Resistance Mechanisms to 2,4-D in Six Different Dicotyledonous Weeds Around the World
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material
2.3. Ethylene Production Fast Screening
2.4. Response of Dicotyledonous Weed Species to 2,4-D Pre-Treated with or without Malathion
2.5. 14C-2,4-D Absorption and Translocation in Dicotyledonous Weeds
2.6. 2,4-D Metabolism in Dicotyledonous Weeds
2.7. Data Analysis
3. Results
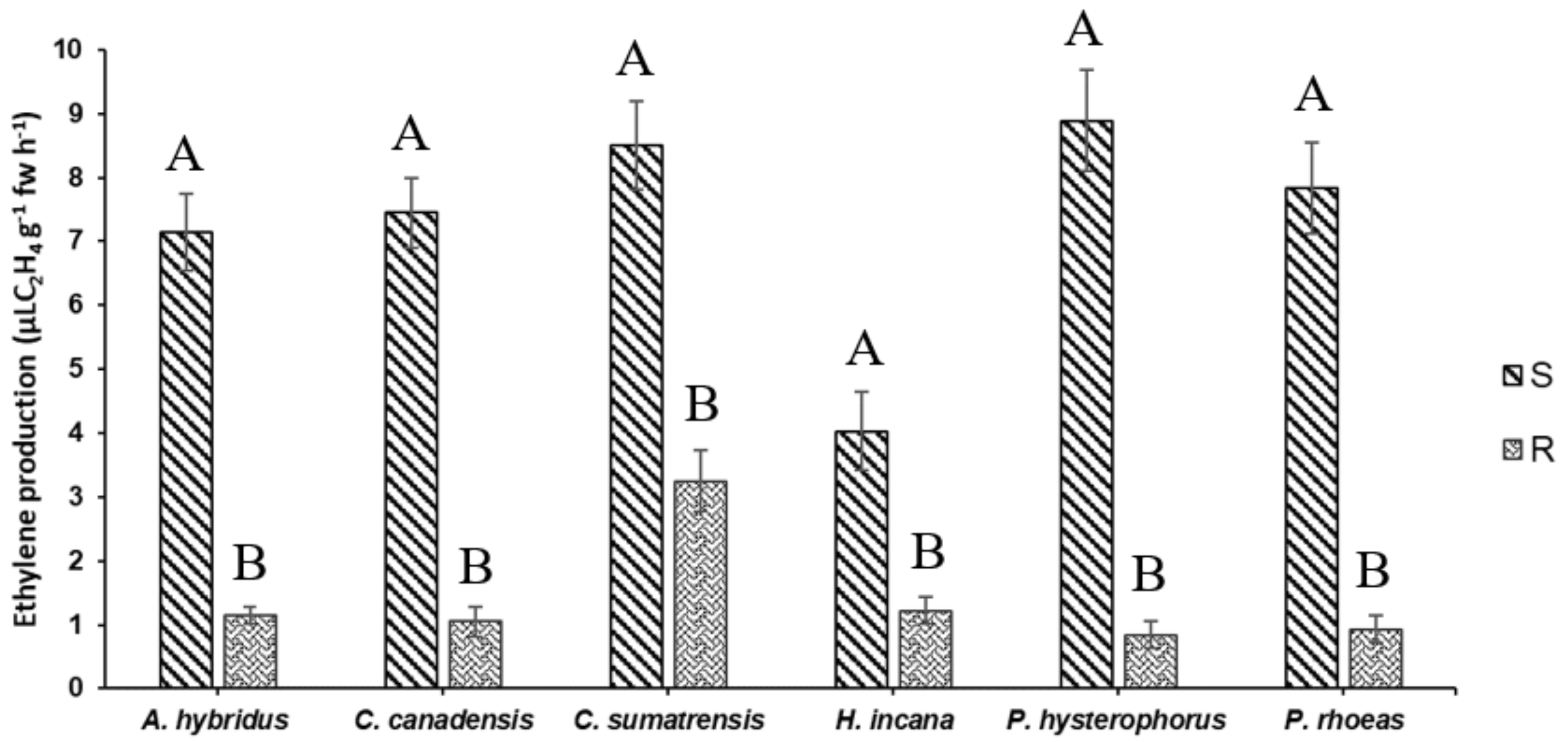
3.1. Ethylene Production Fast Screening
3.2. Response of Dicotyledonous Weed Species to 2,4-D Pre-Treated with or without Malathion
3.3. 14C-2,4-D Absorption and Translocation in Dicotyledonous Weeds
3.4. 2,4-D Plant Metabolism
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mithila, J.; Hall, J.C.; Johnson, W.G.; Kelley, K.B.; Riechers, D.E. Evolution of resistance to auxinic herbicides: Historical perspectives, mechanisms of resistance, and implications for broadleaf weed management in agronomic crops. Weed Sci. 2011, 59, 445–457. [Google Scholar] [CrossRef] [Green Version]
- Peterson, M.A.; McMaster, S.A.; Riechers, D.E.; Skelton, J.; Stahlman, P.W. 2,4-D past, present, and future: A review. Weed Technol. 2016, 30, 303–345. [Google Scholar] [CrossRef]
- Grossmann, K. Auxin herbicides: Current status of mechanism and mode of action. Pest Manag. Sci. 2010, 66, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Gervais, J.A.; Luukinen, B.; Buhl, K.; Stone, D.; 2,4-D Technical Fact Sheet. National Pesticide Information Center, Oregon State University Extension Service. 2008. Available online: http://npic.orst.edu/factsheets/2,4-DTech.pdf (accessed on 7 November 2019).
