Evolving Multiple Resistance to EPSPS, GS, ALS, PSI, PPO, and Synthetic Auxin Herbicides in Dominican Republic Parthenium hysterophorus Populations. A Physiological and Biochemical Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chemicals
2.3. Fast Screening Assays
2.4. Dose–Response Assays
2.5. EPSPS Enzyme Activity
2.6. GS Enzyme Activity
2.7. ALS Enzyme Activity
2.8. 14C-Paraquat Absorption and Translocation
2.9. Proto IX Levels
2.10. 2,4-D and Ethylene Production
2.11. Statistical Analysis
3. Results
3.1. Fast Screening Assays
3.2. Dose–Response Assays
3.3. EPSPS Enzyme Activity
3.4. GS Enzyme Activity
3.5. ALS Enzyme Activity
3.6. 14C-Paraquat Absorption and Translocation
3.7. Proto IX Levels
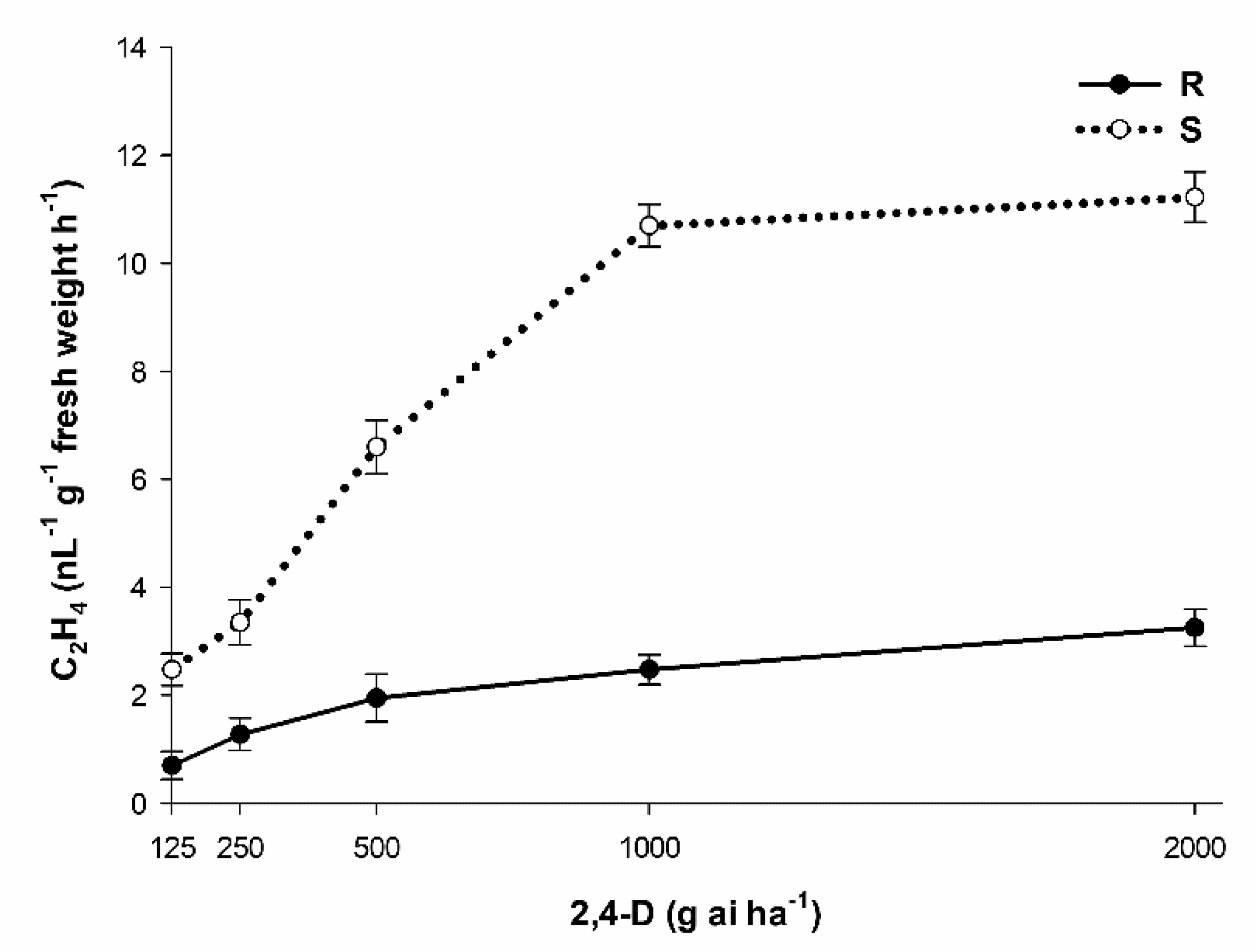
3.8. 2,4-D and Ethylene Production
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Labrada, R.; Caseley, J.C.; Parker, C. Malezas de hoja ancha. In Manejo de Malezas Para Países en Desarrollo; FAO, Ed.; FAO: Rome, Italy, 1996. [Google Scholar]
- Kaur, M.; Aggarwal, N.K.; Kumar, V.; Dhiman, R. Effects and Management of Parthenium hysterophorus: A Weed of Global Significance. Int. Sch. Res. Not. 2014, 2014, 1–12. [Google Scholar]
- GISD Global Invasive Species Database. Available online: http://www.iucngisd.org/gisd/species.php?sc=153 (accessed on 29 November 2019).
