Resistance Mechanisms to 2,4-D in Six Different Dicotyledonous Weeds Around the World
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material
2.3. Ethylene Production Fast Screening
2.4. Response of Dicotyledonous Weed Species to 2,4-D Pre-Treated with or without Malathion
2.5. 14C-2,4-D Absorption and Translocation in Dicotyledonous Weeds
2.6. 2,4-D Metabolism in Dicotyledonous Weeds
2.7. Data Analysis
3. Results
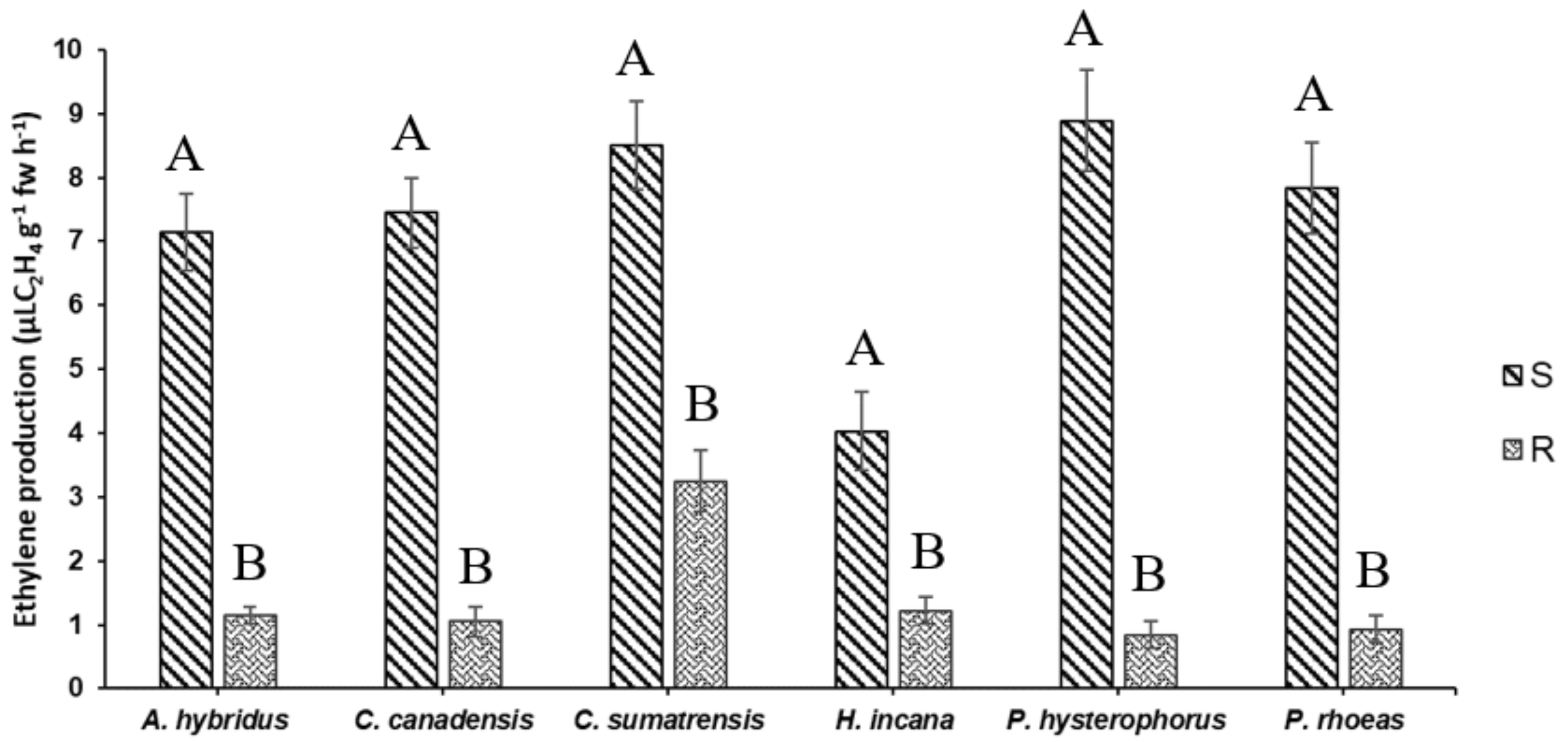
3.1. Ethylene Production Fast Screening
3.2. Response of Dicotyledonous Weed Species to 2,4-D Pre-Treated with or without Malathion
3.3. 14C-2,4-D Absorption and Translocation in Dicotyledonous Weeds
3.4. 2,4-D Plant Metabolism
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mithila, J.; Hall, J.C.; Johnson, W.G.; Kelley, K.B.; Riechers, D.E. Evolution of resistance to auxinic herbicides: Historical perspectives, mechanisms of resistance, and implications for broadleaf weed management in agronomic crops. Weed Sci. 2011, 59, 445–457. [Google Scholar] [CrossRef]
- Peterson, M.A.; McMaster, S.A.; Riechers, D.E.; Skelton, J.; Stahlman, P.W. 2,4-D past, present, and future: A review. Weed Technol. 2016, 30, 303–345. [Google Scholar] [CrossRef]
- Grossmann, K. Auxin herbicides: Current status of mechanism and mode of action. Pest Manag. Sci. 2010, 66, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Gervais, J.A.; Luukinen, B.; Buhl, K.; Stone, D.; 2,4-D Technical Fact Sheet. National Pesticide Information Center, Oregon State University Extension Service. 2008. Available online: http://npic.orst.edu/factsheets/2,4-DTech.pdf (accessed on 7 November 2019).
