A Phenotypic Search on Graft Compatibility in Grapevine
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Details
2.2. Growth Parameters
2.3. Internal Characterization of the Union
2.4. Affinity Coefficients (ACs)
2.5. Chlorophyll Fluorescence and Pigments Content
2.6. Statistical Analysis
3. Results
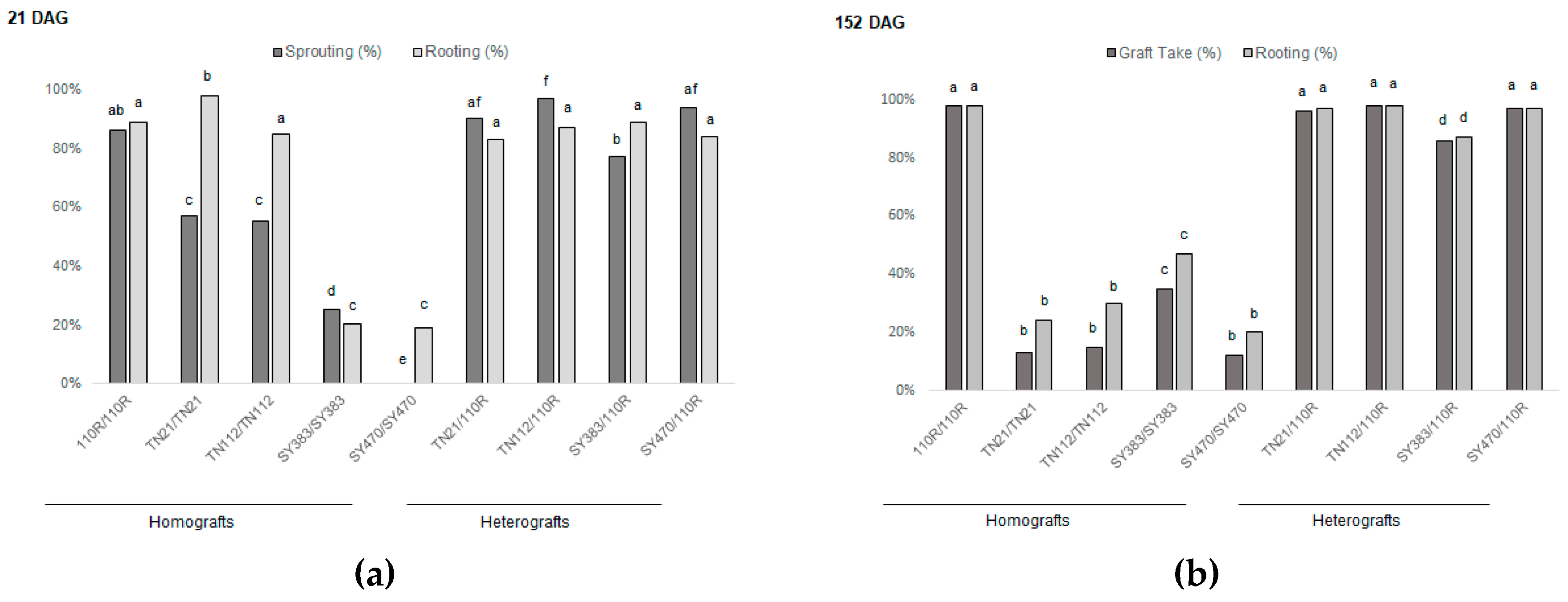
3.1. Grafting onto 110R Rootstock Leads to Higher Graft Take Rates
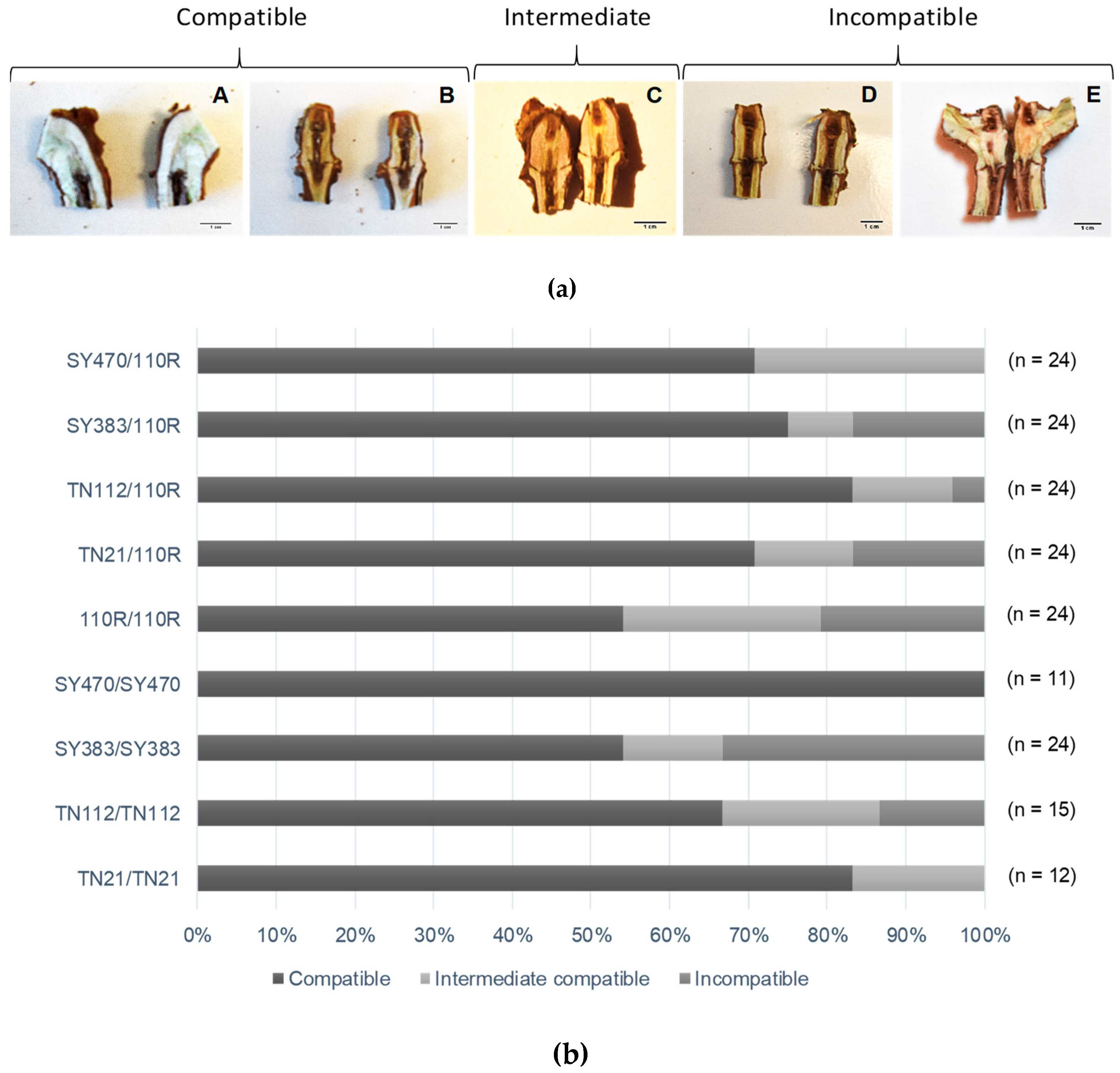
3.2. Graft Unions Are Frequently Incomplete at Five Months after Grafting
3.3. Affinity Coefficients (ACs) Calculated for the Same Graft Combination Vary According to the Formula Used
3.4. Chlorophylls Analysis Is a More Sensitive Indicator of Stress than the Analysis of Chlorophyll Fluorescence
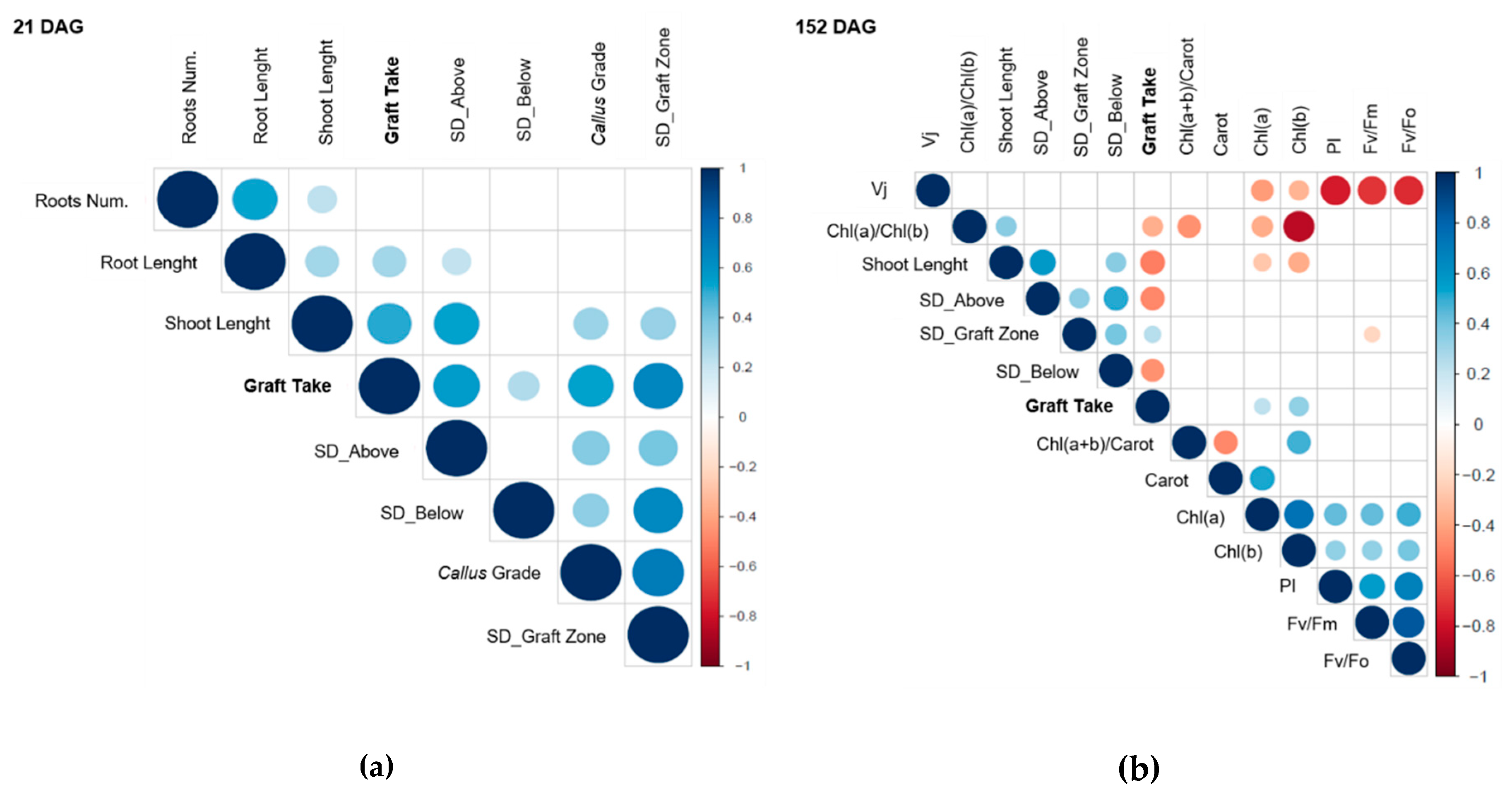
3.5. Graft Take Correlates with Callus Formation and with the Improvement of Scion–Rootstock Translocation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, J.; Kubota, C.; Tsao, S.J.; Bie, Z.; Echevarria, P.H.; Morra, L.; Oda, M. Scientia horticulturae current status of vegetable grafting: Diffusion, grafting techniques, automation. Sci. Hortic. (Amst.) 2010, 127, 93–105. [Google Scholar] [CrossRef]
- Ollat, N.; Bordenave, L.; Tandonnet, J.P.; Boursiquot, J.M.; Marguerit, E. Grapevine rootstocks: Origins and perspectives. Acta Hortic. 2016, 1136, 11–22. [Google Scholar] [CrossRef]
- Pina, A.; Cookson, S.; Calatayud, A.; Trinchera, A.; Errea, P. Chapter 5—Physiological and molecular mechanisms underlying graft compatibility. In Vegetable Grafting Principles and Practices; Colla, G., Perez-Alfocea, F., Schwarz, D., Eds.; CABI Oxfordshire: Wallingford, UK, 2017; pp. 132–154. ISBN 13 978 1 78639 058 5. [Google Scholar]
