Abstract
Tomato (Solanum lycopersicum L.) is a high-value crop that has potential to enhance its P-use efficiency. While phosphorus (P) is an essential nutrient, supplies are finite and much of the P supply in agricultural soils is not bioavailable after application due to reactions such as soil adsorption, immobilization, or precipitation. Low-P bioavailability results in reduced growth, so plants may mobilize soil-bound P by altering root morphology, exuding root-derived compounds, or forming symbiosis with microorganisms. This review discusses (i) the significance of P in plants and agroecosystems, (ii) within-plant response to changing P bioavailabilities, and (iii) strategies to enhance P-acquisition efficiency (PAE). Phosphorus forms fluctuate in the soil and potential approaches to increase the bioavailable pool of P may focus on processes such as desorption, mineralization, or dissolving precipitated P-compounds. To enhance these processes, roots may alter their spatial arrangement, exude protons to acidify the rhizosphere, exude carboxylates to solubilize bound-P, exude phosphatase to mineralize organic P, or enhance symbiosis with native microbes. High PAE allows for use of accumulated soil P as opposed to relying on fertilizer application to meet crop demand.
1. Introduction
Phosphorus (P) is one of 17 essential elements that plants require to develop and function, so flexibility in plant metabolism and bioenergetics may help crops cope with low-P conditions [1,2]. Phosphorus is taken up as acid orthophosphate anions (primarily as H2PO4− at pH < 7.2 or as HPO42− at pH > 7.2) and is an integral part of the chemical structures of (i) adenosine mono-, di-, tri-phosphate (AMP: C10H14N5O7P, ADP: C10H15N5O10P2, ATP: C10H16N5O13P3), (ii) nucleic acids, and (iii) phospholipids. Phosphorus is involved in the regulation of metabolic pathways such as energy transfer, protein activation, and amino acid synthesis [1,2].
Phosphorus deficiency can negatively impact crop yield and, in severe deficiency, can lead to death. However, basing nutrient management on visual deficiency symptoms is unreliable. For example, anthocyanin accumulation is typically associated with a deficiency in P, but may not be a quality indicator on its own. In their review of foliar anthocyanin, Close and Beadle [3] outline a range of contexts for accumulation of this compound; anthocyanin may accumulate in response to ultraviolet (UV) radiation, browsing herbivores, or pathogenic fungi in addition to nutrient deficiency. Instead of visual symptom reliance, soil and tissue testing may provide a reliable analysis of soil P content to establish fertility guidelines. Optimal plant P concentrations typically range from 0.1% to 0.5%, averaging approximately 0.2% [2]. The critical leaf concentration associated with a 10% reduction in dry matter yield for perennial ryegrass (Lolium perenne L.) is 0.0021% [4] and for dwarf saltwort (Salicornia bigelovii Torr.) is 0.00078% [5]. The critical value for P may decline with age as shown by purple bush bean (Macroptilium atropurpureum Moc. and Sessé ex DC), where the critical concentration decreased from 0.03% at 41 days to 0.01% at 77 days [6]. The range of shoot P content tends to be higher on average for crops inoculated with mycorrhizal fungi (0.3–0.4%) compared to uninfected plants (0.1–0.3%) [7]. P-toxicity symptoms typically manifest when leaf-P concentration exceeds 1% dry weight [8], and this has been shown with tomato [9]. Fageria et al. [10] outlines P toxicity symptoms as increased red speckling in sorghum (Sorghum bicolor L.) and reduced yield in sorghum and cotton (Gossypium hirsutum L.).
Crops may cope with low-P by enhancing P-use efficiency (PUE). High PUE necessitates both elevated P acquisition by the roots and enhanced use of P in processes resulting in healthy growth. PUE may be described as the sum of P-acquisition efficiency (PAE) (ability to acquire P from the soil) and P-utilization efficiency (PUtE) (ability to internally use P to result in better growth). A higher PUE could be achieved by selecting for traits that boost either PAE or PUtE. To genetically improve a crop’s P-use efficiency, there needs to be an emphasis on enhancing root biology which could lead to reduced fertilization and expanding agriculture to low-P soils [11].
Tomato (Solanum lycopersicum L.) is a high-value perennial vegetable crop in its native habitat, but is commonly grown as an annual. Tomato is a member of the family Solanaceae, the subfamily Solanoideae, and the tribe Solaneae. The taxonomy has fluctuated between the Linnaeus identification of Solanum lycopersicum in 1753 and the Miller identification of Lycopersicum esculentum in 1768 [12]. The leaves are alternate in arrangement, simple in complexity, and pinnate in venation. Tomato plants possess a showy single flower that is bisexual, radially symmetric, and yellow. The corolla of the flower may fuse forming a corolla tube. The corolla typically contains five connate petals. The calyx has five persistent, connate sepals. The five distinct stamens that open by slits are adnate to the corolla and alternate with petals. Each flower has two carpels and a superior ovary; the placentation is axile. Tomato fruits are multi-seeded berries with small, flat seeds [13]. Many tomatoes are self-pollinating; others may cross-pollinate by anemophily (wind pollination) [14].
Tomato is a nutrient-dense and healthy vegetable. Tomato fresh tissue contains 5.0–7.5% dry matter that is mostly composed of fructose (25%) and glucose (22%) [15]. Tomato also contains four major carotenoids: alpha-carotene, beta-carotene, lutein, and lycopene [14,16,17]. Tomato health benefits include decreasing the risk of cancers (e.g., pancreatic and esophageal) and cardiovascular disease [16]. Tomato is one of the most often cultivated vegetable crops worldwide due to its numerous health benefits and high demand [18]. Worldwide tomato production was across over 5.8 million ha with a production quantity of nearly 244 million tonnes in 2018. The top five tomato producing countries in 2018 were China (approximately 60.6 million tonnes), India (approximately 19.4 million tonnes), United States (approximately 12.6 million tonnes), Turkey (approximately 12.2 million tonnes), and Egypt (approximately 6.6 million tonnes) [19].
There are few polymorphisms in tomato, but there is high morphological variation that is readily detected in self-pollinated varieties. Goals of tomato breeding programs vary widely by location and individual needs, but overarching goals have tended to be the optimization of yield in the 1970s, shelf-life in the 1980s, taste in the 1990s, and nutritional quality in the 2000s. A prominent issue for tomato breeders today is selecting for resistance to pests and pathogens [20]. While an important issue, tomato breeding goals tend to not focus as much on enhancing nutrient-use efficiency. There may be genotypic diversity in tomato regarding P-use efficiency as exemplified by Coltman et al. [21] who noticed an increase in dry weight of 77% among efficient tomato compared to inefficient tomato when growing in low-P conditions.
Crop PAE augmentation seems possible through regulating traits such as microbial symbiosis, root hair development, or organic acid exudation. An enhanced PAE could allow crops to survive in soil by mobilizing P that would otherwise be unavailable to the plant. The purpose of this review is to comprehensively analyze literature on low-P stress-induced mechanisms to better understand traits and practices that may enhance tomato PAE. This review delineates (i) the role of P in an agroecosystem, (ii) tomato plant response mechanisms to low-P stress, and (iii) PAE enhancing strategies. Potential strategies to heighten PAE include rapid P sensing, optimization of root growth to exploit a given volume of soil, exudation of root-derived compounds, and microbial symbiosis. Understanding the structure–function relationships of morphological and physiological adaptations to low-P stress opens opportunities to increase PAE and agricultural sustainability.
2. Phosphorus in an Agroecosystem
2.1. Phosphorus Pools and Sources
As opposed to the cycles of other nutrients like nitrogen (N) [22], sulfur (S) [23], and carbon (C) [24], there is no gaseous form of P available for fixation. Therefore, P is typically supplied to plants through fertilizer application either by foliar application to the leaves and stems or to the ground, usually by banding. Although foliar fertilization is typically used for micronutrients [25], there are still benefits to using foliar sources of P. Salt-stressed tomato (60 mM NaCl applied via foliar application twice each week) recovered from P-deficiency, increased dry matter production, and increased chlorophyll concentration as a result of foliar application of supplemental P [26]. Similarly, common bean was also shown to optimize performance in saline soil when supplied with foliar P fertilizer (10 mM MAP) [27]. Additionally, Mosali et al. [28] concluded from their experiments with winter wheat (Triticum aestivum L.) that low rates of foliar P application may ameliorate mid-season (Feekes 10.54) P-deficiency and ultimately, lead to greater yield. Phosphorus in conventional fertilizer is categorized based on solubility: (i) water soluble P, (ii) citrate soluble P (iii) citrate insoluble P, (iv) bioavailable or phytoavailable P, and (v) total P (often expressed as P2O5) [2]. Fertilizers tend to resupply bioavailable pools of P through dissociating into orthophosphate and the dominant cation in the fertilizer.
Calcium phosphates (single/triple superphosphate) were the most used inorganic P fertilizers until the 1970s when ammonium phosphates (mono/di/urea ammonium phosphate, ammonium polyphosphate) became more popular. Potassium phosphates, including mono- and dipotassium phosphate, are highly water soluble and have high P and K concentrations. Phosphoric acid is another popular inorganic fertilizer source derived from reacting rock phosphate with sulfuric acid. Phosphorous acid (HPO32−), also known as Phi, phosphite, or phosphonate, is an isotere of phosphate where an oxygen (O) is replaced by hydrogen (H) [29]. Unlike phosphoric acid, phosphorous acid has been shown to repress P limitation responses such as root hair initiation [29,30]. Plants lack an ABC-type Phi uptake system, a complex that is present in the genomes of organisms able to oxidize Phi to phosphate. Soil bacteria including Desulfotignum phosphitoxidans, D. balticum, and D. toluenicum are able to oxidize Phi (via oxidase) to phosphate [31]. Phi is typically used as a fungicide and may have preventative efficacy towards Phytophthora vectored diseases [32]. Beneficial effects of Phi on tomato growth have been observed, but only when Phi was supplied with phosphate at equal P concentrations of 20 mg/L [33,34]. Rock phosphate is the primary material used to manufacture P fertilizers. Rock-phosphate typically contains 25% to 36% P2O5 with citrate solubility ranging from 3–20% total P. Finely ground rock phosphate reacts as follows [2]:
While the most cost-effective current means of producing P fertilizers is mining phosphate rock, researchers such as Cordell et al. [35] have noted that the rising price and scarcity of phosphate rock coupled with eutrophication concerns necessitates increased agricultural efficiency (increasing crop yields per unit input of P). These researchers call for integrated nutrient management plans that meet crop demand through utilization of a range of inorganic and organic sources and improving timing and rates of fertilization, enhancing chemical and physical properties of soil, and stimulating microbial symbiosis in the rhizosphere [35]. We must, therefore, shift reliance from nonrenewable mineral resources and instead to accumulated soil P supplies. Soil P concentration typically ranges from 0.003 ppm to 3 ppm, usually averaging 0.05 ppm [2]. Depending on soil test results (Mehlich 3 soil extraction method), UF IFAS recommended P fertilization as P2O5 for tomato production is 120–150 lb/A for low, 100 lb/A for medium, and 0 lb/A for high soil test index [25].
Organic P amendments have variable P content ranging from 0.1% (lawn clippings) to 7% (biosolids) dry matter [2]. Decomposable plant residue may be turned into stable organic humus through the activities of fungi, bacteria, protozoa, and other decomposing organisms. The range of organic P is variable, but typically represents 50% total soil P. Organic P content decreases with depth and with decreasing soil organic carbon [2].
2.2. Soil Phosphorus Fluxes
Common reactions of the P cycle include chelation, precipitation, adsorption, and mineralization. A chelating agent is a multi-dentate ligand that is able to donate pairs of electrons to metal ions and form stable metal complexes. Natural (such as citrate) or synthetic (such as Ethylenediaminetetraacetic acid (EDTA)) chelating agents interact with P-binding sites to liberate bound P [36,37]. A precipitation reaction occurs when a cation and anion interact in aqueous forms to produce a solid or a semi-crystalline structure such as aluminum (Al) or iron (Fe) phosphates [38]. Solution phosphate may precipitate with mineral nutrients such as calcium (Ca) [36]:
While adsorption removes P from pools available for plant uptake, it also removes P from bodies of water, thereby controlling for eutrophication in water bodies. Solution phosphate may adsorb onto metal oxides and metal hydroxides. Removal efficiency depends on environmental conditions (pH, ion strength, competitive ions, dosage, phosphate concentration, temperature) and sorbent chemical (functional groups, metal content, stability) and physical (particle size, specific surface area) characteristics [39,40]. Phosphate adsorption to hydrous Al-oxide was shown to be favored at low pH values [41]. Solution pH may influence the form of bioavailable P as (i) H3PO4 below pH 2.12, (ii) H2PO4− between pH 2.12 and 7.21, (iii) HPO42− between pH 7.21 and 12.44, and (iv) PO43− above pH 12.44 [2]. Adsorption isotherms may help explain interactions between sorbent and adsorbate. Common isotherms include the Langmuir and Freunlich equations. The Langmuir model assumes adsorption occurs at homogenous sites in monolayer adsorption processes, whereas the Freundlich model assumes adsorption occurs at heterogenous sites. Phosphate adsorbents for metal oxides/hydroxides fitting a pseudo-first-order, a pseudo-second order, and an Elovich kinetic model include sepiolite/Al oxide hydroxide, ferric sludge/iron activated carbon fiber, and Mn-Al oxide/Al- and Fe-montmorillonite, respectively [40]. Solution phosphate may also adsorb onto Ca-minerals such as calcite. Electron probe micro-analysis revealed that coral-like growths of Ca-phosphates (predominantly dicalcium phosphate with octa-calcium phosphate present) accumulate on calcite surfaces with exchangeability of reacted P ranging from 30% to 100% depending on amount of P present [42].
Mineralization and immobilization are some of the main reactions involved with microbes turning over residue for nutrient cycling. Immobilization occurs when inorganic compounds become incorporated into organic forms. Phosphatase catalyzes mineralization of organic to inorganic P forms [2]:
Soil P can be taken up by roots, leached into waterways, adsorbed onto mineral surfaces, or immobilized into organic forms. Soil P reaches the roots by mass flow (less often) and diffusion (more often) [2]. Soil P can be resupplied by fertilizer application, organic amendment application, residue decomposition, or primary mineral weathering [2].
