Fire Blight Monitoring in Pear Orchards by Unmanned Airborne Vehicles (UAV) Systems Carrying Spectral Sensors
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Ground Data Collection by Visual Inspections
2.3. Image Collection by UAV
2.4. Hyperspectral Data Analysis
2.4.1. Preprocessing of Hyperspectral Images
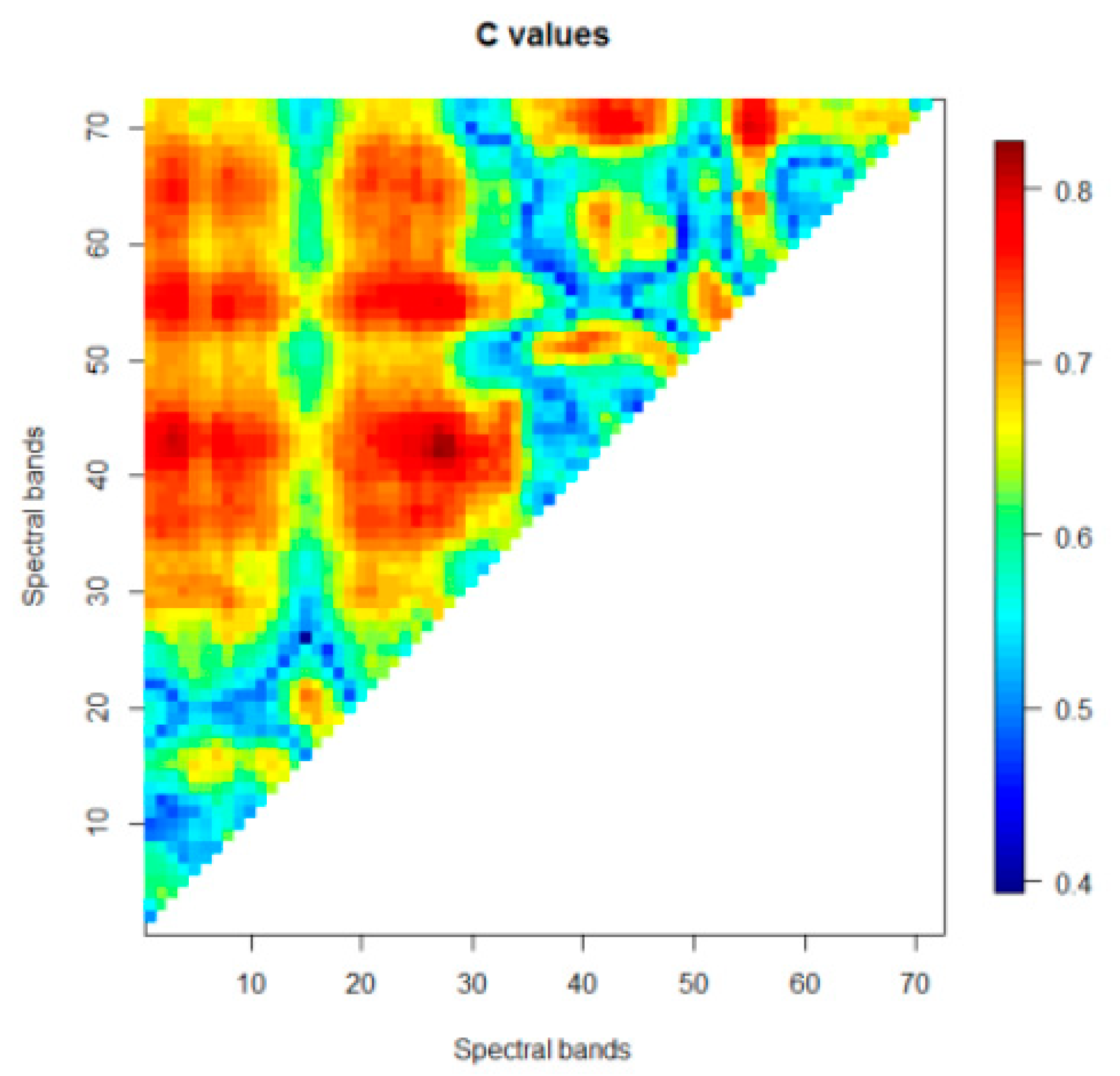
2.4.2. Selection of Spectral Reflectance Bands
3. Results
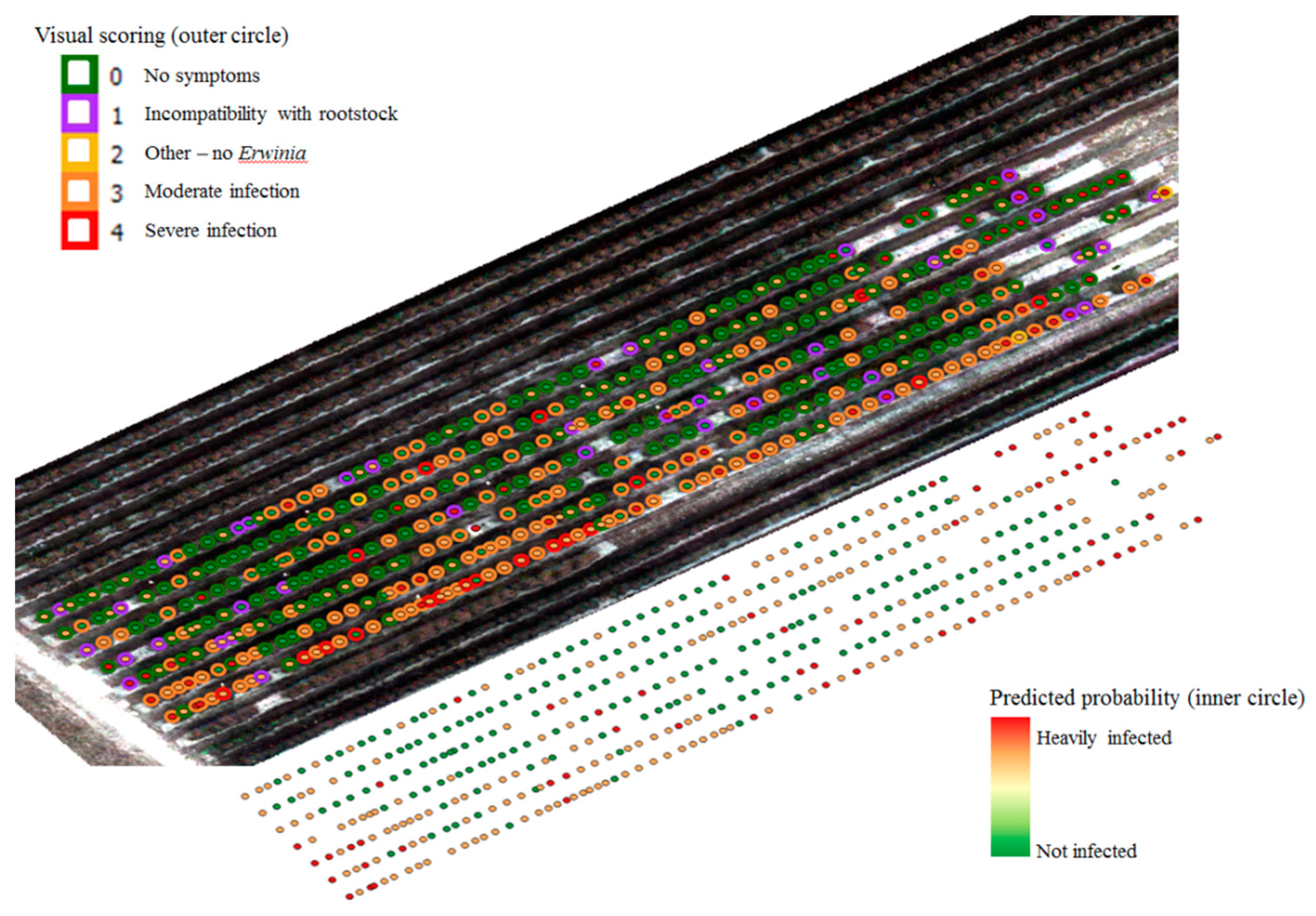
3.1. Ground Data Selection by Visual Inspection
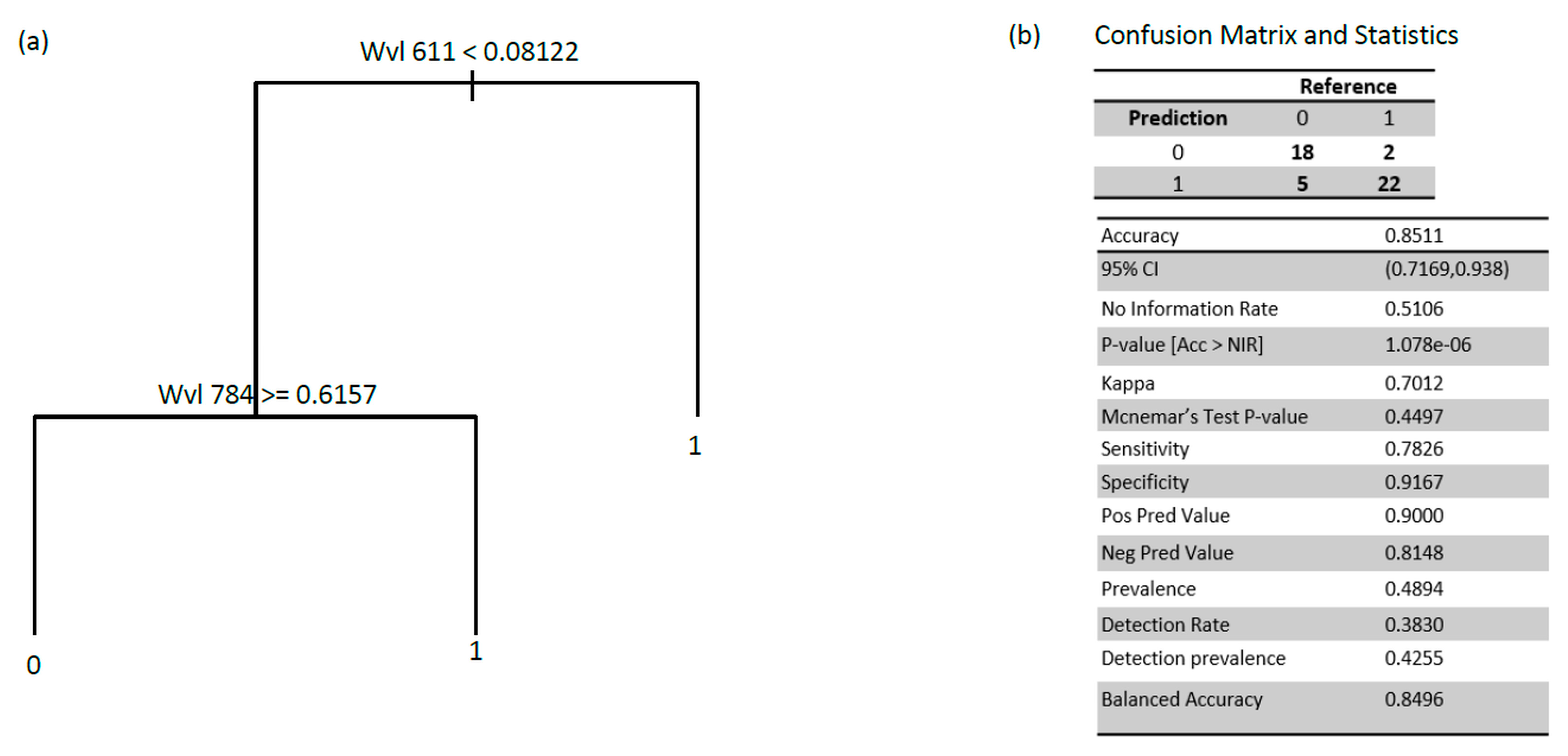
3.2. Hyperspectral Data Analyses for Fire Blight Detection
3.3. Application of Selected Wavelengths on the Entire Data Set
4. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van der Zwet, T.; Orolaza-Halbrendt, N.; Zeller, W. Fire Blight: History, Biology, and Managemen; APS Press: St. Paul, MN, USA, 2012. [Google Scholar]
- Vanneste, J.L. Genetic diversity and host range of Erwinia amylovora? In Fire Blight: The Disease and its Causative agent, Erwinia Amylovora; Vanneste, J.L., Ed.; CABI Publishing: New York, NY, USA, 2000; pp. 55–86. [Google Scholar]
- Thomson, S. Epidemiology of fire blight. In Fire blight: The Disease and its Causative agent, Erwinia Amylovora; Vanneste, J.L., Ed.; CABI Publishing: New York, NY, USA, 2000; pp. 9–36. [Google Scholar]
- Vanneste, J.L. What is fire blight? Who is Erwinia amylovora? How to control it? In Fire Blight: The Disease and its Causative Agent, Erwinia Amylovora; Vanneste, J.L., Ed.; CABI Publishing: New York, NY, USA, 2000; pp. 1–6. [Google Scholar]
