Influence of Substrates in the Development of Bean and in Pathogenicity of Rhizoctonia solani JG Kühn
Abstract
1. Introduction
2. Materials and Methods
2.1. Rhizoctonia Solani Isolate
2.2. Substrates and Bioassays
2.3. Statistical Analysis
3. Results
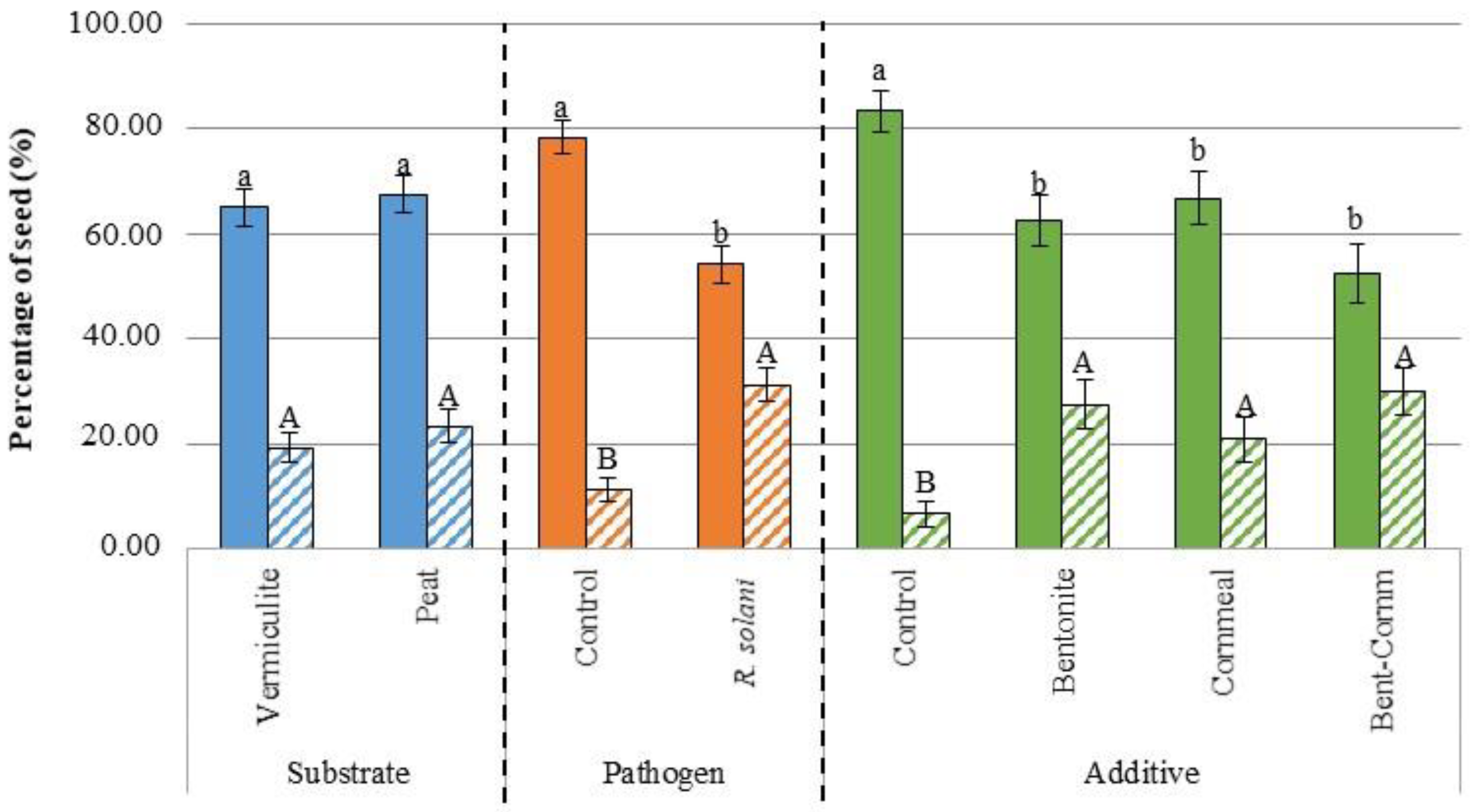
3.1. Germination
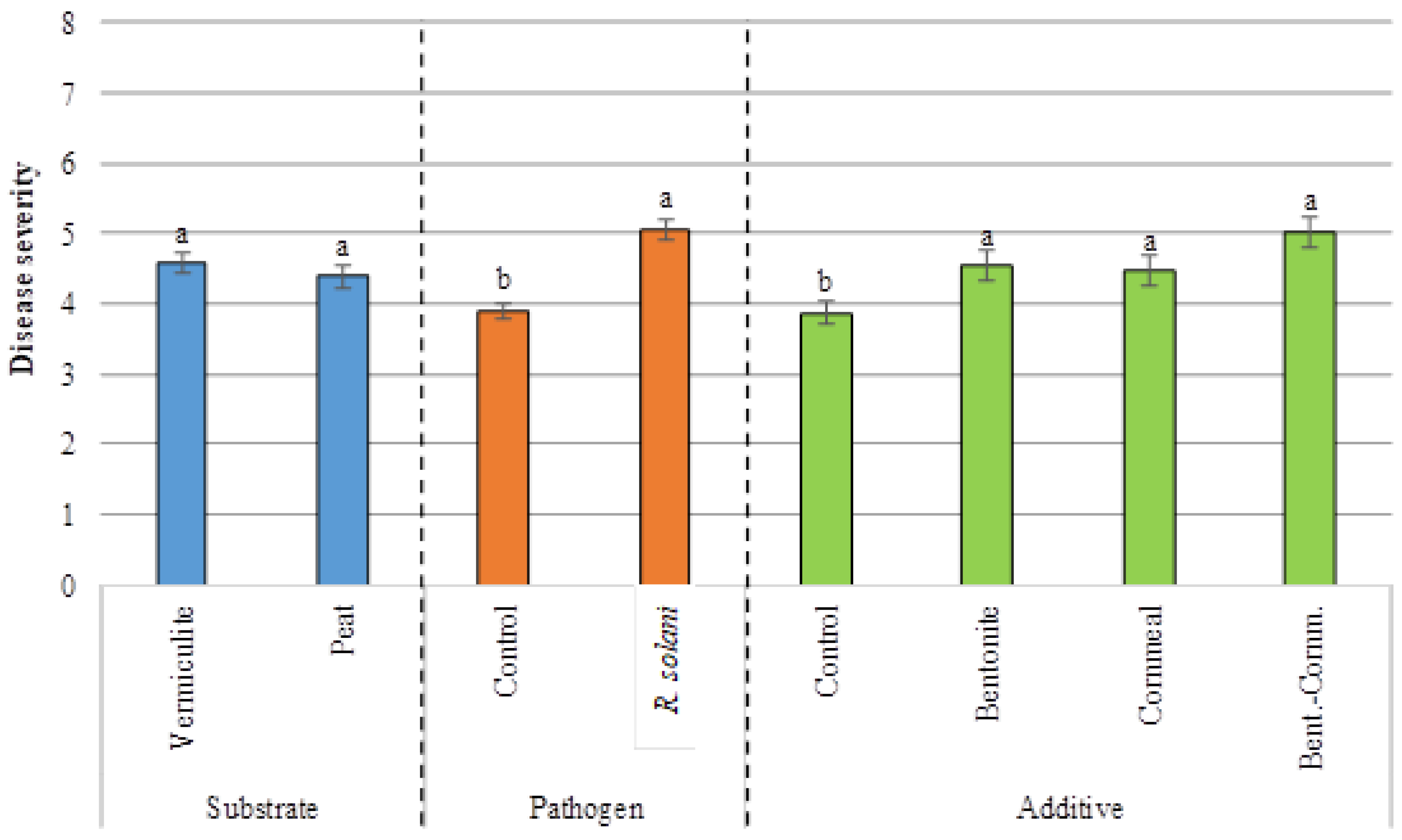
3.2. Disease Severity
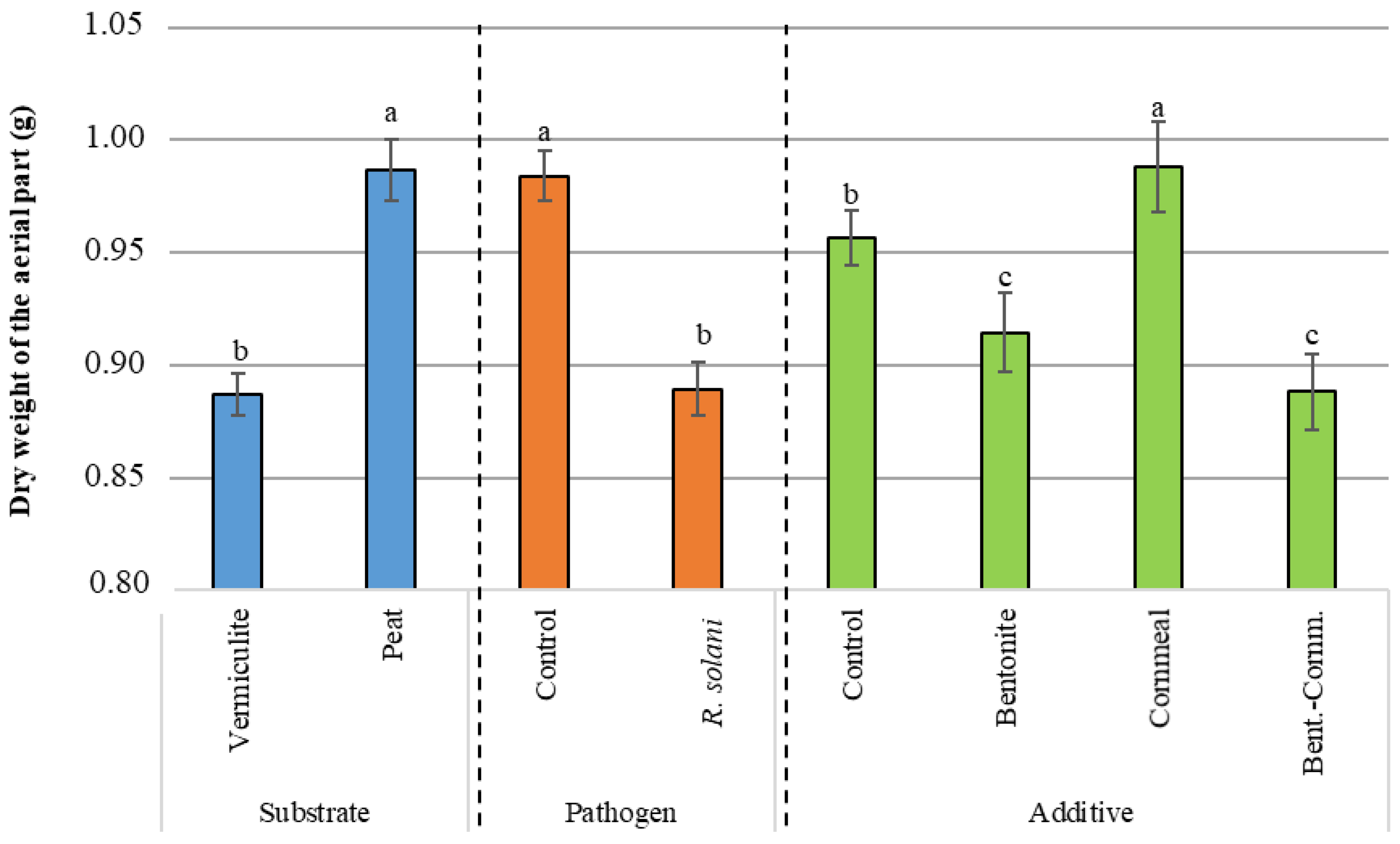
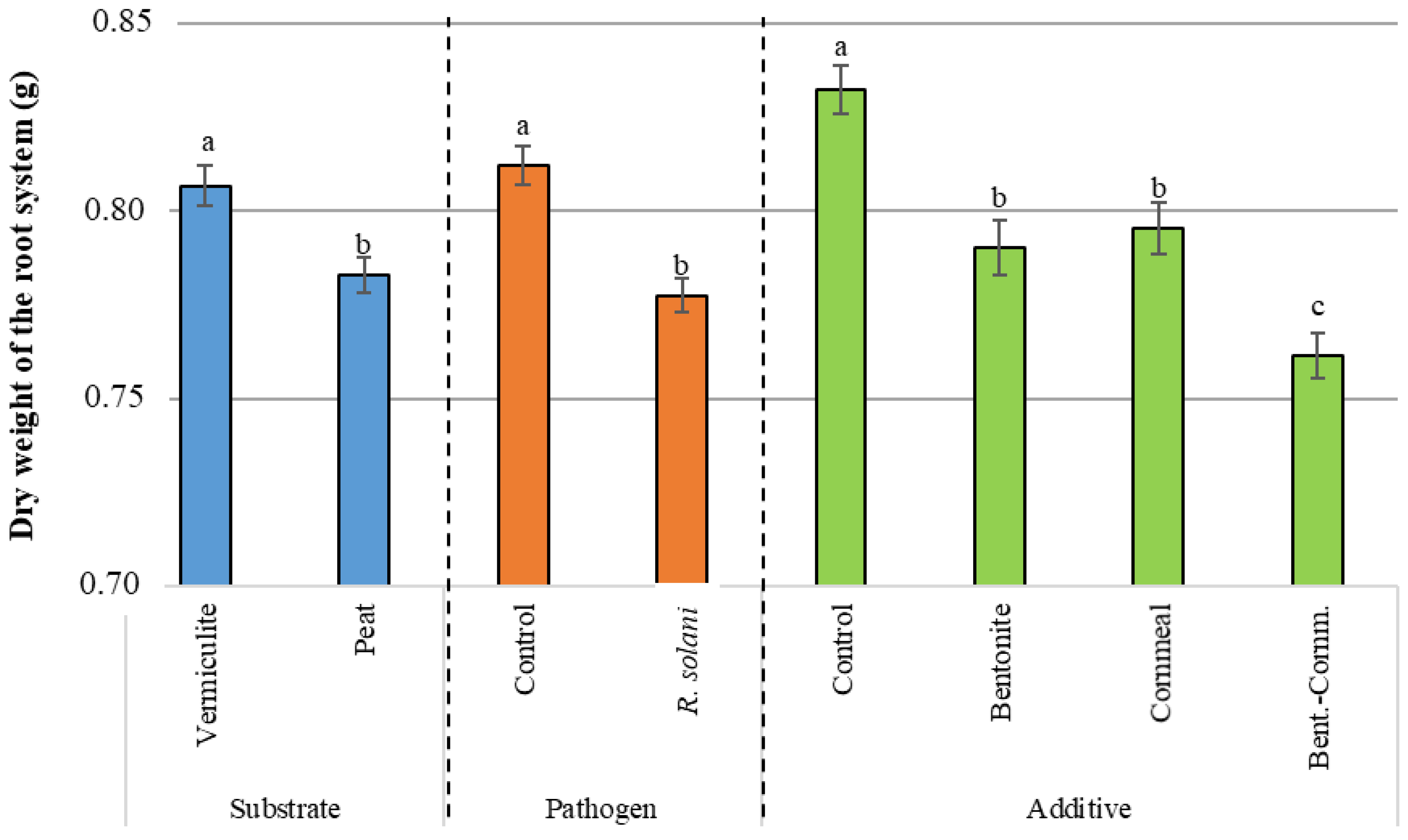
3.3. Dry Weight
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Food and Agriculture Organization (FAO). IPPC ISPM 40 International movement of growing media in association with plants for planting. In International Standards for Phytosanitary Measures; FAO: Roma, Italy, 2017. [Google Scholar]
- Dodd, S.L.; Hill, R.A.; Stewart, A. A duplex-PCR bioassay to detect a Trichoderma virens biocontrol isolate in non-sterile soil. Soil Biol. Biochem. 2004, 36, 1955–1965. [Google Scholar] [CrossRef]
- Lima, L.K.S.; De Jesus, O.N.; Soares, T.L.; De Oliveira, S.A.S.; Haddad, F.; Girardi, E.A. Water deficit increases the susceptibility of yellow passion fruit seedlings to Fusarium wilt in controlled conditions. Sci. Hortic. (Amst.) 2019, 243, 609–621. [Google Scholar] [CrossRef]
- Kaderabek, L.E.; Jackson, B.E.; Fonteno, W.C. Changes in the physical, chemical, and hydrologic properties of pine bark over twelve months of aging. Acta Hortic. 2017, 1174, 313–318. [Google Scholar] [CrossRef]
- Caron, J.; Michel, J.C. Overcoming physical limitations in alternative growing media with and without peat. In Acta Horticulturae; International Society for Horticultural Science: Leuven, Belgium, 2017; Volume 1168, pp. 413–422. [Google Scholar]
- Bowles, T.M.; Acosta-Martínez, V.; Calderón, F.; Jackson, L.E. Soil enzyme activities, microbial communities, and carbon and nitrogen availability in organic agroecosystems across an intensively-managed agricultural landscape. Soil Biol. Biochem. 2014, 68, 252–262. [Google Scholar] [CrossRef]
- Burke, D.J.; Weintraub, M.N.; Hewins, C.R.; Kalisz, S. Relationship between soil enzyme activities, nutrient cycling and soil fungal communities in a northern hardwood forest. Soil Biol. Biochem. 2011, 43, 795–803. [Google Scholar] [CrossRef]
