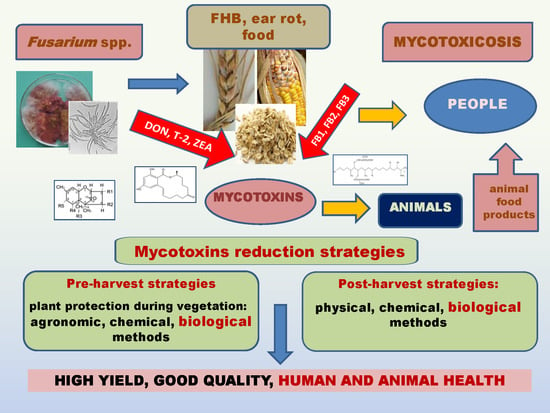
Fusarium Head Blight, Mycotoxins and Strategies for Their Reduction
Abstract
1. Introduction
2. Occurrence and Harmfulness of Fusarium spp. for Cereals
3. Fusarium Mycotoxins and Their Impact on Animal and Human Health
3.1. Trichotecenes
3.2. Fumonisins
3.3. Zearalenone
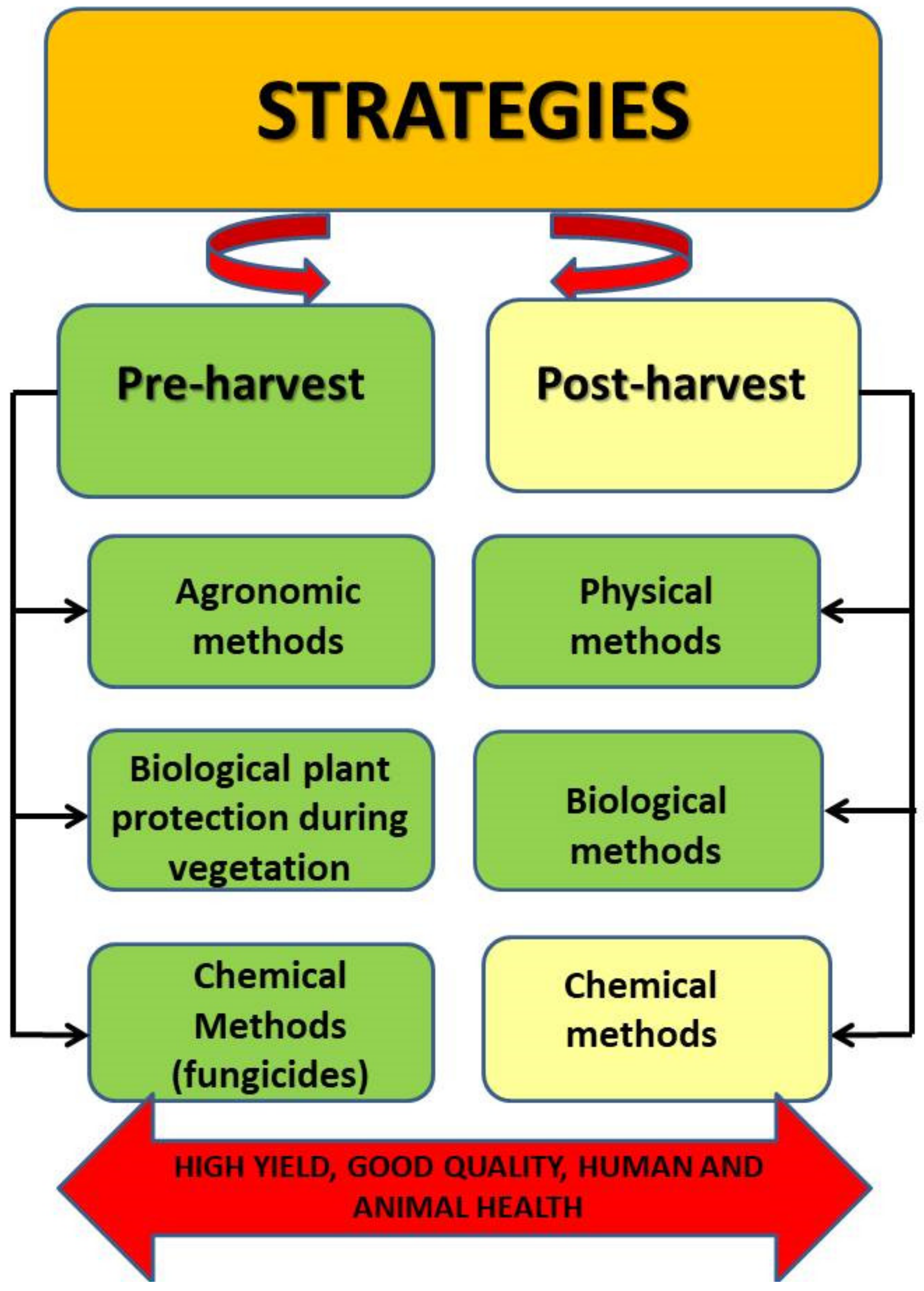
4. Strategies for Reduction of Fusariosis and Mycotoxins
4.1. Pre-Harvest Strategies
4.1.1. Agronomic Methods
• Crop Rotation
• Tillage and Fertilization
• Seeds, Sowing Date and Weather Conditions
4.1.2. Resistance Breeding and Varieties Selection
4.1.3. Biological Plant Protection during Vegetation
4.1.4. Chemical Method
4.2. Post-Harvest Strategies
4.2.1. Physical Methods
4.2.2. Biological Methods
4.2.3. Chemical Methods
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bottallico, A.; Perrone, G. Toxigenic Fusarium species and mycotoxins associated with head blight in small-grain cereals in Europe. J. Plant. Pathol. 2002, 108, 611–624. [Google Scholar] [CrossRef]
- Logrieco, A.; Bottalico, A.; Mule, G.; Morietti, A.; Perrone, G. Epidemiology of toxigenic fungi and their associated mycotoxins for some miediterranean crops. Eur. J. Plant. Pathol. 2003, 109, 557–645. Available online: https://www.researchgate.net/publication/225231648 (accessed on 6 February 2020). [CrossRef]
- Desjardins, A.E. Fusarium-Mycotoxins Chemistry Genetics and Biology; APS Press: St. Paul, MN, USA, 2006. [Google Scholar]
- IARC; WHO. Fumonisin B1. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Some Traditional Herbal Medicines, Some Mycotoxins, Naphthalene and Styrene; IARC Press: Lyon, France, 2002; Volume 82, pp. 301–366. [Google Scholar]
- CAST. Mycotoxins: Risks in Plant, Animal and Human Systems; Report No. 139; Council for Agricultural Science and Technology: Ames, IA, USA, 2003. [Google Scholar]
- Maresca, M. From the gut to the brain: Journey and pathophysiological effects of the food-associated trichothecene mycotoxin deoxynivalenol. Toxins 2013, 5, 784–820. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission regulation (EU) 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union. 2006, 364, 5–24. [Google Scholar]
- EFSA Panel on Contaminants in the Food Chain. Scientific opinion on the risks to human and animal health related to the presence of beauvericin and enniatins in food and feed. EFSA J. 2014, 12, 3802. [Google Scholar] [CrossRef]
- Bryła, M.; Waśkiewicz, A.; Podolska, G.; Szymczyk, K.; Jędrzejczak, R.; Damaziak, K.; Sułek, A. Occurrence of 26 mycotoxins in the grain of cereals cultivated in Poland. Toxins 2016, 8, 160. [Google Scholar] [CrossRef] [PubMed]
- Stanciu, O.; Juan, C.; Miere, D.; Loghin, F.; Mañes, J. Presence of enniatins and beauvericin in Romanian wheat samples: From raw material to products for direct human consumption. Toxins 2017, 9, 189. [Google Scholar] [CrossRef]
- Nganje, W.E.; Kaitibie, S.; Wilson, W.W.; Leistritz, F.L.; Bangsund, D.A. Economic impacts of Fusarium head blight in wheat and barley: 1993–2001. Agribus. Appl. Econ. Rep. 2004, 538, 1–53. [Google Scholar] [CrossRef]
- Salgado, J.D.; Wallhead, M.; Madden, L.V.; Paul, P.A. Grain harvesting strategies to minimize grain quality losses due to Fusarium head blight in wheat. Plant. Dis. 2011, 95, 1448–1457. [Google Scholar] [CrossRef]
- Salgado, J.D.; Madden, L.V.; Paul, P.A. Efficacy and economics of integrating in-field and harvesting strategies to manage Fusarium head blight of wheat. Plant. Dis. 2014, 98, 1407–1421. [Google Scholar] [CrossRef]
- Kamle, M.; Mahato, D.K.; Devi, S.; Lee, K.E.; Kang, S.G.; Kumar, P. Fumonisins: Impact on agriculture, food, and human health and their management strategies. Toxins 2019, 11, 328. [Google Scholar] [CrossRef] [PubMed]
- Piacentini, K.C.; Rocha, L.O.; Savi, G.D.; Carnielli-Queiroz, L.; De Carvalho Fontes, L.; Correa, B. Assessment of toxigenic Fusarium species and their mycotoxins in brewing barley grains. Toxins 2019, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Kikot, G.E.; Moschini, R.; Consolo, V.F.; Rojo, R.; Salerno, G.; Hours, R.A.; Gasoni, L.; Arambarri, A.M.; Alconada, T.M. Occurrence of different species of Fusarium from wheat in relation to disease levels predicted by a weather-based model in Argentina pampas region. Mycopathologia 2011, 171, 139–149. [Google Scholar] [CrossRef] [PubMed]
- McMullen, M.; Bergstrom, G.C.; De Wolf, E.; Dill-Macky, R.; Hershman, D.; Shaner, G.; Van Sanford, D. A unified effort to fight an enemy of wheat and barley: Fusarium head blight. Plant. Dis. 2012, 96, 1712–1728. [Google Scholar] [CrossRef]