- Song, Y. Insight into the mode of action of 2,4-dichlorophenoxyacetic acid (2,4-D) as an herbicide. J. Integr. Plant Biol. 2014, 56, 106–113. [Google Scholar] [CrossRef]
- Weintraub, R.L.; Yeatman, J.N.; Brown, J.W.; Throne, J.A.; Skoss, J.D.; Conover, J.R. Studies on entry of 2,4-D into leaves. Proc. Northeast. Weed Control Conf. 1954, 8, 5–10. [Google Scholar]
- Matthes, M.S.; Best, N.B.; Robil, J.M.; Malcomber, S.; Gallavotti, A.; McSteen, P. Auxin EvoDevo: Conservation and diversification of genes regulating auxin biosynthesis, transport, and signaling. Mol. Plant 2019, 12, 298–320. [Google Scholar] [CrossRef]
- Busi, R.; Goggin, D.E.; Heap, I.M.; Horak, M.J.; Jugulam, M.; Masters, R.A.; Napier, R.M.; Riar, D.S.; Satchivi, N.M.; Torra, J.; et al. Weed resistance to synthetic auxin herbicides. Pest Manag. Sci. 2018, 74, 2265–2276. [Google Scholar] [CrossRef]
- Hilton, H.W. Herbicide tolerant strains of weeds. In Hawaiian Sugar Planters’ Association Annual Report; University of Hawaii, Manoa Library: Honolulu, HI, USA, 1957; p. 69. [Google Scholar]
- Switzer, C.M. The existence of 2, 4-D- resistant strains of wild carrot. Proc. Northeast. Weed Control Conf. 1957, 11, 315–318. [Google Scholar]
- Heap, I. The International Survey of Herbicide Resistant Weeds. Annual Report Internet. 2019. Available online: http://www.weedscience.org (accessed on 8 November 2019).