- Li, Y.; Niu, W.; Zhang, M.; Wang, J.; Zhang, Z. Artificial soil aeration increases soil bacterial diversity and tomato root performance under greenhouse conditions. Land Degrad. Dev. 2020. [Google Scholar] [CrossRef]
- Paradelo, R.; Basanta, R.; Barral, M.T. Water-holding capacity and plant growth in compost-based substrates modified with polyacrylamide, guar gum or bentonite. Sci. Hortic. (Amst.) 2019, 243, 344–349. [Google Scholar] [CrossRef]
- Owen, J.S., Jr.; Warren, S.L.; Bilderback, T.E.; Albano, J.P. Phosphorus rate, leaching fraction and substrate influence on influent quantity, effluent nutrient content and response of a containerized woody ornamental crop. HortScience 2008, 43, 906–912. [Google Scholar] [CrossRef]
- Puértolas, A.; Boa, E.; Bonants, P.J.M.; Woodward, S. Survival of Phytophthora cinnamomi and Fusarium verticillioides in commercial potting substrates for ornamental plants. J. Phytopathol. 2018, 166, 484–493. [Google Scholar] [CrossRef]
- Termorshuizen, A.J.; Van Rijn, E.; Van der Gaag, D.J.; Alabouvette, C.; Chen, Y.; Lagerlöf, J.; Malandrakis, A.A.; Paplomatas, E.J.; Rämert, B.; Ryckeboer, J.; et al. Suppressiveness of 18 composts against 7 pathosystems: Variability in pathogen response. Soil Biol. Biochem. 2006, 38, 2461–2477. [Google Scholar] [CrossRef]
- Hoitink, H.; Boehm, M. Biocontrol within the context of soil microbial communities: A substrate-dependent phenomenon. Annu. Rev. Phytopathol. 1999, 37, 427–446. [Google Scholar] [CrossRef] [PubMed]
- Van Overbeek, L.S.; Senechkin, I.V.; Van Bruggen, A.H.C. Variation in microbial responses and Rhizoctonia solani AG2-2IIIB growth in soil under different organic amendment regimes. Can. J. Plant Pathol. 2012, 34, 268–276. [Google Scholar] [CrossRef]
- Martínez-Medina, A.; Roldán, A.; Pascual, J.A. Performance of a Trichoderma harzianum bentonite-vermiculite formulation against Fusarium wilt in seedling nursery melon plants. HortScience 2009, 44, 2025–2027. [Google Scholar] [CrossRef]
- Bernal-Vicente, A.; Ros, M.; Pascual, J.A. Increased effectiveness of the Trichoderma harzianum isolate T-78 against Fusarium wilt on melon plants under nursery conditions. J. Sci. Food Agric. 2009, 89, 827–833. [Google Scholar] [CrossRef]
