Genotype-Dependent Differences between Cereals in Response to Manganese Excess in the Environment
Abstract
1. Introduction
2. Materials and Methods
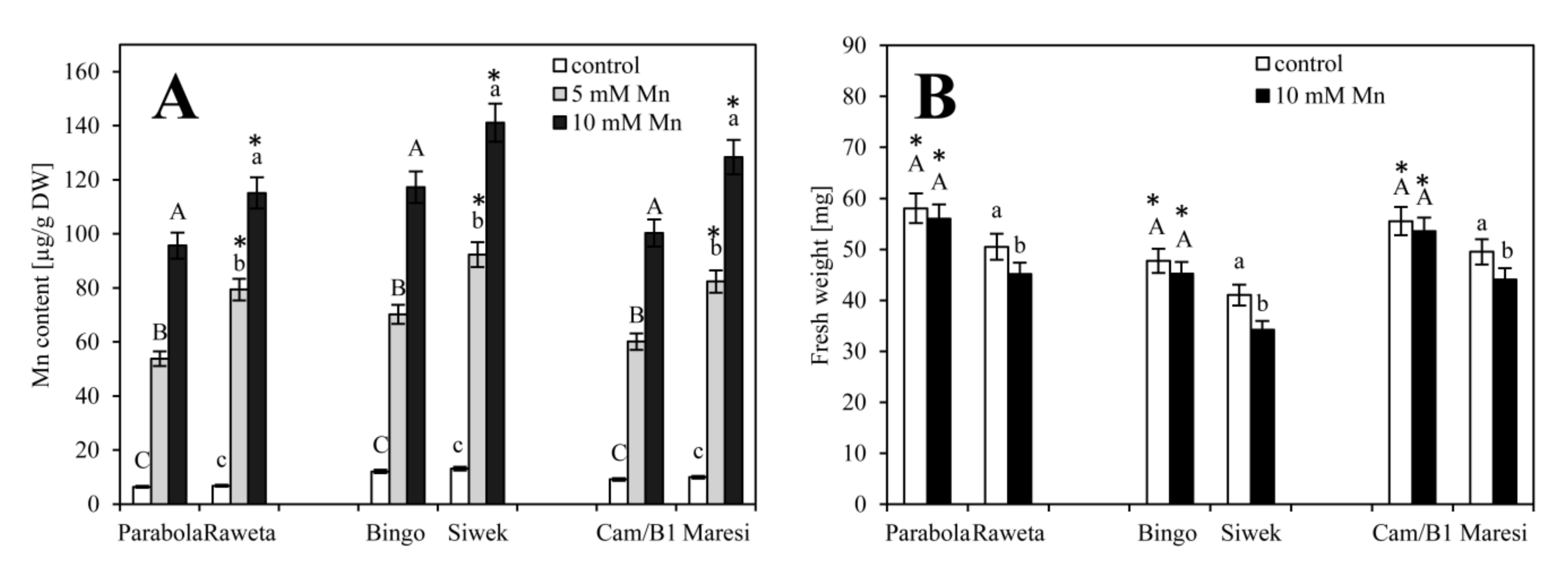
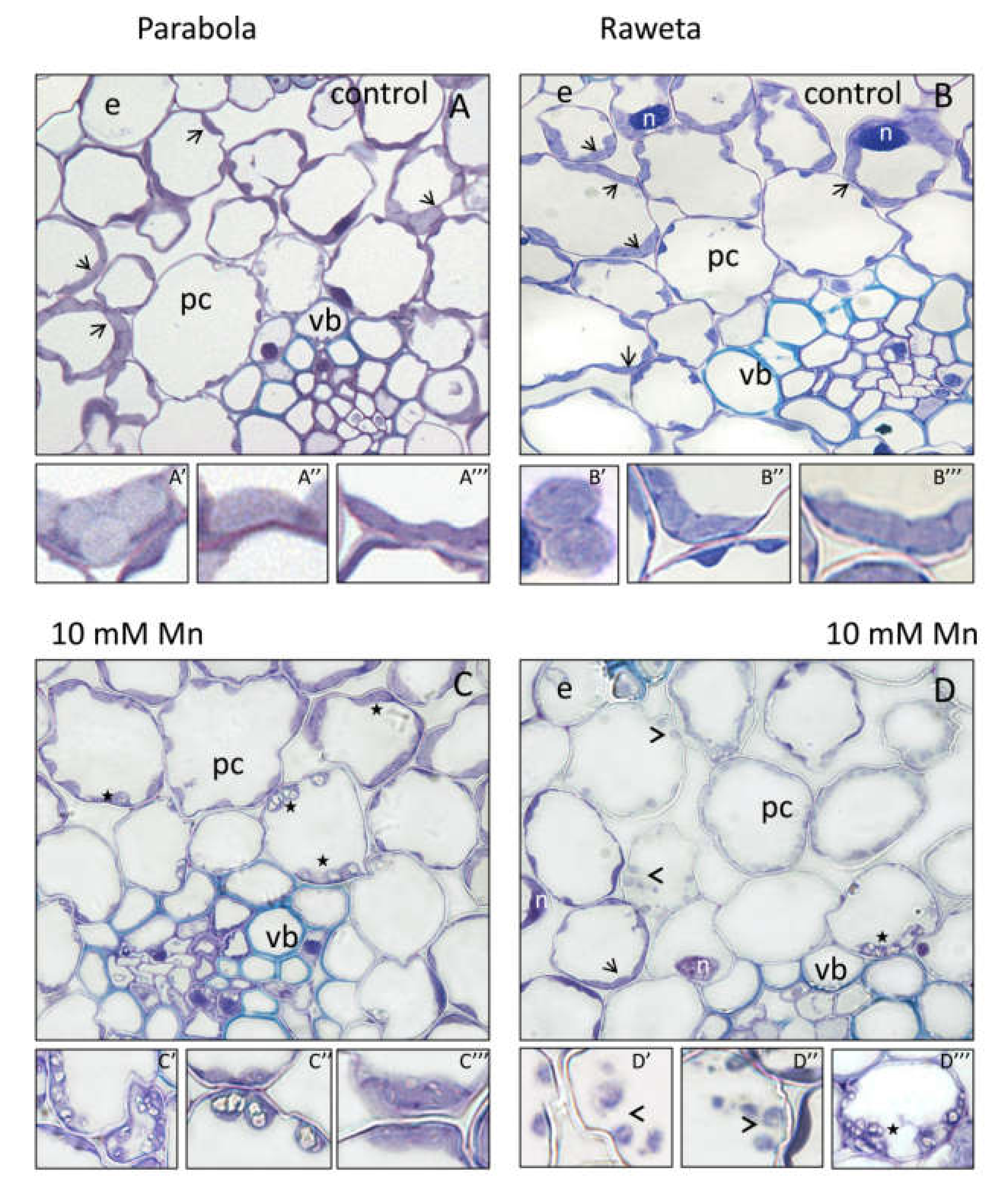
2.1. Determination of Manganese Content via Biochemistry and Microscopy Assays
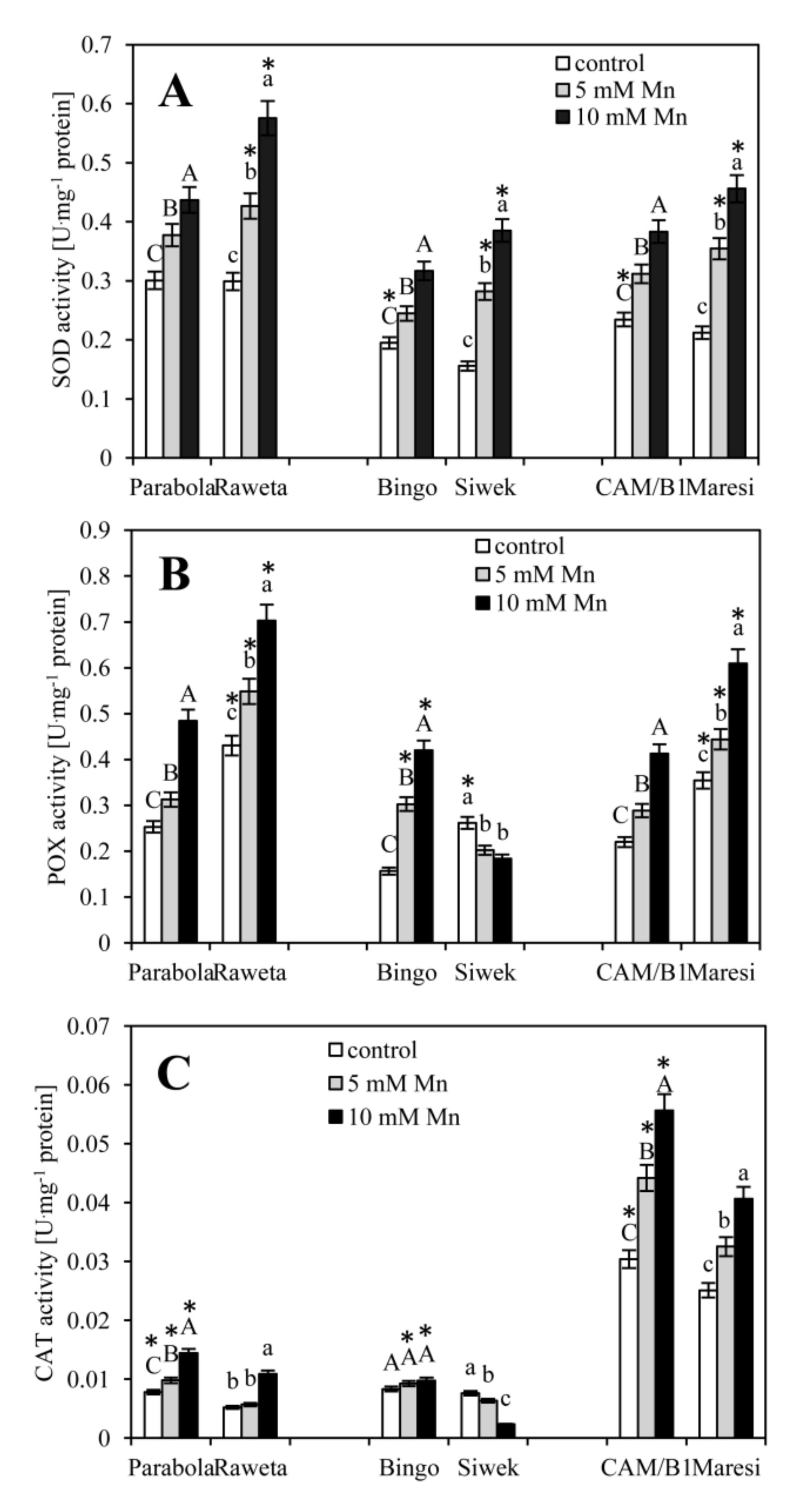
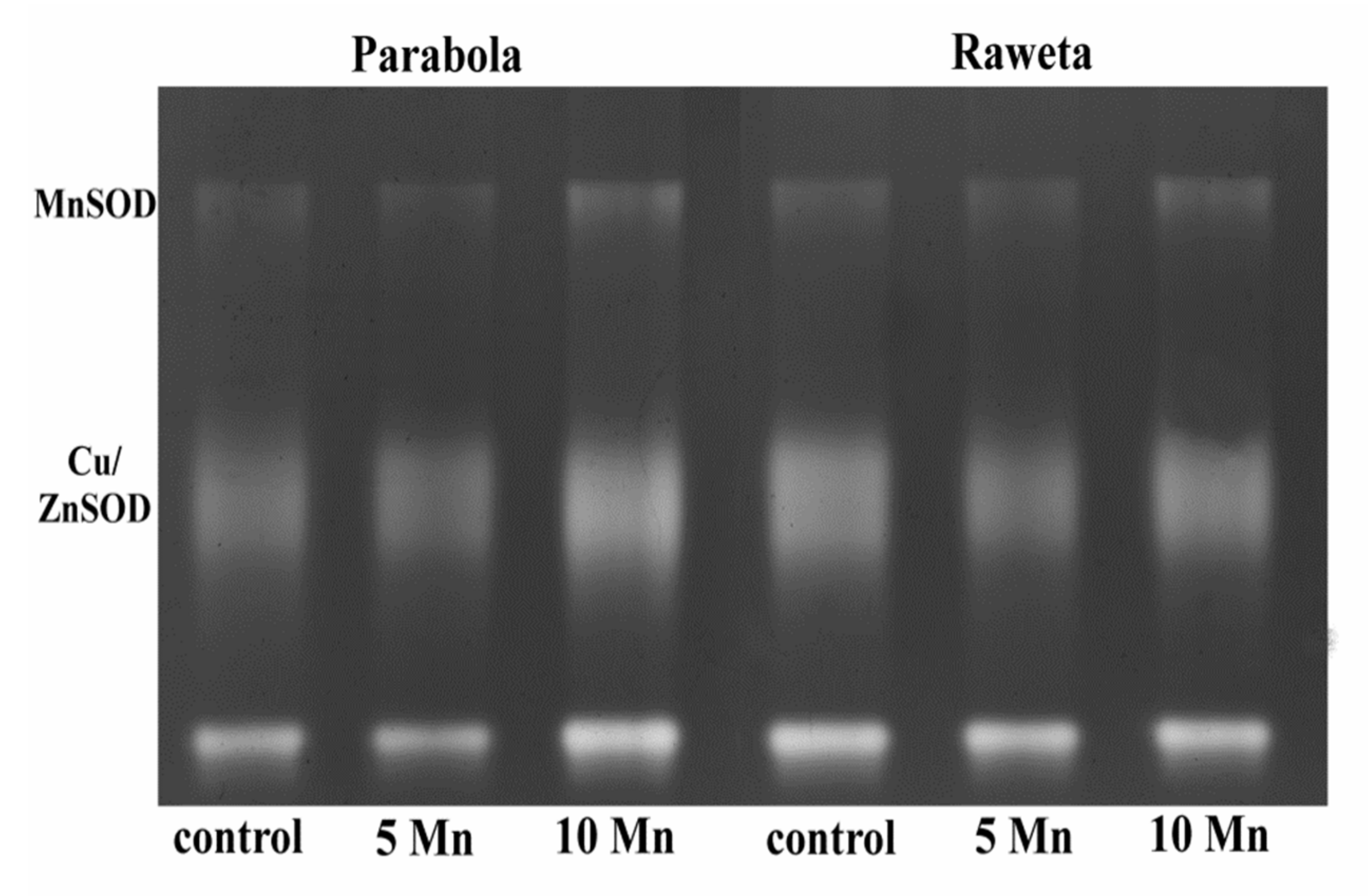
2.2. Antioxidant Enzyme Assays and Detection of Their Isoforms
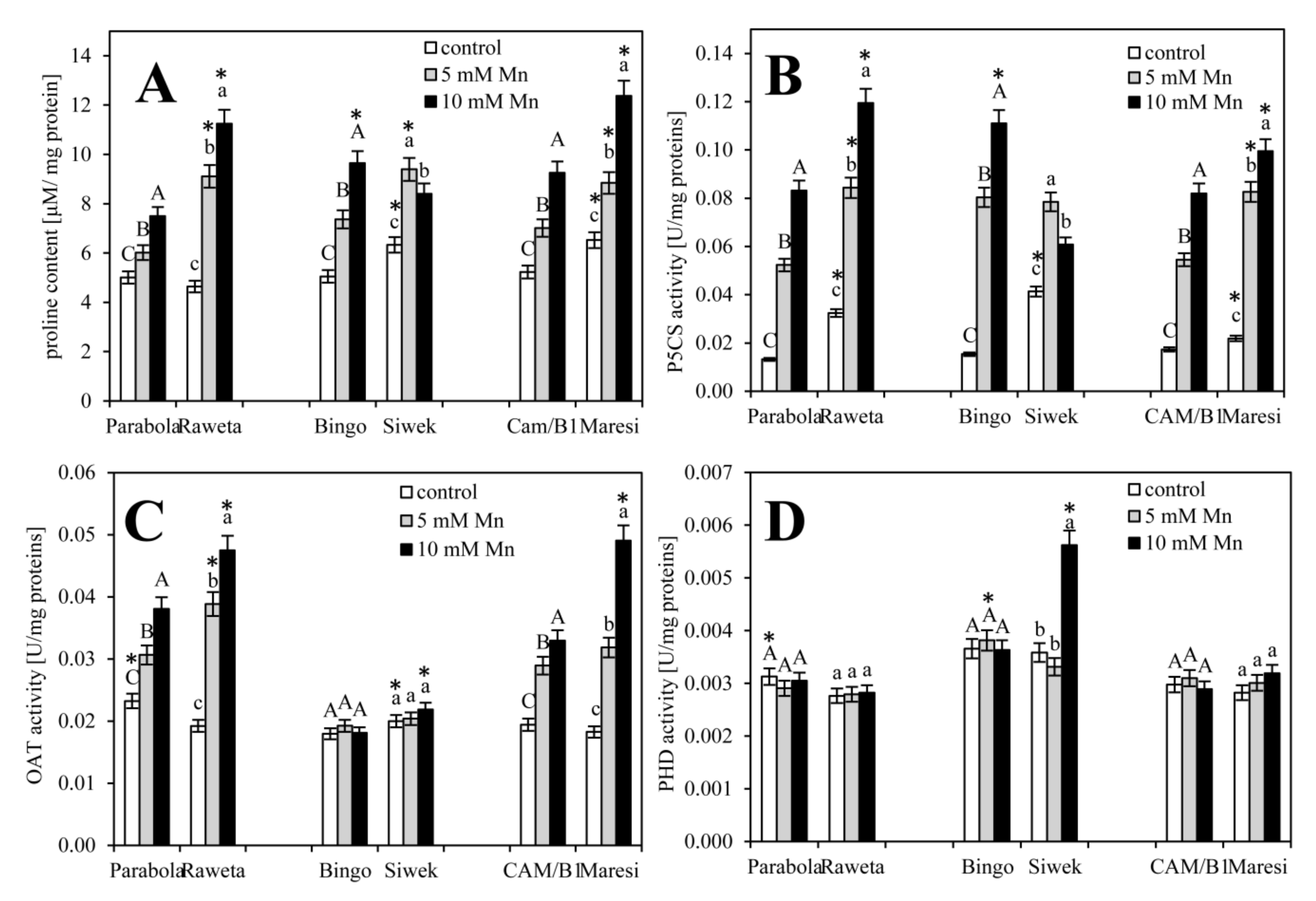
2.3. Analysis of Proline Metabolism
2.4. Carbohydrate and Salicylic Acid Content
2.5. Statistical Analysis of Data
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Brito, C.; Dinis, L.T.; Moutinho-Pereira, J.; Correia, C.M. Drought stress effects and olive tree acclimation under a changing climate. Plants 2019, 8, 232. [Google Scholar] [CrossRef] [PubMed]
- Keunen, E.; Remans, T.; Bohler, S.; Vangronsveld, J.; Cuypers, A. Metal-induced oxidative stress and plant mitochondria. Int. J. Mol. Sci. 2011, 12, 6894–6918. [Google Scholar] [CrossRef] [PubMed]
- Subba, P.; Mukhopadhyay, M.; Mahato, S.K.; Bhutia, K.D.; Mondal, T.K.; Ghosh, S.K. Zinc stress induces physiological, ultra-structural and biochemical changes in mandarin orange (Citrus reticulata Blanco) seedlings. Physiol. Mol. Biol. Plants 2014, 20, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Küpper, H.; Götz, B.; Mijovilovich, A.; Küpper, F.C.; Meyer-Klaucke, W. Complexation and toxicity of copper in higher plants. I. Characterization of copper accumulation, speciation, and toxicity in Crassula helmsii as a new copper accumulator. Plant Physiol. 2009, 151, 702–714. [Google Scholar]
- Schützendübel, A.; Polle, A. Plant responses to abiotic stresses: Heavy metal-induced oxidative stress and protection by mycorrhization. J. Exp. Bot. 2002, 53, 1351–1365. [Google Scholar] [CrossRef] [PubMed]
- Fernando, D.R.; Lynch, J.P. Manganese phytotoxicity: New light on an old problem. Ann. Bot. 2015, 116, 313–319. [Google Scholar] [CrossRef]
