Seed Mineral Composition and Protein Content of Faba Beans (Vicia faba L.) with Contrasting Tannin Contents
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growing Conditions
2.2. Phenotyping
2.2.1. Analysis of Micronutrients with Microwave Digest and ICP-MS Analysis
2.2.2. Protein Content
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Duc, G. Faba bean (Vicia faba L.). Field Crops Res. 1997, 53, 99–109. [Google Scholar] [CrossRef]
- Crépon, K.; Marget, P.; Peyronnet, C.; Carrouée, B.; Arese, P.; Duc, G. Nutritional value of faba bean (Vicia faba L.) seeds for food and feed. Field Crops Res. 2010, 115, 329–339. [Google Scholar] [CrossRef]
- Feedipedia. Faba bean (Vicia faba). 2018. Available online: https://www.feedipedia.org/node/4926 (accessed on 21 January 2020).
- Robinson, G.H.J.; Balk, J.; Domoney, C. Improving pulse crops as a source of protein, starch and micronutrients. Nutr. Bull. 2019, 44, 202–215. [Google Scholar] [CrossRef] [PubMed]
- Khazaei, H.; Subedi, M.; Nickerson, M.; Martínez-Villaluenga, C.; Frias, J.; Vandenberg, A. Seed protein of lentils: Current status, progress, and food applications. Foods 2019, 8, 391. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations. FAOSTAT. Available online: http://faostat.fao.org (accessed on 2 March 2020).
- Stein, A.J. Global impacts of human mineral malnutrition. Plant Soil 2010, 335, 133–154. [Google Scholar] [CrossRef]
- Bouis, H.E.; Hotz, C.; McClafferty, B.; Meenakshi, J.V.; Pfeiffer, W.H. Biofortification: A new tool to reduce micronutrient malnutrition. Food. Nutr. Bull. 2011, 32, S31–S40. [Google Scholar] [CrossRef]
- Garg, M.; Sharma, N.; Sharma, S.; Kapoor, P.; Kumar, A.; Chunduri, V.; Arora, P. Biofortified crops generated by breeding, agronomy, and transgenic approaches are improving lives of millions of people around the world. Front. Nutr. 2018, 5, 12. [Google Scholar] [CrossRef]
- Connorton, J.M.; Balk, J. Iron biofortification of staple crops: Lessons and challenges in plant genetics. Plant Cell Physiol. 2019, 60, 1447–1456. [Google Scholar] [CrossRef]