- Goliński, P.; Waśkiewicz, A.; Wiśniewska, H.; Kiecana, I.; Mielniczuk, E.; Gromadzka, M.; Kostecki, M.; Bocianowski, J.; Rymaniak, E. Reaction of winter wheat (Triticum aestivum L.) cultivars to infection with Fusarium spp. mycotoxins contamination in grain and chaff. Food Add. Contam. A. 2010, 27, 1015–1024. [Google Scholar] [CrossRef]
- Bernhoft, A.; Torp, M.; Clasen, P.-E.; Løes, A.-K. Influence of agronomic and climatic factors on Fusarium infestation and mycotoxin contamination of cereals in Norway. Food Add. Contam. 2012, 29, 1129–1140. [Google Scholar] [CrossRef]
- Xu, X.; Madden, L.V.; Edwards, S.G. Modeling the effects of environmental conditions on HT2 and T2 toxin accumulation in field oat grain. Phytopathology 2014, 104, 57–66. [Google Scholar] [CrossRef]
- Del Ponte, E.M.; Spolti, P.; Ward, T.J.; Gomes, L.B.; Nicolli, C.P.; Kuhnem, P.R.; Silva, C.N.; Tessmann, D.J. Regional and field-specific factors affect the composition of Fusarium head blight pathogens in subtropical no-till wheat agroecosystem of Brazil. Phytopathology 2015, 105, 246–254. [Google Scholar] [CrossRef]
- Hofgaard, I.S.; Aamot, H.U.; Torp, T.; Jestoi, M.; Lattanzio, V.M.T.; Klemsdal, S.S.; Waalwijk, C.; Van der Lee, T.; Brodal, G. Associations between Fusarium species and mycotoxins in oats and spring wheat from farmers’ fields in Norway over a six-year period. World Mycotoxin J. 2016, 9, 365–378. [Google Scholar] [CrossRef]
- Xu, X.M.; Nicholson, P.; Thomsett, M.A.; Simpson, D.; Cooke, B.M.; Doohan, F.M.; Edwards, S.G. Relationship between the fungal complex causing Fusarium head blight of wheat and environmental conditions. Phytopathology 2008, 98, 69–78. [Google Scholar] [CrossRef]
- Popovski, S.; Celar, F.A. The impact of environmental factors on the infection of cereals with Fusarium species and mycotoxin production-A review. Acta Agric. Slov. 2013, 101, 105–116. [Google Scholar] [CrossRef]
- Covarelli, L.; Beccari, G.; Prodi, A.; Generotti, S.; Etruschi, F.; Juan, C.; Ferrer, E.; Mañes, J. Fusarium species, chemotype characterisation and trichothecene contamination of durum and soft wheat in anarea of central Italy. J. Sci. Food Agric. 2015, 95, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Backhouse, D.; Burgess, L.W.; Summerell, B.A. Biogeography of Fusarium. In Fusarium Nelson Memorial Symposium; Summerell, B.A., Leslie, J.F., Backhouse, D., Bryden, W.L., Burgess, L.W., Eds.; APS Press: St Paul, MN, USA, 2012; pp. 122–137. [Google Scholar]
- Tekauz, A.; McCallum, B.; Ames, N.; Mitchell Fetch, J. Fusarium head blight of oat—current status in western Canada. Can. J. Plant. Pathol. 2004, 26, 473–479. [Google Scholar] [CrossRef]
- Chełkowski, J.; Gromadzka, K.; Stępień, Ł.; Lenc, L.; Kostecki, M.; Berthiller, F. Fusarium species, zearalenone and deoxynivalenol content in preharvest scabby wheat heads from Poland. World Mycotox. J. 2012, 5, 133–141. [Google Scholar] [CrossRef]
- Wiśniewska, H.; Stępień, L.; Waśkiewicz, A.; Beszterda, M.; Góral, T.; Belter, J. Toxigenic Fusarium species infecting wheat heads in Poland. Cent. Eur. J. Biol. 2014, 9, 163–172. [Google Scholar] [CrossRef]
- Hellin, P.; Dedeurwaerder, G.; Duvivier, M.; Scauflaire, J.; Huybrechts, B.; Callebaut, A.; Munaut, F.; Legrève, A. Relationship between Fusarium spp. diversity and mycotoxin contents of mature grains in southern Belgium. Food Add. Contam. Part. A 2016, 33, 1228–1240. [Google Scholar] [CrossRef] [PubMed]
- Hietaniemi, V.; Rämö, S.; Yli-Mattila, T.; Jestoi, M.; Peltonen, S.; Kartio, M.; Sieviläinen, E.; Koivisto, T.; Parikka, P. Updated survey of Fusarium species and toxins in Finnish cereal grains. Food Add. Contam. Part. A 2016, 33, 831–848. [Google Scholar] [CrossRef]
- Pascale, M.; Visconti, A.; Chelkowski, J. Ear rot susceptibility and mycotoxin contamination of maize hybrids inoculated with Fusarium species under field conditions. Eur. J. Plant. Pathol. 2002, 108, 645–651. [Google Scholar] [CrossRef]
- Stenglein, S.A.; Dinolfo, M.I.; Bongiorno, F.; Moreno, M.V. Response of wheat (Triticum spp.) and barley (Hordeum vulgare) to Fusarium poae. Agrociencia 2012, 46, 299–306. [Google Scholar]
- Stenglein, S.A.; Dinolfo, M.I.; Barros, G.; Bongiorno, F.; Chulze, S.N.; Moreno, M.V. Fusarium poae pathogenicity and mycotoxin accumulation on selected wheat and barley genotypes at a single location in Argentina. Plant. Dis. 2014, 98, 1733–1738. [Google Scholar] [CrossRef]
- Yli-Mattila, T. Ecology and evolution of toxigenic Fusarium species in cereals in Northern Europe and Asia. J. Plant. Pathol. 2010, 92, 7–18. Available online: https://www.jstor.org/stable/41998764 (accessed on 4 February 2020).