- Song, Y. Insight into the mode of action of 2,4-dichlorophenoxyacetic acid (2,4-D) as an herbicide. J. Integr. Plant Biol. 2014, 56, 106–113. [Google Scholar] [CrossRef]
- Weintraub, R.L.; Yeatman, J.N.; Brown, J.W.; Throne, J.A.; Skoss, J.D.; Conover, J.R. Studies on entry of 2,4-D into leaves. Proc. Northeast. Weed Control Conf. 1954, 8, 5–10. [Google Scholar]
- Matthes, M.S.; Best, N.B.; Robil, J.M.; Malcomber, S.; Gallavotti, A.; McSteen, P. Auxin EvoDevo: Conservation and diversification of genes regulating auxin biosynthesis, transport, and signaling. Mol. Plant 2019, 12, 298–320. [Google Scholar] [CrossRef] [Green Version]
- Busi, R.; Goggin, D.E.; Heap, I.M.; Horak, M.J.; Jugulam, M.; Masters, R.A.; Napier, R.M.; Riar, D.S.; Satchivi, N.M.; Torra, J.; et al. Weed resistance to synthetic auxin herbicides. Pest Manag. Sci. 2018, 74, 2265–2276. [Google Scholar] [CrossRef]
- Hilton, H.W. Herbicide tolerant strains of weeds. In Hawaiian Sugar Planters’ Association Annual Report; University of Hawaii, Manoa Library: Honolulu, HI, USA, 1957; p. 69. [Google Scholar]
- Switzer, C.M. The existence of 2, 4-D- resistant strains of wild carrot. Proc. Northeast. Weed Control Conf. 1957, 11, 315–318. [Google Scholar]
- Heap, I. The International Survey of Herbicide Resistant Weeds. Annual Report Internet. 2019. Available online: http://www.weedscience.org (accessed on 8 November 2019).