- Vrancken, K.; Holtappels, M.; Schoofs, H.; Deckers, T.; Valcke, R. Pathogenicity and infection strategies of the fire blight pathogen Erwinia amylovora in Rosaceae: State of the art. Microbiology 2013, 159, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Piqué, N.; Miñana-Galbis, D.; Merino, S.; Tomás, J.M. Virulence factors of Erwinia amylovora: A Review. Int. J. Mol. Sci. 2015, 16, 12836–12854. [Google Scholar] [CrossRef] [PubMed]
- Norelli, J.L.; Holleran, H.T.; Johnson, W.C.; Robinson, T.L.; Aldwinckle, H.S. Resistance of Geneva and other apple rootstocks to Erwinia amylovora. Plant Dis. 2003, 87, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Billing, E. Fire blight risk assessment systems and models. In Fire Blight: The Disease and its Causative Agent, Erwinia Amylovora; Vanneste, J.L., Ed.; CABI Publishing: New York, NY, USA, 2000. [Google Scholar]
- Santander, R.D.; Biosca, E.G. Erwinia amylovora psychotrophic adaptations: Evidence of pathogenic potential and survival at temperature and low environmental temperatures. PeerJ 2017. [Google Scholar] [CrossRef]
- Momol, M.T.; Norelli, K.L.; Piccioni, D.E.; Momol, E.A.; Gustafson, H.L.; Cummins, J.L.; Aldwinckle, H.S. Internal movement of Erwinia amylovora through symptomless apple scion tissue into the rootstock. Plant. Dis 1998, 82, 646–650. [Google Scholar] [CrossRef]
- Slack, S.M.; Zeng, Q.; Outwater, C.A.; Sundin, G.W. Microbiological examination of Erwinia amylovora exopolysaccharide ooze. Phytopathology 2017, 4, 403–411. [Google Scholar] [CrossRef]