- Adkins, S.; Shabbir, A. Biology, ecology and management of the invasive parthenium weed (Parthenium hysterophorus L.). Pest Manag. Sci. 2014, 70, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- Navie, S.C.; Panetta, F.D.; McFadyen, R.E.; Adkins, S.W. Germinable soil seedbanks of central Queensland rangelands invaded by the exotic weed Parthenium hysterophorus L. Weed Biol. Manag. 2004, 4, 154–167. [Google Scholar] [CrossRef]
- Belz, R.G.; Reinhardt, C.F.; Foxcroft, L.C.; Hurle, K. Residue allelopathy in Parthenium hysterophorus L.-Does parthenin play a leading role? Crop Prot. 2007, 26, 237–245. [Google Scholar] [CrossRef]
- Belz, R.G. Investigating a Potential Auxin-Related Mode of Hormetic/Inhibitory Action of the Phytotoxin Parthenin. J. Chem. Ecol. 2016, 42, 71–83. [Google Scholar] [CrossRef]
- Hassan, G.; Rashid, H.U.; Amin, A.; Khan, I.A.; Shehzad, N. Allelopathic effect of Parthenium hysterophorus on germination and growth of some important crops and weeds of economic importance. Planta Daninha 2018, 36, e018176372. [Google Scholar] [CrossRef]
- Belz, R.G. Stimulation versus inhibition-bioactivity of parthenin, a phytochemical from parthenium hysterophorus L. Dose-Response 2008, 6, 80–96. [Google Scholar] [CrossRef]
- FAO (Food and Agriculture Organization of the United Nations). Trade and Markets: Bananas. Available online: http://www.fao.org/home/en/ (accessed on 15 February 2019).
- FAOSTAT FAO (Food and Agriculture Organization of the United Nations). Statistical Databases. Available online: http://www.fao.org/home/es (accessed on 15 February 2019).