- Schulz, B.; Segobye, K. 2,4-D transport and herbicide resistance in weeds. J. Exp. Bot. 2016, 67, 3177–3179. [Google Scholar] [CrossRef] [Green Version]
- Rey-Caballero, J.; Menendez, J.; Gine-Bordonaba, J.; Salas, M.; Alcántara, R.; Torra, J. Unravelling the resistance mechanisms to 2,4-D (2,4-dichlorophenoxyacetic acid) in corn poppy (Papaver rhoeas). Pestic. Biochem. Physiol. 2016, 133, 67–72. [Google Scholar] [CrossRef] [Green Version]
- Dang, H.T.; Malone, J.M.; Boutsalis, P.; Krishnan, M.; Gill, G.; Preston, C. Reduced translocation in 2,4-D-resistant oriental mustard populations (Sisymbrium orientale L.) from Australia. Pest Manag. Sci. 2018, 74, 1524–1532. [Google Scholar] [CrossRef] [PubMed]
- Goggin, D.E.; Bringans, S.; Ito, J.; Powles, S.B. Plasma membrane receptor-like kinases and transporters are associated with 2,4-D resistance in wild radish. Ann. Bot. in press. [CrossRef] [PubMed]
- Hatzios, K.; Hock, B.; Elstner, E. Metabolism and elimination of toxicants. In Plant Toxicology; Hock, B., Elstner, E.F., Eds.; CRC Press: Boca Raton, FL, USA, 2005; pp. 469–518. [Google Scholar]
- Figueiredo, M.R.; Leibhart, L.J.; Reicher, Z.J.; Tranel, P.J.; Nissen, S.J.; Westra, P.; Bernards, M.L.; Kruger, G.R.; Gaines, T.A.; Jugulam, M. Metabolism of 2,4-dichlorophenoxyacetic acid contributes to resistance in a common waterhemp (Amaranthus tuberculatus) population. Pest Manag. Sci. 2018, 74, 2356–2362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torra, J.; Rojano-Delgado, A.M.; Rey-Caballero, J.; Royo-Esnal, A.; Salas, M.L.; De Prado, R. Enhanced 2,4-D Metabolism in Two Resistant Papaver rhoeas Populations from Spain. Front. Plant Sci. 2017, 8, 1584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LeClere, S.; Wu, C.; Westra, P.; Sammons, R.D. Cross-resistance to dicamba, 2,4-D, and fluroxypyr in Kochia scoparia is endowed by a mutation in an AUX/IAA gene. Proc. Natl. Acad. Sci. USA 2018, 115, E2911–E2920. [Google Scholar] [CrossRef] [Green Version]
- Gray, W.M.; Kepinski, S.; Rouse, D.; Leyser, O.; Estelle, M. Auxin regulates SCFTIR1-dependent degradation of Aux/IAA proteins. Nature 2001, 414, 271–276. [Google Scholar] [CrossRef]
- Goggin, D.E.; Kaur, P.; Owen, M.J.; Powles, S.B. 2,4-D and dicamba resistance mechanisms in wild radish: Subtle, complex and population specific? Ann. Bot. 2018, 122, 627–640. [Google Scholar] [CrossRef]
- Howatt, K.A.; Westra, P.; Nissen, S.J. Ethylene effect on kochia (Kochia scoparia) and emission following dicamba application. Weed Sci. 2006, 54, 31–37. [Google Scholar] [CrossRef]
- Nandula, V.K. Herbicide Resistance Traits in Maize and Soybean: Current Status and Future Outlook. Plants 2019, 8, 337. [Google Scholar] [CrossRef] [Green Version]
- Dellaferrera, I.; Cortés, E.; Panigo, E.; De Prado, R.; Christoffoleti, P.; Perreta, M. First report of Amaranthus hybridus with multiple resistance to 2,4-D, dicamba, and glyphosate. Agronomy 2018, 8, 140. [Google Scholar] [CrossRef] [Green Version]