- Waite, H.; Whitelaw-Weckert, M.; Torley, P. Grapevine propagation: Principles and methods for the production of high-quality grapevine planting material. N. Zeal. J. Crop. Hortic. Sci. 2015, 43, 144–161. [Google Scholar] [CrossRef]
- Assunção, M.; Santos, C.; Brazão, J.; Eiras-Dias, J.E.; Fevereiro, P. Understanding the molecular mechanisms underlying graft success in grapevine. BMC Plant Biol. 2019, 19, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Pina, A.; Errea, P. A review of new advances in mechanism of graft compatibility-incompatibility. Sci. Hortic. (Amst.) 2005, 106, 1–11. [Google Scholar] [CrossRef]
- Hartman, H.T.; Kester, D.E.; Davies, F.T.; Geneve, R.G. Principles of grafting and budding. In Hartmann and Kester’s Plant Propagation: Principles and Practices; Prentice Hall: Upper Saddle River, NJ, USA, 2011; pp. 415–463. [Google Scholar]
- Bianchi, D.; Grossi, D.; Simone Di Lorenzo, G.; Zi Ying, Y.; Rustioni, L.; Brancadoro, L. Phenotyping of the “G series” Vitis hybrids: First screening of the mineral composition. Sci. Hortic. (Amst.) 2020, 264, 109155. [Google Scholar] [CrossRef]
- Bianchi, D.; Grossi, D.; Tincani, D.T.G.; Simone Di Lorenzo, G.; Brancadoro, L.; Rustioni, L. Multi-parameter characterization of water stress tolerance in Vitis hybrids for new rootstock selection. Plant Physiol. Biochem. 2018, 132, 333–340. [Google Scholar] [CrossRef]