- Santos, E.F.; Santini, J.M.K.; Paixão, A.P.; Júnior, E.F.; Lavres, J.; Campos, M.; dos Reis, A.R. Physiological highlights of manganese toxicity symptoms in soybean plants: Mn toxicity responses. Plant Physiol. Bioch. 2017, 113, 6–19. [Google Scholar] [CrossRef]
- Liu, P.; Huang, R.; Hu, X.; Jia, Y.; Li, J.; Luo, J.; Liu, Q.; Luo, L.; Liu, G.; Chen, Z. Physiological responses and proteomic changes reveal insights into Stylosanthes response to manganese toxicity. BMC Plant Biol. 2019, 19, 212. [Google Scholar] [CrossRef]
- Li, J.; Jia, Y.; Dong, R.; Huang, R.; Liu, P.; Li, X.; Chen, Z. Advances in the mechanisms of plant tolerance to manganese toxicity. Int. J. Mol. Sci. 2019, 20, 5096. [Google Scholar] [CrossRef]
- Niu, L.L.; Yang, F.X.; Xu, C.; Yang, H.Y.; Liu, W.P. Status of metal accumulation in farmland soils across China: From distribution to risk assessment. Environ. Pollut. 2013, 176, 55–62. [Google Scholar] [CrossRef]
- Antibachi, D.; Kelepertzis, E.; Kelepertsis, A. Heavy metals in agricultural soils of the Mouriki-Thiva area (Central Greece) and environmental impact implications. Soil Sediment Contam. 2012, 21, 434–450. [Google Scholar] [CrossRef]
- Millaleo, R.; Ivanov, A.G.; Mora, M.L.; Alberdi, M. Manganese as essential and toxic element for plants: Transport, accumulation and resistance mechanisms. J. Soil Sci. Plant Nut. 2010, 10, 476–494. [Google Scholar] [CrossRef]
- Kurdziel, M.; Dłubacz, A.; Wesełucha-Birczyńska, A.; Filek, M.; Łabanowska, M. Stable radicals and biochemical compounds in embryos and endosperm of wheat grains differentiating sensitive and tolerant genotypes–EPR and Raman studies. J. Plant Phys. 2015, 183, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Dubey, R.S. Manganese-excess induces oxidative stress, lowers the pool of antioxidants and elevates activities of key antioxidative enzymes in rice seedlings. Plant Growth Regul. 2011, 64, 1–16. [Google Scholar] [CrossRef]
