The Impact of Priming with Al2O3 Nanoparticles on Growth, Pigments, Osmolytes, and Antioxidant Enzymes of Egyptian Roselle (Hibiscus sabdariffa L.) Cultivar
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Al2O3 Nanoparticles
2.2. Plant Growth Conditions
- The 1st (control) set was priming with distilled water for 12 h.
- The 2nd set was primed with 0.01% Al2O3 NPs for 12 h.
- The 3rd set was primed with 0.05% Al2O3 NPs for 12 h.
- The 4th set was primed with 0.1% Al2O3 NPs for 12 h.
- The 5th set was primed with 0.5% Al2O3 NPs for 12 h.
2.3. Growth Traits
2.4. Photosynthetic Pigments
2.5. Organic Solutes (Soluble Sugar, Soluble Protein, Total Free Amino Acids, and Proline)
2.6. Malondialdehyde (MDA)
2.7. Assays of Antioxidant Enzyme Activities
2.8. Statistical Analysis
3. Results
3.1. Effect of Priming Plants with Different Al2O3 NPs Doses on Growth Traits
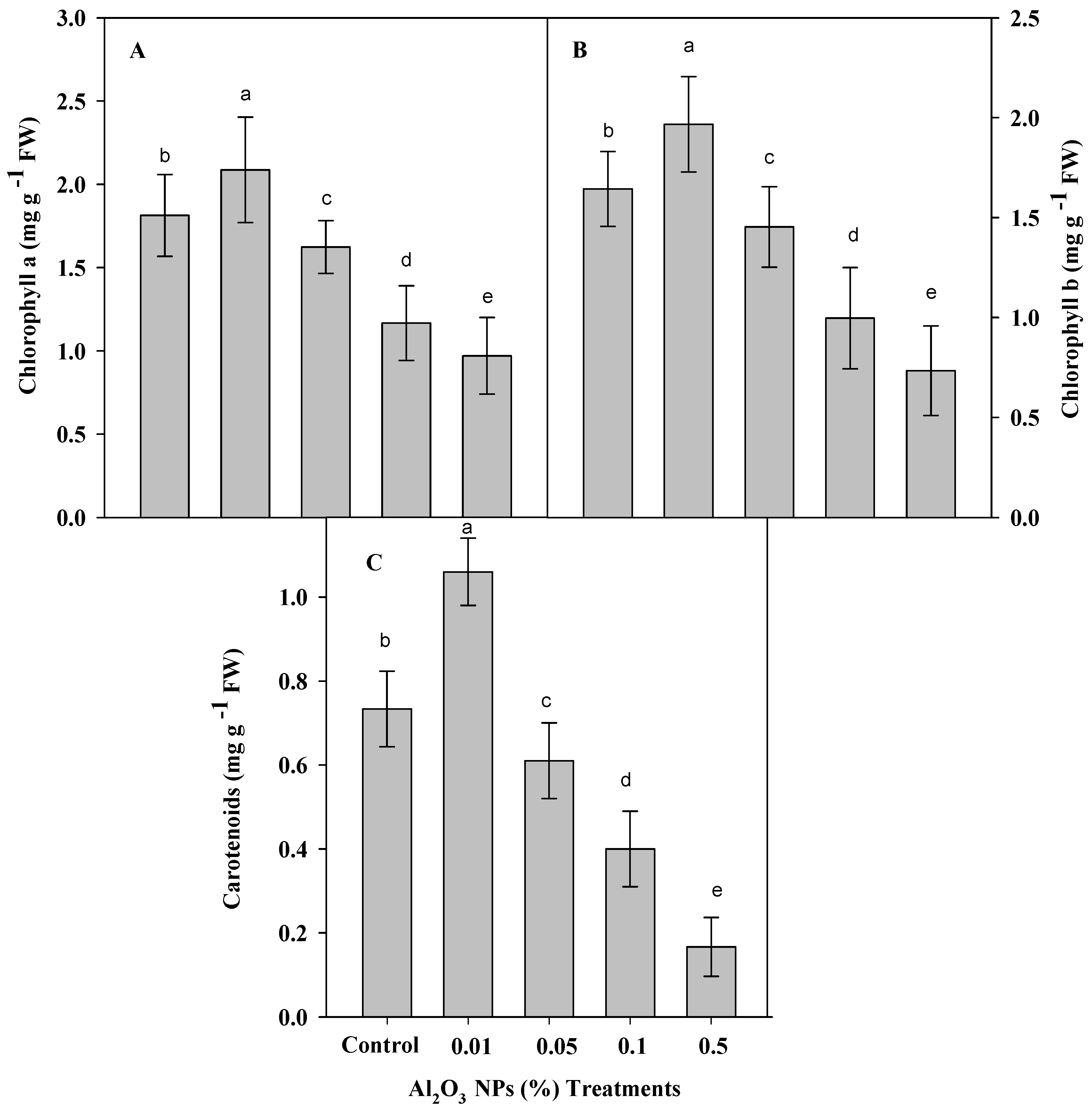
3.2. Effect of Priming Plants by Al2O3 NPs on Contents of Chlorophyll a, b and Carotenoids
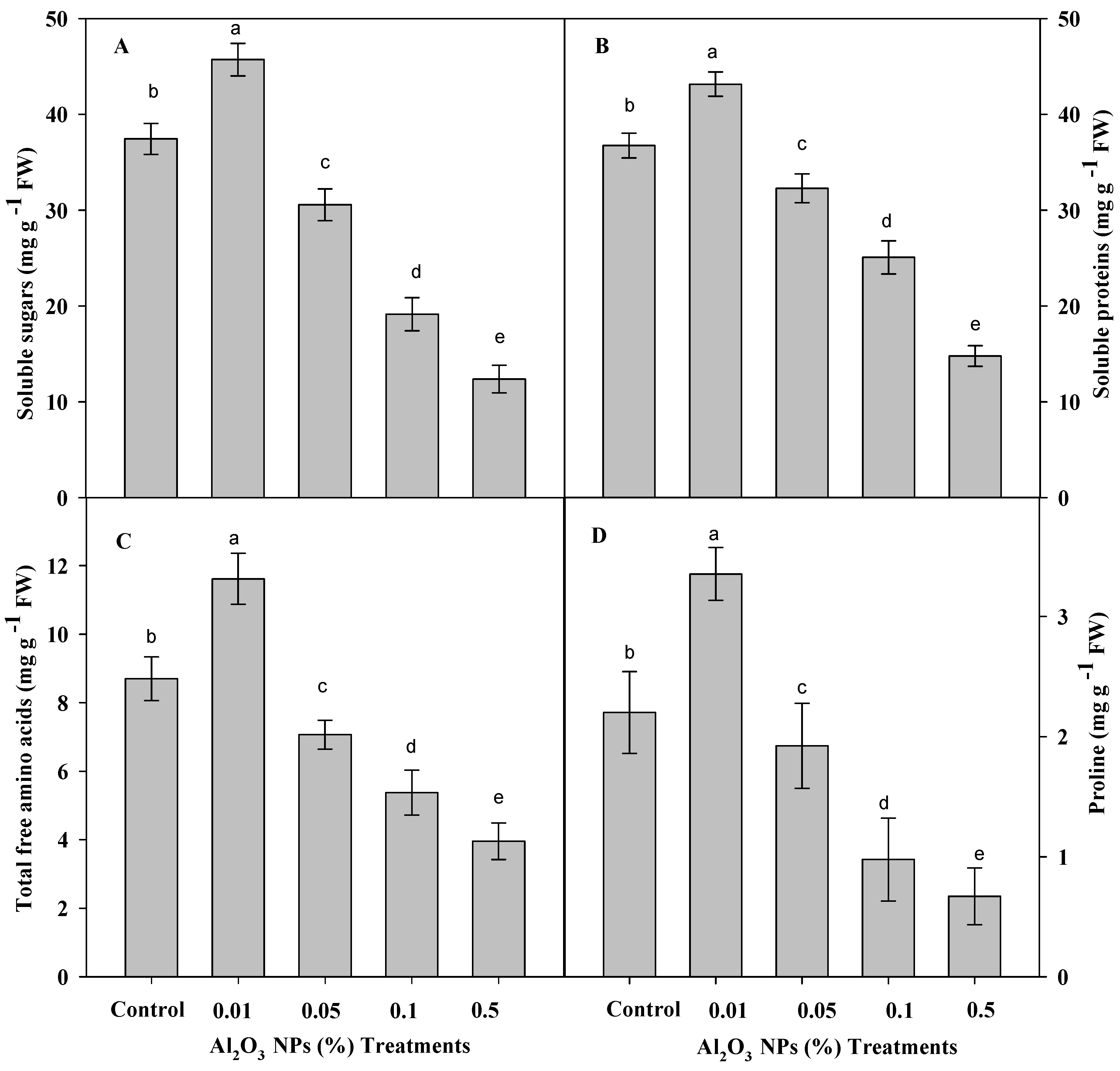
3.3. Effect of Priming with Graded Levels of Al2O3 NPs on Organic Solutes
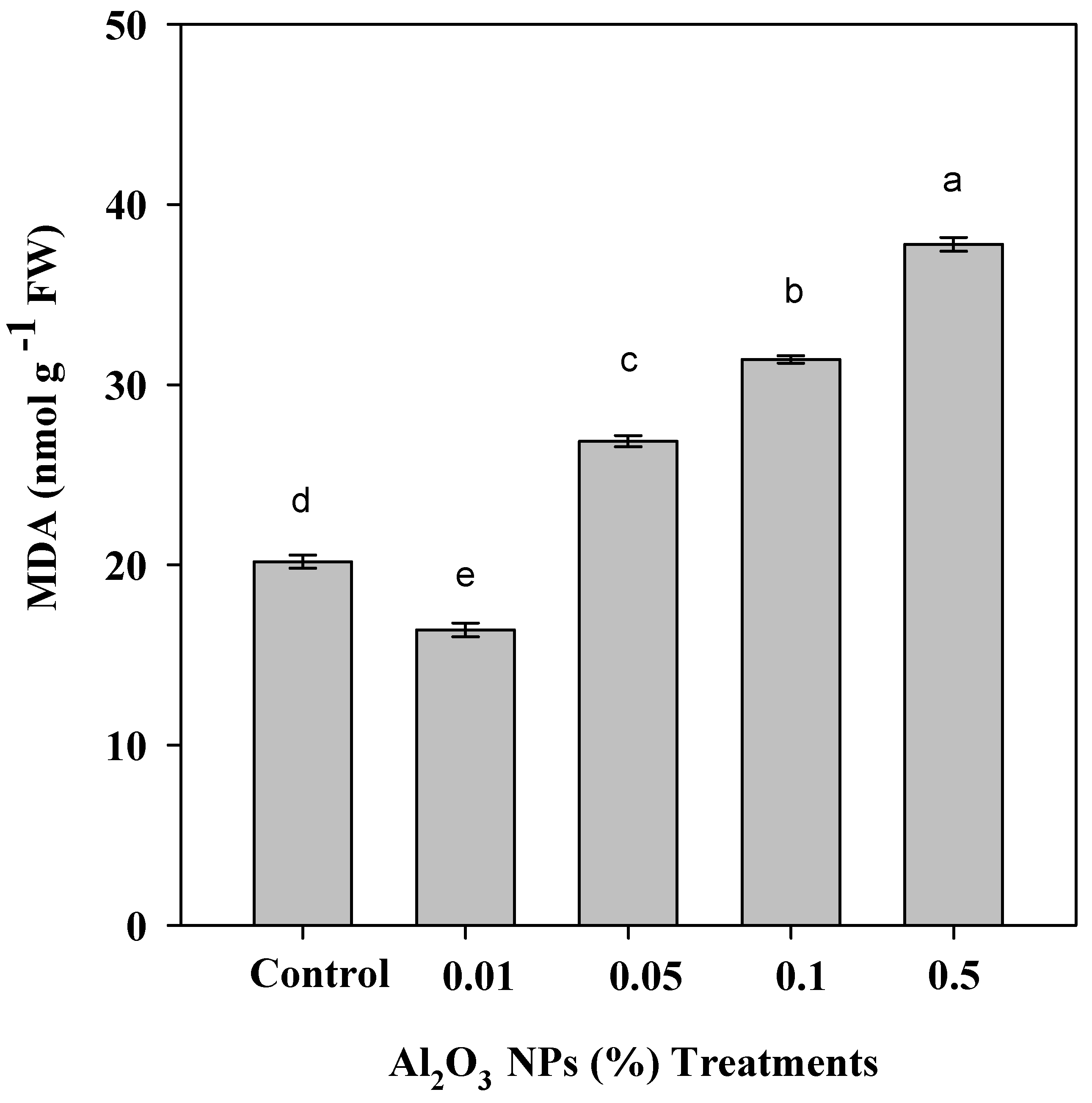
3.4. Effect of Priming with Al2O3 NPs Doses of Roselle Plants on MDA
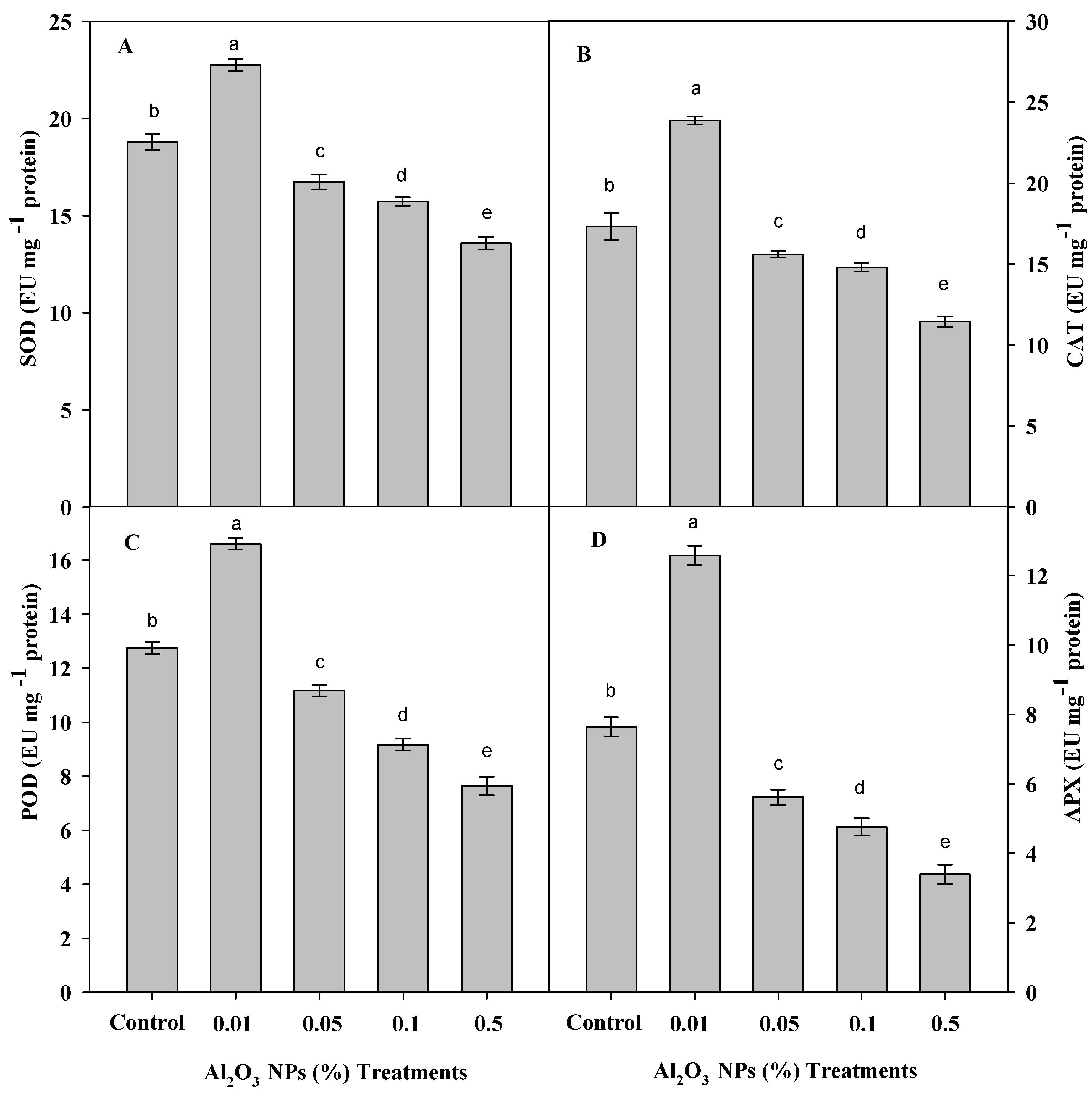
3.5. Effect of Al2O3 NPs Priming on Antioxidant Enzymes of Roselle Plants
3.6. Understanding Interactions between Various Doses of Al2O3 NPs and Variables Through PCA-based Clustering Approach
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Siddiqui, M.H.; Al-Whaibi, M.H.; Firoz, M.; Al-Khaishany, M.Y. Role of Nanoparticles in Plants. In Nanotechnology and Plant Sciences; Springer Science and Business Media LLC: Berlin, Germany, 2015; pp. 19–35. [Google Scholar]
- Hatami, M.; Kariman, K.; Ghorbanpour, M. Engineered nanomaterial-mediated changes in the metabolism of terrestrial plants. Sci. Total. Environ. 2016, 571, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.N.; Mobin, M.; Abbas, Z.K.; Almutairi, K.A.; Siddiqui, Z.H. Role of nanomaterials in plants under challenging environments. Plant Physiol. Biochem. 2017, 110, 194–209. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, D.K.; Shweta, S.; Singh, S.; Pandey, R.; Singh, D.P.; Sharma, N.C.; Prasad, S.M.; Dubey, N.K.; Chauhan, D.K. An overview on manufactured nanoparticles in plants: Uptake, translocation, accumulation and phytotoxicity. Plant Physiol. Biochem. 2017, 110, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, D.K.; Ahmad, P.; Sharma, S.; Chauhan, D.K.; Dubey, N.K. (Eds.) Nanomaterials in Plants, Algae, and Microorganisms: Concepts and Controversies; Academic Press: New York, NY, USA, 2017. [Google Scholar]