- Stenglein, S.A. Fusarium poae: A pathogen that needs more attention. J. Plant. Pathol. 2009, 91, 25–36. [Google Scholar] [CrossRef]
- Kiecana, I.; Cegiełko, M.; Mielniczuk, E.; Perkowski, J. The occurrence of Fusarium poae (Peck) Wollenw. on oat (Avena sativa L.) panicles and its harmfulness. Acta Agrobot. 2002, 65, 169–178. [Google Scholar] [CrossRef][Green Version]
- Parry, D.W.; Jenkinson, P.; McLeoad, L. Fusarium ear blight (scab) in small grain cereals—a review. Plant. Pathol. 1995, 44, 207–238. [Google Scholar] [CrossRef]
- Tekauz, A.; Mitchell Fetch, J.W.; Rossnagel, B.G.; Savard, M.E. Progress in assessing the impact of Fusarium head blight on oat in western Canada and screening of Avena germplasm for resistance. Cer. Res. Comm. 2008, 36, 49–56. [Google Scholar] [CrossRef]
- Tekle, S.; Skinnes, H.; Bjørnstad, A. The germination problem of oat seed lots affected by Fusarium head blight. Eur. J. Plant. Pathol. 2013, 135, 147–158. [Google Scholar] [CrossRef]
- Köhl, J.; de Haas, B.H.; Kastelein, P.; Burgers, S.L.G.E.; Waalwijk, C. Population dynamics of Fusarium spp. and Microdochium nivale in crops and crop residues of winter wheat. Phytopathology 2007, 97, 971–978. [Google Scholar] [CrossRef]
- Shaner, G. Epidemiology of Fusarium head blight of small grain cereals in North America. In Fusarium Head Blight of Wheat and Barley; Kurt, J.L., Bushnell, W.R., Eds.; APS Press: St Paul, MN, USA, 2003; pp. 84–119. [Google Scholar]
- Postic, J.; Cosic, J.; Vrandecic, K.; Jurkovic, D.; Saleh, A.A.; Leslie, J.F. Diversity of Fusarium species isolated from weeds and plant debris in croatia. J. Phytopathol. 2012, 160, 76–81. [Google Scholar] [CrossRef]
- Osborne, L.E.; Stein, J.M. Epidemiology of Fusarium head blight on small-grain cereals. Int. J. Food Microbiol. 2007, 119, 103–108. [Google Scholar] [CrossRef]
- Hjelkrem, A.-G.R.; Torp, T.; Brodal, G.; Aamot, H.U.; Strand, E.; Nordskog, B.; Dill-Macky, R.; Edwards, S.G.; Hofgaard, I.S. DON content in oat grains in Norway related to weather conditions at different growth stages. Eur. J. Plant. Pathol. 2017, 148, 577–594. [Google Scholar] [CrossRef]
- Kiecana, I.; Mielniczuk, E.; Kaczmarek, Z.; Kostecki, M.; Goliński, P. Scab response and moniliformin accumulation in kernels of oat genotypes inoculated with Fusarium avenaceum in Poland. Eur. J. Plant. Pathol. 2002, 108, 245–251. [Google Scholar] [CrossRef]
- Packa, D.; Kulik, T.; Hościk, M. Scanning electron microscopy of Fusarium infected kernels of ancient wheat species. Phytopathologia 2012, 63, 7–19. [Google Scholar]
- Packa, D.; Załuski, D.; Graban, Ł.; Lajszner, W.; Hościk, M. Reakcja diploidalnych, tetraploidalnych I heksaploidalnych pszenic na inokulację Fusarium culmorum (W.G.Smith) Sacc. Polish, J. Agronomy 2013, 12, 38–48. [Google Scholar]
- Eggert, K.; Zörb, C.; Mühling, K.H.; Pawelzik, E. Proteome analysis of Fusarium infection in emmer grains (Triticum dicoccum). Plant. Pathol. 2011, 60, 918–928. [Google Scholar] [CrossRef]
- Pearse, P.G.; Holzgang, G.; Weitzel, C.N.; Fernandez, M.R. Fusarium head blight in barley and oat in Saskatchewan in 2006. Can. Plant. Dis. Surv. 2007, 87, 61–62. [Google Scholar]
- Tamburic-Ilincic, L. Fusarium species and mycotoxins associated with oat in southwestern Ontario, Canada. Can. J. Plant. Sci. 2010, 90, 211–216. [Google Scholar] [CrossRef]
- Uhlig, S.; Jestoi, M.; Parikka, P. Fusarium avenaceum-the North European situation. Int. J. Food Microbiol. 2007, 119, 17–24. [Google Scholar] [CrossRef]
- Infantino, A.; Santori, A.; Shah, D.A. Community structure of the Fusarium complex on wheat seed in Italy. Eur. J. Plant. Pathol. 2012, 132, 499–510. [Google Scholar] [CrossRef]
- Gräfenhan, T.; Patrick, S.K.; Roscoe, M.; Trelka, R.; Gaba, D.; Chan, J.M.; McKendry, T.; Clear, R.M.; Tittlemier, S.A. Fusarium damage in cereal grains from Western Canada. 1. Phylogenetic analysis of moniliformin-producing fusarium species and their natural occurrence in mycotoxin-contaminated wheat, oats, and rye. J. Agric. Food Chem. 2013, 61, 5425–5437. [Google Scholar] [CrossRef]
- Czaban, J.; Wróblewska, B.; Sułek, A.; Mikos, M.; Boguszewska, E.; Podolska, G.; Nieróbca, A. Colonisation of winter wheat grain by Fusarium spp. and mycotoxin content as dependent on a wheat variety, crop rotation, a crop management system and weather conditions. Food Addit Contam. Part. A Chem. Anal. Control. Expo. Risk. Assess. 2015, 32, 874–910. [Google Scholar] [CrossRef]
- Ferrigo, D.; Raiola, A.; Causin, R. Fusarium toxins in cereals: Occurrence, legislation, factors promoting the appearance and their management. Molecules 2016, 21, 627. [Google Scholar] [CrossRef] [PubMed]
- Linkmeyer, A.; Hofer, K.; Rychlik, M.; Herz, M.; Hausladen, H.; Hückelhoven, R.; Hess, M. Influence of inoculum and climatic factors on the severity of Fusarium head blight in German spring and winter barley. Food Add. Contam. Part. A 2016, 33, 489–499. [Google Scholar] [CrossRef]
- Barreto, D.; Carmona, M.; Ferrazini, M.; Zanelli, M.; Perez, B.A. Occurrence and pathogenicity of Fusarium poae in barley in Argentina. Cer. Res. Comm. 2004, 32, 53–60. [Google Scholar] [CrossRef]
- Touati-Hattab, S.; Barreau, C.; Verdal-Bonnin, M.-N.; Chereau, S.; Forget, F.R.; Hadjout, S.; Mekliche, L.; Bouznad, Z. Pathogenicity and trichothecenes production of Fusarium culmorum strains causing head blight on wheat and evaluation of resistance of the varieties cultivated in Algeria. Eur. J. Plant. Pathol. 2016, 145, 797–814. [Google Scholar] [CrossRef]
- Kulik, T.; Jestoi, M. Quantification of Fusarium poae DNA and associated mycotoxins in symptomatically contaminated wheat. Int. J. Food Microbiol. 2009, 130, 233–237. [Google Scholar] [CrossRef]
- Edwards, S.G. Fusarium mycotoxin content of UK organic and conventional oats. Food Add. Cont. 2009, 26, 1063–1069. [Google Scholar] [CrossRef]
- Yli-Mattila, T. Detection of trichothecene-producing Fusarium species in cereals in northen Europe and Asia. Agronomy Res. 2011, 9, 521–526. [Google Scholar]
- Yli-Mattila, T.; Rämö, S.; Hietaniemi, V.; Hussien, T.; Carlobos-Lopez, A.L.; Cumagun, C.J.R. Molecular quantification and genetic diversity of toxigenic Fusarium species in Northern Europe as compared to those in Southern Europe. Microorganisms 2013, 1, 162–174. [Google Scholar] [CrossRef]