- Migliaro, D.; De Lorenzis, G.; Di Lorenzo, G.S.; De Nardi, B.; Gardiman, M.; Failla, O.; Brancadoro, L.; Crespan, M. Grapevine non-vinifera genetic diversity assessed by simple sequence repeat markers as a starting point for new rootstock breeding programs. Am. J. Enol. Vitic. 2019, 70, 390–397. [Google Scholar] [CrossRef]
- Vršič, S.; Pulko, B.; Kocsis, L. Effects of rootstock genotypes on compatibility, biomass, and the yield of Welschriesling. Hortic. Sci. 2016, 43, 92–99. [Google Scholar] [CrossRef]
- Töpfer, R.; Hausmann, L.; Harst, M.; Maul, E.; Zyprian, E.; Eibach, R. New horizons for grapevine breeding. In Methods in Temperate Fruit Breeding; Flachowsky, H., Hanke, M.V., Eds.; Vegetable and cereal science and biotechnology; Global Science Books Ltd., UK: Kagawa, Japan, 2011; pp. 79–100. ISBN 978-4-903313-75-7. [Google Scholar]
- Çelik, Ü. The effects of different grafting methods applied by manual grafting units on grafting success in grapevines. Turk. J. Agric. For. 2000, 24, 499–504. [Google Scholar]
- Gargin, S.; Altindisli, A. A Research on the affinity coefficients of Red Globe grape variety with 140 R, 41 B rootstocks. BIO Web Conf. 2014, 3. [Google Scholar] [CrossRef]
- Vršič, S.; Pulko, B.; Kocsis, L. Factors influencing grafting success and compatibility of grape rootstocks. Sci. Hortic. (Amst.) 2015, 181, 168–173. [Google Scholar] [CrossRef]
- Bahar, E.; Korkutal, I. Using magnetic resonance imaging technique (MRI) to investigate graft connection and its relation to reddening discoloration in grape leaves. JFAE 2010, 8, 293–297. [Google Scholar]
- Milien, M.; Renault-Spilmont, A.S.; Cookson, S.J.; Sarrazin, A.; Verdeil, J.L. Visualization of the 3D structure of the graft union of grapevine using X-ray tomography. Sci. Hortic. (Amst.) 2012, 144, 130–140. [Google Scholar] [CrossRef]