- Rini, C.R.; Sulochana, K.K. Substrate evaluation for multiplication of Trichoderma spp. J. Trop. Agric. 2007, 45, 58–60. [Google Scholar]
- Ramanujam, R.; Prasad, R.D.; Sriram, S.; Rangeswaran, R. Mass production, formulation, quality control and delivery of Trichoderma for plant disease management. J. Plant Prot. Sci. 2010, 2, 1–8. [Google Scholar]
- Chaves-Gómez, J.L.; Cotes-Prado, A.M.; Gómez-Caro, S.; Restrepo-Díaz, H. Physiological response of cape gooseberry seedlings to two organic additives and their mixture under inoculation with Fusarium oxysporum f. sp. physali. HortScience 2020, 55, 55–62. [Google Scholar]
- Klein, E.; Katan, J.; Gamliel, A. Soil suppressiveness to Fusarium disease following organic amendments and solarization. Plant Dis. 2011, 95, 1116–1123. [Google Scholar] [CrossRef]
- Alabouvette, C. Fusarium wilt suppressive soils: An example of disease-suppressive soils. Australas. Plant Pathol. 1999, 28, 57–64. [Google Scholar] [CrossRef]
- McKellar, M.E.; Nelson, E.B. Compost-induced suppression of Pythium damping-off is mediated by fatty-acid-metabolizing seed-colonizing microbial communities. Appl. Environ. Microbiol. 2003, 69, 452–460. [Google Scholar] [CrossRef]
- Bonanomi, G.; Antignani, V.; Capodilupo, M.; Scala, F. Identifying the characteristics of organic soil amendments that suppress soilborne plant diseases. Soil Biol. Biochem. 2010, 42, 136–144. [Google Scholar] [CrossRef]
- Diab, H.G.; Hu, S.; Benson, D.M. Suppression of Rhizoctonia solani on impatiens by enhanced microbial activity in composted swine waste-amended potting mixes. Phytopathology 2003, 93, 1115–1123. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mazzola, M. Mechanisms of natural soil suppressiveness to soilborne diseases. Antonie Leeuwenhoek 2002, 81, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Porch, T.G.; Valentin, S.; Estevez de Jensen, C.; Beaver, J.S. Identification of soil-borne pathogens in a common bean root rot nursery in Isabela, Puerto Rico. J. Agric. Univ. Puerto Rico 2014, 98, 1–14. [Google Scholar]