- Lenc, L. Fusarium head blight (FHB) and Fusarium populations in grain of winter wheat grown in differ end cultivation systems. J. Plant. Prot. Res. 2015, 55, 94–109. [Google Scholar] [CrossRef]
- Munkvold, G.P. Epidemiology of Fusarium diseases and their mycotoxins in maize ears. Eur. J. Plant. Pathol. 2003, 109, 705–713. [Google Scholar] [CrossRef]
- Parsons, M.W.; Munkvold, G.P. Effects of planting date and environmental factors on Fusarium ear rot symptoms and fumonisin B1 accumulation in maize grown in six North American locations. Plant. Pathol. 2012, 61, 1130–1142. [Google Scholar] [CrossRef]
- Pestka, J.J. Deoxynivalenol: Mechanisms of action, human exposure, and toxicological relevance. Arch. Toxicol 2010, 84, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.; Clear, R.M.; Ward, T.J.; Gaba, D.; Tekauz, A.; Turkington, T.K.; Woods, S.M.; Nowicki, T.; O’Donnell, K. Relative aggressiveness and production of 3-or 15-acetyl deoxynivalenol and deoxynivalenol by Fusarium graminearum in spring wheat. Can. J. Plant. Pathol. 2010, 32, 146–152. [Google Scholar] [CrossRef]
- Puri, K.D.; Zhong, S. The 3ADON population of Fusarium graminearum foundin North Dakota is more aggressive and produces a higher level of DON than the prevalent 15ADON population in spring wheat. Phytopathology 2010, 100, 1007–1014. [Google Scholar] [CrossRef]
- Pasquali, M.; Beyer, M.; Logrieco, A.; Audenaert, K.; Balmas, V.; Basler, R.; Boutigny, A.-L.; Chrpová, J.; Czembor, E.; Gagkaeva, T.; et al. A European Database Fusarium graminearum and F. culmorum Trichothecene Genotypes. Front. Microbiol. 2016, 7, 406. [Google Scholar] [CrossRef]
- Carter, J.P.; Rezanoor, H.N.; Holden, D.; Desjardins, A.E.; Plattner, R.D.; Nicholson, P. Variatonin pathogenicity associated with the genetic diversity of Fusarium graminearum. Eur. J. Plant. Pathol. 2002, 108, 573–583. [Google Scholar] [CrossRef]
- Paul, P.A.; Lipps, P.E.; Madden, L.V. Relationship between visual estimates of Fusarium head blight intensity and deoxynivalenol accumulation in harvested wheat grain: A Meta-Analysis. Phytopathology 2005, 95, 1225–1236. [Google Scholar] [CrossRef]
- Baturo-Cieśniewska, A.; Suchorzyńska, M. Verification of the effectiveness of SCAR (sequence characterized amplified region) primers for the identification of Polish strains of Fusarium culmorum and their potential ability to produce B-trichothecenes and zearalenone. Int. J. Food Microbiol. 2011, 148, 168–176. [Google Scholar] [CrossRef]
- Hestbjerg, H.; Nielse, N.K.F.; Thrane, U.; Elmoholt, S. Production of trichothecenes and other secondary metabolites by Fusarium culmorum and Fusarium equiseti on common laboratory media and a soil organic matter agar an ecological interpretatio. J. Agric, Food Chem. 2002, 50, 7593–7599. [Google Scholar] [CrossRef]
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual; Blackwell Publishing: Ames, IA, USA, 2006. [Google Scholar]
- Wang, H.; Hwang, S.F.; Eudes, F.; Chang, K.F.; Howard, R.J.; Turnbul, G.D. Trichothecenes and aggressiveness of Fusarium graminearum causing seedling blight and root rot in cereals. Plant. Pathol. 2006, 55, 224–230. [Google Scholar] [CrossRef]
- Vogelgsang, S.; Sullyok, M.; Hecker, A.; Jenny, E.; Krska, R.; Schuhmacher, R.; Forrer, H.-R. Toxigenicity and pathogenicity of Fusarium poae and Fusarium avenaceum on wheat. Eur. J. Plant. Pathol. 2008, 122, 265–276. [Google Scholar] [CrossRef]
- Beccari, G.; Covarelli, L.; Nicholson, P. Infection process and soft wheat response to root rot and crown rot caused by Fusarium culmorum. Plant. Pathol. 2011, 60, 671–684. [Google Scholar] [CrossRef]
- Wanjiru, W.M.; Zhensheng, K.; Buchenauer, H. Importance of cell wall degrading enzymes produced by Fusarium graminearum during infection of wheat heads. Eur. J. Plant. Pathol. 2002, 108, 803–810. [Google Scholar] [CrossRef]
- Jaroszuk-Ściseł, J.; Kurek, E. Hydrolysis of fungal and plant cell walls by enzymatic complexes from cultures of Fusarium isolates with different aggressiveness to rye (Secale sereale). Arch. Microbiol. 2012, 194, 653–665. [Google Scholar] [CrossRef]
- Hussein, H.S.; Brasel, J.M. Toxicity, metabolism, and impact of mycotoxins on humans and animals. Toxicology 2001, 167, 101–134. [Google Scholar] [CrossRef]
- Denning, D.W.; Driscoll, B.R.; Hogaboam, C.M. The link between fungi and severe astma: A sumary of the evidence. Eur. Respir. J. 2006, 27, 615–626. [Google Scholar] [CrossRef]
- Nabrdalik, M.; Latała, A. Fungi growth in buildings. Rocz. Panstw. Zakl. Hig. 2003, 54, 119–127. [Google Scholar]
- Harris, L.J.; Desjardins, A.E.; Plattner, R.D.; Nicholson, P.; Butler, G.; Young, J.C.; Weston, G.; Proctor, R.H.; Hohn, T.M. Possible role of trichothecene mycotoxins in virulence of Fusarium graminearum on maize. Plant. Dis. 1999, 83, 954–960. [Google Scholar] [CrossRef]
- Desjardins, A.E. Fusarium mycotoxins: Chemistry, genetics and biology. Plant. Pathol. 2007, 56, 337. [Google Scholar]
- Yazar, S.; Omurtag, G.Z. Fumonisins, Trichothecenes and Zearalenone in Cereals Review. Int. J. Mol. Sci. 2008, 9, 2062–2090. [Google Scholar] [CrossRef]
- Foroud, N.A.; Baines, D.; Gagkaeva, T.Y.; Thakor, N.; Badea, A.; Steiner, B.; Bürstmayr, M.; Bürstmayr, H. Trichothecenes in cereal grains-An update. Toxins 2019, 11, 634. [Google Scholar] [CrossRef]
- Suchorzyńska, M.; Misiewicz, A. Mycotoxigenic phythopathogenic fungi of Fusarium genus and their identification by PCR techniques. Post. Microbiol. 2009, 48, 221–230. [Google Scholar]
- Kimura, M.; Tokai, T.; Takahashi-Ando, N.; Ohsato, S.; Fujimura, M. Molecular and Genetic Studies of Fusarium Trichothecene Biosynthesis: Pathways, Genes, and Evolution. Biosci. Biotechnol. Biochem. 2007, 71, 2105–2123. [Google Scholar] [CrossRef] [PubMed]
- Thrane, U.; Adler, A.; Clasen, P.-E.; Galvano, F.; Langseth, W.; Lew, H.; Logrieco, A.; Nielsen, K.F.; Ritieni, A. Diversity in metabolite production by Fusarium langsethiae, Fusarium poae, and Fusarium sporotrichioides. Inter. J. Food Microbiol. 2004, 95, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Kosiak, E.B.; Holst-Jensen, A.; Runtberget, T.; Gonzales Jaen, M.T.; Torp, M. Morphological, chemical and molecular differentiation of Fusarium equiseti isolated from Norwegian cereals. Int. J. Food Microbiol. 2005, 99, 195–206. [Google Scholar] [CrossRef]
