Berry Quality of Grapevine under Water Stress as Affected by Rootstock–Scion Interactions through Gene Expression Regulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
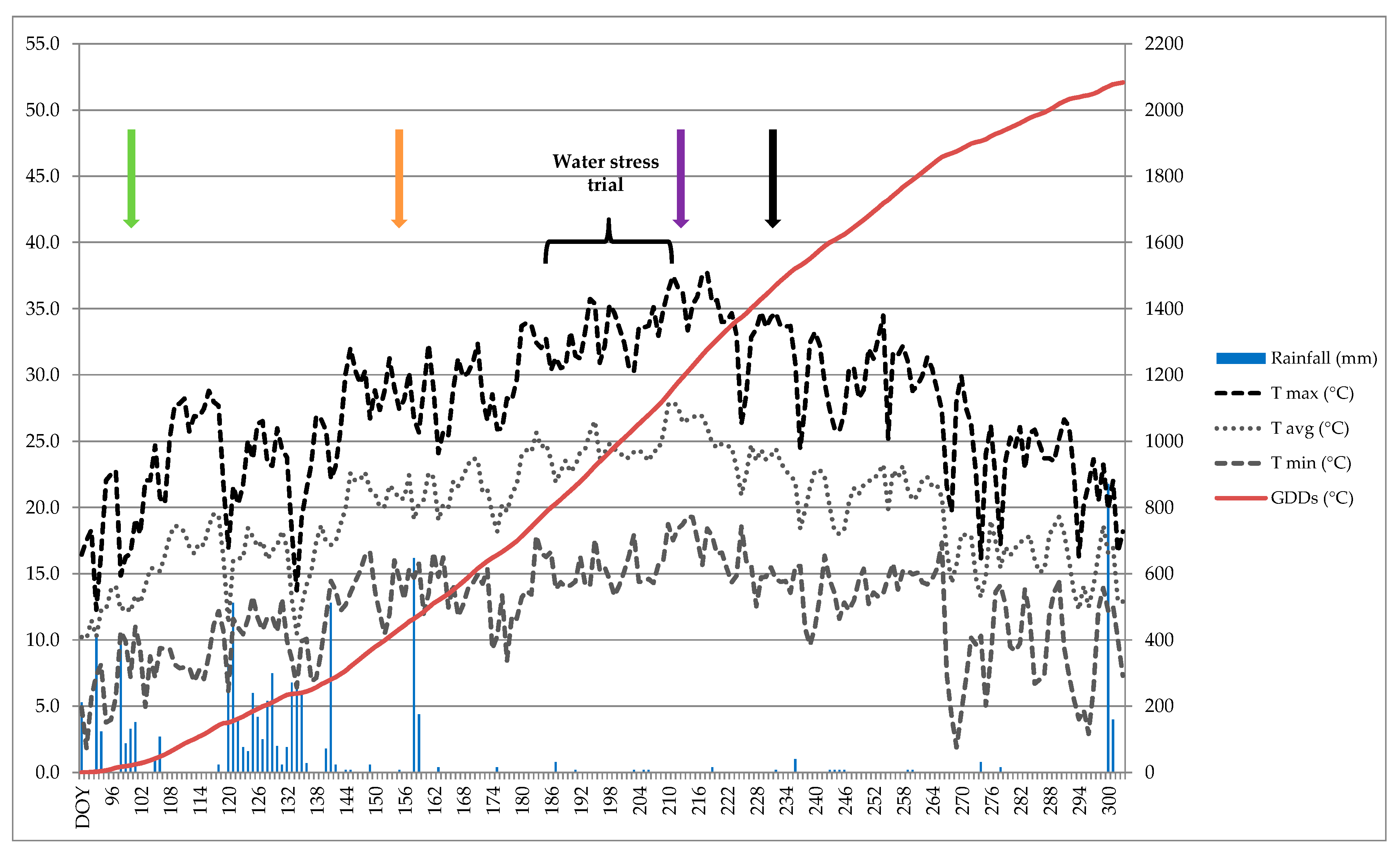
2.2. Weather Conditions and Phenological Surveys
2.3. Irrigation Protocols
2.4. Measurements on Grapevine Physiology
2.5. Grape Production, Berry Characteristics and Pruning Wood Weight
2.6. Technological Maturity and Phenolic Compound Contents
2.7. Grape Sampling for Molecular Analyses
2.8. RNA Extraction and qRT-PCR Analyses
2.9. Statistical Analyses
3. Results
3.1. Weather Conditions and Phenological Surveys
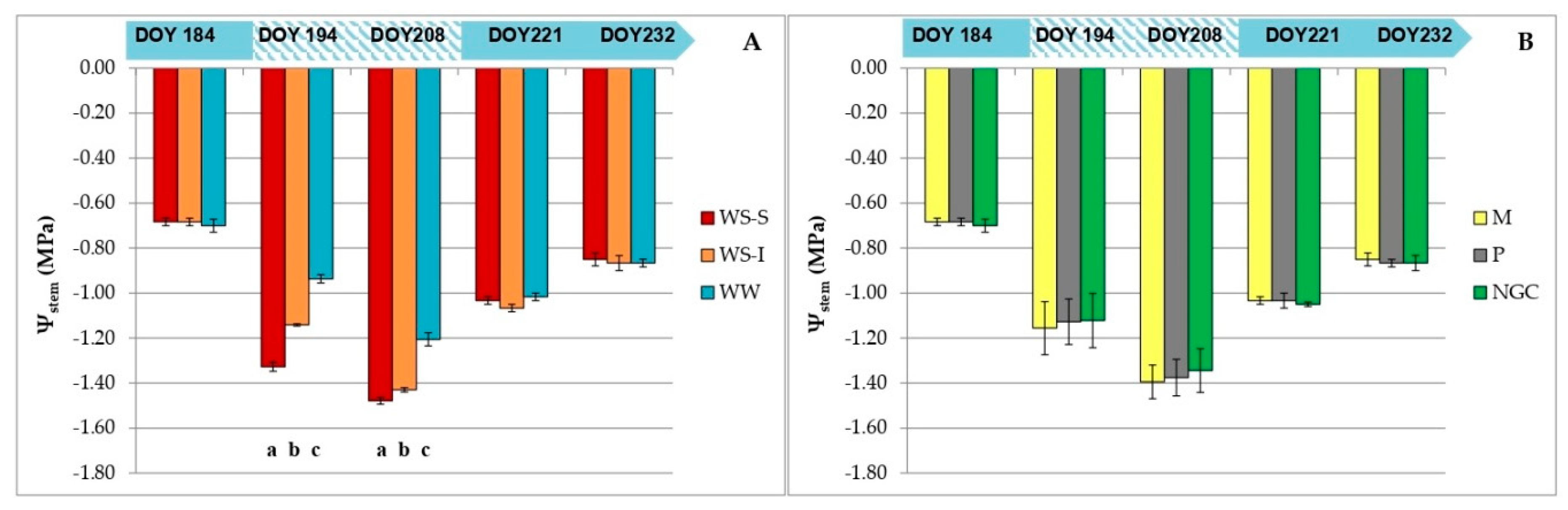
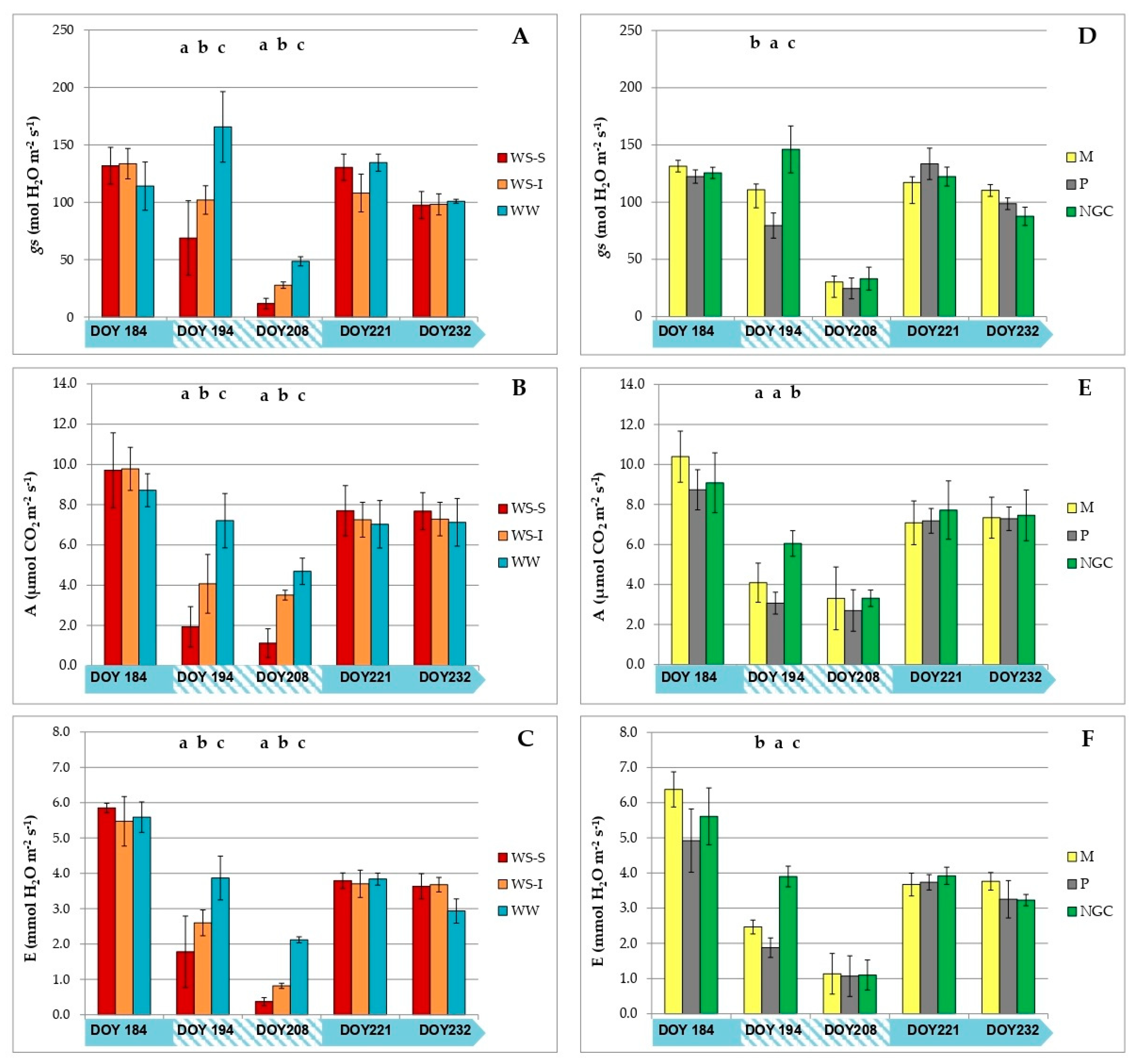
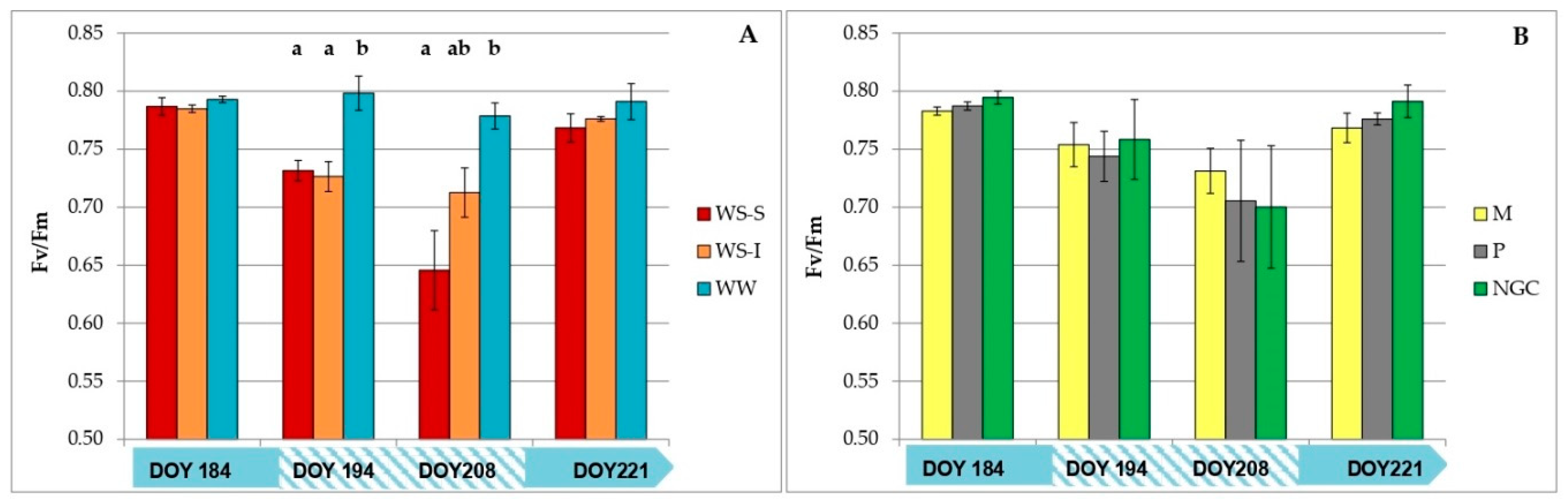
3.2. Measurements on Grapevine Physiology
3.3. Grape Production, Berry Characteristics and Pruning Wood Weight
3.4. Technological Maturity and Phenolic Compound Contents
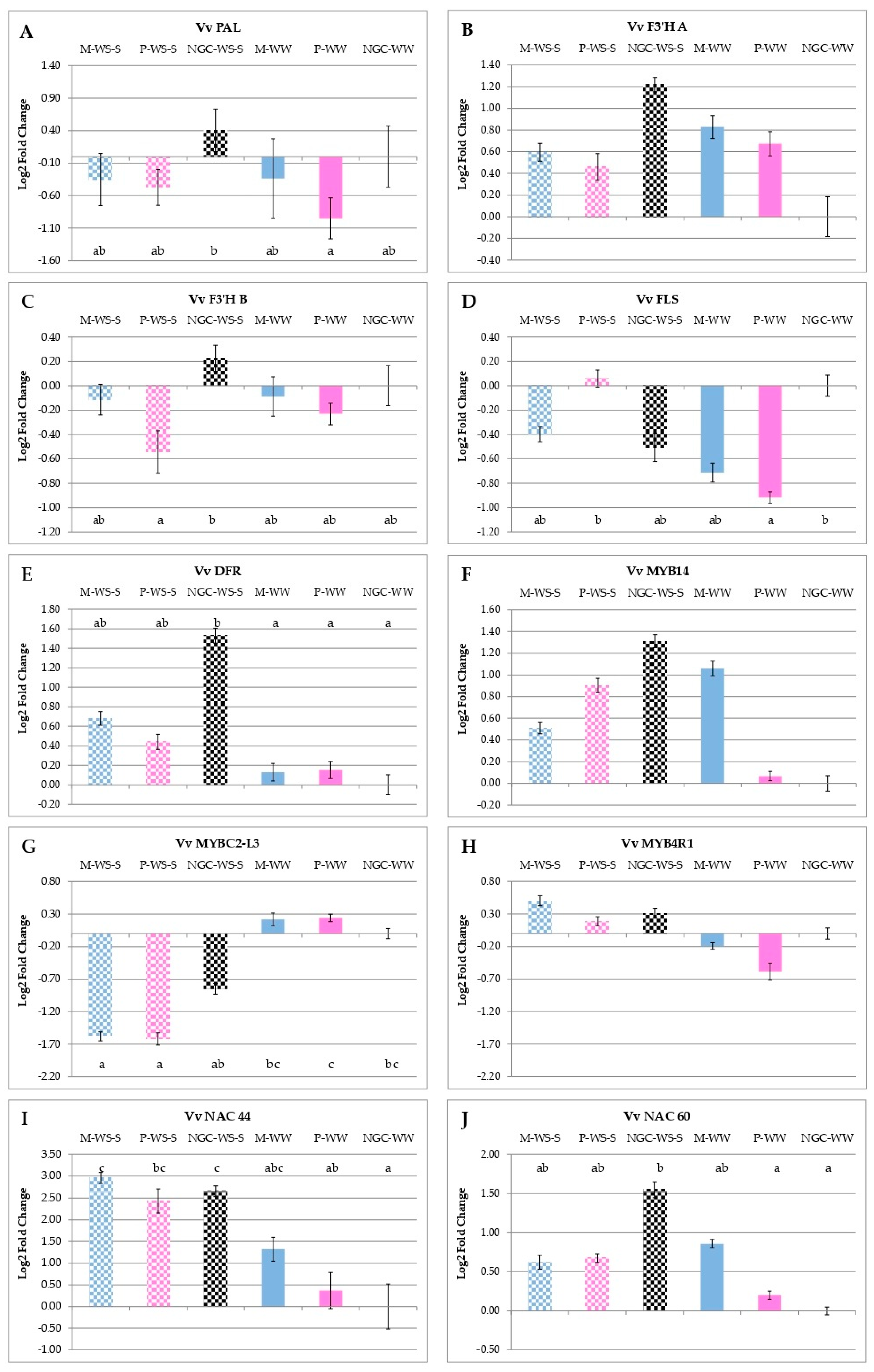
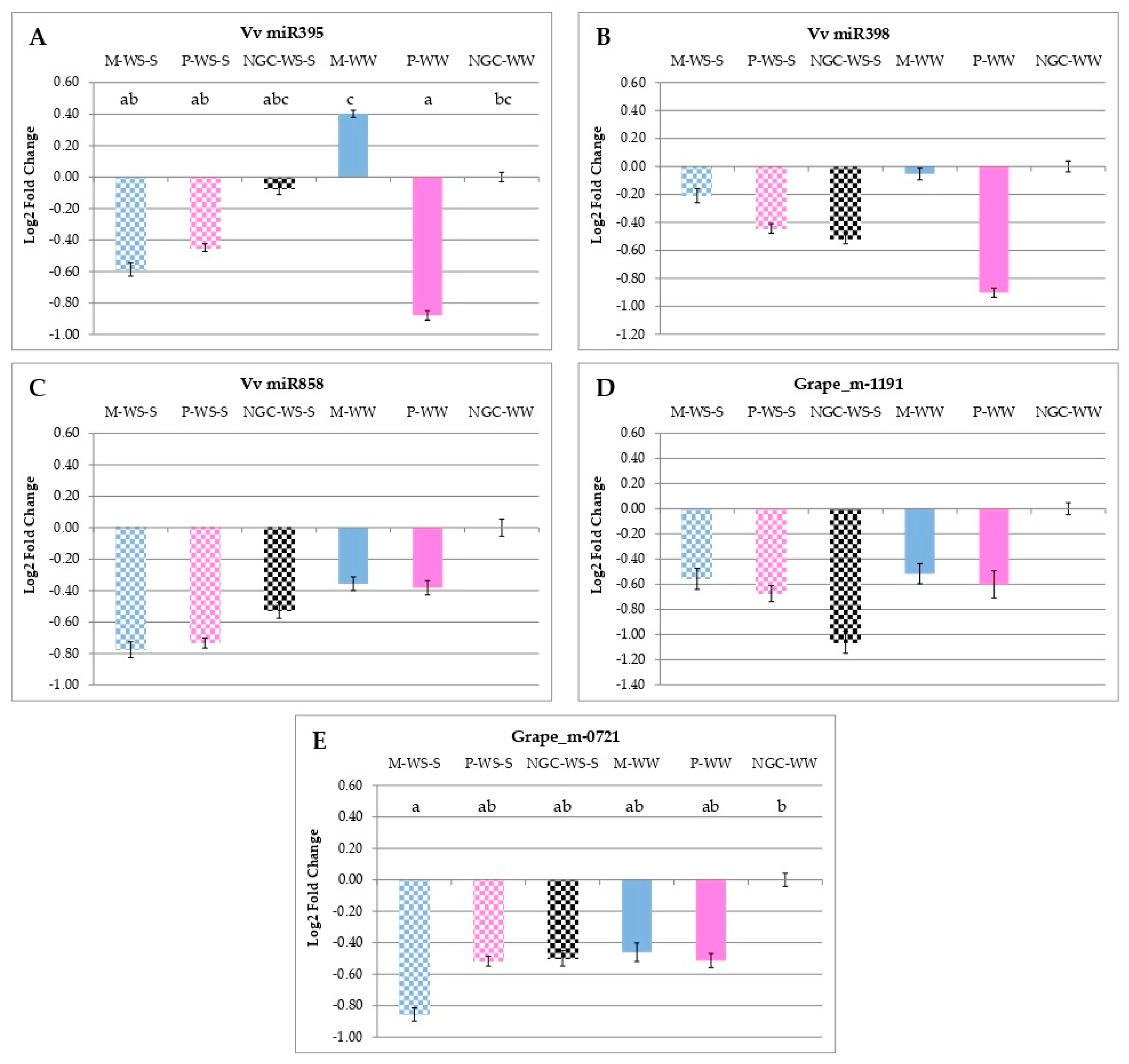
3.5. qRT-PCR Analyses of Gene and miRNA Expressions
4. Discussion
4.1. The Root System and Water Supply Did Not Affect Grape Production, but Water Deficit Had a Strong Influence on the Technological Maturity
4.2. Phenolic Compounds Accumulation was Influenced in Berries Both by Water Supply and Root System
4.3. Early Water Stress Modulated the Expression of Genes and miRNAs Involved in Secondary Metabolism with Lasting Effects, Still Evident at Maturity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jones, G.V.; White, M.A.; Cooper, O.R.; Storchmann, K. Climate change and global wine quality. Clim. Change 2005, 73, 319–343. [Google Scholar] [CrossRef]
- Chaves, M.M.; Zarrouk, O.; Francisco, R.; Costa, J.M.; Santos, T.; Regalado, A.P.; Rodrigues, M.L.; Lopes, C.M. Grapevine under deficit irrigation: Hints from physiological and molecular data. Ann. Bot. 2010, 105, 661–676. [Google Scholar] [CrossRef] [PubMed]
- Goode, J. Viticulture: Fruity with a hint of drought. Nature 2012, 492, 351–352. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Masson-Delmotte, V.; Zhai, P.; Portner, H.-O.; Roberts, D.; Skea, J.; Shula, P.R.; Pirani, A.; Moufouma-Okia, W.; Pean, C.; Pidcock, R.; et al. Global Warming of 1.5 °C. An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2018; ISBN 978-92-9169-151-7. [Google Scholar]
- Peterlunger, E.; Sivilotti, P.; Colussi, V. Water stress increased polyphenolic quality in ‘Merlot’ grapes. Acta Hortic. 2005, 689, 293–300. [Google Scholar] [CrossRef]
- Van Leeuwen, C.; Tregoat, O.; Choné, X.; Bois, B.; Pernet, D.; Gaud-illère, J.P. Vine water status is a key factor in grape ripening and vintage quality for red Bordeaux wine. How can it be assessed for vineyard management purposes? OENO One 2009, 43, 121–134. [Google Scholar] [CrossRef]
- Ferrandino, A.; Lovisolo, C. Abiotic stress effects on grapevine (Vitis vinifera L.): Focus on abscisic acid-mediated consequences on secondary metabolism and berry quality. Environ. Exp. Bot. 2014, 103, 138–147. [Google Scholar] [CrossRef]
- Fraga, H.; Malheiro, C.; Moutinho-Pereira, J.; Santos, J.A. An overview of climate change impacts on European viticulture. Food Energy Secur. 2012, 1, 94–110. [Google Scholar] [CrossRef]
- Palliotti, A.; Tombesi, S.; Silvestroni, O.; Lanari, V.; Gatti, M.; Poni, S. Changes in vineyard establishment and canopy management urged by earlier climate-related grape ripening: A review. Sci. Hortic. 2014, 178, 43–54. [Google Scholar] [CrossRef]