- Zhao, Y.Q.; Tian, Y.I.; Wang, L.M.; Geng, G.M.; Zhao, W.J.; Hu, B.S.; Zhao, Y.F. Fire blight, a fast-approaching threat to apple and pear production in China. J. Integr. Agric. 2019, 18, 815–820. [Google Scholar] [CrossRef]
- Hyon, J.C.; Yeon, J.K.; Yeon-Jeon, L.; Duck, H.P. Survival of Erwinia amylovora on surfaces of materials used in orchards. Res. Plant. Dis. 2019, 25, 89–93. [Google Scholar]
- McManus, P.S.; Jones, A.L. Role of wind-driven rain, aerosols, and contaminated budwood in incidence and spatial pattern of fire blight in an apple nursery. Plant Dis. 1994, 78, 1059–1066. [Google Scholar] [CrossRef]
- Carter, G.A.; Miller, R.L. Early detection of plant stress by digital imaging within narrow stress-sensitive wavebands. Remote Sens. Environ. 1994, 50, 295–302. [Google Scholar] [CrossRef]
- Carter, G.A.; Cibula, W.G.; Miller, R.L. Narrow band reflectance imagery compared with thermal imagery for early detection of plant stress. J. Plant Physiol. 1996, 148, 515–522. [Google Scholar] [CrossRef]
- Filella, I.; Peñuelas, J. The red edge position and shape as indicators of plant chlorophyll content, biomass and hydric status. Int. J. Remote Sens. 1994, 15, 1459–1470. [Google Scholar] [CrossRef]
- Lowe, A.; Harrisonn, N.; French, A.P. Hyperspectral image analysis techniques for the detection and classification of the early onset of plant disease and stress. Plant Methods 2017, 13, 1–12. [Google Scholar] [CrossRef]