- Assunção, M.; Canas, S.; Cruz, S.; Brazão, J.; Zanol, G.C.; Eiras-Dias, J.E. Graft compatibility of Vitis spp.: The role of phenolic acids and flavanols. Sci. Hortic. (Amst.) 2016, 207, 140–145. [Google Scholar] [CrossRef]
- Assunção, M.; Pinheiro, J.; Cruz, S.; Brazão, J.; Queiroz, J.; Eduardo, J.; Dias, E.; Canas, S. Scientia Horticulturae Gallic acid, sinapic acid and catechin as potential chemical markers of Vitis graft success. Sci. Hortic. 2019, 246, 129–135. [Google Scholar] [CrossRef]
- Gökbayrak, Z.; Söylemezoǧlu, G.; Akkurt, M.; Çelik, H. Determination of grafting compatibility of grapevine with electrophoretic methods. Sci. Hortic. (Amst.) 2007, 113, 343–352. [Google Scholar] [CrossRef]
- Cookson, S.; Ollat, N. Grafting with rootstocks induces extensive transcriptional re-programming in the shoot apical meristem of grapevine. BMC Plant Biol. 2013, 13, 147. [Google Scholar] [CrossRef]
- Cookson, S.J.; Clemente Moreno, M.J.; Hevin, C.; Nyamba Mendome, L.Z.; Delrot, S.; Magnin, N.; Trossat-Magnin, C.; Ollat, N. Heterografting with nonself rootstocks induces genes involved in stress responses at the graft interface when compared with autografted controls. J. Exp. Bot. 2014, 65, 2473–2481. [Google Scholar] [CrossRef]
- Renault-Spilmont, A.S.; Grenan, S.; Bousiquot, J.M. Syrah decline. Progrés Agric. Vitic. 2005, 122, 15–16. [Google Scholar]
- Catalogue of Vines Grown in France. Available online: http://plantgrape.plantnet-project.org/en (accessed on 11 March 2020).