- Van Leeuwen, C.; Destrac-Irvine, A. Modified grape composition under climate change conditions requires adaptations in the vineyard. OENO One 2017, 51, 147–154. [Google Scholar] [CrossRef]
- Pavlousek, P. Evaluation of drought tolerance of new grapevine rootstock hybrids. J. Environ. Biol. 2011, 32, 543–549. [Google Scholar]
- Marguerit, E.; Brendel, O.; Lebon, E.; van Leeuwen, C.; Ollat, N. Rootstock control of scion transpiration and its acclimation to water deficit are controlled by different genes. N. Phytol. 2012, 194, 416–429. [Google Scholar] [CrossRef] [PubMed]
- Ollat, N.; Peccoux, A.; Papura, D.; Esmenjaud, D.; Marguerit, E.; Tandonnet, J.P.; Bordenave, L.; Cookson, S.J.; Barrieu, F.; Rossdeutsch, L.; et al. Rootstocks as a component of adaptation to environment. In Grapevine in a Changing Environment: A Molecular and Ecophysiological Perspective; Geros, H., Chaves, M., Medrano, H., Delrot, S., Eds.; Wiley-Blackwell: Oxford, UK, 2015; pp. 68–108. ISBN 978-1-118-73605-0. [Google Scholar]
- Corso, M.; Bonghi, C. Grapevine rootstock effects on abiotic stress tolerance. Plant Sci. Today 2014, 1, 108–113. [Google Scholar] [CrossRef]
- Warschefsky, E.J.; Klein, L.L.; Frank, M.H.; Chitwood, D.H.; Londo, J.P.; von Wettberg, E.J.B.; Miller, A.J. Rootstocks: Diversity, domestication, and impacts on shoot phenotypes. Trends Plant Sci. 2016, 21, 418–437. [Google Scholar] [CrossRef] [PubMed]
- Ollat, N.; Cookson, S.J.; Lauvergeat, V.; Marguerit, E.; Barrieu, F.; Gambetta, G.; Goutouly, J.P.; Tandonnet, J.P.; Vivin, P.; Delrot, S. Grapevines roots: The dark side. Acta Hortic. 2017, 1188, 213–226. [Google Scholar] [CrossRef]
- Koundouras, S.; Hatzidimitriou, E.; Karamolegkou, M.; Dimopoulou, E.; Kallithraka, S.; Tsialtas, J.T.; Zioziou, E.; Nikolau, N.; Kotseridis, Y. Irrigation and rootstock effects on the phenolic concentration and aroma potential of Vitis vinifera L. cv. Cabernet Sauvignon grapes. J. Agric. Food Chem. 2009, 57, 7805–7813. [Google Scholar] [CrossRef]
- Maré, C.; Aprile, A.; Roncaglia, E.; Tocci, E.; Corino, L.G.; De Bellis, G.; Cattivelli, L. Rootstock and soil induce transcriptome modulation of phenylpropanoid pathway in grape leaves. J. Plant Interact. 2013, 8, 334–349. [Google Scholar] [CrossRef]
- Corso, M.; Vannozzi, A.; Maza, E.; Vitulo, N.; Meggio, F.; Pitacco, A.; Telatin, A.; D’Angelo, M.; Feltrin, E.; Negri, A.S.; et al. Comprehensive transcript profiling of two grapevine rootstock genotypes contrasting in drought susceptibility links the phenylpropanoid pathway to enhanced tolerance. J. Exp. Bot. 2015, 66, 5739–5752. [Google Scholar] [CrossRef]
- Berdeja, M.; Nicolas, P.; Kappel, C.; Dai, Z.W.; Hilbert, G.; Peccoux, A.; Lafontaine, M.; Ollat, N.; Gomès, E.; Delrot, S. Water limitation and rootstock genotype interact to alter grape berry metabolism through transcriptome reprogramming. Hortic. Res. 2015, 2, 15012. [Google Scholar] [CrossRef]
- Adams, D.O. Phenolics and Ripening in Grape Berries. Am. J. Enol. Vitic. 2006, 57, 249–256. [Google Scholar]
- Teixeira, A.; Eiras-Dias, J.; Castellarin, S.; Geròs, H. Berry Phenolics of Grapevine under Challenging Environments. Int. J. Mol. Sci. 2013, 14, 18711–18739. [Google Scholar] [CrossRef]
- Waterhouse, A.L. Wine phenolics. Ann. N. Y. Acad. Sci. 2002, 957, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Zombardo, A.; Crosatti, C.; Bagnaresi, P.; Bassolino, L.; Reshef, N.; Puccioni, S.; Faccioli, P.; Tafuri, A.; Delledonne, M.; Fait, A.; et al. Transcriptomic and biochemical investigations support the role of rootstock-scion interaction in grapevine berry quality. BMC Genom. 2020, in press. [Google Scholar]