- Shi, Q.; Zhu, Z. Effects of exogenous salicylic acid on manganese toxicity, element contents and antioxidative system in cucumber. Environ. Exp. Bot. 2008, 63, 317–326. [Google Scholar] [CrossRef]
- Sieprawska, A.; Filek, M.; Tobiasz, A.; Walas, S.; Dudek-Adamska, D.; Grygo-Szymanko, E. Trace elements’ uptake and antioxidant response to excess of manganese in in vitro cells of sensitive and tolerant wheat. Acta Physiol. Plant. 2016, 38, 55. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; Singh, U.; Adisa, I.O.; Bindraban, P.S.; Elmer, W.H.; Gardea-Torresdey, J.L.; White, J.C. Effects of manganese nanoparticle exposure on nutrient acquisition in wheat (Triticum aestivum L.). Agronomy 2018, 8, 158. [Google Scholar] [CrossRef]
- Marschner, P. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: Boston, MA, USA, 2012. [Google Scholar]
- Millaleo, R.; Reyes-Diaz, M.; Alberdi, M.; Ivanov, A.G.; Krol, M.; Huner, N.P. Excess manganese differentially inhibits photosystem I versus II in Arabidopsis thaliana. J. Exp. Bot. 2013, 64, 343–354. [Google Scholar] [CrossRef]
- Socha, A.L.; Guerinot, M.L. Mn-euvering manganese: The role of transporter gene family members in manganese uptake and mobilization in plants. Front. Plant Sci. 2014, 5, 106. [Google Scholar] [CrossRef]
- Sieprawska, A.; Filek, M.; Tobiasz, A.; Bednarska-Kozakiewicz, E.; Walas, S.; Dudek-Adamska, D.; Grygo-Szymanko, E. Response of chloroplasts of tolerant and sensitive wheat genotypes to manganese excess: Structural and biochemical properties. Acta Physiol. Plant. 2017, 39, 6. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Kavi Kishor, P.B.; Sreenivasulu, N. Is proline accumulation per se correlated with stress tolerance or is proline homeostasis a more critical issue? Plant Cell Environ. 2014, 37, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Metwally, A.; Finkemeier, I.; Georgi, M.; Dietz, K.J. Salicylic acid alleviates the cadmium toxicity in barley seedlings. Plant Physiol. 2003, 132, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Dar, M.I.; Naikoo, M.I.; Rehman, F.; Naushin, F.; Khan, F.A. Proline accumulation in plants: Roles in stress tolerance and plant development. In Osmolytes and Plants Acclimation to Changing Environment: Emerging Omics Technologies; Iqbal, N., Nazar, R., Eds.; Springer: New Delhi, India, 2016; pp. 155–166. [Google Scholar]