- Pfeiffer, W.H.; McClafferty, B. HarvestPlus: Breeding crops for better nutrition. Crop Sci. 2007, 47, S88–S105. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Biofortification of crops with seven mineral elements often lacking in human diets–iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol. 2009, 182, 49–84. [Google Scholar] [CrossRef]
- Guzmán-Maldonado, S.H.; Martínez, O.; Acosta-Gallegos, J.A.; Guevara-Lara, F.; Paredes-López, O. Putative quantitative trait loci for physical and chemical components of common bean. Crop Sci. 2003, 43, 1029–1035. [Google Scholar] [CrossRef]
- Grusak, M.A.; Cakmak, I. Methods to improve the crop-delivery of minerals to humans and livestock. In Plant Nutritional Genomics; Broadley, M.R., White, P.J., Eds.; Blackwell: Oxford, UK, 2005; pp. 265–286. [Google Scholar]
- Amarakoon, D.; Thavarajah, D.; McPhee, K.; Thavarajah, P. Iron-, zinc-, and magnesium-rich field peas (Pisum sativum L.) with naturally low phytic acid: A potential food-based solution to global micronutrient malnutrition. J. Food Comps. Anal. 2012, 27, 8–13. [Google Scholar] [CrossRef]
- Ray, H.; Bett, K.; Tar’an, B.; Vandenberg, A.; Thavarajah, D.; Warkentin, T. Mineral micronutrient content of cultivars of field pea, chickpea, common bean, and lentil grown in Saskatchewan, Canada. Crop Sci. 2014, 54, 1698–1708. [Google Scholar] [CrossRef]
- Jha, A.B.; Warkentin, T.D. Biofortification of pulse crops: Status and future perspectives. Plants 2020, 9, 73. [Google Scholar] [CrossRef] [PubMed]
- Baloch, F.S.; Karaköy, T.; Demirbaş, A.; Toklu, F.; Özkan, H.; Hatipoğlu, R. Variation of some seed mineral contents in open pollinated faba bean (Vicia faba L.) landraces from Turkey. Turk. J. Agric. Forest. 2014, 38, 591–602. [Google Scholar] [CrossRef]