- Bagheri, N.; Hosna, M.-M.; Azizi, A.; Ghasemi, A. Detection of Fire Blight disease in pear trees by hyperspectral dat. Eur. J. Remote Sens. 2018, 51, 1–10. [Google Scholar] [CrossRef]
- Delalieux, S.; van Aardt, J.; Keulemans, W.; Schrevens, E.; Coppin, P. Detection of biotic stress (Venturia inaequalis) in apple trees using hyperspectral data: Non-parametric statistical approaches and physiological implications. EJA 2007, 130–143. [Google Scholar] [CrossRef]
- Abdulridha, J.; Ehsani, R.; Abd-Elrahman, A.; Ampatzidis, Y. A Remote Sensing technique for detecting laurel wilt disease in avocado in presence of other biotic and abiotic stresses. Comput. Electron. Agric. 2019, 156, 549–557. [Google Scholar] [CrossRef]
- Garcia-Ruiz, F.; Sankaran, S.; Maja, J.M.; Lee, W.S.; Rasmussen, J.; Ehsani, R. Comparison of two aerial imaging platforms for identification of Huanglongbing-infected citrus trees. Comput. Electron. Agric. 2013, 91, 106–115. [Google Scholar] [CrossRef]
- Romero-Trigueros, C.; Nortes, P.A.; Alarcón, J.J.; Hunink, J.E.; Parra, M.; Contreras, S.; Nicolás, E. Effects of saline reclaimed waters and deficit irrigation on Citrus physiology assessed by UAV remote sensing. Agric. Water Manag. 2017, 183, 60–69. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, Y.; Pu, R.; Gonzalez-Moreno, P.; Yuan, L.; Wu, K.; Huang, W. Monitoring plant diseases and pests through remote sensing technology: A review. Comput. Electron. Agric. 2019, 165, 104943. [Google Scholar] [CrossRef]
