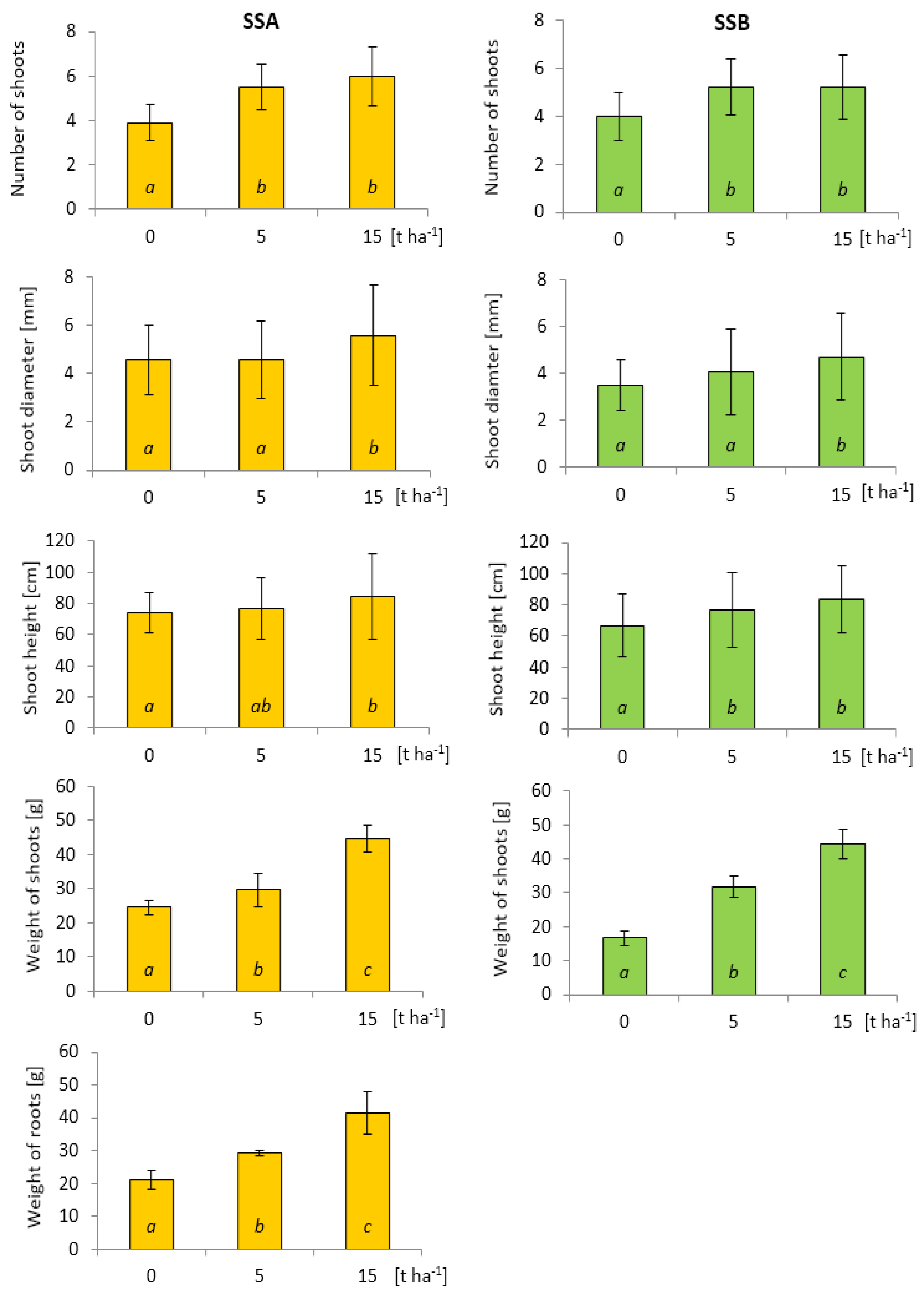
3.2. Evaluation of Growth Parameters and Biomass Yield at the End of the Vegetation Season
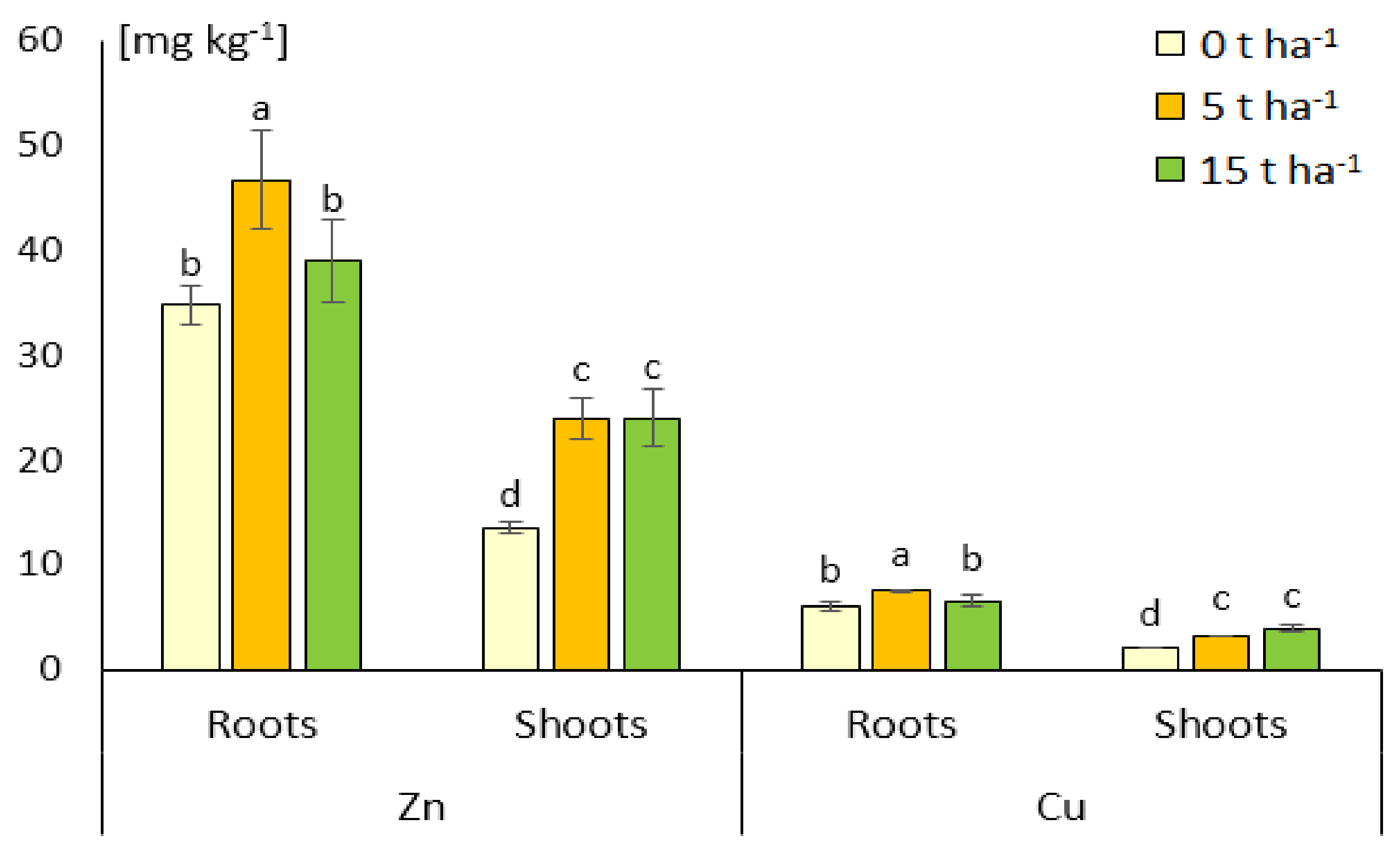
3.3. Metals Accumulation and Distribution in Plants
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kumar, V.; Chopra, A.K.; Kumar, A. A review on sewage sludge (Biosolids) a resource for sustainable agriculture. Arch. Agric. Environ. Sci. 2017, 2, 340–347. [Google Scholar] [CrossRef]
- Eurostat. Available online: https://ec.europa.eu/eurostat (accessed on 4 January 2020).