- Plaza, G. Manejo de Malezas en Frutales. In Manual Para el Cultivo de Frutales en el Trópico; Fischer, G., Ed.; Universidad Nacional de Colombia: Bogotá, Colombia, 2012; pp. 238–251. [Google Scholar]
- Quintero-Pértuz, I.; Carbonó-DelaHoz, E. Panorama del manejo de malezas en cultivos de banano en el departamento del Magdalena, Colombia. Rev. Colomb. Ciencias Hortícolas 2016, 9, 329. [Google Scholar] [CrossRef]
- Pinilla, C.; Garcia, J. Manejo integrado de arvenses en plantaciones de banano (Musa AAA). In Proceedings of the XV Reunión Asociación de Bananero de Colombia (Acorbat); Augura: Cartagena, Colombia, 2002; pp. 222–235. [Google Scholar]
- Martinez, A.M.; Hoyos, L.M. Banano (Musa AAA. Simmonds). In Manual Para el Cultivo de Frutales en el Trópico; Fischer, G., Ed.; Universidad Nacional de Colombia: Bogotá, Colombia, 2012; pp. 349–369. [Google Scholar]
- Rodríguez, A.M.; Agüero, R. Identificación de malezas trepadoras del banano (Musa sp.) en la zona caribe de Costa Rica. Agron. Mesoam. 2000, 11, 123–125. [Google Scholar] [CrossRef]
- De la Cruz, R.; Rojas, C.E.; Lobón, H.; Burgos, C. El papel de las malezas en la reducción de la lixiviación de nutrimentos en cultivos de banano en el trópico húmedo. Manejo Integr. Plagas 2001, 62, 29–37. [Google Scholar]
- Amaya, A.; Santos, M.; Morán, I.; Vargas, P.; Comboza, W.; Lara, E. Malezas Presentes en Cultivos del Cantón Naranjal, Provincia Guayas, Ecuador. Investig. Res. Rev. 2018, 11, 1–16. [Google Scholar]
- Palma-Bautista, C.; Gherekhloo, J.; Domínguez-Martínez, P.A.; Domínguez-Valenzuela, J.A.; Cruz-Hipolito, H.E.; Alcántara-de la Cruz, R.; Rojano-Delgado, A.M.; De Prado, R. Characterization of three glyphosate resistant Parthenium hysterophorus populations collected in citrus groves from Mexico. Pestic. Biochem. Physiol. 2019, 155, 1–7. [Google Scholar] [CrossRef]
- Radosevich, S.R.; Holt, J.S.; Ghersa, C. Weed Ecology: Implications for Management, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 1997; ISBN 0471116068. [Google Scholar]
- Tornisielo, V.L.; Botelho, R.G.; Alves, P.A.D.T.; Bonfleur, E.J.; Monteiro, S.H. Pesticide Tank Mixes: An Environmental Point of View, Herbicides—Current Research and Case Studies in Use; Price, A.J., Kelton, J.A., Eds.; IntechOpen: Rijeka, Croatia, 2013. [Google Scholar]
- Ganie, Z.A.; Jhala, A.J. Interaction of 2,4-D or dicamba with glufosinate for control of glyphosate-resistant giant ragweed (Ambrosia trifida L.) in glufosinate-resistant maize (zea mays L.). Front. Plant Sci. 2017, 8, 1207. [Google Scholar] [CrossRef]
- Bracamonte, E.R.; Fernández-Moreno, P.T.; Bastida, F.; Osuna, M.D.; Alcántara-de la Cruz, R.; Cruz-Hipolito, H.E.; De Prado, R. Identifying Chloris Species from Cuban Citrus Orchards and Determining Their Glyphosate-Resistance Status. Front. Plant Sci. 2017, 8, 1977. [Google Scholar] [CrossRef] [PubMed]
- De Prado, R.; Franco, A.R. Cross-resistance and herbicide metabolism in grass weeds in Europe: Biochemical and physiological aspects. Weed Sci. 2004, 52, 441–447. [Google Scholar] [CrossRef]
- Sammons, R.D.; Gaines, T.A. Glyphosate resistance: State of knowledge. Pest Manag. Sci. 2014, 70, 1367–1377. [Google Scholar] [CrossRef]
- Shaner, D.L.; Lindenmeyer, B.; Ostlie, M.H. What have the mechanisms of resistance to glyphosate taught us? Pest Manag. Sci. 2012, 68, 3–9. [Google Scholar] [CrossRef]
- Alcántara de la Cruz, R.; Barro, F.; Domínguez-Valenzuela, J.A.; De Prado, R. Physiological, morphological and biochemical studies of glyphosate tolerance in Mexican Cologania (Cologania broussonetii (Balb.) DC.). Plant Physiol. Biochem. 2016, 98, 72–80. [Google Scholar] [CrossRef]
- Busi, R.; Vila-Aiub, M.M.; Powles, S.B. Genetic control of a cytochrome P450 metabolism-based herbicide resistance mechanism in Lolium rigidum. Heredity 2011, 106, 817–824. [Google Scholar] [CrossRef]
- Cruz-Hipolito, H.; Osuna, M.D.; Heredia, A.; Ruiz-Santaella, J.P.; De Prado, R. Nontarget mechanims involved in glyphosate tolerance found in Canavalia ensiformis plants. J. Agric. Food Chem. 2009, 57, 4844–4848. [Google Scholar] [CrossRef]
- Cruz-Hipolito, H.; Rojano-Delgado, A.; Domínguez-Valenzuela, J.A.; Heredia, A.; de Castro, M.D.L.; de Prado, R. Glyphosate tolerance by Clitoria ternatea and Neonotonia wightii plants involves differential absorption and translocation of the herbicide. Plant Soil 2011, 347, 221–230. [Google Scholar] [CrossRef]
- González-Torralva, F.; Gil-Humanes, J.; Barro, F.; Brants, I.; De Prado, R. Target site mutation and reduced translocation are present in a glyphosate-resistant Lolium multiflorum Lam. biotype from Spain. Plant Physiol. Biochem. 2012, 58, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Neve, P.; Vila-aiub, M.; Roux, F. Evoltionary-Thinking in Agricultural Weed Managment. New Phytol. 2009, 184, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Heap, I. The International Survey of Herbicide Resistant Weeds. Available online: http://www.weedscience.org (accessed on 29 October 2019).