- Mora, A.D.; Rosario, J.; Rojano-Delgado, A.M.; Palma-Bautista, C.; Torra, J.; Alcántara-de la Cruz, R.; De Prado, R. Multiple Resistance to Synthetic Auxin Herbicides and Glyphosate in Parthenium hysterophorus Occurring in Citrus Orchards. J. Agric. Food Chem. 2019, 67, 10010–10017. [Google Scholar] [CrossRef] [PubMed]
- Kati, V.; Scarabel, L.; Thiery-Lanfranchi, D.; Kioleoglou, V.; Liberopoulou, S.; Délye, C. Multiple resistance of Papaver rhoeas L. to 2,4-D and acetolactate synthase inhibitors in four European countries. Weed Res. 2019, 59, 367–376. [Google Scholar] [CrossRef]
- Rey-Caballero, J.; Menéndez, J.; Osuna, M.D.; Salas, M.; Torra, J. Target-site and non-target-site resistance mechanisms to ALS inhibiting herbicides in Papaver rhoeas. Pestic. Biochem. Physiol. 2017, 138, 57–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tahmasebi, B.K.; Alcántara-de la Cruz, R.; Alcántara, E.; Torra, J.; Domínguez-Valenzuela, J.A.; Cruz-Hipólito, H.E.; Rojano-Delgado, A.M.; De Prado, R. Multiple Resistance Evolution in Bipyridylium-Resistant Epilobium ciliatum After Recurrent Selection. Front. Plant Sci. 2018, 9, 695. [Google Scholar] [CrossRef]
- Bracamonte, E.; Fernández-Moreno, P.T.; Barro, F.; De Prado, R. Glyphosate-resistant Parthenium hysterophorus in the Caribbean Islands: Non target site resistance and target site resistance in relation to resistance levels. Front. Plant Sci. 2016, 7, 1845. [Google Scholar] [CrossRef] [Green Version]
- Riar, D.S.; Burke, I.C.; Yenish, J.P.; Bell, J.; Gill, K. Inheritance and physiological basis for 2,4-D resistance in prickly lettuce (Lactuca serriola L.). J. Agric. Food Chem. 2011, 59, 9417–9423. [Google Scholar] [CrossRef]
- Goggin, D.E.; Cawthray, G.R.; Powles, S.B. 2,4-D resistance in wild radish: Reduced herbicide translocation via inhibition of cellular transport. J. Exp. Bot. 2006, 67, 3223–3235. [Google Scholar] [CrossRef] [Green Version]
- Seefeldt, S.S.; Jensen, J.E.; Fuerst, E.P. Log-Logistic Analysis of Herbicide Dose-Response Relationships. Weed Technol. 1995, 9, 218–227. [Google Scholar] [CrossRef]
- Kohler, E.; Throssell, C.; Reicher, Z. 2,4-D Rate Response, Absorption, and Translocation of Two Ground Ivy (Glechoma hederacea) Populations. Weed Technol. 2004, 18, 917–923. [Google Scholar] [CrossRef]
- Ashworth, M.B.; Walsh, M.J.; Flower, K.C.; Powles, S.B. Recurrent selection with reduced 2, 4-D amine doses results in the rapid evolution of 2, 4-D herbicide resistance in wild radish (Raphanus raphanistrum L.). Pest Manag. Sci. 2016, 72, 2091–2098. [Google Scholar] [CrossRef]
- Busi, R.; Powles, S.B. Inheritance of 2,4-D resistance traits in multiple herbicide- resistant Raphanus raphanistrum populations. Plant Sci. 2017, 257, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, T.; Stephenson, G.R.; McLean, M.D.; Hall, J.C. MCPA (4-chloro-2-ethylphenoxyacetate) resistance in hemp-nettle (Galeopsis tetrahit L.). J. Agric. Food Chem. 2006, 54, 9126–9134. [Google Scholar] [CrossRef] [PubMed]