- Priori, S.; Barbetti, R.; L’Abate, G.; Bucelli, P.; Storchi, P.; Costantini, E.A.C. Natural terroir unit, Siena Province, Tuscany. J. Maps 2014, 10, 466–477. [Google Scholar] [CrossRef]
- Nuzzo, V. Portinnesti. In La Nuova Viticoltura; Palliotti, A., Poni, S., Silvestroni, O., Eds.; Agricole: Bologna, Italy, 2015; pp. 52–71. ISBN 978-88-506-5453-6. [Google Scholar]
- Zombardo, A.; Mica, E.; Puccioni, S.; Bassolino, L.; Perria, R.; Mattii, G.B.; Cattivelli, L.; Storchi, P. Influenza del portinnesto sul metabolismo secondario di uve Pinot nero. Infowine Internet J. Vitic. Enol. 2019, 10, 1–16. [Google Scholar]
- Winkler, A.J.; Cook, J.A.; Kliewer, W.M.; Lider, L.A. General Viticulture, 2nd ed.; University of California Press: Berkeley, CA, USA, 1974; p. 710. ISBN 9780520025912. [Google Scholar]
- Lorenz, D.H.; Eichhorn, K.W.; Bleiholder, H.; Klose, R.; Meier, U.; Weber, E. Phenological growth stages of the grapevine (Vitis vinifera L. spp. vinifera)—Codes and descriptions according to the extended BBCH scale. Aust. J. Grape Wine Res. 1995, 1, 100–110. [Google Scholar] [CrossRef]
- Coombe, B.G.; Mc Carthy, M.G. Dynamics of grape berry growth and physiology of ripening. Aust. J. Grape Wine Res. 2000, 6, 131–135. [Google Scholar] [CrossRef]
- Puccioni, S.; Valentini, P.; Zombardo, A.; Leprini, M.; Perria, R.; Storchi, P.; Priori, S.; Costantini, E.A.C.; Salvi, L.; Cataldo, E.; et al. Interazione suolo-portinnesto in situazione di stress idrico in piante di vite allevate in vaso. Acta Italus Hortus 2016, 19, 95–96. [Google Scholar]
- Naor, A. Midday stem water potential as a plant water stress indicator for irrigation scheduling in fruit trees. Acta Hortic. 2000, 537, 447–454. [Google Scholar] [CrossRef]
- Scholander, P.F.; Bradstreet, E.D.; Hemmingsen, E.A.; Hammel, H.T. Sap pressure in vascular plants. Science 1965, 148, 339–346. [Google Scholar] [CrossRef]
- Kitajima, M.; Butler, W.L. Quenching of chlorophyll fluorescence and primary photochemistry in chloroplasts by dibromothymoquinone. Biochim. Biophys. Acta Bioenerg. 1975, 376, 105–115. [Google Scholar] [CrossRef]
- International Organisation of Vine and Wine. Compendium of International Methods of Analysis of Wines and Musts. Available online: http://www.oiv.int/en/technical-standards-and-documents/methods-of-analysis/compendium-of-international-methods-of-analysis-of-wines-and-musts-2-vol (accessed on 26 March 2020).
- Di Stefano, R.; Cravero, M.C. Metodi per lo studio dei polifenoli dell’uva. Riv. Viticolt. Enol. 1991, 2, 37–45. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventòs, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Method Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Gómez-Alonso, S.; Garcia-Romero, E.; Hermosìn-Gutiérrez, I. HPLC analysis of diverse grape and wine phenolics using direct injection and multidetection by DAD and fluorescence. J. Food Compos. Anal. 2007, 20, 618–626. [Google Scholar] [CrossRef]
- Varkonyi-Gasic, E.; Wu, R.; Wood, M.; Walton, E.F.; Hellens, R.P. Protocol: A highly sensitive RT-PCR method for detection and quantification of microRNAs. Plant Methods 2007, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- De Lorenzis, G.; Squadrito, M.; Rossoni, M.; Di Lorenzo, G.S.; Brancadoro, L.; Scienza, A. Study of intra-varietal diversity in biotypes of Aglianico and Muscat of Alexandria (Vitis vinifera L.) cultivars. Aust. J. Grape Wine Res. 2016, 23, 132–142. [Google Scholar] [CrossRef]