- Bracamonte, E.; Fernández-Moreno, P.T.; Barro, F.; De Prado, R. Glyphosate-resistant Parthenium hysterophorus in the Caribbean Islands: Non target site resistance and target site resistance in relation to resistance levels. Front. Plant Sci. 2016, 7, 1845. [Google Scholar] [CrossRef]
- Fernandez, J.V.; Odero, D.C.; Macdonald, G.E.; Ferrell, J.; Gettys, L.A. Confirmation, Characterization, and Management of Glyphosate-Resistant Ragweed Parthenium (Parthenium hysterophorus L.) in the Everglades Agricultural Area of South Florida. Weed Technol. 2015, 29, 233–242. [Google Scholar] [CrossRef]
- Mora, A.D.; Rosario, J.; Rojano-Delgado, A.M.; Palma-Bautista, C.; Torra, J.; Alcántara-De La Cruz, R.; De Prado, R. Multiple Resistance to Synthetic Auxin Herbicides and Glyphosate in Parthenium hysterophorus Occurring in Citrus Orchards. J. Agric. Food Chem. 2019, 67, 10010–10017. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Tahmasebi, B.K.; Alcántara-de la Cruz, R.; Alcántara, E.; Torra, J.; Domínguez-Valenzuela, J.A.; Cruz-Hipólito, H.E.; Rojano-Delgado, A.M.; De Prado, R. Multiple Resistance Evolution in Bipyridylium-Resistant Epilobium ciliatum After Recurrent Selection. Front. Plant Sci. 2018, 9, 9. [Google Scholar] [CrossRef]
- Hatami, Z.M.; Gherekhloo, J.; Rojano-Delgado, A.M.; Osuna, M.D.; Alcántara, R.; Fernández, P.; Sadeghipour, H.R.; De Prado, R. Multiple Mechanisms Increase Levels of Resistance in Rapistrum rugosum to ALS Herbicides. Front. Plant Sci. 2016, 7, 169. [Google Scholar] [CrossRef]
- Moretti, M.L.; Hanson, B.D. Reduced translocation is involved in resistance to glyphosate and paraquat in Conyza bonariensis and Conyza canadensis from California. Weed Res. 2017, 57, 25–34. [Google Scholar] [CrossRef]
- Dayan, F.E.; Owens, D.K.; Corniani, N.; Silva, F.M.L.; Watson, S.B.; Howell, J.; Shaner, D.L. Biochemical Markers and Enzyme Assays for Herbicide Mode of Action and Resistance Studies. Weed Sci. 2015, 63, 23–63. [Google Scholar] [CrossRef]
- Torra, J.; Rojano-Delgado, A.M.; Rey-Caballero, J.; Royo-Esnal, A.; Salas, M.L.; De Prado, R. Enhanced 2,4-D metabolism in two resistant Papaver rhoeas populations from Spain. Front. Plant Sci. 2017, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Shimabukuro, R.H.; Hoffer, B.L. Induction of ethylene as an indicator of senescence in the mode of action of diclofop-methyl. Pestic. Biochem. Physiol. 1996, 54, 146–158. [Google Scholar] [CrossRef]
- Ritz, C.; Baty, F.; Streibig, J.C.; Gerhard, D. Dose-Response Analysis Using R. PLoS ONE 2015, 10, e0146021. [Google Scholar] [CrossRef]
- Njoroge, J.M. Tolerance of Bidens pilosa L. and Parthenium hysterophorus L. to paraquat (Gramoxone) in Kenya coffee. Kenya Coffee 1991, 56, 999–1001. [Google Scholar]