- Lombardo, S.; Pandino, G.; Pesce, G.R.; Anastasi, U.; Tuttobene, R.; Mauromicale, G. Variation in seed mineral elements profile and yield in field bean (Vicia faba L. var. minor) genotypes. Ital. J. Agron. 2016, 11, 261–267. [Google Scholar] [CrossRef]
- Etemadi, F.; Barker, A.V.; Hashemi, M.; Zandvakili, O.R.; Park, Y. Nutrient accumulation in faba bean varieties. Commun. Soil Sci. Plant Anal. 2018, 49, 2064–2073. [Google Scholar] [CrossRef]
- CEM, MARS 6TH Research Note. Microwave Digestion of Feed Grains. Available online: https://cem.com/media/contenttype/media/literature/MetNote_MARS6_Feed_Grain_3.pdf (accessed on 30 March 2020).
- R Core TeamR. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: http://www.R-project.org/ (accessed on 20 March 2020).
- Falconer, D.S.; Mackay, T.F.C. Introduction to Quantitative Genetics, 4th ed.; Longmans Green: Harlow, UK, 1996. [Google Scholar]
- Khazaei, H.; Podder, R.; Caron, C.T.; Kundu, S.S.; Diapari, M.; Vandenberg, A.; Bett, K.E. Marker–trait association analysis of iron and zinc concentration in lentil (Lens culinaris Medik.) seeds. Plant Genome 2017, 10. [Google Scholar] [CrossRef]
- Food Standards Agency (FSA). McCance and Widdowson’s the Composition of Foods, 6th ed.; Royal Society of Chemistry: Cambridge, UK, 2002. [Google Scholar]
- Jukanti, A.K.; Gaur, P.M.; Gowda, C.L.L.; Chibbar, R.N. Nutritional quality and health benefits of chickpea (Cicer arietinum L.): A review. Br. J. Nutr. 2012, 108, S11–S26. [Google Scholar] [CrossRef]
- Blair, M.W.; Astudillo, C.; Grusak, M.; Graham, R.; Beebe, S. Inheritance of seed iron and zinc content in common bean (Phaseolus vulgaris L.). Mol. Breed. 2009, 23, 197–207. [Google Scholar] [CrossRef]
- McClean, P.E.; Mafi Moghaddam, S.; Lopéz-Millán, A.F.; Brick, M.A.; Kelly, J.D.; Miklas, P.N.; Osorno, J.; Porch, T.G.; Urrea, C.A.; Soltani, A.; et al. Phenotypic diversity for seed mineral concentration in North American dry bean germplasm of Middle American ancestry. Crop Sci. 2017, 57, 3129–3144. [Google Scholar] [CrossRef]