- Schulz, B.; Segobye, K. 2,4-D transport and herbicide resistance in weeds. J. Exp. Bot. 2016, 67, 3177–3179. [Google Scholar] [CrossRef]
- Rey-Caballero, J.; Menendez, J.; Gine-Bordonaba, J.; Salas, M.; Alcántara, R.; Torra, J. Unravelling the resistance mechanisms to 2,4-D (2,4-dichlorophenoxyacetic acid) in corn poppy (Papaver rhoeas). Pestic. Biochem. Physiol. 2016, 133, 67–72. [Google Scholar] [CrossRef]
- Dang, H.T.; Malone, J.M.; Boutsalis, P.; Krishnan, M.; Gill, G.; Preston, C. Reduced translocation in 2,4-D-resistant oriental mustard populations (Sisymbrium orientale L.) from Australia. Pest Manag. Sci. 2018, 74, 1524–1532. [Google Scholar] [CrossRef] [PubMed]
- Goggin, D.E.; Bringans, S.; Ito, J.; Powles, S.B. Plasma membrane receptor-like kinases and transporters are associated with 2,4-D resistance in wild radish. Ann. Bot. in press. [CrossRef] [PubMed]
- Hatzios, K.; Hock, B.; Elstner, E. Metabolism and elimination of toxicants. In Plant Toxicology; Hock, B., Elstner, E.F., Eds.; CRC Press: Boca Raton, FL, USA, 2005; pp. 469–518. [Google Scholar]
- Figueiredo, M.R.; Leibhart, L.J.; Reicher, Z.J.; Tranel, P.J.; Nissen, S.J.; Westra, P.; Bernards, M.L.; Kruger, G.R.; Gaines, T.A.; Jugulam, M. Metabolism of 2,4-dichlorophenoxyacetic acid contributes to resistance in a common waterhemp (Amaranthus tuberculatus) population. Pest Manag. Sci. 2018, 74, 2356–2362. [Google Scholar] [CrossRef] [PubMed]
- Torra, J.; Rojano-Delgado, A.M.; Rey-Caballero, J.; Royo-Esnal, A.; Salas, M.L.; De Prado, R. Enhanced 2,4-D Metabolism in Two Resistant Papaver rhoeas Populations from Spain. Front. Plant Sci. 2017, 8, 1584. [Google Scholar] [CrossRef] [PubMed]
- LeClere, S.; Wu, C.; Westra, P.; Sammons, R.D. Cross-resistance to dicamba, 2,4-D, and fluroxypyr in Kochia scoparia is endowed by a mutation in an AUX/IAA gene. Proc. Natl. Acad. Sci. USA 2018, 115, E2911–E2920. [Google Scholar] [CrossRef]
- Gray, W.M.; Kepinski, S.; Rouse, D.; Leyser, O.; Estelle, M. Auxin regulates SCFTIR1-dependent degradation of Aux/IAA proteins. Nature 2001, 414, 271–276. [Google Scholar] [CrossRef]
- Goggin, D.E.; Kaur, P.; Owen, M.J.; Powles, S.B. 2,4-D and dicamba resistance mechanisms in wild radish: Subtle, complex and population specific? Ann. Bot. 2018, 122, 627–640. [Google Scholar] [CrossRef]
- Howatt, K.A.; Westra, P.; Nissen, S.J. Ethylene effect on kochia (Kochia scoparia) and emission following dicamba application. Weed Sci. 2006, 54, 31–37. [Google Scholar] [CrossRef]
- Nandula, V.K. Herbicide Resistance Traits in Maize and Soybean: Current Status and Future Outlook. Plants 2019, 8, 337. [Google Scholar] [CrossRef]
- Dellaferrera, I.; Cortés, E.; Panigo, E.; De Prado, R.; Christoffoleti, P.; Perreta, M. First report of Amaranthus hybridus with multiple resistance to 2,4-D, dicamba, and glyphosate. Agronomy 2018, 8, 140. [Google Scholar] [CrossRef]