- Poni, S.; Intrieri, C. Grapevine photosynthesis: Effects linked to light radiation and leaf age. Adv. Hort. Sci. 2001, 15, 5–15. [Google Scholar]
- Schultz, H. Climate change and viticulture: A European perspective on climatology, carbon dioxide and UV-B effects. Aust. J. Grape Wine Res. 2000, 6, 2–12. [Google Scholar] [CrossRef]
- Ferrini, F.; Mattii, G.B.; Nicese, F.P. Effect of Temperature on Key Physiological Responses of Grapevine Leaf. Am. J. Enol. Vitic. 1995, 46, 375–379. [Google Scholar]
- Ollat, N.; Diakou-Verdin, P.; Carde, J.P.; Barrieu, F.; Gaudillère, J.P.; Moing, A. Grape berry development: A review. OENO One 2002, 36, 109–131. [Google Scholar] [CrossRef]
- Poni, S.; Lakso, A.N.; Turner, J.R.; Melious, R.E. The effects of pre- and post-veraison water stress on growth and physiology of potted Pinot noir grapevines at varying crop levels. Vitis 1993, 32, 207–214. [Google Scholar]
- Poorter, H.; Bühler, J.; van Dusschoten, D.; Climent, J.; Postma, J.A. Pot size matters: A meta-analysis of the effects of rooting volume on plant growth. Funct. Plant Biol. 2012, 39, 839–850. [Google Scholar] [CrossRef]
- Herrera, J.C.; Savi, T.; Rosner, S.; Forneck, A. The Size matters: Drought Stress Experiments with Potted Vines. In Proceedings of the 4th Xylem International Meeting, Padua, Italy, 25–27 September 2019; p. 64. [Google Scholar]
- Mattivi, F.; Guzzon, R.; Vrhovsek, U.; Stefanini, M.; Velasco, R. Metabolite profiling of Grape: Flavonols and Anthocyanins. J. Agric. Food Chem. 2006, 54, 7692–7702. [Google Scholar] [CrossRef] [PubMed]
- Boss, P.K.; Davies, C.; Robinson, S.P. Analysis of the expression of anthocyanin pathway genes in developing Vitis vinifera L. cv. Shiraz grape berries and the implications for pathway regulation. Plant Physiol. 1996, 111, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Falginella, L.; Castellarin, S.; Testolin, R.; Gambetta, G.; Morgante, M.; Di Gaspero, G. Expansion and subfunctionalisation of flavonoid 3′,5′-hydroxylases in the grapevine lineage. BMC Genom. 2010, 11, 562. [Google Scholar] [CrossRef] [PubMed]
- Bogs, J.; Ebadi, A.; Mc David, D.; Robinson, S.P. Identification of the Flavonoid Hydroxylases from Grapevine and Their Regulation during Fruit Development. Plant Physiol. 2006, 140, 279–291. [Google Scholar] [CrossRef]
- Downey, M.O.; Harvey, J.S.; Robinson, S.P. Synthesis of flavonols and expression of flavonol synthase genes in developing grape berries of Shiraz and Chardonnay (Vitis vinifera L.). Aust. J. Grape Wine Res. 2003, 9, 110–121. [Google Scholar] [CrossRef]
- Cavallini, E.; Matus, J.T.; Finezzo, L.; Zenoni, S.; Loyola, R.; Guzzo, F.; Schlechter, R.; Ageorges, A.; Arce-Johnson, P.; Tornielli, G.B. The phenylpropanoid pathway is controlled at different branches by a set of R2R3-MYBC2 repressors in grapevine. Plant Physiol. 2015, 167, 1448–1470. [Google Scholar] [CrossRef]
- Suzuki, M.; Nakabayashi, R.; Ogata, Y.; Sakurai, N.; Tokimatsu, T.; Goto, S.; Jasinski, M.; Martinoia, E.; Otagaki, S.; Matsumoto, S.; et al. Multiomics in Grape Berry Skin Revealed Specific induction of the Stilbene Synthetic Pathway by Ultraviolet-C irradiation. Plant Physiol. 2015, 168, 47–59. [Google Scholar] [CrossRef]
- Palumbo, M.C.; Zenoni, S.; Fasoli, M.; Massonnet, M.; Farina, L.; Castiglione, F.; Pezzotti, M.; Paci, P. Integrated Network Analysis Identifies Fight-Club Nodes as a Class of Hubs Encompassing Key Putative Switch Genes That Induce Major Transcriptome Reprogramming during Grapevine development. Plant Cell 2014, 26, 4617–4635. [Google Scholar] [CrossRef]