- Raboy, V. Accumulation and storage of phosphate and minerals. In Cellular and Molecular Biology of Plant Seed Development; Larkins, B.A., Vasil, I.K., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1997; pp. 441–447. [Google Scholar]
- Zhou, J.R.; Erdman, J.W., Jr. Phytic acid in health and disease. Crit. Rev. Food Sci. Nutr. 1995, 35, 495–508. [Google Scholar] [CrossRef] [PubMed]
- Shunmugam, A.S.K.; Bock, C.; Arganosa, G.C.; Georges, F.; Gray, G.R.; Warkentin, T.D. Accumulation of phosphorus-containing compounds in developing seeds of low-phytate pea (Pisum sativum L.) mutants. Plants 2015, 4, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Afinah, S.; Yazid, A.M.; Anis Shobirin, M.H.; Shuhaimi, M. Phytase: Application in food industry. Int. Food Res. J. 2010, 17, 13–21. [Google Scholar]
- Multari, S.; Stewart, D.; Russell, W.R. Potential of fava bean as future protein supply to partially replace meat intake in the human diet. Compr. Rev. Food Sci. Food Saf. 2015, 14, 511–522. [Google Scholar] [CrossRef]
- Gupta, R.K.; Gangoliya, S.S.; Singh, N.K. Reduction of phytic acid and enhancement of bioavailable micronutrients in food grains. J. Food Sci. Technol. 2015, 52, 676–684. [Google Scholar] [CrossRef]
- Bond, D.A. In vitro digestibility of the testa in tannin-free field beans (Vicia faba L.). J. Agric. Sci. 1976, 86, 561–566. [Google Scholar] [CrossRef]
- Griffiths, D.W. The inhibition of digestive enzymes by extracts of field bean (Vicia faba). J. Sci. Food Agric. 1979, 30, 458–462. [Google Scholar] [CrossRef]
- Keatinge, J.D.H.; Yang, R.-Y.; Hughes, D.A.J.; Easdown, W.J.; Holmer, R. The importance of vegetables in ensuring both food and nutritional security in attainment of the Millennium Development Goals. Food Secur. 2011, 3, 491–501. [Google Scholar] [CrossRef]
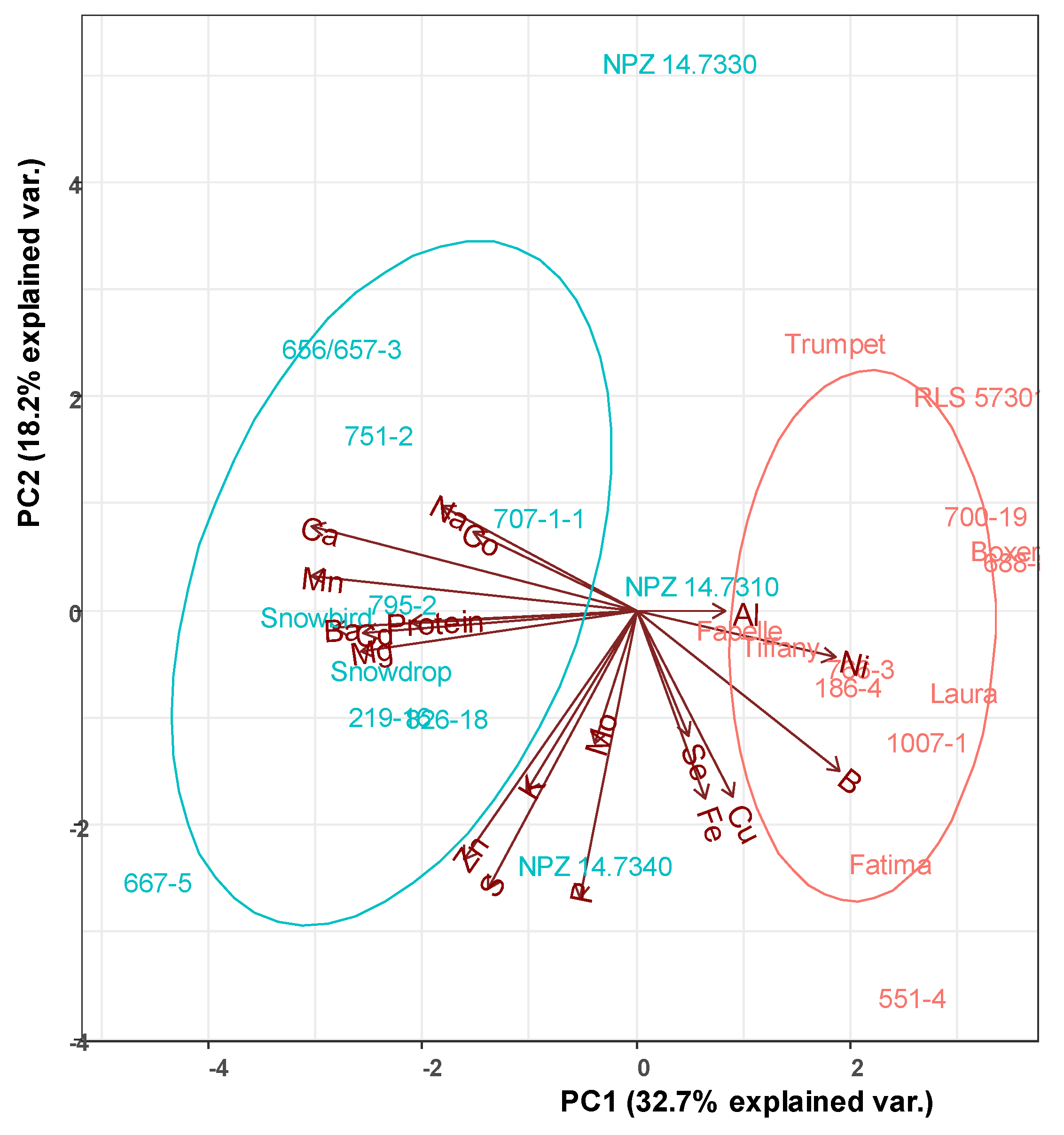
| Genotype | Flower Color | Breeder/Origin |
|---|---|---|
| Snowbird | White | Limagrain, The Netherlands |
| CDC Snowdrop | White | Crop Development Centre (CDC), Canada |
| 219-16 | White | Crop Development Centre (CDC), Canada |