- Hudcová, H.; Vymazal, J.; Rozkošný, M. Present restrictions of sewage sludge application in agriculture within the European Union. Soil Water Res. 2019, 14, 104–120. [Google Scholar] [CrossRef]
- Shaddel, S.; Bakhtiary-Davijany, H.; Kabbe, C.; Dadgar, F.; Østerhus, S.W. Sustainable sewage sludge management: From current practices to emerging nutrient recovery technologies. Sustainability 2019, 11, 3435. [Google Scholar] [CrossRef]
- Suschka, J.; Grübel, K. Nitrogen in the process of waste activated sludge anaerobic digestion. Arch. Environ. Prot. 2014, 40, 123–136. [Google Scholar] [CrossRef]
- Houben, D.; Michel, E.; Nobile, C.; Lambers, H.; Kandeler, E.; Faucon, M.P. Response of phosphorus dynamics to sewage sludge application in an agroecosystem in northern France. Appl. Soil Ecol. 2019, 137, 178–186. [Google Scholar] [CrossRef]
- Kominko, H.; Gorazda, K.; Wzorek, Z. The Possibility of organo-mineral fertilizer production from sewage sludge. Waste Biomass Valori. 2017, 8, 1781–1791. [Google Scholar] [CrossRef]
- Urra, J.; Alkorta, I.; Garbisu, C. Potential Benefits and Risks for Soil Health Derived From the Use of Organic Amendments in Agriculture. Agronomy 2019, 9, 542. [Google Scholar] [CrossRef]
- Xu, G. Analysis of Sewage sludge Recovery System in EU in Perspectives of Nutrients and Energy Recovery Efficiency, and Environmental Impacts. Master’s Thesis, Norwegian University of Science and Technology, Trondheim, Norway, 2014. [Google Scholar]
- Zubala, T.; Patro, M.; Boguta, P. Variability of zinc, copper and lead contents in sludge of the municipal stormwater treatment plant. Environ. Sci. Pollut. Res. 2017, 24, 17145–17152. [Google Scholar] [CrossRef]
- Zennegg, M.; Munoz, M.; Schmid, P.; Gerecke, A.C. Temporal trends of persistent organic pollutants in digested sewage sludge (1993–2012). Environ. Int. 2013, 60, 202–208. [Google Scholar] [CrossRef]
- Vaca, R.; Lugo, J.; Martínez, R.; Esteller, M.V.; Zavaleta, H. Effects of sewage sludge and sewage sludge compost amendment on soil properties and Zea mays L. plants (heavy metals, quality and productivity). Rev. Int. Contam. Ambie. 2011, 27, 303–311. [Google Scholar]
- Dar, M.I.; Naikoo, M.I.; Khan, F.A.; Green, I.D. Assessing the Feasibility of Sewage Sludge Applications for the Cultivation of Brassica Juncea L.: Metal Accumulation, Growth, Biochemical and Yield Responses. Environ. Sci. Renew. Res. 2018, 1, 104. [Google Scholar] [CrossRef]
- Kumar, V.; Chopra, A.K. Agronomical performance of high yielding cultivar of eggplant (Solanum melongena L.) grown in sewage sludge amended soil. Res. Agric. 2016, 1, 1–24. [Google Scholar] [CrossRef][Green Version]
- Shahbazi, F.; Ghasemi, S.; Sodaiezadeh, H.; Ayaseh, K.; Zahani-Ahmadmahmoodi, R. The effect of sewage sludge on heavy metal concentrations in wheat plant (Triticum aestivum L.). Environ. Sci. Pollut. Res. Int. 2017, 24, 15634–15644. [Google Scholar] [CrossRef] [PubMed]