- Adhikari, M.; Negi, B.; Kaushik, N.; Adhikari, A.; Al-Khedhairy, A.A.; Kaushik, N.; Choi, E. T-2 mycotoxin: Toxicological effects and decontamination strategies. Oncotarget 2017, 8, 33933–33952. Available online: http://www.oncotarget.com/index.php?journal=oncotarget&page=article&op=view&path%5B%5D=15422&path%5B%5D=49268 (accessed on 20 February 2020). [CrossRef]
- Pleadin, J.; Vahčić, N.; Perši, N.; Ševelj, D.; Markov, K.; Frece, J. Fusarium mycotoxins’ occurrence in cereals harvested from Croatian fields. Food Control. 2013, 32, 49–54. [Google Scholar] [CrossRef]
- Haikka, H.; Manninen, O.; Hautsalo, J.; Pietilä, L.; Jalli, M.; Veteläinen, M. Genome-wide association Study and genomic prediction for Fusarium graminearum resistance traits in Nordic Oat (Avena sativa L.). Agronomy 2020, 10, 174. [Google Scholar] [CrossRef]
- Milani, J.M. Ecological conditions affecting mycotoxin production in cereals: A review. Veterinarni Medicina 2013, 58, 405–411. Available online: http://vri.cz/docs/vetmed/58-8-405.pdf (accessed on 7 February 2020). [CrossRef]
- Spanic, V.; Marcek, T.; Abicic, I.; Sarkanj, B. Effects of Fusarium head blight on wheat grain and malt infected by Fusarium culmorum. Toxins 2018, 10, 17. [Google Scholar] [CrossRef]
- Scudamore, K.; Baillie, H.; Patel, S.; Edwards, S. Occurrence and fate of Fusarium mycotoxins during commercial processing of oats in the UK. Food Addit. Contam. 2007, 24, 1374–1385. [Google Scholar] [CrossRef] [PubMed]
- Zielonka, Ł.; Gajęcki, M.; Obremski, K.; Zwierzchowski, W. Influence of low doses of deoxynivalenol applied per os on chosen indexes of immune response in swine. Pol. J. Vet. Sci. 2003, 6, 74–77. [Google Scholar] [PubMed]
- Goyarts, T.; Dänicke, S.; Brüssow, K.P.; Valenta, H.; Uberschär, K.H.; Tiemann, U. On the transfer of the Fusarium toxins deoxynivalenol (DON) and zearalenone (ZON) from sows to their fetuses during days 35-70 of gestation. Toxicol Lett. 2007, 171, 38–49. [Google Scholar] [CrossRef] [PubMed]
- WHO. Department of Food Safety and Zoonoses REF, Food Safety Digest. Fumonisins. 2018. Available online: https://www.who.int/foodsafety/FSDigest_Fumonisins_EN.pdf (accessed on 5 February 2020).
- Stockmann-Juvala, H.; Savolainen, K. A review of the toxic effects and mechanisms of action of fumonisin B1. Hum. Exp. Toxicol. 2008, 27, 799–809. [Google Scholar] [CrossRef]
- Gajęcka, M.; Rybarczyk, L.; Zwierzchowski, W.; Jakimiuk, E.; Zielonk, Ł.; Obremski, K.; Gajęcki, M. The effect of experimental, long-term exposure to low-dose zearalenone mycotoxicosis on the histological condition of ovaries in sexually immature gilts. Theriogenology 2011, 75, 1085–1094. [Google Scholar] [CrossRef]
- Damiani, T.; Righetti, L.; Suman, M.; Galaverna, G.; Dall’Asta, C. Analytical issue related to fumonisins: A matter of sample comminution? Food Control. 2019, 95, 1–5. [Google Scholar] [CrossRef]
- Zentai, A.; Szeitzné-Szabó, M.; Mihucz, G.; Szeli, N.; Szabó, A.; Kovács, M. Occurrence and Risk Assessment of Fumonisin B1 and B2 Mycotoxins in Maize-Based Food Products in Hungary. Toxins 2019, 11, 709. [Google Scholar] [CrossRef]
- Bojja, R.S.; Cerny, R.L.; Proctor, R.H.; Du, L. Determining the biosynthetic sequence in the early steps of the fumonisin pathway by use of three gene-disruption mutants of Fusarium verticillioides. J. Agric. Food Chem. 2004, 52, 2855–2860. [Google Scholar] [CrossRef]
- Gelineau-van Waes, J.; Starr, L.; Maddox, J.; Aleman, F.; Voss, K.A.; Wilberding, J.; Riley, R.T. Maternal fumonisin exposure and risk for neural tube defects: Disruption of sphingolipid metabolism and folate transport in an in vivo mouse model. Birth Defects Res. A Clin Mol Teratol. 2005, 73, 487–497. [Google Scholar] [CrossRef]
- Hendricks, K. Fumonisins and neural tube defects in South Texas. Epidemiology 1999, 10, 198–200. [Google Scholar] [CrossRef]
- Venter, P.A.; Christianson, A.L.; Hutamo, C.M.; Makhura, M.P.; Gericke, G.S. Congenital anomalies in rural black South African neonates–a silent epidemic? S. Afr. Med. J. 1995, 85, 15–20. [Google Scholar] [PubMed]
- Marasas, W.F.O.; Riley, R.T.; Hendricks, K.A.; Stevens, V.L.; Sadler, T.W.; Gelineau-van Waes, J.; Missmer, S.A.; Cabrera, J.; Torres, O.; Gelderblom, W.C.; et al. Fumonisins disrupt sphingolipid metabolism, folate transport, and neural tube development in embryo culture and in vivo: A potential risk factor for human neural tube defects among populations consuming fumonisin-contaminated maize. J. Nutr. 2004, 134, 711–716. [Google Scholar] [CrossRef]
- Gelderblom, W.C.; Marasas, W.F.; Lebepe-Mazu, S.; Swanevelder, S.; Vessey, C.J.; Hall Pde, L. Interaction of fumonisin B1 and aflatoxin B1 in a short-term carcinogenesis model in rat liver. Toxicology 2002, 171, 161–173. [Google Scholar] [CrossRef]
- WHO. Safety Evaluation of Certain Mycotoxins in Food: Prepared by the Fiftysixth Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA); World Health Organization: Geneva, Switzerland, 2001. [Google Scholar]
- WHO. Evaluation of Certain Mycotoxins in Food: Fifty-sixth Report of the Joint FAO/WHO Expert Committee on Food Additives; World Health Organization: Geneva, Switzerland, 2002. [Google Scholar]
- Bzducha-Wróbel, A.; Gniewosz, M.; Chlebowska-Śmigiel, A. In vitro and in vivo mycotoxin binding through the bacteria of Lactobacillus and Bifidobacterium species. Med. Weter. 2015, 71, 748–775. [Google Scholar]
- Zhang, G.L.; Feng, Y.L.; Song, J.L.; Zhou, X.S. Zearalenone: A mycotoxin with different toxic effect in domestic and laboratory animals. Front. Genet. 2018, 9, 667. [Google Scholar] [CrossRef]
- Perkowski, J.; Kiecana, I.; Schumacher, V.; Müller, H.-M.; Chełkowski, J.; Goliński, P. Head infection and accumulation of Fusarium toxins in kernels of 12 barley genotypes inoculated with Fusarium graminearum isolates of two chemotypes. Eur. J. Plant. Pathol. 1997, 103, 85–90. [Google Scholar] [CrossRef]
- Zinedine, A.; Soriano, J.M.; Moltó, J.C.; Mañes, J. Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: An oestrogenic mycotoxin. Food Chem. Toxicol. 2007, 45, 1–18. [Google Scholar] [CrossRef]
- Mielniczuk, E.; Kiecana, I.; Cegiełko, M.; Pastucha, A.; Perkowsk, I.J. Harmfulness of Fusarium crookwellense Burgess, Nelson & Toussoun to panicles of selected genotypes of oat (Avena sativa L.) concerning the content of mycotoxins in grain. Prog. Plant. Prot./Post. Ochr. Rośl. 2016, 56, 424–429. [Google Scholar]