- Metereological Dafa for Évora Weather Station. Available online: https://www.meteoblue.com/pt/tempo/archive/export/montemor-o-novo_portugal_2265888 (accessed on 1 October 2018).
- Bouquet, A. Differences observed in the graft compatibility between some cultivars of Muscadine grape (Vitis rotundifolia Michx.) and European grape (Vitis vinifera L. cv. Cabernet Sauvignon). Vitis 1980, 19, 99–104. [Google Scholar]
- Herrero, J. Studies of compatible and incompatible graft combinations with special reference to hardy fruit trees. J. Hortic. Sci. 1951, 26, 186–237. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. ChlorolShylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, 1–8. [Google Scholar] [CrossRef]
- De Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research, R package version 1.3–2; 2020; Available online: https://cran.r-project.org/web/packages/agricolae/agricolae.pdf (accessed on 13 May 2020).
- Harrell, F.E., Jr. Hmisc: Harrell Miscellaneous, R package version 4.4–0; 2020; Available online: https://cran.r-project.org/web/packages/Hmisc/Hmisc.pdf (accessed on 13 May 2020).
- Wei, T.; Simko, V. Visualization of a Correlation Matrix; R package “corrplot” Version 0.84; 2017; Available online: https://cran.r-project.org/web/packages/corrplot/corrplot.pdf (accessed on 13 May 2020).
- Basheer-Salimia, R.; Hamdan, A.J. Grapevine scion-rootstock combinations of palestinian local cultivars and rootstocks resistant to grape phylloxera daktulosphaira vitifoliae (fitch) [phylloxeridae: homoptera]. Dirasat Agric. Sci. 2009, 36, 19–28. [Google Scholar]
- Hamdan, A.S.; Basheer-Salimia, R. Preliminary compatibility between some table-grapevine scion and phylloxera-resistant rootstock cultivars. Jordan J. Agric. Sci. 2010, 6, 1–10. [Google Scholar]
- Robinson, J. The Oxford Companion to Wine, 4th ed.; Oxford University Press: Oxford, UK, 2015; ISBN 9780198705383. [Google Scholar]
- Irisarri, P.; Pina, A.; Errea, P. Evaluación del comportamiento vegetativo y compatibilidad de injerto de variedades de peral sobre los patrones ‘BA-29’ y ‘OHF-87’. ITEA Inf. Tec. Econ. Agrar. 2016, 112, 243–254. [Google Scholar] [CrossRef]
- Moreno, M.A.; Tabuenca, M.C.; Cambra, R.; Adara, A. Plum rootstock for cherries and other stone fruit species. HortScience 1995, 30, 1316–1317. [Google Scholar] [CrossRef]
- Zarrouk, O.; Gogorcena, Y.; Moreno, M.A.; Pinochet, J. Graft compatibility between peach cultivars and Prunus rootstocks. HortScience 2006, 41, 1389–1394. [Google Scholar] [CrossRef]
- Ermel, F.F.; Catesson, J.K.A.M.; Poëssel, J.L. Localized graft incompatibility in pear/quince (Pyrus communis/Cydonia oblonga) combinations: Multivariate analysis of histological data from 5-month-old grafts. Tree Physiol. 1999, 19, 645–654. [Google Scholar] [CrossRef]