| 667-5 | White | Crop Development Centre (CDC), Canada |
| 795-2 | White | Crop Development Centre (CDC), Canada |
| 826-18 | White | Crop Development Centre (CDC), Canada |
| 707-1-1 | White | Crop Development Centre (CDC), Canada |
| 751-2 | White | Crop Development Centre (CDC), Canada |
| 656/657-3 | White | Crop Development Centre (CDC), Canada |
| NPZ 14.7310 | White | Norddeutsche Pflanzenzucht (NPZ), Germany |
| NPZ 14.7330 | White | Norddeutsche Pflanzenzucht (NPZ), Germany |
| NPZ 14.7340 | White | Norddeutsche Pflanzenzucht (NPZ), Germany |
| CDC Fatima | Spotted | Crop Development Centre (CDC), Canada |
| Fabelle | Spotted | Norddeutsche Pflanzenzucht (NPZ), Germany |
| 186-4 | Spotted | Crop Development Centre (CDC), Canada |
| 551-4 | Spotted | Crop Development Centre (CDC), Canada |
| 688-8 | Spotted | Crop Development Centre (CDC), Canada |
| 1007-1 | Spotted | Crop Development Centre (CDC), Canada |
| 700-19 | Spotted | Crop Development Centre (CDC), Canada |
| 766-3 | Spotted | Crop Development Centre (CDC), Canada |
| Boxer | Spotted | Lantmännen SW Seed Hadmersleben, Sweden |
| Laura | Spotted | Lantmännen SW Seed Hadmersleben, Sweden |
| Trumpet | Spotted | Norddeutsche Pflanzenzucht (NPZ), Germany |
| Tiffany | Spotted | Norddeutsche Pflanzenzucht (NPZ), Germany |
| RLS 57301 | Spotted | Norddeutsche Pflanzenzucht (NPZ), Germany |
| Site/Location | |||||||
|---|---|---|---|---|---|---|---|
| Morden | Roblin | Rosthern | S.O.V. 2 | ||||
| Element (ppm DW 1) | Mean ± SD | Mean ± SD | Mean ± SD | H2 | Genotype | E (Site-Year) | G × E |
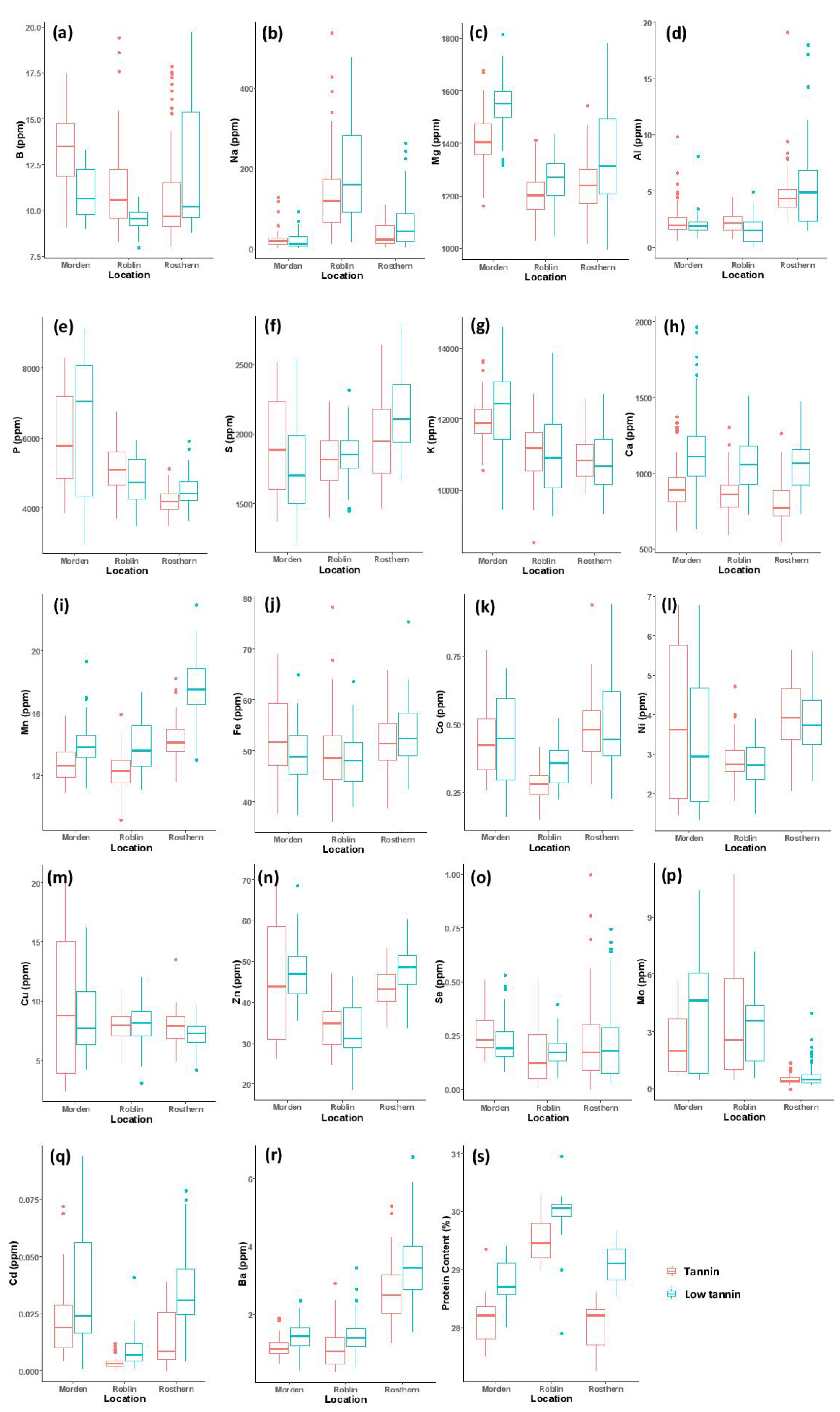
| B | 12.32 ± 1.42 | 10.35 ± 0.98 | 11.73 ± 1.26 | 0.58 | * | *** | ** |
| Na | 21.85 ± 7.38 | 163.74 ± 55.41 | 47.33 ± 22.54 | 0.51 | *** | *** | *** |
| Mg | 1478 ± 91 | 1232 ± 67 | 1292 ± 91 | 0.91 | *** | *** | ns |
| Al | 2.24 ± 0.46 | 1.91 ± 0.49 | 5.05 ± 0.91 | 0.35 | ns | *** | *** |
| P | 6055 ± 305 | 4961 ± 329 | 4339 ± 214 | 0.54 | *** | *** | *** |