- Lag-Brotons, A.; Gómez, I.; Navarro-Pedreño, J.; Mayoral, A.M.; Curt, M.D. Sewage sludge compost use in bioenergy production- A case study on the effects on Cynara cardunculus L energy crop. J. Clean. Prod. 2014, 79, 32–40. [Google Scholar] [CrossRef]
- Wierzbowska, J.; Sienkiewicz, S.; Krzebietke, S.; Sternik, P. Sewage sludge as source of nitrogen and phosphorus for virginia fanpetals. Bulg. J. Agri. Sci. 2016, 22, 722–727. [Google Scholar]
- Kubátová, P.; Hejcman, M.; Száková, J.; Vondráčková, S.; Tlustoš, P. Effects of Sewage sludge application on biomass production and concentrations of Cd, Pb and Zn in shoots of Salix and Populus clones: Improvement of phytoremediation efficiency in contaminated soils. Bioenerg. Res. 2016, 9, 809–819. [Google Scholar] [CrossRef]
- Kołodziej, B.; Antonkiewicz, J.; Stachyra, M.; Bielińska, E.J.; Wiśniewski, J.; Luchowska, K.; Kwiatkowski, C. Use of sewage sludge in bioenergy production—A case study on the effects on sorghum biomass production. Eur. J. Agron. 2015, 69, 63–74. [Google Scholar] [CrossRef]
- Kołodziej, B.; Antonkiewicz, J.; Sugier, D. Miscanthus×giganteus as a biomass feedstock grown on municipal sewage sludge. Ind. Crops Prod. 2016, 81, 72–82. [Google Scholar] [CrossRef]
- Antonkiewicz, J.; Kołodziej, B.; Bielińska, E.J.; Popławska, A. The possibility of using sewage sludge for energy crop cultivation exemplified by reed canary grass and giant miscanthus. Soil Sci. Ann. 2019, 70, 21–33. [Google Scholar] [CrossRef]
- Ondreičková, K.; Gubišová, M.; Gubiš, J.; Klčová, L.; Horník, M. Rhizosphere bacterial communities of Arundo donax grown in soil fertilised with sewage sludge and agricultural by-products. Agriculture 2019, 65, 37–41. [Google Scholar] [CrossRef]
- Ondreičková, K.; Gubišová, M.; Piliarová, M.; Horník, M.; Matušinský, P.; Gubiš, J.; Klčová, L.; Hudcovicová, M.; Kraic, J. Responses of rhizosphere fungal communities to the sewage sludge application into the soil. Microorganisms 2019, 7, 505. [Google Scholar] [CrossRef] [PubMed]
- Alshaal, T.; Elhawat, N.; Domokos-Szabolcsy, É.; Kátai, J.; Márton, L.; Czako, M.; El-Ramady, H.; Fári, M.G. Giant reed (Arundo donax L.): A green technology for clean environment. In Phytoremediation: Management of Environmental Contaminants, 1st ed.; Ansari, A.A., Gill, S.S., Gill, R., Lanza, G.R., Newman, L., Eds.; Springer International Publishing: Basel, Switzerland, 2015; Volume 1, pp. 3–20. [Google Scholar] [CrossRef]
- Dürešová, Z.; Šuňovská, A.; Horník, M.; Pipíška, M.; Gubišová, M.; Gubiš, J.; Hostin, S. Rhizofiltration potential of Arundo donax for cadmium and zinc removal from contaminated wastewater. Chem. Pap. 2014, 68, 1452–1462. [Google Scholar] [CrossRef]
- Gubišová, M.; Čičková, M.; Klčová, L.; Gubiš, J. In vitro tillering—An effective way to multiply high-biomass plant Arundo donax. Ind. Crops Prod. 2016, 81, 123–128. [Google Scholar] [CrossRef]