- Bernards, M.; Crespo, R.; Kruger, G.; Gaussoin, R.; Tranel, P. A Waterhemp (Amaranthus tuberculatus) Population Resistant to 2,4-D. Weed Sci. 2012, 60, 379–384. [Google Scholar] [CrossRef]
| Species | Family | Country/Region | Crop | Survived Field Dose (g ai ha−1) |
|---|---|---|---|---|
| Amaranthus hybridus | Amaranthaceae | Argentina/Colonia Marina | Soybean | 480 |
| Conyza canadensis | Asteraceae | Hungary/Badacsony | Vineyard | 600 |
| C. sumatrensis | Asteraceae | France/Jonquieres | Vineyard | 600 |
| Hirschfeldia incana | Brassicaceae | Argentina/Buenos Aires | Wheat | 480 |
| Parthenium hysterophorus | Asteraceae | Dominican R./Basima | Citrus sinensis | 480 |
| Papaver rhoeas | Papaveraceae | Spain/Baldomar | Winter cereal | 600 |
| Species | Population/ ±Malathion | d | b | GR50 (g ai ha−1) | RF | p-Value |
|---|---|---|---|---|---|---|
| A. hybridus | R-malathion | 99.09 | 2.72 | 572.62 ± 34.17 | 6.06 | <0.001 |
| R+malathion | 99.97 | 2.25 | 294.10 ± 8.77 | 3.26 | <0.001 | |
| S-malathion | 100.25 | 2.35 | 94.41 ± 2.49 | - | <0.001 | |
| S+malathion | 100.49 | 2.51 | 90.12 ± 6.11 | 0.95 | <0.001 | |
| C. canadensis | R-malathion | 100.61 | 3.12 | 797.25 ±31.21 | 16.36 | 0.018 |
| R+malathion | 100.21 | 2.67 | 55.91 ± 3.52 | 1.14 | <0.001 | |
| S-malathion | 100.02 | 3.49 | 48.71 ± 4.82 | - | <0.001 | |
| S+malathion | 100.01 | 3.42 | 44.95 ± 4.85 | 0.92 | <0.001 | |
| H. incana | R-malathion | 101.11 | 3.56 | 563.06 ± 15.55 | 4.17 | <0.001 |
| R+malathion | 100.94 | 3.43 | 553.46 ± 13.65 | 4.10 | <0.001 | |
| S-malathion | 99.11 | 2.36 | 134.74 ± 4.66 | - | <0.001 | |
| S+malathion | 98.69 | 2.84 | 140.91 ± 7.23 | 1.04 | 0.019 | |
| P. hysterophorus | R-malathion | 100.53 | 3.10 | 847.65 ± 30.46 | 9.95 | <0.001 |
| R+malathion | 98.95 | 2.03 | 386.03 ± 12.97 | 4.53 | <0.001 | |
| S-malathion | 100.39 | 2.47 | 85.15 ± 3.61 | - | <0.001 | |
| S+malathion | 100.52 | 2.46 | 86.85 ± 2.65 | 1.01 | <0.001 | |
| P. rhoeas | R-malathion | 100.71 | 2.71 | 875.36 ± 22.06 | 11.03 | <0.001 |
| R+malathion | 100.50 | 2.08 | 399.14 ± 12.92 | 5.03 | <0.001 | |
| S-malathion | 99.75 | 1.76 | 79.34 ± 2.33 | - | <0.001 | |
| S+malathion | 99.88 | 2.73 | 83.26 ± 3.43 | 1.04 | <0.001 | |
| C. sumatrensis | R-malathion | 100.07 | 3.21 | 112.28 ± 4.15 | 4.37 | 0.017 |
| R+malathion | 100.74 | 1.96 | 27.05 ± 2.16 | 1.05 | <0.001 | |
| S-malathion | 99.99 | 1.93 | 25.64 ± 2.29 | - | 0.023 | |
| S+malathion | 100.01 | 2.26 | 22.43 ± 3.06 | 0.87 | <0.001 |
| Species | Populations | Absorption | Translocation | ||
|---|---|---|---|---|---|
| Treated Leaf | Rest of Plant | Root | |||
| A. hybridus | R | 59.1 ± 2.48 A | 97.6 ± 2.54 A | 1.5 ± 1.54 B | 0.9 ± 0.25 A |
| S | 60.1 ± 2.02 A | 65.3 ± 2.18 B | 24.1 ± 3.04 A | 10.6 ± 0.31 A | |