- Rizwan, M.; Ali, S.; Qayyum, M.F.; Ok, Y.S.; Adrees, M.; Ibrahim, M.; Zia-Ur-Rehman, M.; Farid, M.; Abbas, F. Effect of metal and metal oxide nanoparticles on growth and physiology of globally important food crops: A critical review. J. Hazard. Mater. 2017, 322, 2–16. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Ali, B.; Adrees, M.; Arshad, M.; Hussain, A.; Rehman, M.Z.U.; Waris, A.A. Zinc and iron oxide nanoparticles improved the plant growth and reduced the oxidative stress and cadmium concentration in wheat. Chemosphere 2018, 214, 269–277. [Google Scholar] [CrossRef]
- Ahmed, B.; Khan, M.S.; Musarrat, J. Toxicity assessment of metal oxide nano-pollutants on tomato (Solanum lycopersicon): A study on growth dynamics and plant cell death. Environ. Pollut. 2018, 240, 802–816. [Google Scholar] [CrossRef]
- Khan, S.T.; Malik, A. Engineered nanomaterials for water decontamination and purification: From lab to products. J. Hazard. Mater. 2019, 363, 295–308. [Google Scholar] [CrossRef]
- Ahmad, B.; Zaid, A.; Jaleel, H.; Khan, M.M.A.; Ghorbanpour, M. Nanotechnology for Phytoremediation of Heavy Metals: Mechanisms of Nanomaterial-Mediated Alleviation of Toxic Metals. In Advances in Phytonanotechnology; Elsevier BV: Amsterdam, The Netherlands, 2019; pp. 315–327. [Google Scholar]
- Ottoni, C.A.; Neto, M.L.; Léo, P.; Ortolan, B.; Barbieri, E.; De Souza, A.O. Environmental impact of biogenic silver nanoparticles in soil and aquatic organisms. Chemosphere 2020, 239, 124698. [Google Scholar] [CrossRef]
- Corsi, I.; Winther-Nielsen, M.; Sethi, R.; Punta, C.; Della Torre, C.; Libralato, G.; Lofrano, G.; Sabatini, L.; Aiello, M.; Fiordi, L.; et al. Eco-friendly nanotechnologies and nanomaterials for environmental applications: Key issue and consensus recommendations for sustainable and eco safe nanoremediation. Ecotoxicol. Environ. Saf. 2018, 154, 237–244. [Google Scholar] [CrossRef]
- Faisal, M.; Saquib, Q.; Alatar, A.A.; Al-Khedhairy, A.; Hegazy, A.K.; Musarrat, J. Phytotoxic hazards of NiO-nanoparticles in tomato: A study on mechanism of cell death. J. Hazard. Mater. 2013, 250, 318–332. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Mishra, R.K.; Singh, S.; Singh, S.; Vishwakarma, K.; Sharma, S.; Singh, V.P.; Singh, P.K.; Prasad, S.M.; Dubey, N.K.; et al. Nitric Oxide Ameliorates Zinc Oxide Nanoparticles Phytotoxicity in Wheat Seedlings: Implication of the Ascorbate–Glutathione Cycle. Front. Plant Sci. 2017, 8, 11605. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, D.K.; Singh, S.; Singh, S.; Srivastava, P.K.; Singh, D.P.; Singh, S.; Prasad, S.M.; Singh, P.K.; Dubey, N.K.; Pandey, A.C.; et al. Nitric oxide alleviates silver nanoparticles (AgNps)-induced phytotoxicity in Pisum sativum seedlings. Plant Physiol. Biochem. 2017, 110, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aziz, H.; Rizwan, M. Chemically synthesized silver nanoparticles induced physio-chemical and chloroplast ultrastructural changes in broad bean seedlings. Chemosphere 2019, 235, 1066–1072. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, A.; Zivcak, M.; Tripathi, D.; Yadav, S.; Kalaji, H.; Brestic, M. Phytotoxic effect of silver nanoparticles in Triticum aestivum: Improper regulation of photosystem I activity as the reason for oxidative damage in the chloroplast. Photosynth. 2019, 57, 209–216. [Google Scholar] [CrossRef]
- Hurtado-Gallego, J.; Pulido-Reyes, G.; González-Pleiter, M.; Salas, G.; Leganés, F.; Rosal, R.; Fernández-Piñas, F. Toxicity of superparamagnetic iron oxide nanoparticles to the microalga Chlamydomonas reinhardtii. Chemosphere 2020, 238, 124562. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, J.; Dou, R.; Gao, X.; Mao, C.; Wang, L. Assessment of the Phytotoxicity of Metal Oxide Nanoparticles on Two Crop Plants, Maize (Zea mays L.) and Rice (Oryza sativa L.). Int. J. Environ. Res. Public Heal. 2015, 12, 15100–15109. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Li, Q.Q.; Pei, Z.M.; Wang, S. Effects of zinc oxide nanoparticles on the growth, photosynthetic traits, and antioxidative enzymes in tomato plants. Biol. Plant. 2018, 62, 801–808. [Google Scholar] [CrossRef]
- Ghosh, M.; Jana, A.; Sinha, S.; Jothiramajayam, M.; Nag, A.; Chakraborty, A.; Mukherjee, A.; Mukherjee, A. Effects of ZnO nanoparticles in plants: Cytotoxicity, genotoxicity, deregulation of antioxidant defenses, and cell-cycle arrest. Mutat. Res. Toxicol. Environ. Mutagen. 2016, 807, 25–32. [Google Scholar] [CrossRef]
- Iftikhar, A.; Ali, S.; Yasmeen, T.; Arif, M.S.; Zubair, M.; Rizwan, M.; Alhaithloul, H.A.S.; Alayafi, A.A.; Soliman, M.H. Effect of gibberellic acid on growth, photosynthesis and antioxidant defense system of wheat under zinc oxide nanoparticle stress. Environ. Pollut. 2019, 254, 113109. [Google Scholar] [CrossRef]
- Azhar, W.; Khan, A.R.; Muhammad, N.; Liu, B.; Song, G.; Hussain, A.; Yasin, M.U.; Khan, S.; Munir, R.; Gan, Y. Ethylene mediates CuO NP-induced ultrastructural changes and oxidative stress in Arabidopsis thaliana leaves. Environ. Sci. Nano 2020, 7, 938–953. [Google Scholar] [CrossRef]