- Spencer, D.F.; Ksander, G. Estimating Arundo donax ramet recruitment using degree-day based equations. Aquat. Bot. 2006, 85, 282–288. [Google Scholar] [CrossRef]
- Foulkes, M.J.; Murchie, E.H. Optimizing Canopy Physiology Traits to Improve the Nutrient Utilization Efficiency of Crops. In The Molecular and Physiological Basis of Nutrient Use Efficiency in Crops, 1st ed.; Hawkesford, M.J., Barraclough, P., Eds.; John Wiley & Sons: Chichester, West Sussex, UK, 2011; pp. 63–82. [Google Scholar] [CrossRef]
- Cano-Ruiz, J.; Sanz, M.; Curt, M.D.; Plaza, A.; Lobo, M.C.; Maur, P.V. Fertigation of Arundo donax L. with different nitrogen rates for biomass production. Biomass Bioenergy 2020, 133, 105451. [Google Scholar] [CrossRef]
- Mardikis, M.; Christou, M.; Alexopoulou, E. Arundo donax population screening in Greece. In Proceedings of the World Conference and Exhibition on Biomass for Energy and Industry, Sevilla, Spain, 5–9 June 2000; Kyritsis, S., Beenackers, A.A.C.M., Helm, P., Grassi, A., Charamonti, D., Eds.; James & James Science Publisher: London, UK, 2001; pp. 1626–1629. [Google Scholar]
- Sharma, A.; Verma, R.K. Root–Microbe Interactions: Understanding and Exploitation of Microbiome. In Root Biology; Giri, B., Prasad, R., Varma, A., Eds.; Springer: Cham, Switzerland, 2018; Volume 52, pp. 323–339. [Google Scholar] [CrossRef]
- Badri, D.V.; Vivanco, J.M. Regulation and function of root exudates. Plant Cell Environ. 2009, 32, 666–681. [Google Scholar] [CrossRef]
- Pilu, R.; Bucci, A.; Badone, F.C.; Landoni, M. Giant reed (Arundo donax L.): A weed plant or a promising energy crop? Afr. J. Biotech. 2012, 11, 9163–9174. [Google Scholar] [CrossRef]
- Tong, Z.; Quan, G.; Wan, L.; He, F.; Li, X. The Effect of Fertilizers on Biomass and Biodiversity on a Semi-Arid Grassland of Northern China. Sustainability 2019, 11, 2854. [Google Scholar] [CrossRef]
- Malhotra, H.; Sharma, S.; Pandey, R. Phosphorus Nutrition: Plant Growth in Response to Deficiency and Excess. In Plant Nutrients and Abiotic Stress Tolerance, 1st ed.; Hasanuzzaman, M., Fujita, M., Oku, H., Nahar, K., Hawrylak-Nowak, B., Eds.; Springer: Singapore, 2018; pp. 171–190. [Google Scholar] [CrossRef]
- Rigby, H.; Clarke, B.O.; Pritchard, D.L.; Meehan, B.; Beshah, F.; Smith, S.R.; Porter, N.A. A critical review of nitrogen mineralization in biosolids-amended soil, the associated fertilizer value for crop production and potential for emissions to the environment. Sci. Total Environ. 2016, 541, 1310–1338. [Google Scholar] [CrossRef]
- Syed-Hassan, S.S.A.; Wang, Y.; Hu, S.; Su, S.; Xiang, J. Thermochemical processing of sewage sludge to energy and fuel: Fundamentals, challenges and considerations. Renew. Sustain. Energy Rev. 2017, 80, 888–913. [Google Scholar] [CrossRef]
- Christian, D.G.; Poulton, P.R.; Riche, A.B.; Yates, N.; Todd, A.D. The recovery over several seasons of 15N-labelled fertilizer applied to Miscanthus × giganteus ranging from 1 to 3 years old. Biomass Bioenergy 2006, 30, 125–133. [Google Scholar] [CrossRef]