- Jarolmasjed, S.; Sankaran, S.; Marzougui, A.; Kostick, S.; Si, Y.; Quirós Vargas, J.J.; Evans, K. High-Throughput Phenotyping of; Fire Blight Disease Symptoms Using Sensing Techniques in Apple. Front. Plant Sci. 2019, 10, 576. [Google Scholar] [CrossRef]
- Diagnostic protocols for regulated pests. PM 7/20 (2) Erwinia amylovora. EPPO Bull. 2013, 4, 21–45. [CrossRef]
- King, E.O.; Ward, M.; Raney, D.E. Two simple media for the demonstration of pyocyanin and fluorescein. J. Lab. Clin. Med. 1954, 44, 401–407. [Google Scholar]
- Tack, N.; Lambrechts, A.; Soussan, S.; Haspeslagh, L. A compact, high speed, and lowcost hyperspectral imager. In Proceedings of the Volume 8266, Silicon Photonics VII, 82660Q, San Francisco, CA, USA, 9 February 2012. [Google Scholar] [CrossRef]
- Sima, A.; Baeck, P.-J.; Nuyts, D.; Delalieux, S.; Livens, S.; Blommaert, J.; Delauré, B.; Boonen, M. Compact Hyperspectral Imaging System (COSI) for small Remotely Piloted Aircraft Systems (RPAS)—system overview and first performance evaluation results. Int. Arch. Photogramm. Remote Sens. Spatial Inf. Sci. 2016, XLI-B1, 1157–1164. [Google Scholar] [CrossRef]
- Tucker, C.J. Red and photographic infrared linear combinations for monitoring vegetation. Remote Sens. Environ. 1979, 8, 127–150. [Google Scholar] [CrossRef]
- Elvidge, C.D.; Chen, Z. Comparison of broad-band and narrow band red and near infrared vegetation indices. Remote Sens. Environ. 1995, 54, 38–48. [Google Scholar] [CrossRef]
- Blackburn, G.A.; Pitman, J.I. Biophysical controls on the directional spectral reflectance properties of bracken (Pteridium aquilinum) canopies: Results of a field experiment. Int. J. Remote Sens. 1999, 20, 2265–2282. [Google Scholar] [CrossRef]