- Hussain, I.; Singh, N.B.; Singh, A.; Singh, P. Plant-nanoceria interaction: Toxicity, accumulation, translocation and biotransformation. South Afr. J. Bot. 2019, 121, 239–247. [Google Scholar] [CrossRef]
- Alsaeedi, A.; El-Ramady, H.; Alshaal, T.; El-Garawany, M.; Elhawat, N.; Al-Otaibi, A. Silica nanoparticles boost growth and productivity of cucumber under water deficit and salinity stresses by balancing nutrients uptake. Plant Physiol. Biochem. 2019, 139, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pullagurala, V.L.R.; Adisa, I.O.; Rawat, S.; Kalagara, S.; Hernandez-Viezcas, J.A.; Peralta-Videa, J.; Gardea-Torresdey, J.L. ZnO nanoparticles increase photosynthetic pigments and decrease lipid peroxidation in soil grown cilantro (Coriandrum sativum). Plant Physiol. Biochem. 2018, 132, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-C.; Huang, K.-X.; Huang, A.-C.; Ho, Y.-C.; Wang, C.-J. Hibiscus anthocyanins-rich extract inhibited LDL oxidation and oxLDL-mediated macrophages apoptosis. Food Chem. Toxicol. 2006, 44, 1015–1023. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.; Alhmad, M.F.A.; Abdelfattah, K.E. The possible roles of priming with ZnO nanoparticles in mitigation of salinity stress in lupine (Lupinus termis) plants. J. Plant Growth Regul. 2017, 36, 60–70. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.; Srivastava, A.K.; El-Sadek, M.S.A.; Kordrostami, M.; Tran, L.-S.P. Titanium Dioxide Nanoparticles Improve Growth and Enhance Tolerance of Broad Bean Plants under Saline Soil Conditions. Land Degrad. Dev. 2017, 29, 1065–1073. [Google Scholar] [CrossRef]
- Scherrer, P. Nachrichten von der Gesellschaft der Wissenschaftenzu Göttingen, Mathematisch. Phys. Klasse 1918, 2, 98–100. [Google Scholar]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef] [Green Version]
- Irigoyen, J.J.; Emerich, D.W.; Sánchez-Díaz, M. Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativa) plants. Physiol. Plant. 1992, 84, 55–60. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein binding. Ann. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Lee, Y.P.; Takahashi, T. An improved colorimetric determination of amino acids with the use of ninhydrin. Anal. Biochem. 1966, 14, 71–77. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.; Tran, L.-S.P. Impacts of Priming with Silicon on the Growth and Tolerance of Maize Plants to Alkaline Stress. Front. Plant Sci. 2016, 7, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases. I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. Method. Enzymol. 1984, 105, 121–126. [Google Scholar]