- Gaffoor, I.; Trail, F. Characterization of two polyketide synthase genes involved in zearalenone biosynthesis in Gibberella zeae. Appl. Env. Microbiol. 2006, 72, 1793–1799. [Google Scholar] [CrossRef]
- Fink-Gremmels, J.; Malekinejad, H. Clinical effects and biochemical mechanisms associated with exposure to the mycoestrogen zearalenone. Anim. Feed Sci. Technol. 2007, 137, 326–341. [Google Scholar] [CrossRef]
- Rai, A.; Das, M.; Tripathi, A. Occurrence and toxicity of a Fusarium mycotoxin, zearalenone. Crit. Rev. Food Sci. Nutr. 2019, 59, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Lichti, K.; Staudinger, L.J. The mycoestrogen zearalenone induces CYP3A through activation of the pregnane X receptor. Toxicol. Sci. 2006, 91, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Massart, F.; Meucci, V.; Saggese, G.; Soldani, G. High growth rate of girls with precocious puberty exposed to estrogenic mycotoxins. J. Pediatr. 2008, 152, 690–695. [Google Scholar] [CrossRef] [PubMed]
- Vančo, B.; ŠLikova, S.; Šudynová, V. Influence of localities and winter wheat cultivars on deoxynivalenol accumulation and disease damage by Fusarium culmorum. Biol. Bratisl. Sect. Bot. 2007, 62, 62–66. [Google Scholar] [CrossRef]
- Schaafsma, A.W.; Tamburic-Ilinic, L.; Miller, J.D.; Hooker, D.C. Agronomic considerations for reducing deoxynivalenol in wheat grain. Can. J. Plant. Path. 2001, 23, 279–285. [Google Scholar] [CrossRef]
- Bryła, M.; Ksieniewicz-Wożniak, E.; Yoshinari, T.; Waśkiewicz, A.; Szymczyk, K. Contamination of Wheat Cultivated in Various Regions of Poland during 2017 and 2018 Agricultural Seasons with Selected Trichothecenes and Their Modified Forms. Toxins 2019, 11, 88. [Google Scholar] [CrossRef]
- Arseniuk, E.; Foremska, E.; Góral, T.; Chełkowski, J. Fusarium head blight reactions and acumulation odf deoxynivalenol (DON) and some of its derivatives in kernels of wheat, triticale and rye. J. Phytopathol. 1999, 147, 577–590. [Google Scholar] [CrossRef]
- Mendes, G.D.R.L.; Reis, T.A.D.; Corrêa, B.; Badiale-Furlong, E. Mycobiota and occurrence of Fumonisin B1 in wheat harvested in Southern Brazil. Ciênc. Rural. 2015, 45, 1050–1057. [Google Scholar] [CrossRef]
- Stanković, S.; Lević, J.; Ivanović, D.; Krnjaja, V.; Stanković, G.; Tančić, S. Fumonisin B1 and its co-occurrence with other fusariotoxins in naturally-contaminated wheat grain. Food Control. 2012, 23, 384–388. [Google Scholar] [CrossRef]
- Busman, M.; Desjardins, A.; Proctor, R. Analysis of fumonisin contamination and the presence of Fusarium in wheat with kernel black point disease in the United States. Food Addit. Contam. Part. A 2012, 29, 1092–1100. [Google Scholar] [CrossRef]
- Mankevičienė, A.; Butkutė, B.; Dabkevičius, Z.; Supronienė, S. Fusarium mycotoxins in Lithuanian cereals from the 2004–2005 harvests. Ann. Agric. Environ. Med. 2007, 14, 103–107. Available online: http://agro.icm.edu.pl/agro/element/bwmeta1.element.agro-article-c5c595fe-fbb8-43ca-aeaf-d59a82280776/c/23.pdf (accessed on 22 February 2020).
- Müller, H.M.; Reimann, J.; Schumacher, U.; Schwadorf, K. Natural occurrence of Fusarium toxins in oats harvested during five years in an area of Southwest Germany. Food Addit. Contam. Part. A 1998, 15, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Kuzdraliński, A.; Solarska, E.; Mazurkiewicz, J. Mycotoxin content of organic anal conventional oats farm southeastern Poland. Food Control. 2013, 33, 68–72. [Google Scholar] [CrossRef]
- Gromadzka, K.; Górna, K.; Chełkowski, J.; Waśkiewicz, A. Mycotoxins and related Fusarium species in preharvest maize ear rot in Poland. Plant. Soil Environ. 2017, 62, 348–354. [Google Scholar] [CrossRef]
- Schollenberger, M.; Müller, H.M.; Rüfle, M.; Suchy, S.; Planck, S.; Drochner, W. Survey of Fusarium toxins in foodstuffs of plant origin marketed in Germany. Int. J. Food Microbiol. 2005, 97, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Preis, R.; Vargas, E. A method for determining fumonisin B1 in corn using immunoaffinity column clean-up and thin layer chromatography/densitometry. Food Addit. Contam. 2000, 17, 463–468. [Google Scholar] [CrossRef]
- Kpodo, K.; Thrane, U.; Hald, B. Fusaria and fumonisins in maize from Ghana and their co-occurrence with aflatoxins. Int. J. Food Microbiol. 2000, 61, 147–157. [Google Scholar] [CrossRef]
- Tansakul, N.; Jala, P.; Laopiem, S.; Tangmunkhong, P.; Limsuwan, S. Co-occurrence of five Fusarium toxins in corn-dried distiller’s grains with solubles in Thailand and comparison of ELISA and LC-MS/MS for fumonisin analysis. Mycotoxin Res. 2013, 29, 255–260. [Google Scholar] [CrossRef]
- Wagacha, J.M.; Muthomi, J.W. Mycotoxin problem in Africa: Current status, implications to food safety and health and possible management strategies. Int. J. Food Microbiol. 2008, 124, 1–12. [Google Scholar] [CrossRef]
- Blandino, M.; Reyneri, A.; Colombari, G.; Pietri, A. Comparison of integrated field programmes for the reduction of fumonisin contamination in maize kernels. Field Crop. Res. 2009, 111, 284–289. [Google Scholar] [CrossRef]
- Zain, M.E. Impact of mycotoxins on human and animals. J. Saudi Chem. Soc. 2011, 15, 129–144. [Google Scholar] [CrossRef]
- Kangéthe, E.K.; Korhonen, H.; Marimba, K.A.; Nduhiu, G.; Mungatu, J.K.; Okoth, S.A.; Joutsjoki, V.; Wamae, L.W.; Shalo, P. Management and mitigation of health risks associated with the occurrence of mycotoxins along the maize value chain in two counties of Kenya. Food Qual. Saf. 2017, 1, 268–274. [Google Scholar] [CrossRef]
- Janssen, E.M.; Mourits, M.C.M.; Fels-Klerx, H.J.; Oude Lansink, A.G.J.M. Pre-harvest measures against Fusarium spp. Infection and related mycotoxins implemented by Dutch wheat farmers. Crop. Prot. 2019, 122, 9–18. [Google Scholar] [CrossRef]
- Weber, R.; Kita, W. The effects of tillage systems and preceding crop types on the frequency of the incidence of Fusarium culmorum and Fusarium avenaceum on culm bases of some winter wheat cultivars. Acta Agrobot. 2010, 63, 121–128. [Google Scholar] [CrossRef]
- Qiu, J.; Dong, F.; Yu, M.; Xu, J.; Shi, J. Effect of preceding crop on Fusarium species and mycotoxin contamination of wheat grains. J. Sci. Food Agric. 2016, 96, 4536–4541. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.R.; Conner, R.L. Root and crown rot of wheat. Prairie Soils Crops J. 2011, 4, 151–157. Available online: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.690.1240&rep=rep1&type=pdf (accessed on 9 February 2020).