- Mora, A.D.; Rosario, J.; Rojano-Delgado, A.M.; Palma-Bautista, C.; Torra, J.; Alcántara-de la Cruz, R.; De Prado, R. Multiple Resistance to Synthetic Auxin Herbicides and Glyphosate in Parthenium hysterophorus Occurring in Citrus Orchards. J. Agric. Food Chem. 2019, 67, 10010–10017. [Google Scholar] [CrossRef] [PubMed]
- Kati, V.; Scarabel, L.; Thiery-Lanfranchi, D.; Kioleoglou, V.; Liberopoulou, S.; Délye, C. Multiple resistance of Papaver rhoeas L. to 2,4-D and acetolactate synthase inhibitors in four European countries. Weed Res. 2019, 59, 367–376. [Google Scholar] [CrossRef]
- Rey-Caballero, J.; Menéndez, J.; Osuna, M.D.; Salas, M.; Torra, J. Target-site and non-target-site resistance mechanisms to ALS inhibiting herbicides in Papaver rhoeas. Pestic. Biochem. Physiol. 2017, 138, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Tahmasebi, B.K.; Alcántara-de la Cruz, R.; Alcántara, E.; Torra, J.; Domínguez-Valenzuela, J.A.; Cruz-Hipólito, H.E.; Rojano-Delgado, A.M.; De Prado, R. Multiple Resistance Evolution in Bipyridylium-Resistant Epilobium ciliatum After Recurrent Selection. Front. Plant Sci. 2018, 9, 695. [Google Scholar] [CrossRef]
- Bracamonte, E.; Fernández-Moreno, P.T.; Barro, F.; De Prado, R. Glyphosate-resistant Parthenium hysterophorus in the Caribbean Islands: Non target site resistance and target site resistance in relation to resistance levels. Front. Plant Sci. 2016, 7, 1845. [Google Scholar] [CrossRef]
- Riar, D.S.; Burke, I.C.; Yenish, J.P.; Bell, J.; Gill, K. Inheritance and physiological basis for 2,4-D resistance in prickly lettuce (Lactuca serriola L.). J. Agric. Food Chem. 2011, 59, 9417–9423. [Google Scholar] [CrossRef]
- Goggin, D.E.; Cawthray, G.R.; Powles, S.B. 2,4-D resistance in wild radish: Reduced herbicide translocation via inhibition of cellular transport. J. Exp. Bot. 2006, 67, 3223–3235. [Google Scholar] [CrossRef]
- Seefeldt, S.S.; Jensen, J.E.; Fuerst, E.P. Log-Logistic Analysis of Herbicide Dose-Response Relationships. Weed Technol. 1995, 9, 218–227. [Google Scholar] [CrossRef]
- Kohler, E.; Throssell, C.; Reicher, Z. 2,4-D Rate Response, Absorption, and Translocation of Two Ground Ivy (Glechoma hederacea) Populations. Weed Technol. 2004, 18, 917–923. [Google Scholar] [CrossRef]
- Ashworth, M.B.; Walsh, M.J.; Flower, K.C.; Powles, S.B. Recurrent selection with reduced 2, 4-D amine doses results in the rapid evolution of 2, 4-D herbicide resistance in wild radish (Raphanus raphanistrum L.). Pest Manag. Sci. 2016, 72, 2091–2098. [Google Scholar] [CrossRef]
- Busi, R.; Powles, S.B. Inheritance of 2,4-D resistance traits in multiple herbicide- resistant Raphanus raphanistrum populations. Plant Sci. 2017, 257, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, T.; Stephenson, G.R.; McLean, M.D.; Hall, J.C. MCPA (4-chloro-2-ethylphenoxyacetate) resistance in hemp-nettle (Galeopsis tetrahit L.). J. Agric. Food Chem. 2006, 54, 9126–9134. [Google Scholar] [CrossRef] [PubMed]