- Gao, X.; Huete, A.R.; Ni, W.; Miura, T. Optical-biophysical relationships of vegetation spectra without background contamination. Remote Sens. Environ. 2000, 74, 609–620. [Google Scholar] [CrossRef]
- Xue, J.; Su, B. Significant Remote Sensing Vegetation Indices: A Review of Developments and Applications. J. Sens. 2017, 1, 1–17. [Google Scholar] [CrossRef]
- Jiang, Z.; Huete, A.R.; Chen, J.; Chen, U.; Li, J.; Yan, G.; Zhang, X. Analysis of NDVI and scaled difference vegetation index retrievals of vegetation fraction. Remote Sens. Environ. 2006, 101, 366–378. [Google Scholar] [CrossRef]
- Metz, C.E. Basic principles of ROC analysis. Semin. Nucl. Med. 1978, 8, 283–298. [Google Scholar] [CrossRef]
- Newson, R. Parameters behind “non-parametric” statistics: Kendall’s τ and Somers’ D and median differences. Stata J. 2002, 2, 45–64. [Google Scholar] [CrossRef]
- Boyd, K.; Eng, K.H.; Page, C.D. Area under the Precision-Recall Curve: Point Estimates and Confidence Intervals. In Machine Learning and Knowledge Discovery in Databases; volume 8190 of LNCS; Hendrik, B., Kristian, K., Siegfried, N., Filip, Z., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 451–466. [Google Scholar]
- Therneau, T.; Atkinson, B.; Ripley, B. Rpart: Recursive Partitioning and Regression Trees, R Package Version 4.1–10, 2015. Available online: https://CRAN.R-project.org/package=rpart (accessed on 12 April 2019).