- Kawashima, C.G.; Yoshimoto, N.; Maruyama-Nakashita, A.; Tsuchiya, Y.N.; Saito, K.; Takahashi, H.; Dalmay, T. Sulphur starvation induces the expression of microRNA-395 and one of its target genes but in different cell types. Plant J. 2009, 57, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Tavares, S.; Vesentini, D.; Fernandes, J.C.; Ferreira, R.B.; Laureano, O.; Ricardo-Da-Silva, J.M.; Amancio, A. Vitis vinifera secondary metabolism as affected by sulfate depletion: Diagnosis through phenylpropanoid pathway genes and metabolites. Plant Physiol. Biochem. 2013, 66, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Belli Kulhan, J.; Paim Pinto, D.L.; Bertolini, E.; Fasoli, M.; Zenoni, S.; Tornielli, G.B.; Pezzotti, M.; Meyers, B.C.; Farina, L.; Pé, M.E.; et al. miRVine: A micro RNA expression atlas of grapevine based on small RNA sequencing. BMC Genom. 2015, 16, 393. [Google Scholar] [CrossRef] [PubMed]
- Ojeda, H.; Deloire, A.; Carbonneau, A. Influence of water deficits on grape berry growth. Vitis 2001, 40, 141–145. [Google Scholar]
- Keller, M. The Science of Grapevines: Anatomy and Physiology, 1st ed.; Elsevier Academic Press: Burlington, MA, USA, 2010; p. 400. ISBN 978-0123748812. [Google Scholar]
- Jaillon, O.; Aury, J.M.; Noel, B.; Policriti, A.; Clepet, C.; Casagrande, A.; Choisne, N.; Aubourg, S.; Vitulo, N.; Jubin, C.; et al. The grapevine genome sequence suggests ancestral hexaploidization in major angiosperm phyla. Nature 2007, 449, 463–467. [Google Scholar] [CrossRef]
- Velasco, R.; Zharkikh, A.; Troggio, M.; Cartwright, D.A.; Cestaro, A.; Pruss, D.; Pindo, M.; FitzGerald, L.M.; Vezzulli, S.; Reid, J.; et al. A high-quality draft consensus sequence of the genome of a heterozygous grapevine variety. PLoS ONE 2007, 12, e1326. [Google Scholar] [CrossRef]
- Lovisolo, C.; Perrone, I.; Carra, A.; Ferrandino, A.; Flexas, J.; Medrano, H.; Schubert, A. Drought-induced changes in development and function of grapevine (Vitis spp.) organs and in their hydraulic and non-hydraulic interactions at the whole-plant level: A physiological and molecular update. Funct. Plant Biol. 2010, 37, 98–116. [Google Scholar] [CrossRef]
- Palliotti, A.; Tombesi, S.; Frioni, T.; Famiani, F.; Silvestroni, O.; Zamboni, M.; Poni, S. Morpho-structural and physiological response of container-grown Sangiovese and Montepulciano cvv. (Vitis vinifera) to re-watering after a pre-veraison limiting water deficit. Funct. Plant Biol. 2014, 41, 634–647. [Google Scholar] [CrossRef]
- Castellarin, S.D.; Bucchetti, B.; Falginella, L.; Peterlunger, E. Influenza del deficit idrico sulla qualità delle uve: Aspetti fisiologici e molecolari. Acta Italus Hortus 2011, 14, 63–79. [Google Scholar]
- Palliotti, A.; Poni, S. Grapevine under light and heat stresses. In Grapevine in a Changing Environment: A Molecular and Ecophysiological Perspective; Gerós, H., Chaves, M.M., Medrano Gil, H., Delrot, S., Eds.; Wiley Blackwell: Oxford, UK, 2015; pp. 148–178. [Google Scholar] [CrossRef]
- Pulko, B.; Vršič, S.; Valdhuber, J. Influence of Various Rootstocks on the Yield and Grape Composition of Sauvignon Blanc. Czech J. Food Sci. 2012, 30, 467–473. [Google Scholar] [CrossRef]
- Bauerle, T.L.; Smart, D.R.; Bauerle, W.L.; Stockert, C.; Eissenstat, D.M. Root foraging in response to heterogeneous soil moisture in two grapevines that differ in potential growth rate. N. Phytol. 2008, 179, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Ferroni, G.; Scalabrelli, G. Effect of rootstocks on vegetative activity and yield in grapevine. Acta Hortic. 1995, 388, 37–42. [Google Scholar] [CrossRef]