Surface adsorption and precipitation reactions are jointly referred to as P fixation or P retention reactions. Solution P may adsorb onto clay and mineral surfaces and then desorb back into solution. Solution P can also precipitate onto secondary P minerals which can then dissolve back into solution P. Phosphate may precipitate with Al or Fe minerals at low pH and Ca or magnesium (Mg) minerals at high pH [2,43,44]. Ferric iron phosphates dominant at low pH may solubilize to ferrous iron through activity of reductants such as glucose with added C inputs [45]. Fixation reactions may be impacted by clay minerals, soil pH (general P availability being the greatest at pH 6.5), neighboring anion effects (competition for adsorption sites), soil organic matter (organic compounds tend to increase P availability), time (rapid initial reactions followed by slower reactions), temperature (an approximate doubling of mineralization rates with each 10 °C increase in temperature), flooding (bioavailable P increases after flooding), and counter cation characteristics (divalent cations on cation exchange capacity (CEC) increase P adsorption greater than monovalent cations). There is greater adsorption activity in clays saturated with Ca+2 compared to sodium (Na+) because divalent cations increase availability of positively charged clay mineral edges, particularly below soil pH 6.5 [2].
Solution P immobilization to organic P and mineralization to inorganic P is driven by soil microorganisms. Mineralization and immobilization occur simultaneously, and equilibrium is determined by the carbon-to-phosphorus ratio (C:P). When C:P is less than 200:1, between 200:1–300:1, or greater than 300:1, there is net mineralization of organic P, no gain or loss of inorganic P, and net immobilization of inorganic P, respectively [2].
Phosphorus loss from crop removal typically ranges from 11.2 to 44.8 kg P/ha (10–40 lbs P/acre) [2]. Phosphorus losses from leaching by irrigation/rainfall are relatively small because while mobile within the plant, fertilizer P is generally considered immobile in soil [46,47]. The range of the diffusion constant for H2PO4− is 10−12 to 10−15 m2/s. The average rate of diffusion is 0.13 mm/day. Leached dissolved reactive P and erosion of P deposits from acidic waters may result in contamination or eutrophication [48], resulting in nutrient loss from the agroecosystem and possible ecological damage to aquatic ecosystems.
2.3. Phosphorus Bioavailability Across Different Soils
Phosphorus bioavailability is related to soil characteristics such as silt content, clay content, pH, extractable Al, and extractable Fe [49]. An appropriate metric for determination of P sorption is the distribution coefficient, Kd that describes the ratio of contaminant concentration (including cations, anions, radionuclides, redox-sensitive elements) to the contaminant concentration in the surrounding aqueous solution at equilibrium [50]. A greenhouse pot experiment examining biosolid-amended acidic Greek Alfisols found that high-dose application of biosolids (300 t/ha sewage sludge) resulted in increased pH from 5.19 to 6.92 and decreased Kd from 23.3 to 12.9 L/kg [51]. Relatively fertile Alfisols commonly form over calcareous till and tend to have an accumulation of clay [2,52]. This soil type tends to occupy cool and humid regions of the Northern Hemisphere [53]. Alfisols from Southeast Sudan were found to have low available and organic P content with 40% inorganic P forms in the Fe-Al and Ca-P fractions [54].
Andisols develop from volcanic ash, and commonly occur in the Pacific Northwest in the United States [2,53]. In the native volcanic ash-derived Andisol soils of the Mexican highlands, it was found that over 19% of 32P was recovered in bicarbonate organic P and organic sodium hydroxide forms which indicates that organic P cycling and ecological-based management systems are crucial to maintain the supplies of soil organic P and sustain agricultural production [55]. Soils with volcanic origin tend to have strong P fixation with dominant active Al in amorphous clay minerals. For instance, New Zealand andic horizons have high P retention with typically 85% added P becoming sorbed to soil colloids [52]. The Fe-rich Andisols of Hawaii tend to have predominant fixation reactions that may necessitate fertilizer of greater than 1000 k/ha [52].
Aridisol soils tend to form in arid environments such as the western United States with predominant calcium carbonate (CaCO3) compounds [2,53]. Aridisol samples gathered from the North Kordofan state of Sudan were found to have low P content, both available and organic. Most of the inorganic forms were present in the Fe-Al fraction (>50%) as opposed to the Ca-P fraction (20%), so available P was positively related to the Al+Fe-P content of total-P, but negatively correlated with Ca-P content of total-P [54]. Similar results were found for Vertisol samples collected from the Gezira state in Sudan in which there was low organic and available P content. However, the Vertisol samples had the most inorganic P present in the Ca-P fraction (>60%) and less in the Fe-Al fraction (40%) and, therefore, had a more alkaline pH (>8.0) [54]. Vertisols tends to be rich in clay content, which tends to result in shrinking and swelling of the soil with changes in moisture content [2].
Entisols tend to be sandy with no developed horizons and occur in places such as the African Sahara [2,53]. This soil was studied using sequential fractionation and researchers concluded that fertilization greatly impacted P availability where fertilization favored mineralization and redistributed organic and inorganic P fractions, between labile and non-labile pools and, ultimately, resulted in an accumulation of inorganic soil P [56]. While high inorganic P levels in the labile compartment may contribute to increased bioavailable pools, it also represents a risk of P-contamination to waterbodies. Esmail et al. [57] also found high inorganic P content, particularly in Ca-P fractions. These studies investigated Entisols of the Kurdistan region of Iraq and noted predominate fixation reactions from the high CaCO3 content of the regional soils. Esmail et al. [57] also observed high CaCO3 content in Inceptisols and Mollisols in the same region. Inceptisols are characterized by minimal horizon development commonly occurring in Mediterranean soils, while Mollisols are characterized by a prominent dark horizon with high soil organic matter content common to grasslands and the Great Plains of the United States [2,53]. Fertilization rates of 320 kg/ha P2O5 were found to yield both the greatest dry matter accumulation and available P concentration [57]. This study concluded by determining there to be a low P bioavailbility in Inceptisol samples and high P bioavailability in Mollisol samples, which is likely due to the high organic matter content in the Mollisol soil.
Gelisols from permafrost and Histosols from wetlands have high organic matter content, often with a surface organic layer greater than 40 cm [2,52]. Phosphorus tends to be a limiting factor to growth in these soils [58]. Histosols tend to have high water and nutrient holding capacity and form organo-P complexes [52]. Antarctic Gelisols are weakly developed soils whose chemical weathering processes are likely a result of phosphate-containing guano deposits [59].
Acidic Spodosols tend to have high soil organic matter content as well as typical Al and Fe accumulation and are commonly spread across Northern Europe [2,53]. While Spodosols of the Cook Inlet region of Alaska tend to be fertile, P-deficiency may occur from the P-fixing capacity of the allophone-imogolite minerals and Fe-hydroxides [52]. New England Spodosols are characterized by high water retention and abundant poorly crystalline structures with variable charge that are responsible for substantial P fixation [52].
Oxisols and Ultisosls are highly weathered soils that tend to have significant anion exchange capacity and high P fixation potential [52,60]. Oxisols tend to have high Al and Fe content and are the predominant soil order in Brazil [2,53]. Highly weathered Brazilian Oxisol samples were shown to have spatially dependent P availability that could be predicted by Fe-oxide content and magnetic susceptibility [61]. Ultisols occur in the humid regions of the Southeastern United States and Southeastern China, as well as the tropical regions of South America and Africa [53].
2.4. Phosphorus Uptake and Integration
Phosphorus uptake is an energy-mediated process [62]. It is comprised of multiple epidermally located transport systems driven by a proton gradient generated by H+-ATPase across the plasma membrane [63]. The constant Km can help describe the kinetics of phosphate transport across a steep concentration gradient. Numerically, Km equals the concentration of solute that yields half the maximum rate of transport. Low Km values indicate high binding attraction of the transported compound to the transport site, whereas high Km values indicate a lower binding attraction [64]. High-affinity phosphate transporter systems have low Km values typically between 2.5 and 12.3 µM and low affinity systems typically have higher Km values between 50 to 100 µM [65].
While high-affinity transporters are inducible in conditions of low-P, low-affinity transporters are unaffected by P status. In a study with ryegrass (Lolium perenne L.), the functional characterization and expression of two members of the PHT1 family were analyzed and it was determined that LpPHT1;4 was a high-affinity transporter influenced by P status and LpPHT1;1 was a low-affinity transporter not correlated with P status [66].
High-affinity transporters may be suppressed because overexpression of these transporters may lead to an over-accumulation of P [66]. The PHO-4 gene is highly stimulated by the addition of Na+, suggesting that PHO-4 is a Na+/P symporter of fungal origin [67]. Members of the PHT1 family utilize symport (co-transport) as means to move ions across the steep electrochemical gradient caused by coupling ATP hydrolysis with proton transport, and stoichiometry of a proton-phosphate symport system is typically 2 to 4 H+/H2PO4− [68,69].
Table 1 outlines the affinity and location of five phosphate transporter families. Phosphate transporter 1 family (PHT1) is involved in the initial uptake and remobilization of P [70]. Phosphate transporter 2 family (PHT2) impacts P-allocation through moderating P-starvation response genes and the translocation of P within leaves [71]. Phosphate family 3 (PHT3) includes an H+ symport and an OH- antiport [72]. Phosphate family 4 (PHT4) has been shown to mediate P transport in yeast (Saccharomyces cerevisiae) with a great specificity [73]. Phosphate transporter 5 family (PHT5) functions as vacuolar-P transporters to regulate cytoplasmic homeostasis [74].

Table 1.
Notes on the major phosphate transporter families, PHT1, PHT2, PHT3, PHT4, and PHT5.
Tomato high-affinity phosphate transporters LePT1 and LePT2 consist of 12 membrane-spanning regions and have a high degree of sequence identity to other high-affinity phosphate transporters. The percentage of amino acid identity between LePT1/LePT2 and AtPT1 (from Arabidopsis (Arabidopsis thaliana L.)), AtPT2 (from Arabidopsis), STPT1 (from potato), STPT2 (from potato), and PIT1 (from Catharanthus roseus L.) is 78.4/77.2, 82.9/75.9, 95.5/80.0, 78.0/95.0, and 86.4/76.8, respectively [83]. LePT1 and LePT2 are induced by low-P conditions [84]. Increased expression of these transporters was detected when plants were supplied with 100 µM P or less [83]. LePT1 and LePT2 are expressed in roots and LePT1 is also minimally expressed in leaves, stems, and petioles of P-starved (0 µM P) tomato plants. High transcript activities of both transporters were observed in root epidermal cells and lower levels of LePT1 were observed in the central cylinder and LePT1 message accumulation was detected in leaf palisade parenchyma and phloem cells [83].
Yeast has been an important model organism for identifying high-affinity phosphate transporter genes [75]. PHO84 represents a yeast high-affinity phosphate transporter homologous to the PHT1 family of P transporters [76]. As reviewed by Nussaume et al. [65], PHO84 identification allowed for further identification of homologous transporters in other species such as Neurospora crassa, mycorrhizal fungus Glomus versiforme, and Arabidopsis. Homologs have also been identified in potato (Solanum tuberosum L.) [85].
Transporters from the Pht1 family have been found in barley (Hordeum vulgare L.). The HvPHT1;1 barley transporter is induced by P-deficiency and initiates trichoblast expression in the root epidermis [86]. HvPHT1;2 expression in the root hair zone is induced in P-starved conditions [87]. HvPHT1;6 represents a low-affinity P transporter expressed in barley root and shoot to remobilize P. Upregulation of HvPHT1;6 has been associated with greater PUtE [88]. Arabidopsis P transporter gene expression of AtPT2 and tomato expression of TPSI1 are inducible upon low-P and repressible upon sufficient-P. Increases in transcripts of AtPT2 have occurred when external P concentration was below 50 µM. TPSI1 is predominantly expressed in root tissue and other abiotic stresses have little to no impact on expression of this gene [89]. In wheat, TaPT2 represents a high-affinity transporter gene that increases expression in the roots of P-deficient plants [90]. Phosphate transporters show a great degree of conservation among species, underscoring opportunities for identification and selection.
P-uptake is impacted by cross talks with other nutrients such as zinc (Zn) and Fe. Phosphorus deficiency results in an over-accumulation of Zn in shoots and vice versa. The genes PHO1 and PHO1;H1 (for P) and HMA2 and HMA4 (for Zn) have been identified as a set of co-expressed genes responsible for P and Zn loading into the root xylem. There is direct correlation from co-expression analysis for PHO1 and HMA4 and indirect correlation for PHO1;H1 and HMA2, confirming existence of crosstalks between the Zn and P regulatory networks [91,92]. Furthermore, P uptake is enhanced in Fe deficiency, and conversely, P-deficiency enhances Fe availability within plants. Gene expression of FER1, which encodes Fe protein (ferritin) storage responds to low-P as mediated by PHR1 and Fe excess [92].
Once taken up, P may either (i) move via symplastic pathways from the roots to xylem parenchyma cells, (ii) enter the metabolic pool, (iii) become a structural component of the cell, (iv) efflux in high-P conditions, or (v) be stored in the vacuole [62,93]. The rice phosphate transporter OsSPX-MFS3 may be responsible for P efflux from the vacuole; PHT1;5 may be responsible for phloem loading [69]. In P-sufficiency, the majority of root-absorbed P is transported through the xylem to new leaves. In P-deficiency, xylem-supplied P is restricted, and P is supplemented from old leaves. Xylem P concentrations range from 1 mM (in P starved plants) to 7 mM (in plants supplied with 125 µM P). Phosphate concentrations in the phloem range from 0.35 to 0.55 mg/mL [64].
3. Plant Physiological Responses to Low Phosphorus Stress
The standard route for P uptake, translocation, and utilization may need to be altered to allow for growth in stressful conditions of P-deficiency. The following section illustrates the processes that occur in plants when grown in P-starved media.
3.1. Internal Phosphorus Sensing
The first step in a P-deficiency-induced response is sensing when supplies are depleted. Results from a split root study with tomato indicated that transcript levels of tomato phosphate transporters (LePT1 and LePT2) increase from a combination of depleted external P supply and internal P reserves; even when P was supplied only to a portion of the root, phosphate transporter expression did not increase in the portions exposed to P-deficient conditions [83]. Plants sense a within-plant deficiency when vacuolar supplies diminish with a decrease of shoot P supply, resulting in decreased photosynthesis, glycolysis, and respiration [94]. Phosphorus sensing occurs at the root tip, within leaf cells, and through the vascular system. Breeding to fine-tune low-P sensing mechanisms could enhance the overall P-deficiency response and nutrient use efficiency.
Shoot-derived microRNA (miR399) mediates PHO2 mRNA turnover in P-limitation, indicating that microRNAs may work as P-deficiency signaling agents [70]. PHR1 is responsible for upregulation of AtSPX to induce P-starvation coping mechanisms [95]. The PHR1 binding site has related sequences that are highly responsive to P-deficiency including TPSI1 (from tomato), Mt4 (from Medicago truncatula Gaertn), OsPI1 (from rice), and At4 and AtIPS1 (from Arabidopsis) [96,97].