- Rosario, J.; Fuentes, C.; De Prado, R.; Cruz-Hipolito, H. Resistance of Parthenium hysterophorus L. to the glyphosate: A new case of resistance in Colombia. In Proceedings of the XIIIème Colloque International Sur la Biologie des Mauvaises Herbes, Dijon, France, 8–10 September 2009; pp. 1–8. [Google Scholar]
- Jussaume, R.A., Jr.; Ervin, D. Understanding Weed Resistance as a Wicked Problem to Improve Weed Management Decisions. Weed Sci. 2016, 64, 559–569. [Google Scholar] [CrossRef]
- Vencill, W.K.; Nichols, R.L.; Webster, T.M.; Soteres, J.K.; Mallory-smith, C.; Burgos, N.R.; Johnson, W.G.; Mcclelland, M.R. Herbicide Resistance: Toward an Understanding of Resistance Development and the Impact of Herbicide-Resistant Crops. Weed Sci. 2012, 60, 2–30. [Google Scholar] [CrossRef]
- Norsworthy, J.K.; Ward, S.M.; Shaw, D.R.; Llewellyn, R.S.; Nichols, R.L.; Webster, T.M.; Bradley, K.W.; Frisvold, G.; Powles, S.B.; Burgos, N.R.; et al. Reducing the Risks of Herbicide Resistance: Best Management Practices and Recommendations. Weed Sci. 2012, 60, 31–62. [Google Scholar] [CrossRef]
- Gazziero, D.L.P.; Brighenti, A.M.; Voll, E. Resistência cruzada da losna-branca (Parthenium hysterophorus) aos herbicidas inibidores da enzima acetolactato sintase. Planta Daninha Vicosa-MG 2006, 24, 157–162. [Google Scholar] [CrossRef]
- Ghanizadeh, H.; Harrington, K.C.; James, T.K. Glyphosate-resistant Lolium multiflorum and Lolium perenne populations from New Zealand are also resistant to glufosinate and amitrole. Crop Prot. 2015, 78, 1–4. [Google Scholar] [CrossRef]
- Jalaludin, A.; Yu, Q.; Powles, S.B. Multiple resistance across glufosinate, glyphosate, paraquat and ACCase-inhibiting herbicides in an Eleusine indica population. Weed Res. 2015, 55, 82–89. [Google Scholar] [CrossRef]
- Fernández, P.; Alcántara, R.; Osuna, M.D.; Vila-Aiub, M.M.; Prado, R. De Forward selection for multiple resistance across the non-selective glyphosate, glufosinate and oxyfluorfen herbicides in Lolium weed species. Pest Manag. Sci. 2017, 73, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Travlos, I.S.; Cheimona, N.; De Prado, R.; Jhala, A.J.; Chachalis, D.; Tani, E. First case of glufosinate-resistant rigid ryegrass (Lolium rigidum gaud.) in Greece. Agronomy 2018, 8, 35. [Google Scholar] [CrossRef]
- Wang, H.; Guo, W.; Zhang, L.; Zhao, K.; Ge, L.; Lv, X.; Liu, W.; Wang, J. Multiple resistance to thifensulfuronmethyl and fomesafen in redroot pigweed (Amaranthus retroflexus L.) from China. Chil. J. Agric. Res. 2017, 77, 311–317. [Google Scholar] [CrossRef]
- Rousonelos, S.L.; Lee, R.M.; Moreira, M.S.; VanGessel, M.J.; Tranel, P.J. Characterization of a Common Ragweed (Ambrosia artemisiifolia) Population Resistant to ALS- and PPO-Inhibiting Herbicides. Weed Sci. 2012, 60, 335–344. [Google Scholar] [CrossRef]