- Dill-Macky, R.; Jones, R.K. The effect of previous crop residues and tillage on Fusarium head blight of wheat. Plant. Disease 2000, 84, 71–76. [Google Scholar] [CrossRef]
- Horoszkiewicz-Janka, J.; Jajor, E.; Korbas, M. Usage of biopreparations as seed dressings in legume cultivation. J. Res. Appl. Agric. Eng. 2012, 57, 162–166. Available online: http://yadda.icm.edu.pl/yadda/element/bwmeta1.element.baztech-article-BAR8-0019-0088/c/httpwww_pimr_poznan_plbiul2012330hj.pdf (accessed on 26 February 2020).
- Martyniuk, S.; Oroń, J.; Mączka, M. Charakterystyka mikroorganizmów występujących na kłosach pszenicy ozimej uprawianej w systemie konwencjonalnym i ekologicznym. Prog. Plant. Prot. Post. Ochr. Rośl. 2009, 49, 1309–1316. [Google Scholar]
- Kraska, P.; Mielniczuk, E. The occurrence of fungi on the stem base and roots of spring wheat (Triticum aestivum L.) grown in monoculture depending on tillage sys-tems and catch crops. Acta Agrobot. 2012, 65, 1–79. [Google Scholar] [CrossRef]
- Matušinsky, P.; Váňová, M.; Tvarůžek, L.; PolIšenská, I.; Janeček, M.; Mutný, V. Soil management technologies and mycotoxin contamination of wheat and barley grain. Cereal Res. Commun. 2016, 44, 320–329. [Google Scholar] [CrossRef][Green Version]
- Weber, R. Treat and the ways of reducing fusariosis in wheat. Post. Nauk Rol. 2007, 59, 19–31. Available online: https://instytucja.pan.pl/images/stories/pliki/wydzialy/wydzial_v/dwum_pnr/pnr2-07.pdf (accessed on 10 February 2020).
- Klix, M.B. Major Mycotoxin Producin Fusarium Species in Wheat-Factors Affecting the Species Complex Composition and Disease Management; Cuvillier Verlag: Göttingen, Germany, 2007; pp. 17–32. [Google Scholar]
- Steinkellner, S.; Langer, I. Impact of tillage on the incidence of Fusarium spp. in soil. Plant. Soil 2004, 267, 13–22. [Google Scholar] [CrossRef]
- Gajęcki, M.; Jakimiuk, E.; Gajęcka, M.; Motyka, J.; Obremski, K. Praktyczne metody zmniejszania aktywności mikotoksyn w paszach. Magazyn Weterynaryjny Monografia 2010, 605–610. Available online: http://www.konferencjaswinie.pl/referaty/PRAKTYCZNE_METODY.pdf (accessed on 9 February 2020).
- Yi, C.; Kaul, H.P.; Kübler, E.; Schwadorf, K.; Aufhammer, I. Head blight (Fusarium graminearum) and deoxynivalenol concentration in winter wheat as affected by pre-crop soil tillage and nitrogen fertilisation. Pflanzen 2001, 108, 217–230. [Google Scholar]
- Podolska, G.; Bryła, M.; Sułek, A.; Waśkiewicz, A.; Szymczyk, K.; Jędrzejczak, R. Influence of the cultivar and nitrogen fertilisation level on the mycotoxin contamination in winter wheat. Quality Assurance and Saf. Crop. Foods 2017, 9, 451–461. [Google Scholar] [CrossRef]
- Siqueira, C.D.S.; Barrocas, E.N.; Machado, J.D.C.; Silva, U.A.D.; Dias, I.E. Effects of Stenocarpella maydis in seeds and in the initial development of corn. J. Seed Sci. 2014, 36, 79–86. [Google Scholar] [CrossRef]
- Jard, G.; Liboz, T.; Mathieu, F.; Guyonvarc’h, A.; Lebrihi, A. Review of mycotoxin reduction in food and feed: From prevention in the field to detoxification by adsorption or transformation. Food Addit. Contam. Part. A 2011, 28, 1590–1609. [Google Scholar] [CrossRef]
- Champeil, A.; Fourbet, J.F.; Dore, T.; Rossignol, L. Influence of cropping system on Fusarium head blight and mycotoxin levels in winter wheat. Crop. Protect. 2004, 23, 531–537. [Google Scholar] [CrossRef]
- Jouany, J.P. Methods for preventing, decontaminating and minimizing the toxicity of mycotoxins in feeds. Anim. Feed Sci. Technol. 2007, 137, 342–362. [Google Scholar] [CrossRef]
- Magan, N.; Aldred, D. Post-harvest control strategies: Minimizing mycotoxins in the food chain. Int. J. Food Microbiol. 2007, 119, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Blandino, M.; Scarpino, V.; Giordano, D.; Sulyok, M.; Krska, M.; Vanara, F.; Reyneri, A. Impact of sowing time, hybrid and environmental conditions on the contamination of maize by emerging mycotoxins and fungal metabolites. Ital. J. Agron. 2017, 12, 928. [Google Scholar] [CrossRef]
- Torelli, E.; Firrao, G.; Bianchi, G.; Saccardo, F.; Locci, R. The influence of local factors on the prediction of fumonisin contamination in maize. J. Sci. Food Agric. 2012, 92, 1808–1814. [Google Scholar] [CrossRef] [PubMed]
- Gautam, P.; Dill-Macky, R. Impact of moisture, host genetics and Fusarium graminearum isolates on Fusarium head blight development and trichothecene accumulation in spring wheat. Mycotoxin Res. 2012, 28, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Edwards, S.G. Influence of agricultural practices on fusarium infection of cereals and subsequent contamination of grain by trichothecene mycotoxins. Toxicol. Lett. 2004, 153, 29–35. [Google Scholar] [CrossRef]
- Buerstmayr, H.; Lemmens, M.; Hartl, L.; Doldi, L.; Steiner, B.; Stierschneider, M.; Ruckenbauer, P. Molecular mapping of QTLs for Fusarium head blight resistance in spring wheat. I. Resistance to fungal spread (Type II resistance). Theor. Appl. Genet. 2002, 104, 84–91. [Google Scholar] [CrossRef]
- Lanubile, A.; Maschietto, V.; Borrelli, V.M.; Stagnati, L.; Logrieco, A.F.; Marocco, A. Molecular basis of resistance to Fusarium Ear Rot in maize. Front. Plant. Sci. 2017, 12, 1774. [Google Scholar] [CrossRef]
- Perincherry, L.; Lalak-Kańczugowska, J.; Stępień, Ł. Fusarium-Produced mycotoxins in plant-pathogen interactions. Toxins 2019, 11, 664. [Google Scholar] [CrossRef]
- Schroeder, H.W.; Christensen, J.J. Factors affecting resistance of wheat to scab by Gibberella zeae. Phytopathology 1963, 53, 831–838. [Google Scholar]