- Amini, S.; Ghobadi, C.; Yamchi, A. Proline accumulation and osmotic stress: An overview of P5CS gene in plants. J. Plant Mol. Breed. 2015, 3, 44–55. [Google Scholar]
- Kameli, A.; Lösel, D.M. Carbohydrates and water status in wheat plants under water stress. New Phytol. 1993, 125, 609–614. [Google Scholar] [CrossRef]
- Tabuchi, A.; Kikui, S.; Matsumoto, H. Differential effects of aluminium on osmotic potential and sugar accumulation in the root cells of Al-resistant and Al-sensitive wheat. Physiol. Plant. 2004, 120, 106–112. [Google Scholar] [CrossRef]
- Sidhu, G.P.S.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Alterations in photosynthetic pigments, protein, and carbohydrate metabolism in a wild plant Coronopus didymus L. (Brassicaceae) under lead stress. Acta Physiol. Plant. 2017, 39, 176. [Google Scholar] [CrossRef]
- Shewry, P.R. Wheat. J. Exp. Bot. 2009, 60, 1537–1553. [Google Scholar] [CrossRef]
- Verstegen, H.; Köneke, O.; Korzun, V.; von Broock, R. The world importance of barley and challenges to further improvements. In Biotechnological Approaches to Barley Improvement; Springer: Berlin/Heidelberg, Germany, 2014; pp. 3–19. [Google Scholar]
- Rasane, P.; Jha, A.; Sabikhi, L.; Kumar, A.; Unnikrishnan, V.S. Nutritional advantages of oats and opportunities for its processing as value added foods-a review. J. Food. Sci. Technol. 2015, 52, 662–675. [Google Scholar] [CrossRef]
- Curtis, T.; Halford, N.G. Food security: The challenge of increasing wheat yield and the importance of not compromising food safety. Ann. App. Biol. 2014, 164, 354–372. [Google Scholar] [CrossRef]