- Keller, M.; Mills, L.J.; Harbertson, J.F. Rootstock Effects on Deficit-Irrigated Winegrapes in a Dry Climate: Vigor, Yield Formation, and Fruit Ripening. Am. J. Enol. Vitic. 2012, 63, 29–39. [Google Scholar] [CrossRef]
- Girona, J.; Mata, M.; Del Campo, J.; Arbonés, A.; Bartra, E.; Marsal, J. The use of midday leaf water potential for scheduling deficit irrigation in vineyards. Irrig. Sci. 2006, 24, 115–127. [Google Scholar] [CrossRef]
- Guidoni, S.; Ferrandino, A.; Novello, V. Effects of seasonal and agronomical practices on skin anthocyanin profile of Nebbiolo grapes. Am. J. Enol. Vitic. 2008, 1, 22–29. [Google Scholar]
- Castellarin, S.D.; Di Gaspero, G.; Marconi, R.; Nonis, A.; Peterlunger, E.; Paillard, S.; Adam-Blondon, A.F.; Testolin, R. Colour variation in red grapevines (Vitis vinifera L.): Genomic organisation, expression of flavonoid 3′-hydroxylase, flavonoid 3′,5′-hydroxylase genes and related metabolite profiling of red cyanidin-/blue delphinidin-based anthocyanins in berry skin. BMC Genom. 2006, 7, 12. [Google Scholar] [CrossRef]
- Castellarin, S.D.; Matthews, M.A.; Di Gaspero, G.; Gambetta, G.A. Water deficits accelerate ripening and induce changes in gene expression regulating flavonoid biosynthesis in grape berries. Planta 2007, 227, 101–112. [Google Scholar] [CrossRef]
- De Freitas, V.A.P.; Fernandes, A.; Oliveira, J.; Teixeira, N.; Mateus, N. A review of the current knowledge of red wine colour. OENO One 2017, 51, 1–21. [Google Scholar] [CrossRef]
- Mijowska, K.; Ochmian, I.; Oszmiański, J. Rootstock effects on polyphenol content in grapes of ‘Regent’ cultivated under cool climate condition. J. Appl. Bot. Food Qual. 2017, 90, 159–164. [Google Scholar] [CrossRef]
- Jeandet, P.; Delaunois, B.; Conreux, A.; Donnez, D.; Nuzzo, V.; Cordelier, S.; Clement, C.; Courot, E. Biosynthesis, metabolism, molecular engineering, and biological functions of stilbene phytoalexins in plants. Bio Fact. 2010, 36, 331–341. [Google Scholar] [CrossRef]
- Pastor, R.F.; Restani, P.; Di Lorenzo, C.; Orgiu, F.; Teissedre, P.L.; Stockley, C.; Ruf, J.C.; Quini, C.I.; Garcia Tejedor, N.; Gargantini, R.; et al. Resveratrol, human health and winemaking perspectives. Crit. Rev. Food Sci. Nutr. 2019, 59, 1237–1255. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zheng, Y.; Xin, H.; Fang, L.; Li, S. Comprehensive analysis of NAC domain transcription factor gene family in Vitis vinifera. Plant Cell Rep. 2013, 32, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, K.; Tran, L.S.; Van Nguyen, D.; Fujita, M.; Maruyama, K.; Todaka, D.; Ito, Y.; Hayashi, N.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Functional analysis of a NAC-type transcription factor OsNAC6 involved in abiotic and biotic stress-responsive gene expression in rice. Plant J. 2007, 51, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Tran, L.S.; Quach, T.N.; Guttikonda, S.K.; Aldrich, D.L.; Kumar, R.; Neelakandan, A.; Valliyodan, B.; Nguyen, H.T. Molecular characterization of stress-inducible GmNAC genes in soybean. Mol. Genet. Genom. 2009, 281, 647–664. [Google Scholar] [CrossRef] [PubMed]
- Chuck, G.; Candela, H.; Hake, S. Big impact by small RNAs in plant development. Curr. Opin. Plant Biol. 2009, 12, 81–86. [Google Scholar] [CrossRef]
- Mica, E.; Piccolo, V.; Delledonne, M.; Ferrarini, A.; Pezzotti, M.; Casati, C.; Del Fabbro, C.; Valle, G.; Policriti, A.; Morgante, M.; et al. Correction: High throughput approaches reveal splicing of primary microRNA transcripts and tissue specific expression of mature microRNAs in Vitis vinifera. BMC Genom. 2010, 11, 109. [Google Scholar] [CrossRef]