- Salas, R.A.; Burgos, N.R.; Tranel, P.J.; Singh, S.; Glasgow, L.; Scott, R.C.; Nichols, R.L. Resistance to PPO-inhibiting herbicide in Palmer amaranth from Arkansas. Pest Manag. Sci. 2016, 72, 864–869. [Google Scholar] [CrossRef] [PubMed]
- Varanasi, V.K.; Brabham, C.; Norsworthy, J.K. Confirmation and Characterization of Non-target site Resistance to Fomesafen in Palmer amaranth (Amaranthus palmeri). Weed Sci. 2018, 66, 702–709. [Google Scholar] [CrossRef]
- Avila-Garcia, W.V.; Mallory-Smith, C. Glyphosate-Resistant Italian Ryegrass (Lolium perenne) Populations also Exhibit Resistance to Glufosinate. Weed Sci. 2011, 59, 305–309. [Google Scholar] [CrossRef]
- Jalaludin, A.; Yu, Q.; Zoellner, P.; Beffa, R.; Powles, S.B. Characterisation of glufosinate resistance mechanisms in Eleusine indica. Pest Manag. Sci. 2017, 73, 1091–1100. [Google Scholar] [CrossRef]
- Domínguez-Mendez, R.; Alcántara-de la Cruz, R.; Rojano-Delgado, A.M.; da Silveira, H.M.; Portugal, J.; Cruz-Hipolito, H.E.; De Prado, R. Stacked traits conferring multiple resistance to imazamox and glufosinate in soft wheat. Pest Manag. Sci. 2019, 75, 648–657. [Google Scholar] [CrossRef]
- Palma-Bautista, C.; Tahmasebi, B.K.; Fernández-Moreno, P.T.; Rojano-Delgado, A.M.; de la Cruz, R.A.; De Prado, R. First case of Conyza canadensis from Hungary with multiple resistance to glyphosate and flazasulfuron. Agronomy 2018, 8, 157. [Google Scholar] [CrossRef]
- Rojano-Delgado, A.M.; Portugal, J.M.; Palma-Bautista, C.; Alcántara-de la Cruz, R.; Torra, J.; Alcántara, E.; De Prado, R. Target site as the main mechanism of resistance to imazamox in a Euphorbia heterophylla biotype. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tehranchian, P.; Nandula, V.; Jugulam, M.; Putta, K.; Jasieniuk, M. Multiple resistance to glyphosate, paraquat and ACCase-inhibiting herbicides in Italian ryegrass populations from California: Confirmation and mechanisms of resistance. Pest Manag. Sci. 2018, 74, 868–877. [Google Scholar] [CrossRef]
- Harvey, B.M.R.; Muldoon, J.; Harper, D.B. Mechanism of paraquat tolerance in perennial ryegrass. I. Uptake, metabolism and translocation of paraquat. Plant Cell Environ. 1978, 1, 203–209. [Google Scholar] [CrossRef]
- Harper, D.B.; Harvey, B.M.R. Mechanism of paraquat tolerance in perennial ryegrass. II. Role of superoxide dismutase, catalase and peroxidase. Plant Cell Environ. 1978, 1, 211–215. [Google Scholar] [CrossRef]
- Kuk, Y.-I.; Burgos, N.R.; Talbert, R.E. Evaluation of rice by-products for weed control. Weed Sci. 2001, 49, 141–147. [Google Scholar] [CrossRef]
- Bhargava, S. Paraquat tolerance in a photomixotrophic culture of Chenopodium rubrum. Plant Cell Rep. 1993, 12, 230–232. [Google Scholar] [CrossRef]
- Christoffoleti, P.J.; De Figueiredo, M.R.A.; Peres, L.E.P.; Nissen, S.; Gaines, T. Auxinic herbicides, mechanisms of action, and weed resistance: A look into recent. Sci. Agric. 2015, 72, 356–362. [Google Scholar] [CrossRef]