Signaling at P-sensing has been explored by Ham et al. [70]. These researchers found that upon sensing low external P, LOW PHOSPHATE ROOT 1 (LPR1) blocks symplastic communication within the stem-cell niche (SCN) in the root apical meristem (RAM), locally regulating growth in low-P conditions. The transcription factor, SENSITIVE TO RHIZOTOXICITY (STOP1), controls ALUMINUM ACTIVATED MALATE TRANSPORTER 1 (ALMT1) expression, which secretes malate into the apoplast. The secreted malate results in reactive oxygen species (ROS) production and callose deposition in SCN, disrupting plasmodesmata mediate direct cell-to-cell communication and primary root growth. Shin et al. [98] reported an increase in ROS in the cortex of root cells in P-deficiency, a finding in agreement with Ham et al. [70]. ROS may act as a signaling molecule. Sensitivity to P-deficiency would be advantageous, and, therefore, investigations into ROS-responsive genotypes could be of interest.
3.2. Phosphorus Reprioritization
Phosphorus deficiency is typically accompanied by within-plant remobilization of P from senescent to developing tissue [71,88]. Robinson et al. [99] furthers this idea through studying delayed leaf senescence in Arabidopsis and ultimately, claimed that P-efficiency can be enhanced through improved remobilization. Within-plant P is mobile and P recycling is influenced by source and sink relationships, with sinks being young leaves and sources being older leaves [68].
Li et al. [100] investigated source to sink relationships in P-deficiency and found that a rice phosphate transporter (OsPht1;8) redistributed P from old to young leaves and from endosperm to embryo in seeds. Phosphorus-efficient Banksia species (Proteaceae) remobilized P resulting in a leaf-P concentration of 0.027 to 0.196 mg/g P dry matter (DM) after an initial concentration of 0.14 to 0.32 mg/g P DM [101]. Remobilization of internal P is an effective way to temporarily ensure maximum use of P reserves. This strategy may result in reduced growth rates, decreased vacuolar P content, and reduced P nucleic acid pools, indicating that it is not sustainable long-term [62]. Proteaceae members are non-mycorrhizal plants that generally have high P-efficiency partly due to their high P-remobilization efficiency [102]. Besford [103] noted P-deficiency induced nutrient remobilization in tomato. This study found mobilization and a rapid net export of P from the leaves of tomato plants transferred from a medium with 2340 ppm (2.34 kg/m3) of superphosphate (Ca(H2PO4) to a medium with no superphosphate. Similarly, Irshad et al. [104] tested cotton (Gossypium sp.) and found that P remobilization enabled efficient cultivars to establish a better rooting system when grown without added P for 30 days after an initial period of optimal P nutrition.
Intracellular acid phosphatase (IAP) is ubiquitous in vascular plants and activity is a helpful marker of P-deficiency. IAP functions to recycle P from expendable intracellular organophosphate pools. A study with tomato suspension cells revealed expression of a low-P induced IAP composed of a 1:1 ratio of 63 and 57 kDa subunits [105]. Because of the IAP-catalyzed P remobilization, tomato seedlings utilized stores of phytic acid (IAP upregulation corresponded to 20 fold reduction in intracellular free phosphate levels) and avoided morphological and biochemical symptoms of P-deficiency during the first 10 days of growth [105].
Membrane lipid composition may change in a P deficit because phospholipids could be used as P reserves [106,107]. Phosphatase drives remobilization of organic P sources by catalyzing the hydrolysis of orthophosphate-monoesters and anhydrides [94]. The PHOSPHATE2 (PHO2) and RNA isomers are principle regulators of within-plant P remobilization; microRNA399 (miR399) targets pho2 that negatively regulates P uptake resulting in increased plant P uptake and microRNA827 (miR827) interacts with the SPX-MSF genes to moderate P sensing and homeostasis [108].
3.3. Cellular Phosphorus Homeostasis
The primary cellular component for P storage is the vacuole [62,109]. Excess P gets stored in organic compounds such as phytic acid in the vacuoles of leaf cells [110]. The vacuole typically stores 85–95% total P [111]. A protoplast study found that P-sufficient vacuoles contained 87–94% total cell-P [112]. Phosphate influx transporters across the tonoplast (vacuolar membrane) are active in conditions of sufficient P, and in low P, vacuolar P efflux is active [69]. Passage of P across the tonoplast regulates cytoplasmic-P levels and buffers against fluctuations of external P and metabolic activities [74]. Glycine betaine (GB)-regulated phosphate homeostasis in tomato transformed with a choline oxidase gene (codA from Arthrobacter globiformis) resulted in a more resistant tomato to low-P stress compared to the wild type in part due to the differential expression of the ‘PHO regulon’ genes to maintained intracellular phosphate homeostasis [113].
Low cytoplasmic-P concentrations cause an inhibition of ATP synthesis, deactivation of RuBisCO (Ribulose-1,5-bisphosphate carboxylase/oxygenase), and accumulation of RuBP (Ribulose-1,5-bisphosphate) [114]. The metabolically active cytoplasmic P pool tends to range between 10 and 15 mM [71].
4. Strategies to Enhance PAE
There is potential to select for high PAE because many genes alter expression in a P deficit. For example, approximately 29% of Arabidopsis genes microarrayed were up- or down-regulated by a factor or two or more during a P deficit [115]. In yeast, 22 genes from a whole genome microarray were shown to be regulated by the PHO pathway [116]. There may be opportunities for screening genotypes effective in strategies that acquire sparingly available P including root morphological responses to low soil-P, exudation of root derived compounds, and microbial symbiosis (Figure 1).
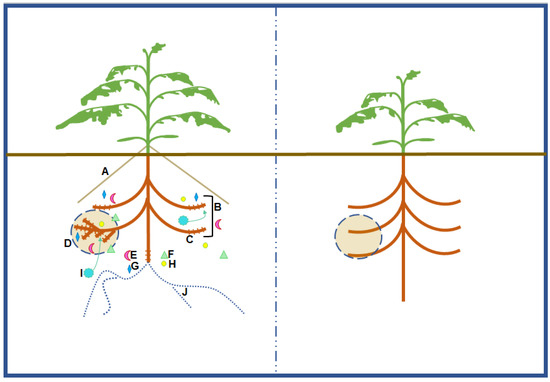
Figure 1.
A tomato plant efficient in phosphorus (P) acquisition. A hypothetical tomato adapted for P-acquisition efficiency (PAE) (left) can be compared to a hypothetical tomato inefficient in P acquisition (right). Root morphological strategies a tomato could employ to cope with a P deficit include adventitious rooting (A), topsoil foraging (B), or root hair growth (C) to better exploit a given volume of soil. Root proliferation in nutrient dense patches (D) exemplifies plasticity of an efficient root system to optimize a heterogeneous supply of soil P. Roots may exude enzymes such as phosphatase (E) to mineralize organic P sources. Roots may also exude organic acids such as citrate, malate, fumarate, or oxalate (F) to chelate bound-P. Reductants such as glucose (G) may reduce ferric iron to mobilize P. Exudation of hydrogen ions (H) acidify the rhizosphere to solubilize calcium phosphates. Rhizosphere acidification can also be achieved through excess cation uptake (I). Symbiosis with mycorrhizal fungi (J) allows for uptake of P transferred from sources unavailable to the root. There are no mechanisms illustrated for the P-inefficient representative. However, any listed strategy could be present in the root system of a P-inefficient tomato, but likely expressed to a lesser degree than a P-efficient representative.
4.1. Root Morphological Responses
Root morphological responses reflect changes in root architecture (spatial configuration), topology (connection and branching), or distribution (position in soil). Roots exhibit great architectural variation among species and genotypes, as demonstrated by soybean with variations in yield, P accumulation, and root morphology and architecture [117]. Lynch and Brown [118] suggested that root architectural traits can be mapped through quantitative trait locus (QTL) tagging (data unpublished) that could allow breeders to select for a more P efficient crop.
Root plasticity. Root plasticity is the ability of a root system to alter its typical structure in response to changing environmental conditions in order to acquire P at minimal metabolic costs. Because there is no single universal least-cost mode of P acquisition, plasticity is important for a root system to adapt to the changing costs of adaptive strategies when external P concentrations fluctuate [119]. An example of root plasticity is topsoil foraging; topsoil foraging occurs when plants concentrate root dispersal to the shallow soil horizons [120]. Computer models have shown that topsoil foraging root systems are more P efficient than a typical root system with an equivalent size. Topsoil foraging traits have been genetically mapped and can be tagged with QTLs in plant breeding programs [118]. Adventitious rooting (roots developed from non-root organs) tends to occur in the topsoil portion of the soil. Adventitious roots require minimal construction cost compared with other root types [121]. A study of common bean found that P-deficiency enhanced adventitious rooting in P-efficient genotypes (G2333 and G19839) but not in P-inefficient genotypes (‘Carioca’ accession G4017 and DOR364) [121]. These fast-growing, small roots help explore the soil. Importantly, Walk et al. [122] noted that an adventitious rooting response is beneficial only when specific respiration rates are similar to those of the basal roots to avoid excess carbohydrate reallocation.
Lynch and Brown [118] concluded that the highly plastic roots directly and positively impacted the P-efficiency of common bean. These plasticity responses included increased presence of adventitious roots and a gravitropic trajectory of 75°–90°. Low levels of ethylene (0.0005–0.0010 µL/L) have been shown to restore a full gravitropic response in tomato [123].
Root plasticity is advantageous because many soils have an unevenly distributed P supply. Therefore, soils such as Oxisols [61] may enhance P bioavailability with plastic root systems (Table 2). Researchers found that biomass and P content were greater for P-efficient wheat and white lupin (Lupinus albus L.) grown in a heterogeneous and localized P supply compared with a uniform P supply [124]. Jing et al. [125] concluded that localized nutrition led to local root proliferation in maize. Plants with high PAE express burgeoning root growth upon sensing nutrient-dense volumes to optimize nutrient influx. Kumar et al. [126] described the advantage of plasticity between root traits as minimizing trade-offs between the costs of maintaining root functional traits and increased nutrient acquisition.

Table 2.
Strategies to enhance phosphorus acquisition across different soil orders.
Lateral root growth to enhance surface area. The lateral root response to P-deficiency varies among crops. While many crops may benefit from enhanced lateral rooting, Lynch and Brown [118] suggested that minimal lateral rooting benefited the P-efficiency of common bean. This response has also not been reported in tomato where high P levels (50 mg/kg) have been shown to increase lateral root number [127]. Tomato root morphology has instead been shown to respond to P-deficiency by increasing root surface area and decreasing total root weight and average root diameter [128]. Although increasing lateral rooting has not yet been reported in tomato, many other crops have been studied that employ this strategy.
The number and length of lateral roots was shown to be greater among higher-yielding and P-efficient accessions of Brassica oleracea L. than lower-yielding accessions [129]. Similarly, greater lateral root branching density with lower average root diameter has been shown to increase P uptake while not substantially increasing root competition in maize [130]. Lateral rooting has been shown to vary greatly in Arabidopsis. Chevalier et al. [131] observed a range of Arabidopsis responses to P-deficiency: 50% reduced length of primary and number of lateral roots, 25% were not sensitive, 16% reduced length of primary root only, and 9% reduced number of lateral roots only (of 73 accessions). Williamson et al. [120] and Reymond et al. [132] both observed an inhibition of primary root elongation and enhancement of lateral rooting in Arabidopsis when grown in low P. Zhu et al. [133] mapped the QTLs for lateral rooting in maize in P-deficiency and found that in low-fertility treatments, six QTLs (flanked by phi001/csu3, scu164a/phi055, nc003/umc36b, bn16.16/umc17, phi070/umc62, bn17.08a/phi121 on chromosomes 1, 1, 2, 3, 6, 8, respectively) were associated with lateral root length response and one QTL (flanked by umc131/nc003 on chromosome 2) was associated with lateral root number. The researchers found that three QTLs are associated with the reduction of primary root length (LPR1 on chromosome 1, LPR2 on chromosome 3, LPR3 on chromosome 4). Ultimately, lateral rooting may be conditionally advantageous to acquire P if root diameter is small. These listed P-efficient crops were able to increase the surface area of their root system, while other crops such as tomato and bean may rely on other strategies.
Root hair production. Root hairs are extensions occupying up to 90% of a root’s surface and they facilitate water and nutrient acquisition [134]. Root hairs have optimal geometry for P capture. Their small radius helps to reduce carbon cost to the plant while also extending soil exploration [135]. Root hairs are fine, which helps with P acquisition. A plant growth model based on P-deficient rice revealed that increasing root fineness by 22% increased P uptake 3-fold [136]. Genetic control of root hair growth has been explored in rice by Giri et al. [137] who found that the auxin influx carrier OsAUX1 controls primary and crown root gravitropic responses and promotes root hair growth at low-P levels (3 µM P). Homologous genes of TRIPTYCHON (TRY) and GLABRA3 (GL3) in Arabidopsis have been identified in tomato by Tominaga-Wada et al. [138] and, respectively, named SlTRY and SlGL3. These genes are dominantly expressed in developing tomato shoots and have been determined to be functionally similar to the CAPRICE (CPC) like MYB transcription factors of Arabidopsis that regulate trichome and root hair development. Furthermore, tomato root hair length was shown to increase from 0.1 mm to 0.2–0.7 mm when phosphate concentration decreased from 100 to 2 µM [139].
Root hairs have high plasticity that enables navigation of soils exposed to environmental changes. Their presence is advantageous; a crop with more densely packed root hairs will be better adapted for an environment with sparingly available P. Bayuelo-Jimenez et al. [140] grew 242 maize accessions in low (23 kg/ha P2O5) and high (97 kg/ha P2O5) P treatments and determined that the dense root hairs on the main root and first order laterals were responsible for the enhanced P-efficiency of high yielding varieties gown on the Mexican highlands with predominant Andisol soils (Table 2). Root hairs develop from epidermal trichoblasts overlaid onto the connection of two cortical cells [134]. More numerous and smaller cortical cells form in P-limitation, leading to a greater density of trichoblasts and, therefore, root hairs [127] and this trend has been seen in maize [141]. Root hair initiation sites are actively and progressively specified as opposed to being determined during trichoblast development. The adaptability and function of root hairs substantiate claims of call-autonomous external-P sensing. Root-hair initiation is regulated by P availability as shown by low-P increasing root hair length and density, increasing trichoblast number, and decreasing distance between root tip and root hair initiation sites [142]. Although root hairs tend to readily respond to a P deficit, barley root hair development was shown to be unaffected by plant Zn status [143]. This trend may be due to a greater reliance of H+-ATPase for P uptake compared with Zn uptake. Plants with root hairs tend to reach maximum biomass at a lower P concentration than root-hairless mutant plants due to their high concentrations H+-ATPase activity [134,144]. Root hair elongation may be driven by a shoot-originating signal, possibly auxin-derived, translocated to the roots upon sensing low-P [145].