- Solofoharivelo, M.C.; Van der Walt, A.P.; Stephan, D.; Burger, J.T.; Murray, S.L. MicroRNAs in fruit trees: Discovery, diversity and future research directions. Plant Biol. 2014, 16, 856–865. [Google Scholar] [CrossRef]
- Pagliarani, C.; Vitali, M.; Ferrero, M.; Vitulo, N.; Incarbone, M.; Lovisolo, C.; Valle, G.; Schubert, A. The accumulation of microRNAs differentially modulated by drought is affected by grafting in grapevine. Plant Physiol. 2017, 173, 2180–2195. [Google Scholar] [CrossRef]
- Yang, Y.; Mao, L.; Jittayasothorn, Y.; Kang, Y.; Jiao, C.; Fei, Z.; Zhong, G.Y. Messenger RNA exchange between scions and rootstocks in grafted grapevines. BMC Plant Biol. 2015, 15, 251. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, Y.; Liu, Z.; Kong, D.; Duan, M.; Luo, L. Genome-wide identification and analysis of drought-responsive microRNAs in Oryza sativa. J. Exp. Bot. 2010, 61, 4157–4168. [Google Scholar] [CrossRef]
- Paim Pinto, D.L.; Brancadoro, L.; Dal Santo, S.; De Lorenzis, G.; Pezzotti, M.; Meyers, B.C.; Pé, M.E.; Mica, E. The influence of genotype and environment on small RNA profiles in grapevine berry. Front. Plant Sci. 2016, 7, 1459. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Fang, J.; Wang, C.; Yin, Y.; Sun, X.; Leng, X.; Song, C. Grapevine microRNAs responsive to exogenous gibberellin. BMC Genom. 2014, 15, 111. [Google Scholar] [CrossRef] [PubMed]
| Yield per Vine | Clusters per Vine | Cluster Weight | Berry Weight | Berry Diameter | Skin Weight | Seed Weight | Seeds per Berry | Pruning Wood Weight | |
|---|---|---|---|---|---|---|---|---|---|
| g | n | g | g | cm | g | g | n | g | |
| Root System | |||||||||
| M | 1028 | 12.4 | 79.31 | 1.26 | 1.00 | 0.129 | 0.032 ab | 2.32 a | 123.0 b |
| P | 872 | 10.3 | 74.95 | 1.20 | 0.99 | 0.128 | 0.034 b | 2.19 a | 176.8 c |
| NGC | 715 | 9.1 | 75.04 | 1.21 | 0.99 | 0.132 | 0.029 a | 2.67 b | 70.2 a |
| Water Protocol | |||||||||
| WS-S | 943 | 11.4 | 79.59 | 1.25 | 0.98 | 0.149 b | 0.031 | 2.29 | 99.5 a |
| WS-I | 768 | 10.2 | 72.81 | 1.20 | 0.99 | 0.127 ab | 0.033 | 2.36 | 97 a |
| WW | 904 | 10.2 | 76.89 | 1.23 | 1.00 | 0.110 a | 0.031 | 2.53 | 173.6 b |
| Root System | ns | ns | ns | ns | ns | ns | * | ** | ** |
| Water Protocol | ns | ns | ns | ns | ns | * | ns | ns | ** |
| A × B | ns | ns | ns | ns | ns | ns | ns | ns | ns |
| Sugars | Titratable Acidity | pH | Skin Anthocyanins | Skin Polyphenols | Seed Polyphenols | |
|---|---|---|---|---|---|---|
| °Brix | g/L tartaric acid | mg/kg grapes | mg/kg grapes | mg/kg grapes | ||
| Root System | ||||||
| M | 20.7 | 5.69 | 3.21 | 871 a | 1556 a | 4117 a |
| P | 20.2 | 5.73 | 3.19 | 1034 b | 1753 b | 4872 b |
| NGC | 21.0 | 5.62 | 3.17 | 945 ab | 1583 a | 4188 a |
| Water Protocol | ||||||
| WS-S | 22.4 c | 5.35 a | 3.30 c | 1146 c | 1738 b | 4782 b |
| WS-I | 21.0 b | 5.32 a | 3.20 b | 1002 b | 1798 b | 4431 b |
| WW | 18.5 a | 6.38 b | 3.07 a | 703 a | 1357 a | 3963 a |
| Root System | ns | ns | ns | * | * | ** |
| Water Protocol | *** | *** | *** | *** | *** | ** |
| A × B | ns | ns | ns | ns | ns | ns |
| Delphindin | Cyanidin | Petunidin | Peonidin | Malvidin | Trisubstituted Anthocyanins | Disubstituted Anthocyanins | Trisubstituted Disubstituted Ratio | |
|---|---|---|---|---|---|---|---|---|
| % | % | % | % | % | % | % | ||
| Root System | ||||||||