- Gullino, M.L.; Gilardi, G.; Bertetti, D.; Garibaldi, A. Emerging soilborne pathogens and trends in their management. Acta Hortic. 2020, 1270, 9–21. [Google Scholar] [CrossRef]
- Jacobs, J.L.; Kelly, J.D.; Wright, E.M.; Varner, G.; Chilvers, M.I. Determining the soilborne pathogens associated with root rot disease complex of dry bean in Michigan. Plant Health Prog. 2019, 20, 122–127. [Google Scholar] [CrossRef]
- Torres, S.V.; Vargas, M.M.; Godoy-Lutz, G.; Porch, T.G.; Beaver, J.S. Isolates of Rhizoctonia solani can produce both web blight and root rot symptoms in common bean (Phaseolus vulgaris L.). Plant Dis. 2016, 100, 1351–1357. [Google Scholar] [CrossRef][Green Version]
- Nerey, Y.; Pannecoucque, J.; Hernandez, H.P.; Diaz, M.; Espinosa, R.; De Vos, S.; Van Beneden, S.; Herrera, L.; Höfte, M. Rhizoctonia spp. causing root and hypocotyl rot in Phaseolus vulgaris in Cuba. J. Phytopathol. 2010, 158, 236–243. [Google Scholar] [CrossRef]
- Peña, P.A.; Steadman, J.R.; Eskridge, K.M.; Urrea, C.A. Identification of sources of resistance to damping-off and early root/hypocotyl damage from Rhizoctonia solani in common bean (Phaseolus vulgaris L.). Crop Prot. 2013, 54, 92–99. [Google Scholar] [CrossRef]
- Guerrero-González, M.L.; Rodríguez-Kessler, M.; Rodríguez-Guerra, R.; González-Chavira, M.; Simpson, J.; Sánchez, F.; Jiménez-Bremont, J.F. Differential expression of Phaseolus vulgaris genes induced during the interaction with Rhizoctonia solani. Plant Cell Rep. 2011, 30, 1465–1473. [Google Scholar] [CrossRef]
- Ajayi-Oyetunde, O.O.; Bradley, C.A. Rhizoctonia solani: Taxonomy, population biology and management of Rhizoctonia seedling disease of soybean. Plant Pathol. 2018, 67, 3–17. [Google Scholar] [CrossRef]
- De Melo, M.P.; Cabral, C.S.; Reis, A.; Matos, K.S.; Martins, P.P.; Beserra Júnior, J.E.A.; Nechet, K.L.; Halfeld-Vieira, B.A. Rhizoctonia solani AG 1-IB and AG 4 HG-I causing leaf blight and root rot in plants from the Lamiaceae family in Brazil. Trop. Plant Pathol. 2018, 43, 152–159. [Google Scholar] [CrossRef]
- Hagedorn, D.J. Rhizoctonia root rot. In Compendium of Bean Diseases; Hall, R., Ed.; American Phytopathological Society (APS): Saint Paul, MN, USA, 1991; p. 13. [Google Scholar]