- Grzesiak, M.; Filek, M.; Barbasz, A.; Kreczmer, B.; Hartikainen, H. Relationships between polyamines, ethylene, osmoprotectants and antioxidant enzymes activities in wheat seedlings after short-term PEG- and NaCl-induced stresses. Plant Growth Regul. 2013, 69, 177–189. [Google Scholar] [CrossRef]
- Łabanowska, M.; Kurdziel, M.; Filek, M.; Wesełucha-Birczyńska, A. The impact of biochemical composition and nature of paramagnetic species in grains on stress tolerance of oat cultivars. J. Plant Physiol. 2016, 199, 52–66. [Google Scholar] [CrossRef]
- Filek, M.; Łabanowska, M.; Kurdziel, M.; Wesełucha-Birczyńska, A.; Bednarska-Kozakiewicz, E. Structural and biochemical response of chloroplasts in tolerant and sensitive barley genotypes to drought stress. J. Plant Phys. 2016, 207, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Tobiasz, A.; Walas, S.; Filek, M.; Mrowiec, H.; Samsel, K.; Sieprawska, A.; Hartikainen, H. Effect of selenium on distribution of macro-and micro-elements to different tissues during wheat ontogeny. Biol. Plant. 2014, 58, 370–374. [Google Scholar] [CrossRef]
- McCord, J.M.; Fridovich, I. Superoxide dismutase an enzymatic function for erythrocuprein (hemocuprein). J. Biol. Chem. 1969, 244, 6049–6055. [Google Scholar] [PubMed]
- Lück, H. Methods of Enzymatic Analysis, 1st ed.; Bergmeyer, H.U., Ed.; Verlag Chemie GmbH: Weinheim/Bergstr, Germany; Academic Press: Cambridge, MA, USA, 1963; Volume 1, pp. 895–897. [Google Scholar]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Gawrońska, K.; Gołębiowska-Pikania, G. The effects of cold-hardening and Microdochium nivale infection on oxidative stress and antioxidative protection of the two contrasting genotypes of winter triticale. Eur. Food Res. Technol. 2016, 242, 1267–1276. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Lűtts, S.; Majerus, V.; Kinet, J.M. NaCl effects on proline metabolism in rice (Oryza sativa) seedlings. Physiol. Plant. 1999, 105, 450–458. [Google Scholar] [CrossRef]