- Maehly, A.C.; Chance, B. The assay of catalase and peroxidase. In Methods in Biochemistry Analysis; Glick, D., Ed.; Interscience Publishers: New York, NY, USA, 1954; Volume 1, pp. 357–425. [Google Scholar]
- Chen, G.; Asada, K. Inactivation of ascorbate peroxidase by thoils requires hydrogen peroxide. Plant Cell Physiol. 1992, 33, 117–123. [Google Scholar]
- Hajiboland, R.; Bastani, S.; Bahrami-Rad, S.; Poschenrieder, C. Interactions between aluminum and boron in tea (Camellia sinensis) plants. Acta Physiol. Plant. 2015, 37, 54. [Google Scholar] [CrossRef]
- Kidd, P.S.; Proctor, J. Effects of aluminium on the growth and mineral composition of Betula pendula Roth. J. Exp. Bot. 2000, 51, 1057–1066. [Google Scholar] [CrossRef] [Green Version]
- Burklew, C.E.; Ashlock, J.; Winfrey, W.B.; Zhang, B. Effects of Aluminum Oxide Nanoparticles on the Growth, Development, and microRNA Expression of Tobacco (Nicotiana tabacum). PLoS ONE 2012, 7, e34783. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.; Fan, X.; Li, X.; Zhang, Z.; Sun, L.; Fu, Z.; Lavoie, M.; Pan, X.; Qian, H. Distinct physiological and molecular responses in Arabidopsis thaliana exposed to aluminum oxide nanoparticles and ionic aluminum. Environ. Pollut. 2017, 228, 517–527. [Google Scholar] [CrossRef]
- Cruz, F.J.R.; de Almeida, H.J.; dos Santos, D.M.M. Growth, nutritional status and nitrogen metabolism in Vigna unguiculata L. Walp is affected by aluminum. Aust. J. Crop Sci. 2014, 8, 1132. [Google Scholar]
- Yanık, F.; Vardar, F. Toxic Effects of Aluminum Oxide (Al2O3) Nanoparticles on Root Growth and Development in Triticum aestivum. Water Air Soil Pollut. 2015, 226, 296. [Google Scholar] [CrossRef]
- Sadiq, I.M.; Pakrashi, S.; Chandrasekaran, N.; Mukherjee, A. Studies on toxicity of aluminum oxide (Al2O3) nanoparticles to microalgae species: Scenedesmus sp. and Chlorella sp. J. Nanoparticle Res. 2011, 13, 3287–3299. [Google Scholar] [CrossRef]
- Yang, L.; Watts, D.J. Particle surface characteristics may play an important role in phytotoxicity of alumina nanoparticles. Toxicol. Lett. 2005, 158, 122–132. [Google Scholar] [CrossRef]
- Griffitt, R.J.; Luo, J.; Gao, J.; Bonzongo, J.C.; Barber, D.S. Effects of particle composition and species on toxicity of metallic nanomaterials in aquatic organisms. Environ. Toxicol. Chem. Inter. J. 2008, 27, 1972–1978. [Google Scholar] [CrossRef]
- Chen, L.-S.; Qi, Y.-P.; Liu, X.-H. Effects of Aluminum on Light Energy Utilization and Photoprotective Systems in Citrus Leaves. Ann. Bot. 2005, 96, 35–41. [Google Scholar] [CrossRef]