- Pereira, I.S.I.A.L.; Picolotto, L.; Fachinello, J.C. Incompatibilidade de enxertia induz aumento da suscetibilidade de cultivares de pessegueiro à Xanthomonas arboricola pv. Pruni. Cienc. Rural 2015, 45, 1147–1153. [Google Scholar] [CrossRef][Green Version]
- Pereira, I.D.S.; Antunes, L.E.C.; Picolotto, L.; Fachinello, J.C. Revista brasileira de engenharia agrícola e ambiental fluorescence of chlorophyll a and photosynthetic pigments in atriplex nummularia under abiotic stresses fluorescência da clorofila a e pigmentos fotossintéticos em atriplex nummularia sob estresses abi. Cienc. Rural 2017, 21, 232–237. [Google Scholar]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Chapter 12—Analysis of the chlorophyll a fluorescence transient. In Chlorophyll A Fluorescence: A Signature of Photosynthesis; Springer: Dordrecht, The Netherlands, 2004; pp. 321–322. [Google Scholar]
- Pavlović, D.; Nikolić, B.; Đurović, S.; Waisi, H.; Anđelković, A. Chlorophyll as a measure of plant health: Agroecological aspects. Pestic. Phytomed. (Belgrade) 2014, 29, 21–34. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Davis, A.R.; Perkins-veazie, P.; Box, P.O.; West, H.; Levi, A.; King, S.R. Grafting effects on vegetable quality. Hortscience 2008, 43, 1670–1672. [Google Scholar] [CrossRef]
- Warschefsky, E.J.; Klein, L.L.; Frank, M.H.; Chitwood, D.H.; Londo, J.P.; von Wettberg, E.J.B.; Miller, A.J. Rootstocks: Diversity, domestication, and impacts on shoot phenotypes. Trends Plant Sci. 2016, 21, 418–437. [Google Scholar] [CrossRef]
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef]
- Calatayud, Á.; San, A.; Pascual, B.; Vicente, J.; López-galarza, S. Scientia horticulturae use of chlorophyll fluorescence imaging as diagnostic technique to predict compatibility in melon graft. Sci. Hortic. (Amst.) 2013, 149, 13–18. [Google Scholar] [CrossRef]
- Ito, H.; Tatsuyuki, O.; Tanaka, A. Conversion of Chlorophyll b to Chlorophyll a via 7-Hydroxymethyl Chlorophyll. J. Biol. Chem. 1996, 271, 1475–1479. [Google Scholar] [CrossRef]
- Rustioni, L.; Grossi, D.; Brancadoro, L.; Failla, O. Iron, magnesium, nitrogen and potassium deficiency symptom discrimination by reflectance spectroscopy in grapevine leaves. Sci. Hortic. (Amst.) 2018, 241, 152–159. [Google Scholar] [CrossRef]
- Pina, A.; Errea, P.; Martens, H.J. Graft union formation and cell-to-cell communication via plasmodesmata in compatible and incompatible stem unions of Prunus spp. Sci. Hortic. (Amst.) 2012, 143, 144–150. [Google Scholar] [CrossRef]