The P-inefficient root hairless barley mutant (brb) has been extensively studied for root-hair traits. This inefficiency is likely due to its smaller root surface area compared with the root hair covered wild type [88]. Wild-type barley root hair length increased in P-deficiency [146]. Similar results were found in investigations with Arabidopsis. Bates and Lynch [147] compared growth and P accumulation of wild-type Arabidopsis with root hairless mutants (rhd6 and rhd2). Root hairless mutants were shown to acquire less P than plants with a root hair response. Phosphorus-efficient Arabidopsis have longer root hairs at greater density, resulting in higher rates of nutrient uptake per unit root length [148]. Arabidopsis root-hair density is stimulated by low-P and suppressed logarithmically in response to an increase in P supply [144,149]. The presence or absence of root hairs did not affect growth in nutrient-sufficient conditions [147,150].
Denser root hair patches may lead to overlapping zones of depletion among root hairs [149]. Increasing the length of root hairs could alleviate this issue, and in P-starvation (<1 mmol/m3), root-hair length can exceed 1 mm [144]. However, longer root hairs come at a metabolic cost to the plant [146]. This cost is small because of the size of root hairs, their ephemeral nature, and the comparative benefit of growing root hairs. Root hairs can extend the total zone of depletion and explore a greater volume of soil. This enhanced exploration of the soil from higher root length density is important for nutrients such as P that diffuse slowly through the soil [119]. The Arabidopsis root hairless mutant rhd6 has been used to study hormonal effects on root hair development. Application of indole-3-acetic acid (IAA) at 30 nM suppressed mutant defects and increased root hair elongation [151]. Auxin was also able to restore root hair growth in the ethylene insensitive Arabidopsis mutant (ein2-1) [152]. Application of exogenous auxin may be beneficial to P acquisition because of its ability to restore root hair production.
4.2. Exudation of Root Derived Compounds
The rhizosphere is a biologically and chemically active area surrounding the root from which plants take up nutrients. Roots exude compounds into the rhizosphere including protons, hydroxide, organic anions, enzymes (such as phosphatase), sugars, vitamins, amino acids, purines, gaseous molecules (such as H2), root border cells, and phytosiderophores [153], which help to produce bioavailable P.
Proton exudation and rhizosphere acidification. The advantage of rhizosphere acidification may be dependent on initial soil pH and buffering capacity. Phosphorus bioavailability in high-pH soils such as Entisols or Inceptisols buffered with CaCO3 benefits from high Ca uptake and proton exudation to maintain electro-neutrality [154] (Table 2). However, in initially acidic soil, exuded protons could interact with the dominant Al-phosphates and may result in Al toxicity in plants [155]. Studies have shown that decreasing soil-pH resulted in increased exchangeable Al and decreased exchangeable Ca [156]. Calcium phosphates (including Ca3(PO4)2; CaHPO4−•2H2O) are dominant in the soil with increasing pH [157], but have negligible precipitation in solutions of pH 4.4 [158]. Therefore, in non-acidic soils dominated by Ca-phosphates, proton secretion is a significant process that enhances P-bioavailability.
H+-ATPase is the driving force behind rhizosphere acidification. In the plasma membrane, this enzyme couples ATP hydrolysis with proton transport, establishing electrochemical gradients across the plasma membrane [63,159]. Upregulation of plasma membrane H+-ATPase is largely beneficial to P uptake. A study with soybean (Glycine max L.) showed that plants treated with fusicoccin (plasma membrane H+-ATPase activator) increased P uptake by 35%, but plants treated with vanadate (plasma membrane H+-ATPase inhibitor) suppressed P uptake [160].
Hydrogen ion efflux is genetically controlled. In tomato, the gene TFT7 (a member of the 14-3-3 gene family) was found to activate H+-ATPase and subsequent H+ release in low-P conditions (2 µM P). After one day, TFT7 expression increased 2.5 times compared with P-sufficient plants [161]. The activity of 14-3-3 proteins and auxin regulatory pathways were shown to modulate H+ efflux by affecting the AHA2 or AHA7 genes in Arabidopsis; AHA7 moderates H+ exudation in the root hair zone and AHA2 regulates primary root elongation and mediates H+ exudation in the root elongation zone [162]. Wild-type tomato was shown to exude H+ at approximately 0.3 µM/hr/10g FW at high P (200 µM P) and approximately 0.8 µM/hr/10g FW at low P (10 µM P) [84]. This study also found that transgenic tomato treated with General Regulatory Factor 9 (GRF9) (an expression vector with Arabidopsis 14-3-3 protein cDNA) exuded more H+ (approximately 1.6 µM/hr/10g FW at low P), and its high higher H+-ATPase activity helped these transgenic lines accumulate more shoot-P. Hydrogen-ion exudation as a coping mechanism for a P-deficit has been observed in numerous species such as bean [163], white lupin [164], and tea (Camellia sinensis L.) [165]. The tea genotypes, TRI 2023, TRI 2025 and S 106 accumulated approximately 3.3, 2.6 and 1.1 mg P/plant and decreased rhizosphere pH by 0.30, 0.19 and 0.17 units, respectively. Compared with P-sufficient white lupin, P-deficient samples increased (i) ATPase activity, (ii) plasma membrane H+-ATPase concentration, (iii) H+ pumping activity, and (iv) H+ plasma membrane permeability.
Nitrogen exists in the soil primarily as nitrate (NO3−) and ammonium (NH4+) [155]. Rhizosphere pH increases during NO3− nutrition and decreases during NH4+ nutrition [2]. In a study with maize, Jing et al. [125] found that localized application of P with ammonium decreased rhizosphere pH by 3 units and subsequently increased leaf expansion by 20–50%, root length 23–30%, and plant growth rate 18–77%. Understanding how different N sources impact P-bioavailability can help manage P-deficient crops. Other management strategies to enhance plasma membrane H+-ATPase may include attracting earthworms (Eisenia foetida) or applying humic acid to the soil. Earthworms produce humic substances, and isolated humic acid from earthworm compost has been shown to enhance root growth of maize and stimulate expression of plasma membrane H+-ATPase [166]. Additionally, auxin may impact H+-ATPase activity. Application of IAA to endogenous auxin-depleted Arabidopsis increased activity of the H+-ATPase enzyme through phosphorylation of threonine [167].
Organic acid exudation. Organic acids/anions (OAs) (including malic, fumaric, oxalic, oxalo-acetic, succinic, α-cetoglutaric, isocitric, citric, aconitic, formic, piscidic, shikimic) are important metabolites with typically one or more carboxylic group that dissociates in the cytosol of root cells. The high exudation of carboxylates from ephemeral cluster roots is largely why researchers consider members of Proteaceae to be highly P-use efficient [102]. OAs complex with metal cations and displace anions like phosphate from the soil matrix [168,169]. OA exudation significantly helps plants cope with Al toxicity and P-deficiency in acid conditions, which could benefit soils such as Spodosols (Table 2). Exudation is regulated by membrane-localized transporters including Aluminum Activated Malate Transporter (ALMT), Multidrug and Toxic Compound Extrusion (MATE), and plasma membrane H+-ATPase [170]. In P-deficient proteoid roots of white lupin, there was an increase of ATPase activity, Vmax and Km, H+-ATPase concentration in the plasma membrane, H+ pumping activity, pH gradient, and passive H+ permeability in the plasma membrane [164]. Lupin and other members of the Lupinus genus exude high concentrations of carboxylates, which accounts for their great efficiency in P-use and may also account for their role as an aggressive invasive species [171]. Exudation of OAs may also increase P bioavailability by enhancing soil microbial activity. The C compounds in OAs stimulate microflora which may help plants acquire P through symbiosis [155].
The dominant OA exuded may be species-specific. Citric acid has been reported to be dominantly exuded in tomato. There has been an accumulation of citric acid observed in P-deficient roots of tomato and an increase in PEP carboxylase needed for citrate biosynthesis [172]. Luo et al. [173] found that citric acid exuded by tomato roots increased phosphate solubility in the rhizosphere. Tomato roots were shown to predominantly exude fumarate, citrate, and succinate at 0 M P and predominantly exude succinate and citrate at 0.5 M P [174].
Citric and malic acid may be the most frequently involved OA in responding to low-P conditions [155]. Citric and malic acid exuded from sweet potato (Ipomoea batatas L.) benefited root growth in low-P [169]. Citric and oxalic acid exudation increased in mungo bean (Vigna mungo L.) [168] and maize [169] in response to low-P stress. Similarly, P-sorption decreased with addition of citric acid [175]. However, citric acid exudation may not be beneficial in soils with high a concentration of adsorbed Ca and 2:1 clay minerals. Citric acid addition at 10 µmol/kg decreased P availability in chromic Cambisol and Luvisol samples, but increased availability in Ferralsol samples [176].
Selecting to enhance organic acid exudation could prove beneficial in searching for a more P-efficient tomato as long as considerations have been made to account for soil type and OA species specificity. Managing soils to see the advantages of OA exudation on P bioavailability would include adding chelates to the soil. Chelating agents are intricate organic acids, so adding chelating agents may help solubilize bound-P supplies. Repeated addition of synthetic ethylenediamine di(o-hydroxy-phenylacetic) acid (EDDHA), a strong Fe-chelating agent, increased P uptake and growth of big bluestem (Andropogon gerardii Vitman) in both laboratory conditions and in a prairie soil water slurry [177]. Furthermore, fertilization practices may impact OA effects. Fertilizing maize with monoammonium phosphate (MAP) coated with peat humic organic acid increased agronomic efficiency 11% and apparent P recovery 41% compared to conventional MAP fertilization [178]. The greater P content may be a result of the slower release from coating the fertilizer and from the solubilization effects from the organic acid coating. It was also found that fertilization with greater rates of NO3− compared to NH4+ resulted in greater exudation of citrate, malate, and fumarate in tomato plants [179].
Phosphatase exudation. Phosphatase enzymes enhance soil-P bioavailability through catalyzing hydrolysis of phytic acid (inositol hexaphosphate (IP6)) thereby converting stores on unavailable organic P to available inorganic P, which may help plants growing in soils with high organic P content such as Gelisols and Histosols (Table 2). Having the ability to mineralize organic P is invaluable; 30% to 80% of total P in an agroecosystem is in organic forms, unable to be used by the plant before mineralization [2]. Both acid and alkaline phosphatase are active over a range of orthophosphoric acid monoesters [180]. Warming soils may benefit phosphatase activity. In a study with Erica multiflora, increasing soil and air temperature by an average of 1 °C resulted in a 68% increase in soil acid phosphatase and 22% increase in alkaline phosphatase activity [181].
Acid phosphatase is a type of phosphatase present primarily in the mitochondria and is exuded at low pH to make organic P bioavailable [180]. Two monomeric secreted acid phosphatase (SAP) isozymes have been identified in tomato (84 kDa SAP1 and 57 kDa SAP2) that mobilize external organophosphates [105]. Chickpea roots supplied with phytate (organic P) exuded acid phosphatase to a greater degree than those supplied with KH2PO4 or Ca(H2PO4)2 (inorganic P) and were able to utilize the organic P source in both hydroponic and soil culture [182]. White lupin has been extensively studied for its high P-efficiency in part because white lupin secretes acid phosphatase when there are minimal surrounding stores of bioavailable P [183]. Playsted et al. [184] concluded that the ecophysiological advantage of a rhizomatous sedge (Caustis blakei Kuk.) can be attributed to the high concentration of carboxylates (citrate released at 0.12 nm/g FW/s) and phosphatases (acid phosphatase released at 150 µmol/g FW/s) exuded from their dauciform roots that grew in organic soil horizons. Dauciform roots as well as cluster roots rigorously scavenge pools of local external P [119].
Reactions of alkaline phosphatase can proceed in both directions, catalyzing the synthesis and the hydrolysis of phosphate esters [185]. This enzyme may be responsible for both hydrolyzing and transporting phosphate at high pH [180,185]. Alkaline phosphatase is primarily present in the microsomes of cells [180]. While many higher plants express high acid phosphatase activity, there is significantly less activity for alkaline phosphatase [185]. However, numerous species of fungi synthesize alkaline phosphatase including Neurospora crassa and Saccharomyces cerevisiae [185]. Treatments with higher alkaline phosphatase activity were observed in soils inoculated with Glomus etunicatum (vesicular arbuscular mycorrhizal (VAM) fungus) and Enterobacter agglomerans (bacterium) and resulted in higher concentrations of soluble P, suggesting a synergistic interaction between the microbes and alkaline phosphatase activity to increase pools of bioavailable P [186]. Fungal production of alkaline phosphatase is dependent on external P supply; medium with high inorganic P content hinders alkaline phosphatase production [185].
4.3. Microbial Symbiosis
Because microbes can produce beneficial exudates and facilitate soil P transfer, soils that favor bacterial growth (as in soils with high soil organic matter such as Alfisols and Mollisols (Table 2) may enhance phosphatase activity as opposed to soils that reduce bacterial growth such as fumigated soils). Numerous organisms including bacteria, fungi, actinomycetes, and algae are able to solubilize P through mineralization [11,187]. Highly weathered soils may benefit from microbial inoculation. In a slightly acidic savanna Ultisol, the dominant native bacteria species Burkholderia cepacia was shown to improve phosphate availability when used as a biofertilizer [60] (Table 2).
Plant roots colonized by arbuscular mycorrhizal (AM) fungi are capable of phosphate uptake through typical direct root epidermal uptake and through mycorrhizal uptake. Mycorrhizal P uptake in tomato is at least partially regulated by plant P status. At high P (3.5 mg P/g dry weight), the mycorrhizal uptake pathway in tomato was repressed almost completely (10% P taken up via mycorrhizal uptake pathway), whereas at low P (1.5 mg P/g dry weight), the mycorrhizal uptake pathway in tomato was dominant (75% P taken up via mycorrhizal uptake pathway) in mycorrhiza colonized conditions [188]. Three phosphate transporters that are mycorrhiza-inducible have been identified in tomato: LePT3, LepPT4, and LePT5 (within Pht1 family). LePT4 has a great degree of sequence identity to StPT4 from potato, another solanaceous crop [189]. The transcripts of these transporters, LePT3, LePT4, and LePT5, were exclusive to arbuscule-containing cells [190]. LePT3 and LePT4 transcripts are reliable markers of a functional mycorrhiza uptake pathway in tomato as shown by their expression in Glomus intraradices colonized roots with symbiotic phosphate transfer [191].