- Bernards, M.; Crespo, R.; Kruger, G.; Gaussoin, R.; Tranel, P. A Waterhemp (Amaranthus tuberculatus) Population Resistant to 2,4-D. Weed Sci. 2012, 60, 379–384. [Google Scholar] [CrossRef]
| Species | Family | Country/Region | Crop | Survived Field Dose (g ai ha−1) |
|---|---|---|---|---|
| Amaranthus hybridus | Amaranthaceae | Argentina/Colonia Marina | Soybean | 480 |
| Conyza canadensis | Asteraceae | Hungary/Badacsony | Vineyard | 600 |
| C. sumatrensis | Asteraceae | France/Jonquieres | Vineyard | 600 |
| Hirschfeldia incana | Brassicaceae | Argentina/Buenos Aires | Wheat | 480 |
| Parthenium hysterophorus | Asteraceae | Dominican R./Basima | Citrus sinensis | 480 |
| Papaver rhoeas | Papaveraceae | Spain/Baldomar | Winter cereal | 600 |
| Species | Population/ ±Malathion | d | b | GR50 (g ai ha−1) | RF | p-Value |
|---|---|---|---|---|---|---|
| A. hybridus | R-malathion | 99.09 | 2.72 | 572.62 ± 34.17 | 6.06 | <0.001 |
| R+malathion | 99.97 | 2.25 | 294.10 ± 8.77 | 3.26 | <0.001 | |
| S-malathion | 100.25 | 2.35 | 94.41 ± 2.49 | - | <0.001 | |
| S+malathion | 100.49 | 2.51 | 90.12 ± 6.11 | 0.95 | <0.001 | |
| C. canadensis | R-malathion | 100.61 | 3.12 | 797.25 ±31.21 | 16.36 | 0.018 |
| R+malathion | 100.21 | 2.67 | 55.91 ± 3.52 | 1.14 | <0.001 | |
| S-malathion | 100.02 | 3.49 | 48.71 ± 4.82 | - | <0.001 | |
| S+malathion | 100.01 | 3.42 | 44.95 ± 4.85 | 0.92 | <0.001 | |
| H. incana | R-malathion | 101.11 | 3.56 | 563.06 ± 15.55 | 4.17 | <0.001 |
| R+malathion | 100.94 | 3.43 | 553.46 ± 13.65 | 4.10 | <0.001 | |
| S-malathion | 99.11 | 2.36 | 134.74 ± 4.66 | - | <0.001 | |
| S+malathion | 98.69 | 2.84 | 140.91 ± 7.23 | 1.04 | 0.019 | |
| P. hysterophorus | R-malathion | 100.53 | 3.10 | 847.65 ± 30.46 | 9.95 | <0.001 |
| R+malathion | 98.95 | 2.03 | 386.03 ± 12.97 | 4.53 | <0.001 | |
| S-malathion | 100.39 | 2.47 | 85.15 ± 3.61 | - | <0.001 | |
| S+malathion | 100.52 | 2.46 | 86.85 ± 2.65 | 1.01 | <0.001 | |
| P. rhoeas | R-malathion | 100.71 | 2.71 | 875.36 ± 22.06 | 11.03 | <0.001 |
| R+malathion | 100.50 | 2.08 | 399.14 ± 12.92 | 5.03 | <0.001 | |
| S-malathion | 99.75 | 1.76 | 79.34 ± 2.33 | - | <0.001 | |
| S+malathion | 99.88 | 2.73 | 83.26 ± 3.43 | 1.04 | <0.001 | |
| C. sumatrensis | R-malathion | 100.07 | 3.21 | 112.28 ± 4.15 | 4.37 | 0.017 |
| R+malathion | 100.74 | 1.96 | 27.05 ± 2.16 | 1.05 | <0.001 | |
| S-malathion | 99.99 | 1.93 | 25.64 ± 2.29 | - | 0.023 | |
| S+malathion | 100.01 | 2.26 | 22.43 ± 3.06 | 0.87 | <0.001 |
| Species | Populations | Absorption | Translocation | ||
|---|---|---|---|---|---|
| Treated Leaf | Rest of Plant | Root | |||
| A. hybridus | R | 59.1 ± 2.48 A | 97.6 ± 2.54 A | 1.5 ± 1.54 B | 0.9 ± 0.25 A |
| S | 60.1 ± 2.02 A | 65.3 ± 2.18 B | 24.1 ± 3.04 A | 10.6 ± 0.31 A | |
| C. canadensis | R | 69.4 ± 5.70 A | 97.2 ± 2.1 A | 1.8 ± 1.1 A | 1.0 ± 0.3 A |