- Stines, A.P.; Naylor, D.J.; Høj, P.B.; Heeswijck, R. Proline accumulation in developing grapevine fruit occurs independently of changes in the levels of Δ1-pyrroline-5-carboxylate synthetase mRNA or protein. Plant Physiol. 1999, 120, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Vogel, R.H.; Kopac, M.J. Some properties of ornithine-γ-transaminase from neurospora. Biochem. Biophys. Acta 1960, 37, 539–540. [Google Scholar] [CrossRef]
- Janeczko, A.; Biesaga-Kościelniak, J.; Oklestkova, J.; Filek, M.; Dziurka, M.; Szarek-Łukaszewska, G.; Kościelniak, J. Role of 24- epibrassinolide in wheat production: Physiological effects and uptake. J. Agron. Crop Sci. 2010, 196, 311–321. [Google Scholar] [CrossRef]
- Warrier, R.R.; Paul, M.; Vineetha, M.V. Estimation of salicylic acid in Eucalyptus leaves using spectrophotometric methods. Genet. Plant Physiol. 2013, 3, 90–97. [Google Scholar]
- Izaguirre-Mayoral, M.L.; Sinclair, T.R. Soybean genotypic difference in growth, nutrient accumulation and ultrastructure in response to manganese and iron supply in solution culture. Ann. Bot. 2005, 96, 149–158. [Google Scholar] [CrossRef]
- Chatterjee, N.; Nagarajan, S. Evaluation of water binding, seed coat permeability and germination characteristics of wheat seeds equilibrated at different relative humidities. Indian J. Biochem. Biophys. 2006, 43, 233–238. [Google Scholar]
- Kornaś, A.; Filek, M.; Sieprawska, A.; Bednarska-Kozakiewicz, E.; Gawrońska, K.; Miszalski, Z. Foliar application of selenium for protection against the first stages of mycotoxin infection of crop plant leaves. J. Sci. Food Agric. 2019, 99, 482–485. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Manivannan, P.; Wahid, A.; Farooq, M.; Al-Juburi, H.J.; Somasundaram, R.; Panneerselvam, R. Drought stress in plants: A review on morphological characteristics and pigments composition. Int. J. Agric. Biol. 2009, 11, 100–105. [Google Scholar]
- Rady, M.M. Effect of 24-epibrassinolide on growth, yield, antioxidant system and cadmium content of bean (Phaseolus vulgaris L.) plants under salinity and cadmium stress. Sci. Hortic. 2011, 129, 232–237. [Google Scholar] [CrossRef]