| S | 1828 ± 136 | 1833 ± 82 | 2047 ± 125 | 0.81 | *** | *** | *** |
| K | 12055 ± 504 | 11049 ± 507 | 10842 ± 343 | 0.86 | *** | *** | *** |
| Ca | 1030 ± 175 | 955 ± 125 | 928 ± 157 | 0.90 | *** | ** | * |
| Mn | 13.38 ± 0.83 | 13.03 ± 1.02 | 15.87 ± 1.79 | 0.87 | *** | *** | ** |
| Fe | 50.87 ± 4.09 | 48.57 ± 3.59 | 52.54 ± 3.15 | 0.77 | *** | *** | *** |
| Co | 0.438 ± 0.283 | 0.316 ± 0.044 | 0.495 ± 0.066 | 0.65 | ** | *** | ns |
| Ni | 3.55 ± 0.45 | 2.79 ± 0.26 | 3.85 ± 0.30 | 0.71 | *** | *** | ** |
| Cu | 9.08 ± 0.86 | 8.00 ± 0.69 | 7.53 ± 0.51 | 0.31 | ** | *** | *** |
| Zn | 46.13 ± 3.16 | 33.77 ± 2.53 | 45.50 ± 3.09 | 0.52 | *** | *** | * |
| Se | 0.238 ± 0.036 | 0.169 ± 0.027 | 0.233 ± 0.068 | 0.50 | ns | *** | *** |
| Mo | 3.04 ± 0.72 | 3.70 ± 1.03 | 0.60 ± 0.22 | 0.48 | ** | *** | *** |
| Cd | 0.027 ± 0.012 | 0.006 ± 0.004 | 0.024 ± 0.013 | 0.47 | *** | *** | *** |
| Ba | 1.18 ± 0.27 | 1.16 ± 0.28 | 3.04 ± 0.56 | 0.39 | *** | *** | ** |
| Protein content (%) | 28.43 ± 0.56 | 29.68 ± 0.61 | 28.55 ± 0.66 | 0.81 | *** | * | * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khazaei, H.; Vandenberg, A. Seed Mineral Composition and Protein Content of Faba Beans (Vicia faba L.) with Contrasting Tannin Contents. Agronomy 2020, 10, 511. https://doi.org/10.3390/agronomy10040511
Khazaei H, Vandenberg A. Seed Mineral Composition and Protein Content of Faba Beans (Vicia faba L.) with Contrasting Tannin Contents. Agronomy. 2020; 10(4):511. https://doi.org/10.3390/agronomy10040511
Chicago/Turabian StyleKhazaei, Hamid, and Albert Vandenberg. 2020. "Seed Mineral Composition and Protein Content of Faba Beans (Vicia faba L.) with Contrasting Tannin Contents" Agronomy 10, no. 4: 511. https://doi.org/10.3390/agronomy10040511
APA StyleKhazaei, H., & Vandenberg, A. (2020). Seed Mineral Composition and Protein Content of Faba Beans (Vicia faba L.) with Contrasting Tannin Contents. Agronomy, 10(4), 511. https://doi.org/10.3390/agronomy10040511