Calcium phosphates may solubilize when interacting with OAs exuded by microorganisms [192]. When compared with symbiotic bacteria, mycorrhizal fungi are able to secrete more OAs and diffuse greater distances through their hyphae thereby further enhancing P availability [187,193]. Mycorrhizal fungi acquire P more rapidly from a lower concentration than their plant host because they possess an accumulation mechanism with a higher affinity than that of plant roots [32]. Inoculation of mycorrhizal fungi benefits P-efficiency by reducing P loss as shown by studies inoculating Glomus mosseae into rice paddies [194]. These associations are so helpful in P acquisition that other P adaptations become less pronounced in plants with mycorrhizal symbiosis [195].
Root exudates may stimulate microbial growth and favor an environment with symbiotic relationships. Hyphal growth of arbuscular mycorrhizal (AM) fungi is stimulated by flavonoids that can be exuded from roots [187]. Roots may secrete a branching factor (BF) to stimulates hyphae branching during AM fungi spore germination. In lotus (Nelumbo spp.) and sorghum, the active BF was a strigolactone [196]. Exploration of the soil from microbial root extensions scavenges the soil widely, converse to the exploitation from cluster or dauciform root exudates [119].
Symbiosis occurs at a C cost to the host (typically below 10% photosynthetically fixed C) [197]. Therefore, overly stressed plants may become more stressed with symbiosis, so ensuring that proper management techniques are in place (including the 4-Rs of nutrient stewardship and integrated pest management) would allow for an environment where breeding for enhanced microbial symbiosis and inoculation with beneficial microbes would enhance PAE.
5. Conclusions
Phosphorus is a constituent of ATP/ADP, phospholipids, and nucleic acids, enabling growth and development in plants. However, P tends to be scarcely available in agroecosystems due to processes such as precipitation, adsorption, or immobilization. Roots may alter characteristics of the rhizosphere to facilitate P-bioavailability-enhancing reactions such as mineralization, desorption, and chelation. These strategies may include alterations to root structure such as plasticity in low-P stress responses and root spatial arrangement. Enhanced adventitious root growth, expansion of fine roots, and longer and denser root hairs benefit root P acquisition by increasing root surface area. Physiological adaptations such as increased exudation of protons, organic acids, reductants, and phosphatases help to solubilize bound-P. Enhancement of microbial symbiosis also greatly enhances P acquisition from the microbes exploiting greater volumes of soil and taking up phosphate at low concentrations. Plants that employ these strategies are capable of healthy growth in conditions of low bioavailable P. Understanding the morphological and physiological responses to low-P stress can help provide the tools necessary to understand and screen P-efficient crops and enhance agricultural sustainability. It is important to select for crops with high PAE to optimally utilize the soil-P supply. It seems plausible to select for a tomato with greater PAE because of the numerous genetically regulated responses. Management strategies to enhance P cycling may also increase pools of bioavailable P. Enhancing P acquisition may reduce the necessity for off-farm inputs and extend the longevity of indispensable phosphate rock reserves.
Author Contributions
M.D. and G.L. conceived and designed the manuscript. M.D., G.L., E.S., and T.O. wrote and approved the revision of the manuscript. All authors have read and agree to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
Harry Klee at the University of Florida/IFAS reviewed and improved the manuscript before submission.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Plaxton, W.C.; Tran, H.T. Metabolic adaptations of phosphate-starved plants. Plant Physiol. 2011, 156, 1006–1015. [Google Scholar] [CrossRef] [PubMed]
- Havlin, J.L.; Tisdale, S.L.; Nelson, W.L.; Beaton, J.D. Phosphorus. In Soil Fertility and Fertilizers, 8th ed.; Lawrensen, W., Gohn, J., Eds.; Pearson Inc.: Upper Saddle River, NJ, USA, 2014; pp. 185–221. ISBN 978-0-13-503373-9. [Google Scholar]
- Close, D.C.; Beadle, C.L. The ecophysiology of foliar anthocyanin. Bot. Rev. 2003, 69, 149–161. [Google Scholar] [CrossRef]
- Smith, G.S.; Cornforth, I.S.; Henderson, H.V. Critical leaf concentrations for deficiencies of nitrogen, potassium, phosphorus, Sulphur, and magnesium in perennial ryegrass. New Phytol. 1985, 101, 393–409. [Google Scholar] [CrossRef]
- Alsaeedi, A.H.; Elprince, A.M. Critical phosphorus levels for Salicornia growth. Agron. J. 1999, 92, 336–345. [Google Scholar] [CrossRef]
- Johansen, C.; Merkley, K.E.; Dolby, G.R. Critical phosphorus concentrations in parts of Macroptilium atropurpureum cv. Siratro and Desmodium intortum cv. Greenleaf as affected by plant age. Aust. J. Agric. Res. 1980, 31, 693–702. [Google Scholar] [CrossRef]
- Stribley, D.P.; Tinker, P.B.; Rayner, J.H. Relation of internal phosphorus concentration and plant weight in plants infected by vesicular-arbuscular mycorrhizas. New Phytol. 1980, 86, 261–266. [Google Scholar] [CrossRef]
- Haneklaus, S.H.; Schnug, E. Assessing the plant phosphorus status. In Phosphorus in Agriculture: 100% Zero; Schnug, E., De Kok, L., Eds.; Springer: Dordrecht, The Netherlands, 2016; pp. 95–125. ISBN 978-94-017-7612-7. [Google Scholar]
- Jones, J.B., Jr. Phosphorus toxicity in tomato plants: When and how does it occur? Commun. Soil Sci. Plant Anal. 1998, 29, 1779–1784. [Google Scholar] [CrossRef]
- Fageria, N.K.; Wright, R.J.; Baligar, V.C. Rice cultivar evaluation for phosphorus use efficiency. Plant Soil 1988, 111, 105–109. [Google Scholar] [CrossRef]
- Wang, X.; Yan, X.; Liao, H. Genetic improvement for phosphorus efficiency in soybean: A radical approach. Ann. Bot. 2010, 106, 215–222. [Google Scholar] [CrossRef]
- Costa, J.M.; Csizinszky, A.A.; Dorais, M.A.; Jones, J.B.; Huevelink, E.; Lindhout, P.; Peet, M.M.; Saltveit, M.E.; Schuster, D.J.; van Lenteren, J.C.; et al. Tomatoes; CABI: Cambridge, MA, USA, 2005; ISBN 0-85199-396-6. [Google Scholar]
- Castner, J.L. Solanaceae. In Photographic Atlas of Botany and Guide to Plant Identification; Feline Press: Gainesville, FL, USA, 2004; pp. 214–215. ISBN 0-9625150-0-0. [Google Scholar]
- Kelley, W.T.; Boyhan, G.E.; Harrison, K.A.; Sumner, P.E.; Langston, D.B.; Sparks, A.N.; Culpepper, S.; Hurst, W.C.; Fonsah, E.G. Commercial Tomato Production Handbook; University of Georgia Extension: Athens, GA, USA, 2010. [Google Scholar]
- Davies, J.N.; Hobson, G.E.; McGlasson, W.B. The constituents of tomato fruit: The influence of environment, nutrition, and genotype. Crit. Rev. Food Sci. Nutr. 1981, 15, 205–280. [Google Scholar] [CrossRef]
- Bhowmik, D.; Kumar, K.S.; Paswan, S.; Srivastava, S. Tomato—A natural medicine and its health benefits. J. Pharmacogn. Phytochem. 2012, 1, 33–43. [Google Scholar]
- Rao, A.V.; Waseem, Z.; Agarwal, S. Lycopene content of tomatoes and tomato products and their contribution to dietary lycopene. Food Res. Int. 1998, 31, 737–741. [Google Scholar] [CrossRef]
- Gerszberg, A.; Hnatuszko-Konka, K. Tomato tolerance to abiotic stress: A review of most often engineered target sequences. Plant Growth Regul. 2017, 83, 175–198. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO) of the United Nations. Crops, Tomatoes; Food and Agriculture Organization (FAO) of the United Nations: Rome, Italy, 2019. [Google Scholar]
- Bai, Y.; Lindhout, P. Domestication and breeding of tomatoes: What have we gained and what can we gain in the future? Ann. Bot. 2007, 100, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- Coltman, R.; Gerloff, G.; Gabelman, W. Intraspecific variation in growth, phosphorus acquisition and phosphorus utilization in tomatoes under phosphorus-deficiency stress. In Proceedings of the 9th International Plant Nutrition Colloquium, Coventry, UK, 22–27 August 1982. [Google Scholar]
- Soderlund, R.; Svensson, B.H. The global nitrogen cycle. Ecol. Bull. 1976, 7, 23–73. [Google Scholar]
- Fuentes-Lara, L.O.; Medrano-Macías, J.; Pérez-Labrada, F.; Rivas-Martínez, E.N.; García-Enciso, E.L.; González-Morales, S.; Juárez-Maldonado, A.; Rincón-Sánchez, F.; Benavides-Mendoza, A. From elemental sulfur to hydrogen sulfide in agricultural soils and plants. Molecules 2019, 24, 2282. [Google Scholar] [CrossRef]
- Tang, H.; Liu, Y.; Li, X.; Muhammad, A.; Huang, G. Carbon sequestration of cropland and paddy soils in China: Potential, driving factors, and mechanisms. Greenh. Gases Sci. Technol. 2019, 9, 872–885. [Google Scholar] [CrossRef]
- Liu, G.D.; Simonne, E.H.; Morgan, K.T.; Hochmuth, G.J.; Agehara, S.; Mylavarapu, R. Fertilizer management for vegetable production in Florida. In Vegetable Production Handbook of Florida, 23rd ed.; Chapter 2; Dittmar, P., Paret, M., Freeman, J., Smith, H., Eds.; University of Florida Institute of Food and Agricultural Sciences: Gainesville, FL, USA, 2019; pp. 3–9. [Google Scholar]
- Kaya, C.; Kirnak, H.; Higgs, D. Enhancement of growth and normal growth parameters by foliar application of potassium and phosphorus in tomato cultivars grown at high (NaCl) salinity. J. Plant Nutr. 2001, 24, 357–367. [Google Scholar] [CrossRef]
- Rady, M.M.; El-Shewy, A.A.; El-Yazal, M.A.S.; Abdelaal, K.E.S. Response of salt-stressed common bean plant performances to foliar application of phosphorus (MAP). Int. Lett. Nat. Sci. 2018, 72, 7–20. [Google Scholar] [CrossRef]
- Mosali, J.; Desta, K.; Teal, R.K.; Freeman, K.W.; Martin, K.L.; Lawles, J.W.; Raun, W.R. Effect of foliar application of phosphorus on winter wheat grain yield, phosphorus uptake, and use efficiency. J. Plant Nutr. 2006, 29, 2147–2163. [Google Scholar] [CrossRef]
- Ticconi, C.A.; Abel, S. Short on phosphate: Plant surveillance and countermeasures. Trends Plant Sci. 2004, 9, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Poehlein, A.; Daniel, R.; Schink, B.; Simeonova, D.D. Life based on phosphite: A genome-guided analysis of Desulfotignum phosphitoxidans. BMC Genom. 2013, 14, 753. [Google Scholar] [CrossRef] [PubMed]
- Rolando, C.; Gaskin, R.; Horgan, D.; Williams, N.; Bader, M.K.F. The use of adjuvants to improve uptake of phosphorous acid applied to Pinus radiata needles for control of foliar Phytophthora diseases. N. Z. J. Sci. 2014, 44, 8. [Google Scholar] [CrossRef]
- Bertsch, F.; Ramírez, F.; Henríquez, C. Evaluación del fosfito como fuente fertilizante de fósforo vía radical y foliar. Agron. Costarric. 2009, 33, 249–265. [Google Scholar]
- Gómez-Merino, F.C.; Trejo-Téllez, L.I. Biostimulant activity of phosphite in horticulture. Sci. Hortic. 2015, 196, 82–90. [Google Scholar] [CrossRef]
- Ozturk, L.; Eker, S.; Torun, B.; Cakmak, I. Variation in phosphorus efficiency among 73 bread and durum wheat genotypes grown in a phosphorus-deficient calcareous soil. Plant Soil 2005, 269, 69–80. [Google Scholar] [CrossRef]
- Cordell, D.; Schmid-Neset, T.; White, S.; Drangert, J.-O. Preferred future phosphorus scenarios: A framework for meeting long-term phosphorus needs for global food demand. In International Conference on Nutrient Recovery from Wastewater Streams; Ashlet, K., Mavinic, D., Koch, F., Eds.; IWA Publishing: London, UK, 2009; pp. 23–43. ISBN 9781843392323. [Google Scholar]
- Harris, D.C.; Lucy, C.A. Quantitataive Chemical Analysis, 9th ed.; Schultz, L., Murphy, B., Bristow, A., Eds.; W.H. Freeman and Company: New York, NY, USA, 2016; ISBN 978-1-4641-3538-5. [Google Scholar]
- Edwards, C.L.; Maguire, R.O.; Whitehurst, G.B.; Thomason, W.E.; Alley, M.M. Using synthetic chelating agents to decrease phosphorus binding in soils. Soil Sci. 2016, 181, 377–385. [Google Scholar] [CrossRef]
- Qin, Z.; Shober, A.L.; Scheckel, K.G.; Penn, C.J.; Turner, K.C. Mechanisms of phosphorus removal by phosphorus sorbing materials. J. Environ. Qual. 2018, 47, 1232–1241. [Google Scholar] [CrossRef]
- Fang, H.; Cui, Z.; He, G.; Huang, L.; Chen, M. Phosphorus adsorption onto clay minerals and iron oxide with consideration of heterogenous particle morphology. Sci. Total Environ. 2017, 605–606, 357–367. [Google Scholar] [CrossRef]
- Li, M.; Liu, J.; Xu, Y.; Guangren, Q. Phosphate adsorption on metal oxides and metal hydroxides: A comparative review. Environ. Rev. 2016, 24, 319–332. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, Y.Q.; Babatunde, A.O.; Wang, L.; Ren, Y.X.; Han, Y. Characteristics and mechanisms of phosphate adsorption on dewatered alum sludge. Sep. Purif. Technol. 2006, 51, 193–200. [Google Scholar] [CrossRef]
- Freeman, J.S.; Rowell, D.L. The adsorption and precipitation of phosphate onto calcite. J. Soil Sci. 1981, 32, 75–84. [Google Scholar] [CrossRef]
- Péret, B.; Clément, M.; Nussaume, L.; Desnos, T. Root developmental adaptation to phosphate starvation: Better safe than sorry. Trends Plant Sci. 2011, 16, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.L. Improving nutrient use efficiency. Turk. J. Agr. 2008, 32, 177–182. [Google Scholar]
- Chacon, N.; Silver, W.L.; Dubinsky, E.A.; Cusack, D.F. Iron reduction and soil phosphorus solubilization in humid tropical forests soils: The roles of labile carbon pools and an electron shuttle compounds. Biogeochemistry 2006, 78, 67–84. [Google Scholar] [CrossRef]
- Wang, E.; Bell, M.; Luo, Z.; Moody, P.; Probert, M.E. Modelling crop response to phosphorus inputs and phosphorus use efficiency in a crop rotation. Field Crops Res. 2014, 155, 120–132. [Google Scholar] [CrossRef]
- Schröder, J.J.; Smit, A.L.; Cordell, D.; Rosemarin, A. Improved phosphorus use efficiency in agriculture: A key requirement for its sustainable use. Chemosphere 2011, 84, 822–831. [Google Scholar] [CrossRef]
- Odom, H.T.; Kangas, P.; Best, G.R.; Rushton, B.T.; Leibowitz, S.; Butner, J.R. Studies on Phosphate Mining, Reclamation, and Energy; Center of Wetlands, University of Florida: Gainesville, FL, USA, 1981. [Google Scholar]
- Ballard, R.; Fiskell, J.G.A. Phosphorus retention in coastal plain forest soils: I. Relationship to soil properties. Soil Sci. Soc. Am. J. 1974, 38, 250–255. [Google Scholar] [CrossRef]
- Wilhelm, R.G.; Beam, P.; Krupka, K.M.; Kaplan, D.I.; Whelan, G.; Serne, R.J.; Mattigod, S.V. Understanding Variation in Partition Coefficient, Kd, Values: The Kd Model, Methods of Measurement, and Application of Chemical Reaction Codes; EPA 402-R-99-004A; United States Environmental Protection Agency and Office of Air and Radiation: Washington, DC, USA, 1999.