- Mayo-Prieto, S.; Porteous-Álvarez, A.J.; Rodríguez-González, Á.; Gutiérrez, S.; Casquero, P.A. Evaluation of substrates and additives to Trichoderma harzianum development by qPCR quantification. Agron. J. 2020. [Google Scholar] [CrossRef]
- Mayo, S.; Gutierrez, S.; Malmierca, M.G.; Lorenzana, A.; Campelo, M.P.; Hermosa, R.; Casquero, P.A. Influence of Rhizoctonia solani and Trichoderma spp. in growth of bean (Phaseolus vulgaris L.) and in the induction of plant defense-related genes. Front. Plant Sci. 2015, 6, 685. [Google Scholar] [CrossRef]
- Rigaud, J.R.; Puppo, A. Indole 3 acetic acid catabolism by soybean bacteroids. J. Gen. Microbiol. 1975, 88, 223–228. [Google Scholar] [CrossRef]
- Gomez, A.A.; Gomez, K.A. Statistical Procedures for Agricultural Research, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 1984; Volume 6, ISBN 978-0-471-87092-0. [Google Scholar]
- Valenciano, J.B.; Casquero, P.A.; Boto, J.A.; Marcelo, V. Evaluation of the occurrence of root rots on bean plants (Phaseolus vulgaris) using different sowing methods and with different techniques of pesticide application. N. Z. J. Crop Hortic. Sci. 2006, 34, 291–298. [Google Scholar] [CrossRef]
- Steel, R.; Torrie, J.H. Bioestadística. Principios y Procedimientos; McGraw Hill Latinomamericana: Bogotá, Colombia, 1985; Volume 2. [Google Scholar]
- Anderson, J.P.; Lichtenzveig, J.; Oliver, R.P.; Singh, K.B. Medicago truncatula as a model host for studying legume infecting Rhizoctonia solani and identification of a locus affecting resistance to root canker. Plant Pathol. 2013, 62, 908–921. [Google Scholar] [CrossRef]
- Icishahayo, D.; Sibiya, J.; Dimbi, S.; Madakadze, I.C.; Manyangarirwa, W.; Chipindu, B.; Tenywa, J.S.; Joubert, G.D.; Marais, D.; Rubaihayo, P.R. Assessment of quality and health of field bean seeds home-saved by smallholder farmers. In Proceedings of the 9th African Crop Science, Conference Proceedings, Cape Town, South Africa, 28 September–2 October 2009; pp. 609–615. [Google Scholar]
- Ahmadzadeh, M.; Sharifi Tehrani, A. Evaluation of fluorescent pseudomonads for plant growth promotion, antifungal activity against Rhizoctonia solani on common bean, and biocontrol potential. Biol. Control 2009, 48, 101–107. [Google Scholar] [CrossRef]