- Silva, S.; Pinto, G.; Dias, M.C.; Correia, C.; Moutinho-Pereira, J.; Pinto-Carnide, O.; Santos, C. Aluminium long-term stress differently affects photosynthesis in rye genotypes. Plant Physiol. Biochem. 2012, 54, 105–112. [Google Scholar] [CrossRef]
- Cárcamo, M.P.; Reyes-Díaz, M.; Rengel, Z.; Alberdi, M.; Omena-Garcia, R.P.; Nunes-Nesi, A.; Inostroza-Blancheteau, C. Aluminum stress differentially affects physiological performance and metabolic compounds in cultivars of highbush blueberry. Sci. Rep. 2019, 9, 11275. [Google Scholar] [CrossRef] [Green Version]
- Pirzadah, T.B.; Malik, B.; Tahir, I.; Rehman, R.U.; Hakeem, K.R.; Alharby, H.F. Aluminium stress modulates the osmolytes and enzyme defense system in Fagopyrum species. Plant Physiol. Biochem. 2019, 144, 178–186. [Google Scholar] [CrossRef]
- Zaid, A.; Wani, S.H. Reactive Oxygen Species Generation, Scavenging and Signaling in Plant Defense Responses. In Bioactive Molecules in Plant Defense; Springer Science and Business Media LLC: Berlin, Germany, 2019; pp. 111–132. [Google Scholar]
- Guo, P.; Qi, Y.-P.; Cai, Y.-T.; Yang, T.-Y.; Yang, L.-T.; Huang, Z.-R.; Chen, L.-S. Aluminum effects on photosynthesis, reactive oxygen species and methylglyoxal detoxification in two Citrus species differing in aluminum tolerance. Tree Physiol. 2018, 38, 1548–1565. [Google Scholar] [CrossRef] [Green Version]
- Yanık, F.; Vardar, F. Oxidative stress response to aluminum oxide (Al2O3) nanoparticles in Triticum aestivum. Biologia 2018, 73, 129–135. [Google Scholar] [CrossRef]
- Hajiboland, R.; Bahrami Rad, S.; Barceló, J.; Poschenrieder, C. Mechanisms of aluminum-induced growth stimulation in tea Camellia sinensis. J. Plant Nutr. Soil. Sci. 2013, 176, 616–625. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, Y.; Ding, Z.; Song, L.; Li, Y.; Ma, D. Aluminum induced metabolic responses in two tea cultivars. Plant Physiol. Biochem. 2016, 101, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, U.; Tomioka, R.; Kojima, M.; Sakakibara, H.; Takenaka, C. Aluminum effect on starch, soluble sugar, and phytohormone in roots of Quercus serrata Thunb. seedlings. Trees 2015, 30, 405–413. [Google Scholar] [CrossRef]