- Shaheen, S.; Tsadilas, C. Phosphorus sorption and availability to canola grown in an Alfisol amended with various soil amendments. Commun. Soil Sci. Plant Anal. 2013, 44, 89–103. [Google Scholar] [CrossRef]
- Ditzler, C.A. Soil properties and classification (soil taxonomy). In The Soils of the USA; West, L.T., Singer, M.J., Hartemink, A.E., Eds.; Springer International Publishing: Gewerbestrasse/Cham, Switzerland, 2017; pp. 29–42. ISBN 978-3-319-41868-1. [Google Scholar]
- United States Department of Agriculture (USDA) Natural Resource Conservation Service (NRCS). Global Soil Regions Map. 2005. Available online: https://www.nrcs.usda.gov/wps/portal/nrcs/detail/soils/use/worldsoils/?cid=nrcs142p2_054013 (accessed on 10 April 2020).
- Ahmed, A.W.A.M.; Elsheikh, M.A.; El Mahi, Y.E.G. Relationship between phosphorus fractions of some selected Sudanese soil orders to phosphate availability. Eurasian J. Soil Sci. 2018, 7, 224–229. [Google Scholar] [CrossRef]
- Bayuelo-Jiménez, J.S.; Muraoka, T.; de la Cruz-Torres, E.; Quintero-Ponce, E.; Paredes-Gutiérrez, L.C.; Zaman, M. Phosphorus fractions and dynamics as affected by land—Use changes in the Central Mexican highlands. Soil Use Manag. 2020, 36, 240–249. [Google Scholar] [CrossRef]
- Alovisi, A.M.T.; Alovisi, A.A.; Serra, A.P.; Tokura, L.K.; Davide, L.M.C.; Lournte, E.R.P.; da Silva, R.S.; Tokura, W.I.; de Souza, D.A.; do Mar, G.D. Phosphorus fractions and their transformation in Entisol. J. Agric. Sci. 2019, 11, 485–493. [Google Scholar] [CrossRef]
- Esmail, A.O.; Sheikh-Abdullah, S.M.; Maruf, M.T. Phosphorus availability in Entisols, Inceptisols, and Mollisols of Iraqi Kurdistan. Soil Sci. 2019, 184, 95–100. [Google Scholar] [CrossRef]
- Kolka, R.; Bridgham, S.D.; Ping, C.-L. Soils of Peatlands: Histosols and Gelisols. In Wetland Soils: Genesis, Hydrology, Landscapes, and Classification, 2nd ed.; Vepraskas, M.J., Craft, C.B., Eds.; CRC Press: Boca Raton, FL, USA, 2016; pp. 277–310. ISBN 978-1-4398-9800-0. [Google Scholar]
- Haus, N.W.; Wilhelm, K.R.; Bockheim, J.G.; Fournelle, J.; Miller, M. A case for chemical weathering soils of Hurd Peninsula, Livingston Island, South Shetland Islands, Antarctica. Geoderma 2016, 263, 185–194. [Google Scholar] [CrossRef]
- Mora, E.; Toro, M.; Flores, E.; Lopez-Hernandez, D. Plant growth promoting abilities of phosphate solubilizing bacteria native from a high P-sorbing Ultisol. Ann. Adv. Agric. Sci. 2017, 1. [Google Scholar] [CrossRef]
- Camargo, L.A.; Marques, J., Jr.; Pereira, G.T.; Alleoni, L.R.F.; Bahia, A.D.S.; Teixeira, D.D.B. Pedotransfer functions to assess adsorbed phosphate using iron oxide content and magnetic susceptibility in an Oxisol. Soil Use Manag. 2016, 32, 172–182. [Google Scholar] [CrossRef]
- Rausch, C.; Bucher, M. Molecular mechanisms of phosphate transport in plants. Planta 2002, 216, 23–37. [Google Scholar] [CrossRef]
- Duby, G.; Boutry, M. The plasma membrane proton pump ATPase: A highly regulated P-type ATPase with multiple physiological roles. Pflug. Arch. Eur. J. Physiol. 2009, 457, 645–655. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E.; Moller, I.M.; Murphy, A. Translocation in the Phloem. In Plant Physiology and Development, 6th ed.; Sinauer Associates Inc.: Sunderland, MA, USA, 2015; pp. 285–316. ISBN 978-1-60535-255-8. [Google Scholar]
- Nussaume, L.; Kanno, S.; Javot, H.; Marin, E.; Nakanishi, T.M.; Thibaud, M.-C. Phosphate import in plants: Focus on the PHT1 transporters. Front. Plant Sci. 2011, 2, 83. [Google Scholar] [CrossRef]
- Parra-Almuna, L.; Pontigo, S.; Larama, G.; Cumming, J.R.; Perez-Tienda, J.; Ferrol, N.; de la Luz Mora, M. Expression analysis and functional characterization of two PHT1 family phosphate transporters in ryegrass. Planta 2020, 251, 6. [Google Scholar] [CrossRef]
- Versaw, W.K.; Metzenberg, R.L. Repressible cation-phosphate symporters in Neurospora crassa. Proc. Natl. Acad. Sci. USA 1995, 92, 3884–3887. [Google Scholar] [CrossRef] [PubMed]
- Sakano, K. Proton/Phosphate stoichiometry in uptake of inorganic phosphate by cultured cells of Catharanthus roseus (L.) G. Don. Plant Physiol. 1990, 93, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Luan, M.; Tang, R.; Tang, Y.; Tian, W.; Hou, C.; Zhao, F.; Lan, W.; Luan, S. Transport and homeostasis of potassium and phosphate limiting factors for sustainable crop production. J. Exp. Bot. 2017, 68, 3091–3105. [Google Scholar] [CrossRef] [PubMed]
- Ham, B.-K.; Chen, J.; Yan, Y.; Lucas, W.J. Insights into plant phosphate sensing and signaling. Curr. Opin. Biotechnol. 2018, 49, 1–9. [Google Scholar] [CrossRef]
- Versaw, W.K.; Harrison, M.J. A chloroplast phosphate transporter, PHT2; 1, influences allocation of phosphate within the plant and phosphate-starvation responses. Plant Cell 2002, 14, 1751–1766. [Google Scholar] [CrossRef]
- Zhang, C.; Meng, S.; Li, M.; Zhao, Z. Genomic identification and expression analysis of the phosphate transporter gene family in poplar. Front. Plant Sci. 2016, 7, 1398. [Google Scholar] [CrossRef]
- Guo, B.; Jin, Y.; Wussler, C.; Blancaflor, E.B.; Motes, C.M.; Versaw, W.K. Functional analysis of the Arabidopsis PHT4 family of intracellular phosphate transporters. New Phytol. 2008, 177, 889–898. [Google Scholar] [CrossRef]
- Liu, T.-Y.; Huang, T.-K.; Yang, S.-Y.; Hong, Y.-T.; Huang, S.-M.; Wang, F.-N.; Chiang, S.-F.; Tsai, S.-Y.; Lu, W.-C.; Chiou, T.J. Identification of plant vacuolar transporters mediating phosphate storage. Natl. Commun. 2016, 7, 11095. [Google Scholar] [CrossRef]
- Teng, W.; Zhao, Y.-Y.; Zhao, X.-Q.; He, X.; Ma, W.-Y.; Deng, Y.; Chen, X.P.; Tong, Y.-P. Genome-wide identification, characterization, and expression analysis of PHT1 phosphate transporters in wheat. Front. Plant Sci. 2017, 8, 543. [Google Scholar] [CrossRef]
- Rae, A.L.; Cybinski, D.H.; Jarmey, J.M.; Smith, F.W. Characterization of two phosphate transporters from barley; Evidence for diverse function and kinetic properties among members of the Pht1 family. Plant Mol. Biol. 2003, 53, 27–36. [Google Scholar] [CrossRef]
- Chen, A.; Chen, X.; Wang, H.; Liao, D.; Gu, M.; Qu, H.; Sun, S.; Xu, G. Genome-wide investigation and expression analysis suggest diverse roles and genetic redundancy of Pht1 family genes in response to Pi deficiency in tomato. BMC Plant Biol. 2014, 14, 61. [Google Scholar] [CrossRef] [PubMed]
- Mudge, S.R.; Rae, A.L.; Diatloff, E.; Smith, F.W. Expression analysis suggests novel roles for members of the Pht1 family of phosphate transporters in Arabidopsis. Plant J. 2002, 31, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.; Shin, H.S.; Dewbre, G.R.; Harrison, M.J. Phosphate transport in Arabidopsis: Pht1;1 and Pht1;4 play a major role in phosphate acquisition from both low- and high-phosphate environments. Plant J. 2004, 39, 629–642. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.; Haimovich, G.A.L.; Pick, U.R.I. Phosphate and sulfate uptake in the halotolerant alga Dunaliella are driven by Na+-symport mechanism. J. Plant Physiol. 2001, 158, 1519–1525. [Google Scholar] [CrossRef]
- Hürlimann, H.C.; Stadler-Waibel, M.; Werner, T.P.; Freimoser, F.M. Pho91 is a vacuolar phosphate transporter that regulates phosphate and polyphosphate metabolism in Saccharomyces cerevisiae. Mol. Biol. Cell 2007, 18, 4438–4445. [Google Scholar] [CrossRef]
- Wang, C.; Yue, W.; Ying, Y.; Wang, S.; Secco, D.; Liu, Y.; Whelan, J.; Tyerman, S.D.; Shou, H. Rice SPX-Major Facility Superfamily 3, A vacuolar phosphate efflux transporter, is involved in maintaining phosphate homeostasis in rice. Plant Physiol. 2015, 169, 2822–2831. [Google Scholar]
- Liu, C.; Muchhal, U.S.; Uthappa, M.; Kononowicz, A.K.; Ragothama, K.G. Tomato phosphate transporter genes are differently regulated in plant tissues by phosphorus. Plant Physiol. 1998, 116, 91–99. [Google Scholar] [CrossRef]
- Zhang, L.; Li, G.; Li, Y.; Min, J.; Kronzucker, H.J.; Shi, W. Tomato plants ectopically expressing Arabidopsis GRF9 show enhanced resistance to phosphate deficiency and improved fruit production in the field. J. Plant Physiol. 2018, 226, 31–39. [Google Scholar] [CrossRef]
- Leggewie, G.; Willmitzer, L.; Riesmeier, J.W. Two cDNAs from potato are able to complement a phosphate uptake-deficient yeast mutant: Identification of phosphate transporters from higher plants. Plant Cell 1997, 9, 381–392. [Google Scholar]
- Schünmann, P.H.D.; Richardson, A.E.; Smith, F.W.; Delhaize, E. Characterization of promoter expression patterns derived from the Pht1 phosphate transporter genes of barley (Hordeum vulgare L.). J. Exp. Bot. 2004, 55, 855–865. [Google Scholar] [CrossRef]
- Preuss, C.P.; Huang, C.Y.; Tyerman, S.D. Proton-coupled high-affinity phosphate transport revealed from heterologous characterization in Xenopus of barley-root plasma membrane transporter, HvPHT1;1. Plant Cell Environ. 2011, 34, 681–689. [Google Scholar] [CrossRef]
- Huang, C.Y.; Shirley, N.; Genc, Y.; Shi, B.; Langridge, P. Phosphate utilization efficiency correlates with expression of low-affinity phosphate transporters and noncoding RNA, IPS1, in barley. Plant Physiol. 2011, 156, 1217–1229. [Google Scholar] [CrossRef] [PubMed]
- Mukatira, U.T.; Liu, C.; Varadarajan, D.K.; Raghothama, K.G. Negative regulation of phosphate starvation-induced genes. Plant Physiol. 2001, 127, 1854–1862. [Google Scholar] [CrossRef]
- Tittarelli, A.; Milla, L.; Vargas, F.; Morales, A.; Neupert, C.; Meisel, L.; Salvo-G, H.; Penaloza, E.; Munoz, G.; Corcuera, L.J. Isolation and comparative analysis of the wheat TaPT2 promoter: Identification in silico of new putative regulatory motifs conserved between monocots and dicots. J. Exp. Bot. 2007, 58, 2573–2582. [Google Scholar] [CrossRef] [PubMed]
- Kisko, M.; Bouain, N.; Rouached, A.; Choudhary, S.P.; Rouched, H. Molecular mechanisms of phosphate and zinc signaling crosstalk in plants: Phosphate and zinc loading into root xylem in Arabidopsis. Environ. Exp. Bot. 2015, 114, 57–64. [Google Scholar] [CrossRef]
- Xie, X.; Hu, W.; Fan, X.; Chen, H.; Tang, M. Interactions between phosphorus, zinc, and iron homeostasis in nonmycorrhizal and mycorrhizal plants. Front. Plant Sci. 2019, 10, 1172. [Google Scholar] [CrossRef]
- Vance, C.P.; Uhde-Stone, C.; Allan, D.L. Phosphorus acquisition and use: Critical adaptations by plants for securing a nonrenewable resource. New Phytol. 2003, 157, 423–447. [Google Scholar] [CrossRef]
- Sánchez-Calderón, L.; Chacon-López, A.; Pérez-Torres, C.-A.; Herrera-Estrella, L. Phosphorus: Plant strategies to cope with its scarcity. In Cell Biology of Metals and Nutrients; Springer: Berlin/Heidelberg, Germany, 2010; pp. 173–198. ISBN 978-3-642-10613-2. [Google Scholar]
- Rouached, H.; Arpat, A.B.; Poirier, Y. Regulation of phosphate starvation responses in plants: Signaling players and cross-talks. Mol. Plant 2010, 3, 288–299. [Google Scholar] [CrossRef]
- Hammond, J.P.; Broadley, M.R.; White, P.J. Genetic responses to phosphorus deficiency. Ann. Bot. 2004, 94, 323–332. [Google Scholar] [CrossRef]
- Rubio, V.; Linhares, F.; Solano, R.; Martín, A.C.; Iglesias, J.; Leyva, A.; Paz-Ares, J. A conserved MYB transcription factor involved in phosphate starvation signaling both in vascular plants and in unicellular algae. Genes Dev. 2001, 15, 2122–2133. [Google Scholar] [CrossRef]
- Shin, R.; Berg, R.H.; Schachtman, D.P. Reactive oxygen species and root hairs in Arabidopsis root response to nitrogen, phosphorus and potassium deficiency. Plant Cell Physiol. 2005, 46, 1350–1357. [Google Scholar] [CrossRef]