- Mayo, S.; Cominelli, E.; Sparvoli, F.; González-López, O.; Rodríguez-González, A.; Gutiérrez, S.; Casquero, P.A. Development of a qPCR strategy to select bean genes involved in plant defense response and regulated by the Trichoderma velutinum—Rhizoctonia solani interaction. Front. Plant Sci. 2016, 7, 1109. [Google Scholar] [CrossRef]
- Cohen, R.; Burger, Y.; Horev, C.; Saar, U.; Raviv, M. Peat in the inoculation medium induces Fusarium susceptibility in melons. Plant Breed. 2008, 127, 424–428. [Google Scholar] [CrossRef]
- Campelo, M.P.; Casado, R.A.; Lorenzana, A.; Casquero, P.A.; Reinoso, B.; González, A.J. Patogenicidad de aislamientos locales de Botrytis cinerea, Rhizoctonia solani, Sclerotinia sclerotiorum y Trichothecium roseum sobre tres variedades de alubia de León. In Proceedings of the XV Congreso Nacional de la Sociedad Española de Fitopatología, Vitoria, España, 27 September–1 October 2010; p. 181. [Google Scholar]
- Minoshima, H.; Jackson, L.E.; Cavagnaro, T.R.; Sánchez-Moreno, S.; Ferris, H.; Temple, S.R.; Goyal, S.; Mitchell, J.P. Soil food webs and carbon dynamics in response to conservation tillage in California. Soil Sci. Soc. Am. J. 2007, 71, 952–963. [Google Scholar] [CrossRef]
- Harries, E.; Berruezo, L.A.; Galván, M.Z.; Rajal, V.B.; Mercado Cárdenas, G.E. Soil properties related to suppression of Rhizoctonia solani on tobacco fields from northwest Argentina. Plant Pathol. 2020, 69, 77–86. [Google Scholar] [CrossRef]
- Van Agtmaal, M.; Straathof, A.L.; Termorshuizen, A.; Lievens, B.; Hoffland, E.; De Boer, W. Volatile-mediated suppression of plant pathogens is related to soil properties and microbial community composition. Soil Biol. Biochem. 2018, 117, 164–174. [Google Scholar] [CrossRef]
- Watanabe, K.; Matsui, M.; Honjo, H.; Becker, J.O.; Fukui, R. Effects of soil pH on Rhizoctonia damping-off of sugar beet and disease suppression induced by soil amendment with crop residues. Plant Soil 2011, 347, 255–268. [Google Scholar] [CrossRef]