- Mithila, J.; Hall, J.C.; Johnson, W.G.; Kelley, K.B.; Riechers, D.E. Evolution of Resistance to Auxinic Herbicides: Historical Perspectives, Mechanisms of Resistance, and Implications for Broadleaf Weed Management in Agronomic Crops. Weed Sci. 2011, 59, 445–457. [Google Scholar] [CrossRef]
- Busi, R.; Goggin, D.E.; Heap, I.M.; Horak, M.J.; Jugulam, M.; Masters, R.A.; Napier, R.M.; Riar, D.S.; Satchivi, N.M.; Torra, J.; et al. Weed resistance to synthetic auxin herbicides. Pest Manag. Sci. 2018, 74, 2265–2276. [Google Scholar] [CrossRef]
- Grossmann, K. Auxin herbicides: Current status of mechanism and mode of action. Pest Manag. Sci. 2010, 66, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Rey-Caballero, J.; Menéndez, J.; Giné-Bordonaba, J.; Salas, M.; Alcántara, R.; Torra, J. Unravelling the resistance mechanisms to 2,4-D (2,4-dichlorophenoxyacetic acid) in corn poppy (Papaver rhoeas). Pestic. Biochem. Physiol. 2016, 133, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Romero-Puertas, M.C.; McCarthy, I.; Gomez, M.; Sandalio, L.M.; Corpas, F.J.; Del Rio, L.A.; Palma, J.M. Reactive oxygen species-mediated enzymatic systems involved in the oxidative action of 2,4-dichlorophenoxyacetic acid. Plant Cell Environ. 2004, 27, 1135–1148. [Google Scholar] [CrossRef]
| Herbicide | Company | Commercial Product | MOA (HRAC) | Field Dose (g ai ha−1) |
|---|---|---|---|---|
| Glyphosate a | Monsanto | 36% w/v SL, Roundup® | EPSPS inhibitor | 720 |
| Glufosinate | Bayer CropScience | 15% w/v SL, Finale® | GS inhibitor | 750 |
| Flazasulfuron | Syngenta | 25 % w/w, Terafit® WG. | ALS inhibitor | 50 |
| Paraquat | Syngenta | 25% SL, Gramosone® | PS I inhibitor | 500 |
| Fomesafen | Syngenta | 25% w/v, Flex® 25 SL | PPO inhibitor | 375 |
| 2,4-D | Nufarm | 60% w/v, U26 D Complet® SL | Synthetic auxin | 400 |
| Population | Glyphosate | Glufosinate | Flazasulfuron | Paraquat | Fomesafen | 2,4-D |
|---|---|---|---|---|---|---|
| R | 100% | 65% | 80% | 100% | 60% | 60% |
| S | 0% | 0% | 0% | 100% | 0% | 0% |
| Herbicide | Population | d | b | GR50 (g ai ha−1) | p-value | RF |
|---|---|---|---|---|---|---|
| Glyphosate b | R | 99.19 | 2.24 | 1459.6 ± 134.1 | <0.001 | 27.1 |
| S | 99.61 | 1.44 | 53.8 ± 5.7 | <0.001 | - | |
| Glufosinate | R | 99.29 | 2.85 | 490.4 ± 26.7 | <0.001 | 7.3 |
| S | 98.95 | 3.11 | 66.8 ± 6.2 | 0.022 | - | |
| Flazasulfuron | R | 99.62 | 2.49 | 64.3 ± 5.8 | <0.001 | 15.3 |
| S | 98.49 | 0.07 | 4.2 ± 1.1 | <0.001 | - | |
| Paraquat | R | 98.46 | 1.82 | 356.5 ± 24.4 | 0.012 | 0.98 |
| S | 98.96 | 1.55 | 360.7 ± 29.3 | 0.017 | - | |
| Fomesafen | R | 99.37 | 0.71 | 1637.1 ± 163.7 | <0.001 | 12.6 |
| S | 99.03 | 3.64 | 129.7 ± 17.5 | <0.001 | - | |
| 2,4-D | R | 99.79 | 2.70 | 479.8 ± 31.4 | <0.001 | 4.9 |