- Robinson, W.D.; Carson, I.; Ying, S.; Ellis, K.; Plaxton, W.C. Eliminating the purple acid phosphatase AtPAP26 in Arabidopsis thaliana delays leaf senescence and impairs phosphorus remobilization. New Phytol. 2012, 196, 1024–1029. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, J.; Zhang, X.; Fan, H.; Gu, M.; Qu, H.; Xu, G. Phosphate transporter OsPht1;8 in rice plays in important role in phosphorus redistribution from source to sink organs and allocation between embryo and endosperm of seeds. Plant Sci. 2015, 230, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Denton, M.D.; Veneklaas, E.J.; Freimoser, F.M.; Lambers, H. Banksia species (Proteaceae) from severely phosphorus-impoverished soils exhibit extreme efficiency in the use and re-mobilization of phosphorus. Plant Cell Environ. 2007, 30, 1557–1565. [Google Scholar] [CrossRef]
- Lambers, H.; Clode, P.L.; Hawkins, H.J.; Laliberté, E.; Oliveira, R.S.; Reddell, P.; Shane, M.W.; Stitt, M.; Weston, P. Metabolic adaptations of the non-mycotrophic Proteaceae to soils with low phosphorus availability. Annu. Plant Rev. 2018, 15, 289–335. [Google Scholar] [CrossRef]
- Besford, R. Uptake and distribution of phosphorus in tomato plants. Plant Soil 1979, 51, 331–340. [Google Scholar] [CrossRef]
- Irshad, M.; Gill, M.A.; Aziz, T.; Ahmed, I. Growth response of cotton cultivars to zinc deficiency stress in chelator-buffered nutrient solution. Pak. J. Bot. 2004, 36, 373–380. [Google Scholar]
- Bozzo, G.G.; Dunn, E.L.; Plaxton, W.C. Differential synthesis of phosphate—Starvation inducible purple acid phosphatase isozymes in tomato (Lycopersicon esculentum) suspension cells and seedlings. Plant Cell Environ. 2005, 29, 303–313. [Google Scholar] [CrossRef]
- Ha, S.; Tran, L.-S. Understanding plant responses to phosphorus starvation for improvement of plant tolerance to phosphorus deficiency by biotechnological approaches. Crit. Rev. Biotechnol. 2014, 34, 16–30. [Google Scholar] [CrossRef]
- Puga, M.I.; Rojas-Triana, M.; de Lorenzo, L.; Leyva, A.; Rubio, V.; Paz-Ares, J. Novel signals in the regulation of Pi starvation responses in plants: Facts and promises. Curr. Opin. Plant Biol. 2017, 39, 40–49. [Google Scholar] [CrossRef]
- Hackenberg, M.; Shi, B.-J.; Gustafson, P.; Langridge, P. Characterization of phosphorus-regulated miR399 and miR827 and their isomirs in barley under phosphorus-sufficient and phosphorus-deficient conditions. BMC Plant Biol. 2013, 13, 214. [Google Scholar] [CrossRef]
- Veneklaas, E.J.; Lambers, H.; Bragg, J.; Finnegan, P.M.; Lovelock, C.E.; Plaxton, W.C.; Price, C.A.; Scheible, W.R.; Shane, M.W.; White, P.J. Opportunities for improving phosphorus--use efficiency in crop plants. New Phytol. 2012, 195, 306–320. [Google Scholar] [CrossRef]
- Haran, S.; Logendra, S.; Seskar, M.; Bratanova, M.; Raskin, I. Characterization of Arabidopsis acid phosphatase promoter and regulation of acid phosphatase expression. Plant Physiol. 2000, 124, 615–626. [Google Scholar] [CrossRef] [PubMed]
- Poirier, Y.; Bucher, M. Phosphate transport and homeostasis in Arabidopsis. Arabidopsis Book 2002, 1, e0024. [Google Scholar] [CrossRef]
- Mimura, T.; Dietz, K.J.; Kaiser, W.; Schramm, M.; Kaiser, G.; Heber, U. Phosphate transport across biomembranes and cytosolic phosphate homeostasis in barley leaves. Planta 1990, 180, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, T.; Wang, M.; Liu, Y.; Brestic, M.; Chen, T.H.H.; Yang, X. Genetic engineering of the biosynthesis of glycine betaine modulates phosphate homeostasis by regulating phosphate acquisition in tomato. Front. Plant Sci. 2019, 9, 1995. [Google Scholar] [CrossRef] [PubMed]
- Groot, C.C.; van den Boogaard, R.; Marcelis, L.F.M.; Harbinson, J.; Lambers, H. Contrasting effects of N and P deprivation on the regulation of photosynthesis in tomato plants in relation to feedback limitation. J. Exp. Bot. 2003, 54, 1957–1967. [Google Scholar] [CrossRef]
- Wu, P.; Ma, L.; Hou, X.; Wang, M.; Wu, Y.; Liu, F.; Deng, X.W. Phosphate starvation triggers distinct alterations of genome expression in arabidopsis roots and leaves. Plant Physiol. 2003, 132, 1260–1271. [Google Scholar] [CrossRef]
- Ogawa, N.; DeRisi, J.; Brown, P.O. New components of a system for phosphate accumulation and polyphosphate metabolism in Saccharomyces cerevisiae revealed by genomic expression analysis. Mol. Biol. Cell 2000, 11, 4309–4321. [Google Scholar] [CrossRef]
- He, J.; Jin, Y.; Du, Y.-L.; Wang, T.; Turner, N.C.; Yang, R.P.; Siddique, K.H.M.; Li, F.M. Genotypic variation in yield, yield components, root morphology and architecture, in soybean in relation to water and phosphorus supply. Front. Plant Sci. 2017, 8, 1499. [Google Scholar] [CrossRef]
- Lynch, J.P.; Brown, K.M. Topsoil foraging—An architectural adaptation of plants to low phosphorus availability. Plant Soil 2001, 237, 225–237. [Google Scholar] [CrossRef]
- Raven, J.A.; Lambers, H.; Smith, S.E.; Westoby, M. Costs of acquiring phosphorus by vascular land plants: Patterns and implications for plant coexistence. New Phytol. 2018, 217, 1420–1427. [Google Scholar] [CrossRef] [PubMed]
- Williamson, L.C.; Ribrioux, S.P.; Fitter, A.H.; Leyser, H.O. Phosphate availability regulates root system architecture in Arabidopsis. Plant Physiol. 2001, 126, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.R.; Ochoa, I.; Nielsen, K.L.; Beck, D.; Lynch, J.P. Genetic variation for adventitious rooting in response to low phosphorus availability: Potential utility for phosphorus acquisition from stratified soils. Funct. Plant Biol. 2003, 30, 973–985. [Google Scholar] [CrossRef]
- Walk, T.C.; Jaramillo, R.; Lynch, J.P. Architectural tradeoffs between adventitious and basal roots for phosphorus acquisition. Plant Soil 2006, 279, 347–366. [Google Scholar] [CrossRef]
- Madlung, A.; Behringer, F.J.; Lomax, T.L. Ethylene plays multiple nonprimary roles in modulating the gravitropic response in tomato. Plant Physiol. 1999, 120, 897–906. [Google Scholar] [CrossRef]
- Ma, Q.; Rengel, Z.; Siddique, K.H. Wheat and white lupin differ in root proliferation and phosphorus use efficiency under heterogeneous soil P supply. Crop Pasture Sci. 2011, 62, 467–473. [Google Scholar] [CrossRef]
- Jing, J.; Rui, Y.; Zhang, F.; Rengel, Z.; Shen, J. Localized application of phosphorus and ammonium improves growth of maize seedlings by stimulating root proliferation and rhizosphere acidification. Field Crops Res. 2010, 119, 355–364. [Google Scholar] [CrossRef]
- Kumar, A.; Shahbaz, M.; Koirala, M.; Blagodatskaya, E.; Seidel, S.J.; Kuzyakov, Y.; Pausch, J. Root trait plasticity and plant nutrient acquisition in phosphorus limited soil. J. Plant Nutr. Soil Sci. 2019, 182, 945–952. [Google Scholar] [CrossRef]
- Jiang, X.; Chen, W.L.; Xu, C.X.; Zhu, H.H.; Yao, Q. Influences of arbuscular mycorrhizal fungus and phosphorus level on the lateral root formation of tomato seedlings. J. Appl. Ecol. 2015, 26, 1186–1192. [Google Scholar]
- Garcia, M.; Ascencio, J. Root morphology and acid phosphatase activity in tomato plants during development of and recovery from phosphorus stress. J. Plant Nutr. 1992, 15, 2491–2503. [Google Scholar] [CrossRef]
- Hammond, J.P.; Broadley, M.R.; White, P.J.; King, G.J.; Bowen, H.C.; Hayden, R.; Meacham, M.C.; Mead, A.; Overs, T.; Spracklen, W.P. Shoot yield drives phosphorus use efficiency in Brassica oleracea and correlates with root architecture traits. J. Exp. Bot. 2009, 60, 1953–1968. [Google Scholar] [CrossRef] [PubMed]
- Postma, J.A.; Dathe, A.; Lynch, J.P. The optimal lateral root branching density for maize depends on nitrogen and phosphorus availability. Plant Physiol. 2014, 166, 590–602. [Google Scholar] [CrossRef]
- Chevalier, F.; Pata, M.; Nacry, P.; Doumas, P.; Rossignol, M. Effects of phosphate availability on the root system architecture: Large-scale analysis of the natural variation between Arabidopsis accessions. Plant Cell Environ. 2003, 26, 1839–1850. [Google Scholar] [CrossRef]
- Reymond, M.; Svistoonoff, S.; Loudet, O.; Nussaume, L.; Desnos, T. Identification of QTL controlling root growth response to phosphate starvation in Arabidopsis thaliana. Plant Cell Environ. 2006, 29, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Kaeppler, S.M.; Lynch, J.P. Mapping of QTLs for lateral root branching and length in maize (Zea mays L.) under differential phosphorus supply. Theor. Appl. Genet. 2005, 111, 688–695. [Google Scholar] [CrossRef]
- Gilroy, S.; Jones, D.L. Through form to function: Root hair development and nutrient uptake. Trends Plant Sci. 2000, 5, 56–60. [Google Scholar] [CrossRef]
- Gahoonia, T.S.; Nielsen, N.E. Root traits as tools for creating phosphorus efficient crop varieties. Plant Soil 2004, 260, 47–57. [Google Scholar] [CrossRef]
- Wissuwa, M. How do plants achieve tolerance to phosphorus deficiency? Small causes with big effects. Plant Physiol. 2003, 133, 1947–1958. [Google Scholar] [CrossRef]
- Giri, J.; Bhosale, R.; Huang, G.; Pandey, B.K.; Parker, H.; Zappala, S.; Yang, J.; Dievart, A.; Bureau, C.; Ljung, K.; et al. Rice auxin influx carrier OsAUX1 facilitates root hair elongation in response to low external phosphate. Nat. Commun. 2018, 9, 1408. [Google Scholar] [CrossRef]
- Tominaga-Wada, R.; Nukumizu, Y.; Sato, S.; Wada, T. Control of plant trichome and root-hair development by a tomato (Solanum lycopersicum) R3 MYB transcription factor. PLoS ONE 2013, 8, e54019. [Google Scholar] [CrossRef] [PubMed]
- Foehse, D.; Jungk, A. Influence of phosphate and nitrate supply on root hair formation of rape, spinach and tomato plants. Plant Soil 1983, 74, 359–368. [Google Scholar] [CrossRef]
- Bayuelo-Jiménez, J.S.; Gallardo-Valdéz, M.; Pérez-Decelis, V.A.; Magdaleno-Armas, L.; Ochoa, I.; Lynch, J.P. Genotypic variation for root traits of maize (Zea mays L.) from the Purhepecha Plateau under contrasting phosphorus availability. Field Crops Res. 2011, 121, 350–362. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Lynch, J.P.; Brown, K.M. Ethylene and phosphorus availability have interacting yet distinct effects on root hair development. J. Exp. Bot. 2003, 54, 2351–2361. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Walk, T.C.; Marcus, A.; Lynch, J.P. Morphological synergism in root hair length, density, initiation and geometry for phosphorus acquisition in Arabidopsis thaliana: A modeling approach. Plant Soil 2001, 236, 221–235. [Google Scholar] [CrossRef]
- Genc, Y.; Huang, C.Y.; Langridge, P. A study of the role of root morphological traits in growth of barley in zinc-deficient soil. J. Exp. Bot. 2007, 58, 2775–2784. [Google Scholar] [CrossRef] [PubMed]
- Bates, T.R.; Lynch, J.P. Plant growth and phosphorus accumulation of wild type and two root hair mutants of Arabidopsis thaliana (Brassicaceae). Am. J. Bot. 2000, 87, 958–963. [Google Scholar] [CrossRef] [PubMed]
- Lambers, H.; Shane, M.W.; Cramer, M.D.; Pearse, S.J.; Veneklaas, E.J. Root structure and functioning for efficient acquisition of phosphorus: Matching morphological and physiological traits. Ann. Bot. 2006, 98, 693–713. [Google Scholar] [CrossRef]
- Brown, L.; George, T.; Thompson, J.; Wright, G.; Lyon, J.; Dupuy, L.; Hubbard, S.; White, P. What are the implications of variation in root hair length on tolerance to phosphorus deficiency in combination with water stress in barley (Hordeum vulgare)? Ann. Bot. 2012, 110, 319–328. [Google Scholar] [CrossRef]
- Bates, T.R.; Lynch, J.P. The efficiency of Arabidopsis thaliana (Brassicaceae) root hairs in phosphorus acquisition. Am. J. Bot. 2000, 87, 964–970. [Google Scholar] [CrossRef]
- Narang, R.A.; Bruene, A.; Altmann, T. Analysis of phosphate acquisition efficiency in different Arabidopsis accessions. Plant Physiol. 2000, 124, 1786–1799. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Bielenberg, D.; Brown, K.M.; Lynch, J.P. Regulation of root hair density by phosphorus availability in Arabidopsis thaliana. Plant Cell Environ. 2001, 24, 459–467. [Google Scholar] [CrossRef]