- Anees, M.; Edel-Hermann, V.; Steinberg, C. Build up of patches caused by Rhizoctonia solani. Soil Biol. Biochem. 2010, 42, 1661–1672. [Google Scholar] [CrossRef]
- Otten, W.; Gilligan, C.A. Soil structure and soil-borne diseases: Using epidemiological concepts to scale from fungal spread to plant epidemics. Eur. J. Soil Sci. 2006, 57, 26–37. [Google Scholar] [CrossRef]
- Gill, J.S.; Sivasithamparam, K.; Smettem, K.R.J. Soil types with different texture affects development of Rhizoctonia root rot of wheat seedlings. Plant Soil 2000, 221, 113–120. [Google Scholar] [CrossRef]
- Larena, I.; Melgarejo, P.; De Cal, A. Production, survival, and evaluation of solid-substrate inocula of Penicillium oxalicum, a biocontrol agent against Fusarium wilt of tomato. Phytopathology 2002, 92, 863–869. [Google Scholar] [CrossRef]
- Ghini, R.; Fortes, N.L.P.; Navas-Cortés, J.A.; Silva, C.A.; Bettiol, W. Combined effects of soil biotic and abiotic factors, influenced by sewage sludge incorporation, on the incidence of corn stalk rot. PLoS ONE 2016, 11, e0155536. [Google Scholar] [CrossRef]
- Löbmann, M.T.; Vetukuri, R.R.; de Zinger, L.; Alsanius, B.W.; Grenville-Briggs, L.J.; Walter, A.J. The occurrence of pathogen suppressive soils in Sweden in relation to soil biota, soil properties, and farming practices. Appl. Soil Ecol. 2016, 107, 57–65. [Google Scholar] [CrossRef]
- Baker, R. Mechanisms of biological control of soil-borne pathogens. Annu. Rev. Phytopathol. 1968, 6, 263–294. [Google Scholar] [CrossRef]
- MacNish, G.C. The use of undisturbed soil cores to study methods of controlling Rhizoctonia patch of cereals. Plant Pathol. 1984, 33, 355–359. [Google Scholar] [CrossRef]
| Substrate | Treatments | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| Peat (%) | 100 | - | 95 | - | 98 | - | 93 | - |
| Vermiculite (%) | - | 100 | - | 95 | - | 98 | - | 93 |
| Bentonite (%) | - | - | 5 | 5 | - | - | 5 | 5 |
| Cornmeal (%) | - | - | - | - | 2 | 2 | 2 | 2 |
| Source of Variation | d.f.1 | Germination (%) | Disease Severity | Dry Weight Aerial Part (g) | Dry Weight Root System (g) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 12 | Day 17 | Day 24 | |||||||||||
| Pathogen | 1 | 23,361.122 | *** | 16,673.610 | *** | 18,062.500 | *** | 35.685 | *** | 1.071 | *** | 30.719 | *** |