- Sharma, P.; Dubey, R.S. Drought induces oxidative stress and enhances the activities of antioxidant enzymes in growing rice seedlings. Plant Growth Regul. 2005, 46, 209–221. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Filek, M.; Łabanowska, M.; Kurdziel, M.; Sieprawska, A. Electron paramagnetic resonance (EPR) spectroscopy 24-epibrasinoide and selenium against zearalenone-stimulation of the oxidative stress in germinating grains of wheat. Toxins 2017, 9, 178. [Google Scholar] [CrossRef] [PubMed]
- Alscher, R.G.; Erturk, N.; Heath, L.S. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot. 2002, 53, 1331–1341. [Google Scholar] [CrossRef]
- Thalmann, M.; Santelia, D. Starch as a determinant of plant fitness under abiotic stress. New Phytol. 2017, 214, 943–951. [Google Scholar] [CrossRef]
- Shakirova, F.M.; Sakhabutdinova, A.R.; Bezrukova, M.V.; Fatkhutdinova, R.A.; Fatkhutdinova, D.R. Changes in the hormonal status of wheat seedlings induced by salicylic acid and salinity. Plant Sci. 2003, 164, 317–322. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, S.; Wang, P.; Hou, J.; Qian, J.; Ao, Y.; Lu, J.; Li, L. Salicylic acid involved in the regulation of nutrient elements uptake and oxidative stress in Vallisneria natans (Lour.) Hara under Pb stress. Chemosphere 2011, 84, 136–142. [Google Scholar] [CrossRef]
- Zhang, F.Q.; Wang, Y.S.; Lou, Z.P.; Dong, J.D. Effect of heavy metal stress on antioxidative enzymes and lipid peroxidation in leaves and roots of two mangrove plant seedlings (Kandelia candel and Bruguiera gymnorrhiza). Chemosphere 2007, 67, 44–50. [Google Scholar] [CrossRef]
| Cultivar | Salicylic Acid [μg·g−1 FW] | Carbohydrates [mg·g−1 DW] | Starch [mg·g−1 DW] | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | 5 mM Mn | 10 mM Mn | Control | 5 mM Mn | 10 mM Mn | Control | 5 mM Mn | 10 mM Mn | ||
| Wheat | Parabola | 0.29 ± 0.09 c | 0.90 ± 0.12 a,* | 0.64 ± 0.11 b | 1.33 ± 0.02 b | 1.44 ± 0.02 a | 1.48 ± 0.03 a | 2.16 ± 0.02 b,* | 2.17 ± 0.02 b | 2.74 ± 0.03 a |