| M | 4.47 b | 2.21 b | 6.25 b | 29.15 b | 57.93 a | 68.64 a | 31.36 b | 2.21 a |
| P | 3.69 a | 1.43 a | 5.56 a | 23.96 a | 65.36 b | 74.61 b | 25.39 a | 3.19 b |
| NGC | 3.77 a | 2.03 b | 5.56 a | 28.71 b | 59.94 a | 69.26 a | 30.74 b | 2.41 a |
| Water Protocol | ||||||||
| WS-S | 3.61 a | 1.92 | 5.40 a | 30.24 b | 58.83 a | 67.84 a | 32.16 b | 2.20 a |
| WS-I | 4.15 b | 1.85 | 6.03 b | 26.16 a | 61.81 ab | 71.99 b | 28.01 a | 2.74 ab |
| WW | 4.15 b | 1.90 | 5.94 b | 25.42 a | 62.59 b | 72.68 b | 27.32 a | 2.87 b |
| Root System | * | *** | * | * | * | * | * | * |
| Water Protocol | * | ns | * | * | * | * | * | * |
| A × B | ns | ns | ns | ns | ns | ns | ns | ns |
| Procynidin B1 | Epigallocatechin | Catechin | Epicatechin | Quercetin | Myricetin | Kaempferol | Isorhamnetin | |
| HPLC Area | HPLC Area | HPLC Area | HPLC Area | HPLC Area | HPLC Area | HPLC Area | HPLC Area | |
| Root System | ||||||||
| M | 62 b | 49 | 26 | 35 b | 2262 | 1195 | 205 | 114 |
| P | 53 ab | 43 | 34 | 26 a | 1992 | 1056 | 177 | 112 |
| NGC | 45 a | 38 | 24 | 26 a | 2154 | 985 | 205 | 110 |
| Water Protocol | ||||||||
| WS-S | 55 | 41 | 21 | 33 | 1957 | 1080 | 212 | 118 |
| WS-I | 51 | 41 | 25 | 28 | 2066 | 1017 | 170 | 104 |
| WW | 54 | 47 | 37 | 26 | 2385 | 1139 | 206 | 113 |
| Root System | * | ns | ns | * | ns | ns | ns | ns |
| Water Protocol | ns | ns | ns | ns | ns | ns | ns | ns |
| A × B | ns | ns | ns | ns | ns | ns | ns | ns |
| Protocatechuic Acid | Trans-Caftaric Acid | Cis-Coutaric Acid | Trans-Coutaric Acid | Trans-Fertaric Acid | Polydatin | Resveratrol | Trans-ε-Viniferin | |
| HPLC Area | HPLC Area | HPLC Area | HPLC Area | HPLC Area | HPLC Area | HPLC Area | HPLC Area | |
| Root System | ||||||||
| M | 18 | 27 b | 155 | 112 | 39 b | 355 | 128 | 7 b |
| P | 15 | 24 ab | 113 | 94 | 29 ab | 287 | 101 | 5 a |
| NGC | 16 | 22 a | 111 | 98 | 26 a | 373 | 106 | 6 ab |
| Water Protocol | ||||||||
| WS-S | 19 | 25 | 120 | 104 | 34 | 348 | 137 b | 8 b |
| WS-I | 16 | 24 | 124 | 98 | 28 | 320 | 86 a | 5 a |
| WW | 14 | 24 | 135 | 103 | 31 | 347 | 112 ab | 6 ab |
| Root System | ns | * | ns | ns | * | ns | ns | * |
| Water Protocol | ns | ns | ns | ns | ns | ns | * | * |
| A × B | ns | ns | ns | ns | ns | ns | ns | ns |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zombardo, A.; Mica, E.; Puccioni, S.; Perria, R.; Valentini, P.; Mattii, G.B.; Cattivelli, L.; Storchi, P. Berry Quality of Grapevine under Water Stress as Affected by Rootstock–Scion Interactions through Gene Expression Regulation. Agronomy 2020, 10, 680. https://doi.org/10.3390/agronomy10050680
Zombardo A, Mica E, Puccioni S, Perria R, Valentini P, Mattii GB, Cattivelli L, Storchi P. Berry Quality of Grapevine under Water Stress as Affected by Rootstock–Scion Interactions through Gene Expression Regulation. Agronomy. 2020; 10(5):680. https://doi.org/10.3390/agronomy10050680
Chicago/Turabian StyleZombardo, Alessandra, Erica Mica, Sergio Puccioni, Rita Perria, Paolo Valentini, Giovan Battista Mattii, Luigi Cattivelli, and Paolo Storchi. 2020. "Berry Quality of Grapevine under Water Stress as Affected by Rootstock–Scion Interactions through Gene Expression Regulation" Agronomy 10, no. 5: 680. https://doi.org/10.3390/agronomy10050680
APA StyleZombardo, A., Mica, E., Puccioni, S., Perria, R., Valentini, P., Mattii, G. B., Cattivelli, L., & Storchi, P. (2020). Berry Quality of Grapevine under Water Stress as Affected by Rootstock–Scion Interactions through Gene Expression Regulation. Agronomy, 10(5), 680. https://doi.org/10.3390/agronomy10050680