| C. canadensis | R | 69.4 ± 5.70 A | 97.2 ± 2.1 A | 1.8 ± 1.1 A | 1.0 ± 0.3 A |
| S | 73.8 ± 6.3 A | 98.1 ± 4.3 A | 1.2 ± 0.9 A | 0.7 ± 0.4 A | |
| H. incana | R | 64.7 ± 6.2 A | 96.6 ± 2.6 A | 2.3 ± 0.8 B | 1.1 ± 0.5 B |
| S | 70.5 ± 6.9A | 64.9 ± 5.6 B | 25.7 ± 2.1 A | 9.4 ± 0.7 A | |
| P. hysterophorus | R | 62.4 ± 3.2 A | 98.2 ± 2.3 B | 1.2 ± 0.2 B | 0.6 ±0.2 B |
| S | 61.9 ± 5.6 A | 58.3 ± 5.4 A | 25.6 ± 2.6 A | 16.1 ± 3.4 A | |
| P. rhoeas | R | 66.73 ± 4.2 A | 95.6 ± 4.4 A | 2.6 ± 0.9 B | 1.8 ± 0.7 B |
| S | 65.8 ± 3.2 A | 70.3 ± 3.2 B | 23.2 ± 1.2 A | 6.5 ± 0.4 A | |
| C. sumatrensis | R | 78.9 ±3.2 A | 96.8 ± 3.6 A | 3.1 ± 1.5 A | 0.1 ± 0.1 A |
| S | 80.1 ± 2.4 A | 97.6 ± 1.8 A | 1.8 ± 0.9 A | 0.6 ± 0.5 A | |
| Species | Populations | Foliar | Root | ||
|---|---|---|---|---|---|
| 2,4-D | Metabolites Non-Toxic | 2,4-D | Metabolites Non-Toxic | ||
| A. hybridus | R | 46.5 ± 4.5 B | 51.8 ± 3.3 | ND a | 1.7 ± 0.4 |
| S | 87.4 ± 3.6 A | ND | 12.58 ± 1.8 | ND | |
| p-value | 0.0001 | - | - | - | |
| C. canadensis | R | 36.2 ± 5.1 B | 62.2 ± 2.9 | ND | 1.6 ± 0.3 |
| S | 97.6 ± 1.8 A | ND | 2.4 ± 0.9 | ND | |
| p-value | 0.0001 | - | - | - | |
| H. incana | R | 98.4 ± 1.2 A | ND | 1.6 ± 0.5 B | ND |
| S | 89.9 ± 2.1 B | ND | 10.1 ± 1.2 A | ND | |
| p-value | 0.0037 | - | 0.0143 | - | |
| P. hysterophorus | R | 38.8 ± 6.1 B | 59.3 ± 3.5 | ND | 0.8 ± 0.2 |
| S | 86.2 ± 5.6 A | ND | 13.8 ± 2.9 | ND | |
| p-value | 0.0010 | - | - | - | |
| P. rhoeas | R | 38.2 ± 2.7 B | 58.6 ± 2.1 | 1.3 ± 0.6 B | 1.9 ± 0.7 |
| S | 91.2 ± 1.4 A | ND | 8.8 ± 0.8 A | ND | |
| p-value | 0.0001 | - | 0.0004 | - | |
| C. sumatrensis | R | 59.4 ± 4.3 B | 39.7 ± 2.7 | 0.9 ± 0.2 B | ND |
| S | 96.9 ± 2.4 A | ND | 3.1 ± 1.0 A | ND | |
| p-value | 0.0005 | - | 0.0109 | - | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palma-Bautista, C.; Rojano-Delgado, A.M.; Dellaferrera, I.; Rosario, J.M.; Vigna, M.R.; Torra, J.; de Prado, R. Resistance Mechanisms to 2,4-D in Six Different Dicotyledonous Weeds Around the World. Agronomy 2020, 10, 566. https://doi.org/10.3390/agronomy10040566
Palma-Bautista C, Rojano-Delgado AM, Dellaferrera I, Rosario JM, Vigna MR, Torra J, de Prado R. Resistance Mechanisms to 2,4-D in Six Different Dicotyledonous Weeds Around the World. Agronomy. 2020; 10(4):566. https://doi.org/10.3390/agronomy10040566
Chicago/Turabian StylePalma-Bautista, Candelario, Antonia M. Rojano-Delgado, Ignacio Dellaferrera, Jesús M. Rosario, Mario R. Vigna, Joel Torra, and Rafael de Prado. 2020. "Resistance Mechanisms to 2,4-D in Six Different Dicotyledonous Weeds Around the World" Agronomy 10, no. 4: 566. https://doi.org/10.3390/agronomy10040566
APA StylePalma-Bautista, C., Rojano-Delgado, A. M., Dellaferrera, I., Rosario, J. M., Vigna, M. R., Torra, J., & de Prado, R. (2020). Resistance Mechanisms to 2,4-D in Six Different Dicotyledonous Weeds Around the World. Agronomy, 10(4), 566. https://doi.org/10.3390/agronomy10040566