- Andrews, P.K.; Serrano Marquez, C. Volume 15—Graft incompatibility. In Horticultural Reviews; Janick, J., Ed.; John Wiley & Sons, Inc.: Oxford, UK, 1993; pp. 183–232. ISBN 978-0-471-57338-8. [Google Scholar]
- Pereira, I.D.S.; Antunes, L.E.C.; Picolotto, L.; Fachinello, J.C. Incompatibilidade de enxertia em Prunus Graft incompatibility in Prunus. Cienc. Rural 2014, 449, 1519–1526. [Google Scholar] [CrossRef][Green Version]
- Shtein, I.; Hayat, Y.; Munitz, S.; Harcavi, E.; Akerman, M.; Drori, E.; Schwartz, A.; Netzer, Y. From structural constraints to hydraulic function in three Vitis rootstocks. Trees-Struct. Funct. 2017, 31, 851–861. [Google Scholar] [CrossRef]
| 21 DAG | 152 DAG | ||||
|---|---|---|---|---|---|
| Callus Grade (0–4) | Roots Number | Root Length (cm) | Shoot Length (cm) | Shoot Length (cm) | |
| Graft Combination | *** | *** | *** | *** | *** |
| 110R/110R | 3.9 ± 0.1 abc | 4.1 ± 0.6 a | 1.6 ± 0.3 a | 4.3 ± 0.6 a | 78 ± 6.2 cd |
| TN21/TN21 | 3.0 ± 0.3 d | 5.3 ± 0.9 a | 1.6 ± 0.2 a | 1.9 ± 0.5 bcd | 95 ± 7.4 bc |
| TN112/TN112 | 1.5 ± 0.2 e | 5.7 ± 1.2 a | 1.1 ± 0.2 a | 0.9 ± 0.3 cde | 127 ± 8.2 ab |
| SY383/SY383 | 3.4 ± 0.2 bcd | 0.5 ± 0.2 b | 0.2 ± 0.1 b | 0.4 ± 0.1 de | 78 ± 3.8 cd |
| SY470/SY470 | 3.3 ± 0.3 cd | 0.8 ± 0.3 b | 0.3 ± 0.2 b | 0.0 ± 0.0 e | 139 ± 9.0 a |
| TN21/110R | 4.0 ± 0.0 a | 3.0 ± 0.5 a | 1.3 ± 0.3 a | 2.9 ± 0.4 ab | 80 ± 7.7 cd |
| TN112/110R | 4.0 ± 0.0 ab | 4.1 ± 0.8 a | 1.8 ± 0.4 a | 3.5 ± 0.4 a | 63 ± 4.1 d |
| SY383/110R | 3.9 ± 0.1 abc | 3.8 ± 0.7 a | 1.9 ± 0.3 a | 2.1 ± 0.4 abc | 66 ± 3.9 d |
| SY470/110R | 4.0 ± 0.0 a | 3.9 ± 0.6 a | 1.8 ± 0.3 a | 4.3 ± 0.5 a | 78 ± 4.6 cd |
| Graft Type | *** | ns | ns | *** | *** |
| Homograft | 3.0 ± 0.1 a | 3.3 ± 0.4 | 1.0 ± 0.1 | 1.5 ± 0.2 a | 97 ± 3.9 a |
| Heterograft | 4.0 ± 0.0 b | 3.7 ± 0.3 | 1.7 ± 0.1 | 3.2 ± 0.2 b | 72 ± 2.7 b |
| 21 DAG | 152 DAG | |||||
|---|---|---|---|---|---|---|
| SD Above | SD Graft Union | SD Below | SD Above | SD Graft Union | SD Below | |
| Graft Combination | *** | *** | ** | *** | *** | *** |
| 110R/110R | 3.21 ± 0.18 ab | 14.26 ± 0.35 a | 9.47 ± 0.29 ab | 5.08 ± 0.37 c | 18.18 ± 0.88 ab | 10.86 ± 0.44 bc |
| TN21/TN21 | 1.55 ± 0.32 cd | 10.94 ± 0.38 cd | 8.82 ± 0.17 ab | 6.88 ± 0.48 abc | 15.67 ± 0.68 bc | 11.01 ± 0.46 abc |
| TN112/TN112 | 1.46 ± 0.41 bcd | 9.38 ± 0.29 d | 8.42 ± 0.15 b | 6.77 ± 0.40 bc | 13.88 ± 1.04 c | 11.45 ± 0.47 abc |
| SY383/SY383 | 1.46 ± 0.46 bcd | 12.01 ± 0.49 bc | 9.70 ± 0.29 a | 7.59 ± 0.40 ab | 17.80 ± 0.51 ab | 12.90 ± 0.44 ab |
| SY470/SY470 | 0.00 ± 0.00 d | 11.20 ± 0.44 cd | 11.20 ± 0.44 ab | 10.61 ± 0.90 a | 18.98 ± 0.79 ab | 14.0 ± 0.72 a |
| TN21/110R | 3.55 ± 0.27 a | 13.9 ± 0.27 a | 13.90 ± 0.27 a | 5.33 ± 0.33 c | 17.99 ± 0.62 ab | 10.24 ± 0.37 c |