| S | 73.8 ± 6.3 A | 98.1 ± 4.3 A | 1.2 ± 0.9 A | 0.7 ± 0.4 A | |
| H. incana | R | 64.7 ± 6.2 A | 96.6 ± 2.6 A | 2.3 ± 0.8 B | 1.1 ± 0.5 B |
| S | 70.5 ± 6.9A | 64.9 ± 5.6 B | 25.7 ± 2.1 A | 9.4 ± 0.7 A | |
| P. hysterophorus | R | 62.4 ± 3.2 A | 98.2 ± 2.3 B | 1.2 ± 0.2 B | 0.6 ±0.2 B |
| S | 61.9 ± 5.6 A | 58.3 ± 5.4 A | 25.6 ± 2.6 A | 16.1 ± 3.4 A | |
| P. rhoeas | R | 66.73 ± 4.2 A | 95.6 ± 4.4 A | 2.6 ± 0.9 B | 1.8 ± 0.7 B |
| S | 65.8 ± 3.2 A | 70.3 ± 3.2 B | 23.2 ± 1.2 A | 6.5 ± 0.4 A | |
| C. sumatrensis | R | 78.9 ±3.2 A | 96.8 ± 3.6 A | 3.1 ± 1.5 A | 0.1 ± 0.1 A |
| S | 80.1 ± 2.4 A | 97.6 ± 1.8 A | 1.8 ± 0.9 A | 0.6 ± 0.5 A | |
| Species | Populations | Foliar | Root | ||
|---|---|---|---|---|---|
| 2,4-D | Metabolites Non-Toxic | 2,4-D | Metabolites Non-Toxic | ||
| A. hybridus | R | 46.5 ± 4.5 B | 51.8 ± 3.3 | ND a | 1.7 ± 0.4 |
| S | 87.4 ± 3.6 A | ND | 12.58 ± 1.8 | ND | |
| p-value | 0.0001 | - | - | - | |
| C. canadensis | R | 36.2 ± 5.1 B | 62.2 ± 2.9 | ND | 1.6 ± 0.3 |
| S | 97.6 ± 1.8 A | ND | 2.4 ± 0.9 | ND | |
| p-value | 0.0001 | - | - | - | |
| H. incana | R | 98.4 ± 1.2 A | ND | 1.6 ± 0.5 B | ND |
| S | 89.9 ± 2.1 B | ND | 10.1 ± 1.2 A | ND | |
| p-value | 0.0037 | - | 0.0143 | - | |
| P. hysterophorus | R | 38.8 ± 6.1 B | 59.3 ± 3.5 | ND | 0.8 ± 0.2 |
| S | 86.2 ± 5.6 A | ND | 13.8 ± 2.9 | ND | |
| p-value | 0.0010 | - | - | - | |
| P. rhoeas | R | 38.2 ± 2.7 B | 58.6 ± 2.1 | 1.3 ± 0.6 B | 1.9 ± 0.7 |
| S | 91.2 ± 1.4 A | ND | 8.8 ± 0.8 A | ND | |
| p-value | 0.0001 | - | 0.0004 | - | |
| C. sumatrensis | R | 59.4 ± 4.3 B | 39.7 ± 2.7 | 0.9 ± 0.2 B | ND |
| S | 96.9 ± 2.4 A | ND | 3.1 ± 1.0 A | ND | |
| p-value | 0.0005 | - | 0.0109 | - | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palma-Bautista, C.; Rojano-Delgado, A.M.; Dellaferrera, I.; Rosario, J.M.; Vigna, M.R.; Torra, J.; de Prado, R. Resistance Mechanisms to 2,4-D in Six Different Dicotyledonous Weeds Around the World. Agronomy 2020, 10, 566. https://doi.org/10.3390/agronomy10040566
Palma-Bautista C, Rojano-Delgado AM, Dellaferrera I, Rosario JM, Vigna MR, Torra J, de Prado R. Resistance Mechanisms to 2,4-D in Six Different Dicotyledonous Weeds Around the World. Agronomy. 2020; 10(4):566. https://doi.org/10.3390/agronomy10040566
Chicago/Turabian StylePalma-Bautista, Candelario, Antonia M. Rojano-Delgado, Ignacio Dellaferrera, Jesús M. Rosario, Mario R. Vigna, Joel Torra, and Rafael de Prado. 2020. "Resistance Mechanisms to 2,4-D in Six Different Dicotyledonous Weeds Around the World" Agronomy 10, no. 4: 566. https://doi.org/10.3390/agronomy10040566
APA StylePalma-Bautista, C., Rojano-Delgado, A. M., Dellaferrera, I., Rosario, J. M., Vigna, M. R., Torra, J., & de Prado, R. (2020). Resistance Mechanisms to 2,4-D in Six Different Dicotyledonous Weeds Around the World. Agronomy, 10(4), 566. https://doi.org/10.3390/agronomy10040566