- Moreno-Alvarado, M.; García-Morales, S.; Trejo-Téllez, L.I.; Hidalgo-Contreras, J.V.; Gómez-Merino, F.C. Aluminum Enhances Growth and Sugar Concentration, Alters Macronutrient Status and Regulates the Expression of NAC Transcription Factors in Rice. Front. Plant Sci. 2017, 8, 709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasanuzzaman, M.; Nahar, K.; Khan, M.I.R.; Al Mahmud, J.; Alam, M.M.; Fujita, M. Regulation of Reactive Oxygen Species Metabolism and Glyoxalase Systems by Exogenous Osmolytes Confers Thermotolerance in Brassica napus. GesundePflanz. 2019, 72, 3–16. [Google Scholar] [CrossRef]
- Zaid, A.; Mohammad, F.; Wani, S.H.; Siddique, K.M. Salicylic acid enhances nickel stress tolerance by up-regulating antioxidant defense and glyoxalase systems in mustard plants. Ecotoxicol. Environ. Saf. 2019, 180, 575–587. [Google Scholar] [CrossRef]
- Rajasheker, G.; Jawahar, G.; Jalaja, N.; Kumar, S.A.; Kumari, P.H.; Punita, D.L.; Karumanchi, A.R.; Reddy, P.S.; Rathnagiri, P.; Sreenivasulu, N.; et al. Role and Regulation of Osmolytes and ABA Interaction in Salt and Drought Stress Tolerance. In Plant Signaling Molecules; Elsevier BV: Amsterdam, The Netherlands, 2019; pp. 417–436. [Google Scholar]
- Sharma, A.; Shahzad, B.; Kumar, V.; Kohli, S.K.; Sidhu, G.P.S.; Bali, A.S.; Handa, N.; Kapoor, D.; Bhardwaj, R.; Zheng, B. Phytohormones Regulate Accumulation of Osmolytes Under Abiotic Stress. Biomolecules 2019, 9, 285. [Google Scholar] [CrossRef] [Green Version]
- Wani, S.H.; Singh, N.B.; Haribhushan, A.; Mir, J.I. Compatible Solute Engineering in Plants for Abiotic Stress Tolerance—Role of Glycine Betaine. Curr. Genom. 2013, 14, 157–165. [Google Scholar] [CrossRef]
- Talaat, N.B.; Shawky, B. 24-Epibrassinolide alleviates salt-induced inhibition of productivity by increasing nutrients and compatible solutes accumulation and enhancing antioxidant system in wheat (Triticum aestivum L.). Acta Physiol. Plant. 2012, 35, 729–740. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Akram, N.A.; Ashraf, M. Osmoprotection in plants under abiotic stresses: new insights into a classical phenomenon. Planta 2019, 251, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yalcinkaya, T.; Uzilday, B.; Ozgur, R.; Turkan, I.; Mano, J. Lipid peroxidation-derived reactive carbonyl species (RCS): Their interaction with ROS and cellular redox during environmental stresses. Environ. Exp. Bot. 2019, 165, 139–149. [Google Scholar] [CrossRef]
- Zaouali, W.; Mahmoudi, H.; Ben Salah, I.; Mejri, F.; Casabianca, H.; Hosni, K.; Ouerghi, Z. Copper-induced changes in growth, photosynthesis, antioxidative system activities and lipid metabolism of cilantro (Coriandrum sativum L.). Biologia 2020, 75, 367–380. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.; Chaoxing, H. Does the inoculation with Glomus mosseae improve salt tolerance in pepper plants? J. Plant Growth Regul. 2014, 33, 644–653. [Google Scholar] [CrossRef]