- Li, T.; Lin, G.; Zhang, X.; Chen, Y.; Zhang, S.; Chen, B. Relative importance of an arbuscular mycorrhizal fungus (Rhizophagus intraradices) and root hairs in plant drought tolerance. Mycorrhiza 2014, 24, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Masucci, J.D.; Schiefelbein, J.W. Hormones act downstream of TTG and GL2 to promote root hair outgrowth during epidermis development in the Arabidopsis root. Plant Cell. 1996, 8, 1505–1517. [Google Scholar] [CrossRef]
- Rahman, A.; Hosokawa, S.; Oono, Y.; Amakawa, T.; Goto, N.; Tsurumi, S. Auxin and ethylene response interactions during Arabidopsis root hair development dissected by auxin influx modulators. Plant Physiol. 2002, 130, 1908–1917. [Google Scholar] [CrossRef]
- Dakora, F.D.; Phillips, D.A. Root exudates as mediators of mineral acquisition in low-nutrient environments. In Food Security in Nutrient-Stressed Environments: Exploiting Plants’ Genetic Capabilities; Adu-Gyamfi, J.J., Ed.; Springer: Dordrecht, The Netherlands, 2002; pp. 201–213. ISBN 978-94-017-1570-6. [Google Scholar]
- Perez, M.J.; Smyth, T.J.; Israel, D.W. Comparative effects of two forage species on ehizospehre acidification and solubilization of phosphate rocks of different reactivity. J. Plant Nutr. 2007, 30, 1421–1439. [Google Scholar] [CrossRef]
- Hinsinger, P. Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: A review. Plant Soil 2001, 237, 173–195. [Google Scholar] [CrossRef]
- Calba, H.; Firdaus, C.P.; Thee, C.; Poss, R.; Jaillard, B. The dynamics of protons, aluminum, and calcium in the rhizosphere of maize cultivated in tropical acid soils: Experimental study and modelling. Plant Soil 2004, 260, 33–46. [Google Scholar] [CrossRef]
- Wang, L.; Nancollas, G.H. Calcium orthophosphates: Crystallization and dissolution. Chem. Rev. 2008, 108, 4628–4669. [Google Scholar] [CrossRef]
- Chow, L.C.; Eanes, E. Solubility of calcium phosphates. Monogr. Oral Sci. 2001, 18, 94–111. [Google Scholar]
- Houmani, H.; Rabhi, M.; Abdelly, C.; Debez, A. Implication of rhizosphere acidification in nutrient uptake by plants: Cases of potassium (K), phosphorus (P), and iron (Fe). In Crop Production and Global Environmental Issues; Springer: Dordrecht, The Netherlands, 2015; pp. 103–122. [Google Scholar]
- Shen, H.; Chen, J.; Wang, Z.; Yang, C.; Sasaki, T.; Yamamoto, Y.; Matsumoto, H.; Yan, X. Root plasma membrane H+-ATPase is involved in the adaptation of soybean to phosphorus starvation. J. Exp. Bot. 2006, 57, 1353–1362. [Google Scholar] [CrossRef]
- Xu, W.; Shi, W.; Jia, L.; Liang, J.; Zhang, J. TFT6 and TFT7, two different members of tomato 14-3-3 gene family, play distinct roles in plant adaption to low phosphorus stress. Plant Cell Environ. 2012, 35, 1393–1406. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Zhang, D.; Song, T.; Xu, F.; Lin, S.; Xu, W.; Li, Q.; Zhu, Y.; Liang, J. Arabidopsis plasma membrane H+-ATPase genes AHA2 and AHA7 have distinct and overlapping roles in the modulation of root tip H+ efflux in response to low-phosphorus stress. J. Exp. Bot. 2017, 68, 1731–1741. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Drevon, J.; Jaillard, B.; Souche, G.; Hinsinger, P. Proton release of two genotypes of bean (Phaseolus vulgaris L.) as affected by N nutrition and P deficiency. Plant Soil 2004, 260, 59–68. [Google Scholar] [CrossRef]
- Yan, F.; Zhu, Y.; Muller, C.; Zorb, C.; Schubert, S. Adaptation of H+-Pumping and plasma membrane H+ ATPase activity in proteoid roots of white lupin under phosphate deficiency. Plant Physiol. 2002, 129, 50–63. [Google Scholar] [CrossRef]
- Zoysa, A.; Loganathan, P.; Hedley, M. Phosphorus utilization efficiency and depletion of phosphate fractions in the rhizosphere of three tea (Camellia sinensis L.) clones. Nutr. Cycl. Agroecosyst. 1999, 53, 189–201. [Google Scholar] [CrossRef]
- Canellas, L.P.; Olivares, F.L.; Okorokova-Facanha, A.L. Humic acids isolated from eartheorm compost enhance root elongation, lateral root emergence, and plasma membrance H+-ATPase activity in maize roots. Plant Physiol. 2002, 130, 1951–1957. [Google Scholar] [CrossRef]
- Takahashi, K.; Hayashi, K.; Kinoshita, T. Auxin activates the plasma membrane H+-ATPase by phosphorylation during hypocotyl elongation in Arabidopsis. Cell Biol. Signal Transduct. 2012, 159, 632–641. [Google Scholar] [CrossRef]
- Jakkeral, S.A.; Kajjidoni, S. Root exudation of organic acids in selected genotypes under phosphorus deficient condition in blackgram (Vigna mungo L. Hepper). Karnataka J. Agr. Sci. 2011, 24, 316–319. [Google Scholar]
- Minemba, D.; Gleeson, D.B.; Veneklaas, E.; Ryan, M.H. Variation in morphological and physiological root traits and organic acid exudation of three sweet potato (Ipomoea batatas) cultivars under seven phosphorus levels. Sci. Hortic. 2019, 256, 108572. [Google Scholar] [CrossRef]
- Yu, W.; Kan, Q.; Zhang, J.; Zeng, B.; Chen, Q. Role of the plasma membrane H+-ATPase in the regulation of organic acid exudation under aluminum toxicity and phosphorus deficiency. Plant Signal. Behav. 2016, 11, e1106660. [Google Scholar] [CrossRef]
- Lambers, H.; Clements, J.C.; Nelson, M.N. How a phosphorus-acquisition strategy based on carboxylate exudation powers the success and agronomic potential of lupines (Lupinus, Fabaceae). Am. J. Bot. 2013, 100, 263–288. [Google Scholar] [CrossRef] [PubMed]
- Neumann, G.; Römheld, V. Root excretion of carboxylic acids and protons in phosphorus-deficient plants. Plant Soil 1999, 211, 121–130. [Google Scholar] [CrossRef]
- Luo, H.M.; Watanabe, T.; Shinano, T.; Tadano, T. Comparison of aluminum tolerance and phosphate absorption between rape (Brassica napus L.) and Tomato (Lycopersicum esculentum Mill.) in relation to organic acid exudation. Soil Sci. Plant Nutr. 1999, 45, 897–907. [Google Scholar] [CrossRef]
- Imas, P.; Bar-Yosef, B.; Kadkafi, U.; Ganmore-Neumann, R. Phosphate induced carboxylate and proton release by tomato roots. Plant Soil 1997, 191, 35–39. [Google Scholar] [CrossRef]
- De Souza, M.F.; Soares, E.M.B.; Silva, I.R.D.; Novais, R.F.; Silva, M.F.D.O. Competitive sorption and desorption of phosphate and citrate in clayey and sandy loam soils. Rev. Bras. Ciênc. 2014, 38, 1153–1161. [Google Scholar] [CrossRef][Green Version]
- Duputel, M.; Devau, N.; Brossard, M.; Jaillard, B.; Jones, D.L.; Hinsinger, P.; Gérard, F. Citrate adsorption can decrease soluble phosphate concentration in soils: Results of theoretical modeling. Appl. Geochem. 2013, 35, 120–131. [Google Scholar] [CrossRef]
- Jayachandran, K.; Hetrick, B.A.D.; Schwab, A.P. Mycorrhizal mediation of phosphorus availability: Synthetic iron chelate effects on phosphorus solubilization. Soil Sci. Soc. Am. J. 1989, 53, 1701–1706. [Google Scholar] [CrossRef]
- Teixeira, R.D.S.; Ribeiro da Silva, I.; Nogueira de Sousa, R.; Márcio Mattiello, E.; Barros Soares, E.M. Organic acid coated-slow-release phosphorus fertilizers improve P availability and maize growth in a tropical soil. J. Soil Sci. Plant Nutr. 2016, 16, 1097–1112. [Google Scholar] [CrossRef][Green Version]
- Imas, P.; Bar-Yosef, B.; Kafkafi, U.; Ganmore-Neumann, R. Release of carboxylic anions and protons by tomato roots in response to ammonium nitrate ratio and pH in nutrient solution. Plant Soil 1997, 191, 27–34. [Google Scholar] [CrossRef]
- Dixon, M.; Webb, E.C. Enzymes, 2nd ed.; Academic Press: New York, NY, USA, 1964. [Google Scholar]
- Sardans, J.; Peñuelas, J.; Estiarte, M. Warming and drought alter soil phosphatase activity and soil P availability in a Mediterranean shrubland. Plant Soil 2006, 289, 227–238. [Google Scholar] [CrossRef]
- Li, S.M.; Li, L.; Zhang, F.; Tang, C. Acid phosphatase role in chickpea/maize intercropping. Ann. Bot. 2004, 94, 297–303. [Google Scholar] [CrossRef]
- Wasaki, J.; Yamamura, T.; Shinano, T.; Osaki, M. Secreted acid phosphatase is expressed in cluster roots of lupin in response to phosphorus deficiency. Plant Soil 2003, 248, 129–136. [Google Scholar] [CrossRef]
- Playsted, C.W.S.; Johnston, M.E.; Ramage, C.M.; Edwards, D.G.; Cawthray, G.R.; Lambers, H. Functional significance of dauciform roots: Exudation of carboxylates and acid phosphatase under phosphorus deficiency in Caustis blakei (Cyperaceae). New Phytol. 2006, 170, 491–500. [Google Scholar] [CrossRef]
- McComb, R.B.; Bowers, G.N., Jr.; Posen, S. Alkaline Phosphatase; Springer Science and Business Media: New York, NY, USA, 2013; ISBN 978-1-4613-2972-5. [Google Scholar]
- Kim, K.Y.; Jordan, D.; McDonald, G.A. Effect of phosphate-solubilizing bacteria and vesicular-arbuscular mycorrhizae on tomato growth and soil microbial activity. Biol. Fertil. Soils 1997, 26, 79–87. [Google Scholar] [CrossRef]
- Sharma, S.B.; Sayyed, R.Z.; Trivedi, M.H.; Gobi, T.A. Phosphate solubilizing microbes: Sustainable approach for managing phosphorus deficiency in agricultural soils. SpringerPlus 2013, 2, 587. [Google Scholar] [CrossRef] [PubMed]
- Nagy, R.; Drissner, D.; Amrhein, N.; Jakobsen, I.; Bucher, M. Mycorrhizal phosphate uptake pathway in tomato is phosphorus-repressible and transcriptionally regulated. New Phytol. 2009, 181, 950–959. [Google Scholar] [CrossRef]
- Nagy, R.; Karandashov, V.; Chague, V.; Kalinkevich, K.; Tamasloukht, M.; Xu, G.; Jakpbsen, I.; Levy, A.A.; Amrhein, N.; Bucher, M. The characterization of novel mycorrhiza-specific phosphate transporters from Lycopersicon esculentum and Solanum tuberosum uncovers functional redundancy in symbiotic phosphate transport in solanaceous species. Plant J. 2005, 42, 236–250. [Google Scholar] [CrossRef]
- Gomez-Ariza, J.; Balestrini, R.; Novero, M.; Bonfante, P. Cell-specific gene expression of phosphate transporters in mycorrhizal tomato roots. Biol. Fertil. Soils 2009, 45, 845–853. [Google Scholar] [CrossRef]
- Poulsen, K.H.; Nagy, R.; Gao, L.-L.; Smith, S.E.; Bucher, M.; Smith, F.A.; Jakobsen, I. Physiological and molecular evidence for Pi uptake via the symbiotic pathway in a reduced mycorrhizal colonization mutant in tomato associated with a compatible fungus. New Phytol. 2005, 168, 445–454. [Google Scholar] [CrossRef]
- Goldstein, A.; Krishnaraj, P. Phosphate solubilizing microorganisms vs. phosphate mobilizing microorganisms: What separates a phenotype from a trait? In First International Meeting on Microbial Phosphate Solubilization; Velazquez, E., Rodriguez-Barrueco, C., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 203–213. [Google Scholar]
- Alori, E.T.; Glick, B.R.; Babalola, O.O. Microbial phosphorus solubilization and its potential for use in sustainable agriculture. Front. Microbiol. 2017, 8, 971. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, L.; Ma, F.; Zhang, X.; Fu, D. Arbuscular mycorrhiza improved phosphorus efficiency in paddy fields. Ecol. Eng. 2016, 95, 64–72. [Google Scholar] [CrossRef]
- Rengel, Z. Breeding for better symbiosis. In Food Security in Nutrient-Stressed Environments: Exploiting Plants’ Genetic Capabilities; Adu-Gyamfi, J.J., Ed.; Springer: Dordrecht, The Netherlands, 2002; pp. 245–260. [Google Scholar]
- Besserer, A.; Puech-Pagès, V.; Kiefer, P.; Gomez-Roldan, V.; Jauneau, A.; Roy, S.; Portais, J.C.; Roux, C.; Bécard, G.; Séjalon-Delmas, N. Strigolactones stimulate arbuscular mycorrhizal fungi by activating mitochondria. PLoS Biol. 2006, 4, e226. [Google Scholar] [CrossRef] [PubMed]
- Rezacova, V.; Konvalinkova, T.; Jansa, J. Carbon Fluxes in Mycorrhizal Plants. In Mycorrhiza—Eco-Physiology, Secondary Metabolites, Nanomaterials; Varma, A., Prasad, R., Tuteja, N., Eds.; Springer: Cham, Switzerland, 2017; ISBN 978-3-319-57848-4. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).