- Nassi o Di Nasso, N.; Roncucci, N.; Bonari, E. Seasonal Dynamics of Aboveground and Belowground Biomass and Nutrient Accumulation and Remobilization in Giant Reed (Arundo donax L.): A Three-Year Study on Marginal Land. BioEnergy Res. 2013, 6, 725–736. [Google Scholar] [CrossRef]
- Elloumi, N.; Belhaj, D.; Jerbi, B.; Zouari, M.; Kallel, M. Effects of sewage sludge on bio-accumulation of heavy metals in tomato seedlings. Span. J. Agric. Res. 2016, 14, e0807. [Google Scholar] [CrossRef]
- Belhaj, D.; Elloumi, N.; Jerbi, B.; Zouari, M.; Abdallah, F.B.; Ayadi, H.; Kallel, M. Effects of sewage sludge fertilizer on heavy metal accumulation and consequent responses of sunflower (Helianthus annuus). Environ. Sci. Pollut. Res. 2016, 23, 20168–20177. [Google Scholar] [CrossRef] [PubMed]

| PTEs [mg kg−1; d.w.] | |||||||
|---|---|---|---|---|---|---|---|
| Type of SS | As | Cd | Cr | Cu | Ni | Pb | Zn |
| SSA | 8 | <2 | 85 | 654 | 42 | 36 | 1940 |
| SSB | 3 | <1 | 36 | 224 | 22 | 46 | 1269 |
| Macroelements [g kg−1; d.w.] | ||||||
|---|---|---|---|---|---|---|
| Substrate | N | C | Ca | Mg | K | P |
| Soil | 0.958 | 10.3 | 2.94 | 0.280 | 0.193 | 0.097 |
| SSA | 34.7 | 564 | 23.7 | 5.70 | 5.96 | 18.1 |
| SSB | 35.1 | 334 | 36.4 | 6.44 | 2.66 | 16.7 |
| Season | Planting Date | Harvest | Vegetation Period | Precipitation | Average Air Temperature |
|---|---|---|---|---|---|
| 2014 | 15.5.2014 | 25.11.2014 | 194 days | 660 mm | 11.81 °C |
| 2015 | 18.5.2015 | 13.11.2015 | 179 days | 502 mm | 11.20 °C |
| Factor | P Values; Two-Way ANOVA | |||
|---|---|---|---|---|
| Weight of Biomass | No. of Shoots | Height of Shoot | Shoot Diameter | |
| Dose of SS | 0.0000 | 0.0001 | 0.0003 | 0.0000 |
| Type of SS | 0.0834 | 0.2832 | 0.3588 | 0.0002 |
| Interaction | 0.0009 | 0.4929 | 0.4987 | 0.5827 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gubišová, M.; Horník, M.; Hrčková, K.; Gubiš, J.; Jakubcová, A.; Hudcovicová, M.; Ondreičková, K. Sewage Sludge as a Soil Amendment for Growing Biomass Plant Arundo donax L. Agronomy 2020, 10, 678. https://doi.org/10.3390/agronomy10050678
Gubišová M, Horník M, Hrčková K, Gubiš J, Jakubcová A, Hudcovicová M, Ondreičková K. Sewage Sludge as a Soil Amendment for Growing Biomass Plant Arundo donax L. Agronomy. 2020; 10(5):678. https://doi.org/10.3390/agronomy10050678
Chicago/Turabian StyleGubišová, Marcela, Miroslav Horník, Katarína Hrčková, Jozef Gubiš, Andrea Jakubcová, Martina Hudcovicová, and Katarína Ondreičková. 2020. "Sewage Sludge as a Soil Amendment for Growing Biomass Plant Arundo donax L." Agronomy 10, no. 5: 678. https://doi.org/10.3390/agronomy10050678
APA StyleGubišová, M., Horník, M., Hrčková, K., Gubiš, J., Jakubcová, A., Hudcovicová, M., & Ondreičková, K. (2020). Sewage Sludge as a Soil Amendment for Growing Biomass Plant Arundo donax L. Agronomy, 10(5), 678. https://doi.org/10.3390/agronomy10050678