- Zaid, A.; Mohammad, F. Methyl jasmonate and nitrogen interact to alleviate cadmium stress in Mentha arvensis by regulating physio-biochemical damages and ROS detoxification. J. Plant Growth Regul. 2018, 37, 1331–1348. [Google Scholar] [CrossRef]
- De, A.; Chakrabarti, M.; Ghosh, I.; Mukherjee, A. Evaluation of genotoxicity and oxidative stress of aluminium oxide nanoparticles and its bulk form in Allium cepa. Nucleus 2016, 59, 219–225. [Google Scholar] [CrossRef]
- Poborilova, Z.; Opatrilova, R.; Babula, P. Toxicity of aluminium oxide nanoparticles demonstrated using a BY-2 plant cell suspension culture model. Environ. Exp. Bot. 2013, 91, 1–11. [Google Scholar] [CrossRef]
- Zhu, C.Q.; Zhang, J.H.; Sun, L.M.; Zhu, L.F.; Abliz, B.; Hu, W.J.; Zhong, C.; Bai, Z.G.; Sajid, H.; Cao, X.C.; et al. Hydrogen Sulfide Alleviates Aluminum Toxicity via Decreasing Apoplast and Symplast Al Contents in Rice. Front. Plant Sci. 2018, 9, 294. [Google Scholar] [CrossRef] [Green Version]
- Li, X.-M.; Zhou, S.; Fan, W. Effect of Nano-Al2O3 on the Toxicity and Oxidative Stress of Copper towards Scenedesmus obliquus. Int. J. Environ. Res. Public Heal. 2016, 13, 575. [Google Scholar] [CrossRef] [Green Version]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel Latef, A.A.H.; Zaid, A.; Abu Alhmad, M.F.; Abdelfattah, K.E. The Impact of Priming with Al2O3 Nanoparticles on Growth, Pigments, Osmolytes, and Antioxidant Enzymes of Egyptian Roselle (Hibiscus sabdariffa L.) Cultivar. Agronomy 2020, 10, 681. https://doi.org/10.3390/agronomy10050681
Abdel Latef AAH, Zaid A, Abu Alhmad MF, Abdelfattah KE. The Impact of Priming with Al2O3 Nanoparticles on Growth, Pigments, Osmolytes, and Antioxidant Enzymes of Egyptian Roselle (Hibiscus sabdariffa L.) Cultivar. Agronomy. 2020; 10(5):681. https://doi.org/10.3390/agronomy10050681
Chicago/Turabian StyleAbdel Latef, Arafat Abdel Hamed, Abbu Zaid, Mona Fawzy Abu Alhmad, and Khaled Ebnalwaled Abdelfattah. 2020. "The Impact of Priming with Al2O3 Nanoparticles on Growth, Pigments, Osmolytes, and Antioxidant Enzymes of Egyptian Roselle (Hibiscus sabdariffa L.) Cultivar" Agronomy 10, no. 5: 681. https://doi.org/10.3390/agronomy10050681
APA StyleAbdel Latef, A. A. H., Zaid, A., Abu Alhmad, M. F., & Abdelfattah, K. E. (2020). The Impact of Priming with Al2O3 Nanoparticles on Growth, Pigments, Osmolytes, and Antioxidant Enzymes of Egyptian Roselle (Hibiscus sabdariffa L.) Cultivar. Agronomy, 10(5), 681. https://doi.org/10.3390/agronomy10050681