| Raweta | 0.73 ± 0.10 a,* | 0.62 ± 0.08 b | 0.52 ± 0.07 c | 1.68 ± 0.02 c,* | 2.11 ± 0.02 a,* | 2.06 ± 0.04 b,* | 1.95 ± 0.02 c | 2.14 ± 0.02 b | 3.07 ± 0.04 a,* | |
| Oat | Bingo | 0.16 ± 0.03 b | 0.22 ± 0.02 a | 0.17 ± 0.02 b,* | 1.28 ± 0.01 c | 1.27 ± 0.05 b | 1.37 ± 0.02 a | 1.08 ± 0.01 c | 1.36 ± 0.01 b | 1.75 ± 0.01 a |
| Siwek | 0.14 ± 0.02 a | 0.02 ± 0.00 b | 0.02 ± 0.00 b | 1.72 ± 0.02 c,* | 2.40 ± 0.02 a,* | 2.06 ± 0.02 b,* | 1.12 ± 0.01 c,* | 2.00 ± 0.02 b,* | 1.87 ± 0.02 a,* | |
| Barley | CAM/B1 | 0.04 ± 0.00 c | 0.10 ± 0.01 b | 0.34 ± 0.02 a,* | 1.24 ± 0.01 a | 1.28 ± 0.01 a | 1.29 ± 0.01 a | 1.84 ± 0.01 c,* | 1.88 ± 0.01 a | 1.91 ± 0.01 b |
| Maresi | 0.04 ± 0.00 b | 0.19 ± 0.02 a,* | 0.04 ± 0.00 b | 1.36 ± 0.01 b,* | 1.75 ± 0.02 a,* | 1.73 ± 0.03 a,* | 1.64 ± 0.01 c | 2.05 ± 0.02 b,* | 3.03 ± 0.03 a,* | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skórka, M.; Sieprawska, A.; Bednarska-Kozakiewicz, E.; Gawrońska, K.; Kornaś, A.; Telk, A. Genotype-Dependent Differences between Cereals in Response to Manganese Excess in the Environment. Agronomy 2020, 10, 510. https://doi.org/10.3390/agronomy10040510
Skórka M, Sieprawska A, Bednarska-Kozakiewicz E, Gawrońska K, Kornaś A, Telk A. Genotype-Dependent Differences between Cereals in Response to Manganese Excess in the Environment. Agronomy. 2020; 10(4):510. https://doi.org/10.3390/agronomy10040510
Chicago/Turabian StyleSkórka, Magdalena, Apolonia Sieprawska, Elżbieta Bednarska-Kozakiewicz, Katarzyna Gawrońska, Andrzej Kornaś, and Anna Telk. 2020. "Genotype-Dependent Differences between Cereals in Response to Manganese Excess in the Environment" Agronomy 10, no. 4: 510. https://doi.org/10.3390/agronomy10040510
APA StyleSkórka, M., Sieprawska, A., Bednarska-Kozakiewicz, E., Gawrońska, K., Kornaś, A., & Telk, A. (2020). Genotype-Dependent Differences between Cereals in Response to Manganese Excess in the Environment. Agronomy, 10(4), 510. https://doi.org/10.3390/agronomy10040510