| TN112/110R | 4.25 ± 0.30 a | 13.85 ± 0.34 a | 13.85 ± 0.34 ab | 5.39 ± 0.33 c | 17.58 ± 0.63 abc | 10.14 ± 0.46 c |
| SY383/110R | 3.26 ± 0.48 a | 13.31 ± 0.29 ab | 13.31 ± 0.29 a | 5.91 ± 0.35 c | 18.05 ± 0.68 ab | 10.30 ± 0.30 c |
| SY470/110R | 2.85 ± 0.28 abc | 14.09 ± 0.29 a | 14.09 ± 0.29 ab | 6.20 ± 0.28 bc | 19.28 ± 0.52 a | 10.17 ± 0.39 c |
| Graft Type | *** | *** | ** | *** | * | *** |
| Homograft | 1.54 ± 0.17 a | 11.56 ± 0.23 a | 9.05 ± 0.11 a | 7.18 ± 0.28 a | 16.99 ± 0.4 a | 12.04 ± 0.25 a |
| Heterograft | 3.47 ± 0.18 b | 13.79 ± 0.15 b | 9.56 ± 0.14 b | 5.7 ± 0.16 b | 18.23 ± 0.31 b | 10.21 ± 0.19 b |
| Chl(a) (mg/cm2) | Chl(b) (mg/cm2) | Carot (mg/cm2) | Chl(a)/Chl(b) | Chl(a+b)/Carot | |
|---|---|---|---|---|---|
| Graft Combination | ** | *** | ns | *** | *** |
| 110R/110R | 0.023 ± 0.001 a | 0.01 ± 0.001 ab | 0.004 ± 0.000 | 2.6 ± 0.1 a | 9.5 ± 0.9 ab |
| TN21/TN21 | 0.019 ± 0.001 ab | 0.007 ± 0.001 bc | 0.005 ± 0.000 | 2.7 ± 0.1 ab | 5.4 ± 0.2 cd |
| TN112/TN112 | 0.019 ± 0.001 ab | 0.008 ± 0.001 bc | 0.004 ± 0.000 | 2.6 ± 0.1 ab | 6.3 ± 0.5 bcd |
| SY383/SY383 | 0.022 ± 0.001 a | 0.011 ± 0.001 ab | 0.004 ± 0.000 | 2.4 ± 0.1 a | 10.2 ± 3.0 abc |
| SY470/SY470 | 0.016 ± 0.001 b | 0.005 ± 0.000 c | 0.004 ± 0.000 | 2.9 ± 0.1 b | 5.1 ± 0.1 d |
| TN21/110R | 0.022 ± 0.001 a | 0.011 ± 0.001 ab | 0.004 ± 0.000 | 2.1 ± 0.1 a | 8.2 ± 1 abc |
| TN112/110R | 0.022 ± 0.001 a | 0.014 ± 0.001 a | 0.004 ± 0.000 | 1.7 ± 0.1 a | 14.7 ± 4.0 a |
| SY383/110R | 0.022 ± 0.001 a | 0.011 ± 0.001 ab | 0.005 ± 0.000 | 2.1 ± 0.1 a | 7.0 ± 0.7 bcd |
| SY470/110R | 0.020 ± 0.001 ab | 0.009 ± 0.001 ab | 0.004 ± 0.000 | 2.3 ± 0.1 ab | 7.4 ± 0.6 abcd |
| Graft Type | ns | *** | ns | *** | ns |
| Homograft | 0.020 ± 0.001 | 0.009 ± 0.000 a | 0.004 ± 0.000 | 2.6 ± 0.1 a | 8.0 ± 0.9 |
| Heterograft | 0.021 ± 0.001 | 0.011 ± 0.000 b | 0.004 ± 0.000 | 2.1 ± 0.1 b | 9.3 ± 1.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tedesco, S.; Pina, A.; Fevereiro, P.; Kragler, F. A Phenotypic Search on Graft Compatibility in Grapevine. Agronomy 2020, 10, 706. https://doi.org/10.3390/agronomy10050706
Tedesco S, Pina A, Fevereiro P, Kragler F. A Phenotypic Search on Graft Compatibility in Grapevine. Agronomy. 2020; 10(5):706. https://doi.org/10.3390/agronomy10050706
Chicago/Turabian StyleTedesco, Sara, Ana Pina, Pedro Fevereiro, and Friedrich Kragler. 2020. "A Phenotypic Search on Graft Compatibility in Grapevine" Agronomy 10, no. 5: 706. https://doi.org/10.3390/agronomy10050706
APA StyleTedesco, S., Pina, A., Fevereiro, P., & Kragler, F. (2020). A Phenotypic Search on Graft Compatibility in Grapevine. Agronomy, 10(5), 706. https://doi.org/10.3390/agronomy10050706