| S | 98.59 | 1.44 | 97.6 ± 11.2 | <0.001 | - |
| Herbicide | Population | d | b | I50 (µM) | p-value | RF |
|---|---|---|---|---|---|---|
| Glyphosate | S | 99.74 | 0.22 | 21.18 ± 1.41 | <0.001 | - |
| R | 99.78 | 1.01 | 501.48 ± 20.95 | <0.001 | 23.68 | |
| Glufosinate | S | 99.75 | 2.50 | 32.30 ± 0.71 | <0.001 | - |
| R | 98.77 | 2.01 | 946.62 ± 41.92 | <0.001 | 29.31 | |
| Flazasulfuron | S | 98.29 | 0.40 | 46.25 ± 2.28 | <0.001 | - |
| R | 99.12 | 1.30 | 624.44 ± 21.29 | <0.001 | 13.50 |
| Population | HAT | Absorption (%) a | Translocation (% Absorbed) b | ||
|---|---|---|---|---|---|
| TL | TS | TR | |||
| S | 6 | 19.7 ± 1.6 e | 98.2 ± 1.1 ab | 1.7 ± 0.2 d | ND |
| 12 | 29.2 ± 0.9 d | 98.4 ± 0.6 a | 1.6 ± 0.1 d | ND | |
| 24 | 46.6 ± 0.9 c | 95.7 ± 0.9 c | 4.4 ± 0.3 a | ND | |
| 48 | 62.2 ± 1.4 b | 96.1 ± 0.3 bc | 3.6 ± 0.1 b | 0.1* | |
| 96 | 72.1 ± 1.8 a | 96.5 ± 1.1 abc | 3.1 ± 0.2 c | 0.5 * | |
| R | 6 | 17.9 ± 0.9 e | 98.6 ± 0.7 a | 1.2 ± 0.2 d | ND |
| 12 | 30.2 ± 1.2 d | 98.1 ± 0.8 a | 1.8 ± 0.1 d | ND | |
| 24 | 45.8 ± 1.1 c | 95.6 ± 0.8 b | 4.6 ± 0.2 a | ND | |
| 48 | 62.7 ± 1.3 b | 95.4 ± 1 b | 3.7 ± 0.1 b | 1 * | |
| 96 | 71.6 ± 1.3 a | 97.3 ± 0.5 ab | 2.7 ± 0.2 c | 0.4 * | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palma-Bautista, C.; Hoyos, V.; Plaza, G.; Vázquez-García, J.G.; Rosario, J.; Rojano-Delgado, A.M.; De Prado, R. Evolving Multiple Resistance to EPSPS, GS, ALS, PSI, PPO, and Synthetic Auxin Herbicides in Dominican Republic Parthenium hysterophorus Populations. A Physiological and Biochemical Study. Agronomy 2020, 10, 554. https://doi.org/10.3390/agronomy10040554
Palma-Bautista C, Hoyos V, Plaza G, Vázquez-García JG, Rosario J, Rojano-Delgado AM, De Prado R. Evolving Multiple Resistance to EPSPS, GS, ALS, PSI, PPO, and Synthetic Auxin Herbicides in Dominican Republic Parthenium hysterophorus Populations. A Physiological and Biochemical Study. Agronomy. 2020; 10(4):554. https://doi.org/10.3390/agronomy10040554
Chicago/Turabian StylePalma-Bautista, Candelario, Verónica Hoyos, Guido Plaza, José G. Vázquez-García, Jesús Rosario, Antonia M. Rojano-Delgado, and Rafael De Prado. 2020. "Evolving Multiple Resistance to EPSPS, GS, ALS, PSI, PPO, and Synthetic Auxin Herbicides in Dominican Republic Parthenium hysterophorus Populations. A Physiological and Biochemical Study" Agronomy 10, no. 4: 554. https://doi.org/10.3390/agronomy10040554
APA StylePalma-Bautista, C., Hoyos, V., Plaza, G., Vázquez-García, J. G., Rosario, J., Rojano-Delgado, A. M., & De Prado, R. (2020). Evolving Multiple Resistance to EPSPS, GS, ALS, PSI, PPO, and Synthetic Auxin Herbicides in Dominican Republic Parthenium hysterophorus Populations. A Physiological and Biochemical Study. Agronomy, 10(4), 554. https://doi.org/10.3390/agronomy10040554