| Substrate | 1 | 250.001 | 173.611 | 173.611 | 0.928 | 1.200 | *** | 14.306 | *** | ||||
| Additive | 3 | 6601.854 | *** | 6081.019 | *** | 5599.536 | *** | 5.871 | *** | 0.234 | *** | 21.382 | *** |
| Pathogen X Substrate | 1 | 1361.112 | 3673.613 | * | 3062.502 | * | 0.243 | 0.051 | 0.026 | ||||
| Pathogen X Additive | 3 | 1601.852 | 729.167 | 1081.019 | 0.077 | 0.029 | * | 1.683 | |||||
| Substrate X Additive | 3 | 490.741 | 858.796 | 673.611 | 0.487 | 0.078 | 5.571 | *** | |||||
| Pathogen X Substrate X Additive | 3 | 1416.668 | 469.907 | 710.648 | 1.459 | 0.040 | 3.713 | ** | |||||
| Source of Variation 1 | d.f.2 | Germination (%) | Disease Severity | Dry Weight of Aerial Part (g) | Dry Weight of Root System (g) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Control vs. (B + C + BC) | 1 | <0.0001 | *** | <0.0001 | *** | 0.0020 | *** | <0.0001 | *** |
| BC vs. (B + C) | 1 | 0.3115 | 0.0797 | 0.2310 | 0.0013 | *** | |||
| B vs. C | 1 | 0.3855 | 0.7853 | 0.0009 | *** | 0.5666 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mayo-Prieto, S.; Rodríguez-González, Á.; Lorenzana, A.; Gutiérrez, S.; Casquero, P.A. Influence of Substrates in the Development of Bean and in Pathogenicity of Rhizoctonia solani JG Kühn. Agronomy 2020, 10, 707. https://doi.org/10.3390/agronomy10050707
Mayo-Prieto S, Rodríguez-González Á, Lorenzana A, Gutiérrez S, Casquero PA. Influence of Substrates in the Development of Bean and in Pathogenicity of Rhizoctonia solani JG Kühn. Agronomy. 2020; 10(5):707. https://doi.org/10.3390/agronomy10050707
Chicago/Turabian StyleMayo-Prieto, Sara, Álvaro Rodríguez-González, Alicia Lorenzana, Santiago Gutiérrez, and Pedro A. Casquero. 2020. "Influence of Substrates in the Development of Bean and in Pathogenicity of Rhizoctonia solani JG Kühn" Agronomy 10, no. 5: 707. https://doi.org/10.3390/agronomy10050707
APA StyleMayo-Prieto, S., Rodríguez-González, Á., Lorenzana, A., Gutiérrez, S., & Casquero, P. A. (2020). Influence of Substrates in the Development of Bean and in Pathogenicity of Rhizoctonia solani JG Kühn. Agronomy, 10(5), 707. https://doi.org/10.3390/agronomy10050707