- Venables, W.N.; Ripley, B.D. Tree based methods. In Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Ahmad, I.S.; Reid, J.F. Evaluation of colour representations for maize images. J. Agric. Eng. Res. 1996, 63, 185–195. [Google Scholar] [CrossRef]
- Laliberte, A.S.; Rango, A.; Herrick, J.E.; Fredrickson, E.L.; Burkett, L. An object-based image analysis approach for determining fractional cover of senescent and green vegetation with digital plot photography. J. Arid Environ. 2007, 69, 1–14. [Google Scholar] [CrossRef]
- Fahrentrapp, J.; Ria, F.; Geilhausen, M.; Panassiti, B. Detection of Grey Mold leaf infections prior to visual symptom appearance using a five-band multispectral sensor. Front. Plant Sci. 2019, 10, 1–14. [Google Scholar] [CrossRef]
- Ahlawat, V.; Jhorar, O.; Kumar, L.; Backhouse, D. Using hyperspectral remote sensing as a tool for early detection of leaf rust in blueberries. In Proceedings of the 34th International symposium on Remote Sensing of Environment – The GEOSS Era: Towards Operational Evironmental Monintoring, Sydney, Australia, 10–15 April 2011. [Google Scholar]
- Adeline, K.R.M.; Chen, M.; Briotteta, X.; Pang, S.K.; Paparoditis, N. Shadow detection in very high spatial resolution aerial images: A comparative study. ISPRS J. Photogramm. Remote Sens. 2013, 82, 31–38. [Google Scholar] [CrossRef]
- Crepel, C.; Maes, M. Hibernation of the fire blight pathogen Erwinia amylovora in host plants. Meded. Fac. Landbouwk Toegep. Biol. Wet. (Univ. Gent) 2000, 65, 19–25. [Google Scholar]
| Visual FB * Infection Status | No. of Trees Scored Per Orchard Inspection In 2015 | ||||||
|---|---|---|---|---|---|---|---|
| June 12 | June 19 | June 29 | July 7 | August 12 | September 10 | Entire Season | |
| Class 0 (Healthy) | 338 | 323 | 209 | 236 | 161 | 76 | 39 |
| Class 1 (Moderately FB infected) | 72 | 86 | 203 | 155 | 240 | 242 | 263 |
| Class 2 (Severely FB infected) | 28 | 28 | 20 | 19 | 14 | 57 | 78 |
| No. fire blight infected trees | 100 | 114 | 223 | 174 | 254 | 299 | 341 |
| FB* Infection Status | Visual Scoring on 7 July 2015 | Relation with Hyperspectral Data | |||
|---|---|---|---|---|---|
| True Negative | True Positive | False Negative | False Positive | ||
| Healthy | 236 | 126 | / | / | 110 |
| Fire blight infected | 174 | / | 108 | 66 | / |
| Classification Based on the Relation Between Visual Scoring and Hyperspectral COSI-Cam Data of 7 July 2015 | No. of Trees Visually Scored (Entire Season, Until 10 September 2015) | |||
|---|---|---|---|---|
| Class | No. of trees | FB* infected | Healthy | Other |
| True positive | 108 | 107 | 1 | 0 |
| True negative | 126 | 86 | 27 | 13 |
| False positive | 110 | 82 | 11 | 17 |
| False negative | 66 | 66 | 0 | 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schoofs, H.; Delalieux, S.; Deckers, T.; Bylemans, D. Fire Blight Monitoring in Pear Orchards by Unmanned Airborne Vehicles (UAV) Systems Carrying Spectral Sensors. Agronomy 2020, 10, 615. https://doi.org/10.3390/agronomy10050615
Schoofs H, Delalieux S, Deckers T, Bylemans D. Fire Blight Monitoring in Pear Orchards by Unmanned Airborne Vehicles (UAV) Systems Carrying Spectral Sensors. Agronomy. 2020; 10(5):615. https://doi.org/10.3390/agronomy10050615
Chicago/Turabian StyleSchoofs, Hilde, Stephanie Delalieux, Tom Deckers, and Dany Bylemans. 2020. "Fire Blight Monitoring in Pear Orchards by Unmanned Airborne Vehicles (UAV) Systems Carrying Spectral Sensors" Agronomy 10, no. 5: 615. https://doi.org/10.3390/agronomy10050615
APA StyleSchoofs, H., Delalieux, S., Deckers, T., & Bylemans, D. (2020). Fire Blight Monitoring in Pear Orchards by Unmanned Airborne Vehicles (UAV) Systems Carrying Spectral Sensors. Agronomy, 10(5), 615. https://doi.org/10.3390/agronomy10050615