- Steiner, B.; Buerstmayr, M.; Michel, S. Breeding strategies and advances in line selection for Fusarium head blight resistance in wheat. Trop. Plant. Pathol. 2017, 42, 165–174. [Google Scholar] [CrossRef]
- Mesterhazy, A. Types and components of resistance to Fusarium head blight of wheat. Plant. Breed. 1995, 114, 377–386. [Google Scholar] [CrossRef]
- Boutigny, A.L.; Richard-Forget, F.; Barreau, C. Natural mechanisms for cereal resistance to the accumulation of Fusarium trichothecenes. Eur. J. Plant. Pathol. 2008, 121, 411–423. [Google Scholar] [CrossRef]
- Kluger, B.; Bueschl, C.; Lemmens, M.; Michlmayr, H.; Malachova, A.; Koutnik, A.; Maloku, I.; Berthiller, F.; Adam, G.; Krska, R.; et al. Biotransformation of the mycotoxin deoxynivalenol in Fusarium resistant and susceptible near isogenic wheat lines. PLoS ONE 2015, 10, e0119656. [Google Scholar] [CrossRef] [PubMed]
- Lemmens, M.; Steiner, B.; Sulyok, M.; Nicholson, P.; Mesterhazy, A.; Buerstmayr, H. Masked mycotoxins: Does breeding for enhanced Fusarium head blight resistance result in more deoxynivalenol 3-glucoside in new wheat varieties? World Mycotoxin J. 2016, 9, 741–754. [Google Scholar] [CrossRef]
- Bekalu, Z.E.; Krogh Madsen, C.; Dionisio, G.; Bæksted Holme, I.; Jørgensen, L.N.S.; Fomsgaard, I.; Brinch-Pedersen, H. Overexpression of nepenthesin HvNEP-1 in barley endosperm reduces Fusarium head blight and mycotoxin accumulation. Agronomy 2020, 10, 203. [Google Scholar] [CrossRef]
- Steiner, B.; Buerstmayr, M.; Wagner, C.; Danler, A.; Eshonkulov, B.; Ehn, M.; Buerstmayr, H. Fine-mapping of the Fusarium head blight resistance QTL Qfhs.ifa-5A identifies two resistance QTL associated with anther extrusion. Theor Appl Genet. 2019, 132, 2039–2053. [Google Scholar] [CrossRef]
- Machado, A.K.; Brown, N.A.; Urban, M.; Kanyuka, K.; Hammond-Kosack, K.E. RNAi as an emerging approach to control Fusarium head blight disease and mycotoxin contamination in cereals. Pest. Manag. Sci. 2018, 74, 790–799. [Google Scholar] [CrossRef]
- Majumdar, R.; Rajasekaran, K.; Cary, J.W. RNA interference (RNAi) as a potential tool for control of mycotoxin contamination in crop plants: Concepts and considerations. Front. Plant. Sci. 2017, 8, 200. [Google Scholar] [CrossRef]
- Kuźniar, A.; Włodarczyk, K.; Wolińska, A. Agricultural and Other Biotechnological Applications Resulting from Trophic Plant-Endophyte Interactions. Agronomy 2019, 9, 779. [Google Scholar] [CrossRef]
- Khare, E.; Mishra, J.; Arora, N.K. Multifaceted interactions between endophytes and plant: Developments and prospects. Front. Microbiol. 2018, 9, 2732. [Google Scholar] [CrossRef]
- Dutta, D.; Puzari, K.C.; Gogoi, R.; Dutta, P. Endophytes: Exploitation as a tool in plant protection. Braz. Arch. Biol. Technol. 2014, 57, 5. [Google Scholar] [CrossRef]
- Bacon, C.W.; Glenn, A.E.; Yates, I.E. Fusarium verticillioides: Managing the endophytic association with maize for reduced fumonisins accumulation. Toxin Rev. 2008, 27, 411–446. [Google Scholar] [CrossRef]
- Oren, L.; Ezrati, S.; Cohen, D.; Sharon, A. Early events in the Fusarium verticillioides-maize interaction characterized by using a green fluorescent protein-expressing transgenic isolate. Appl. Environ. Microbiol. 2003, 69, 1695–1701. [Google Scholar] [CrossRef] [PubMed]
- Jochum, C.C.; Osborne, L.E.; Yuen, G. Fusarium head blight biological control with Lysobacter enzymogenes strain C3. Biol. Control. 2006, 39, 336–344. [Google Scholar] [CrossRef]
- Khan, N.I.; Schisler, D.A.; Boehm, M.J.; Lipps, P.E.; Slininger, P.J. Field testing of antagonists of Fusarium head blight incited by Gibberella zeae. Biol. Control. 2004, 29, 245–255. [Google Scholar] [CrossRef]
- Woo, S.L.; Ruocco, M.; Vinale, F.; Nigro, M.; Marra, R.; Lombardi, N.; Pascale, A.; Lanzuise, S.; Manganiello, G.; Lorito, M. Trichoderma-based products and their widespread use in agriculture. J. Open Mycol. 2014, 8, 71–126. [Google Scholar] [CrossRef]
- Błaszczyk, L.; Siwulski, M.; Sobieralski, K.; Lisiecka, J.; Jędryczka, M. Trichoderma spp.—application and prospects for use in organic farming and industry. J. Plant. Prot. Res. 2014, 54, 309–317. Available online: http://yadda.icm.edu.pl/yadda/element/bwmeta1.element.agro-5947c63c-7c28-44f6-b087-751fdea87a33/c/JPPR_54_4__01_Blaszczyk.pdf (accessed on 24 February 2020). [CrossRef]
- Buśko, M.; Chełkowski, J.; Popiel, D.; Perkowski, J. Solid substrate bioassay to evaluate impact of Trichoderma on trichothecene mycotoxin production by Fusarium species. J. Sci. Food Agric. 2008, 88, 533–541. [Google Scholar] [CrossRef]
- Popiel, D.; Kwaśna, H.; Chełkowski, J.; Stępień, Ł.; Laskowska, M. Impact of selected antagonistic fungi on Fusarium species—Toxigenic cereal pathogens. Acta Mycol. 2008, 43, 29–40. [Google Scholar] [CrossRef]
- Ferrigo, D.; Raiola, A.; Picollo, E.; Scopel, C.; Causin, R. Trichoderma harzianum T22 induces in maize systemic resistance against fusarium verticillioides. J. Plant. Pathol. 2014, 96, 133–142. [Google Scholar] [CrossRef]
- El-Gremi, S.M.; Draz, I.S.; Youssef, W.A.-E. Biological control of pathogens associated with kernel black point disease of wheat. Crop. Prot. 2017, 91, 13–19. [Google Scholar] [CrossRef]
- Bacon, C.W.; Yates, I.E.; Hinton, D.M.; Meredith, F.J. Biological control of Fusarium moniliforme in maize. Environ. Health Perspect. 2001, 109, 325. [Google Scholar] [CrossRef]
- Utkhede, R.; Smith, F.M. Impact of chemical, biological and cultural treatments on the growth and yield of apple in replant-Disease soil. Aust. Plant. Pathol. 2000, 29, 129–136. [Google Scholar] [CrossRef]
- Coombs, J.T.; Michelsen, P.P.; Franco, C.M.M. Evaluation of endophytic Actinobacteria as antagonists of Gaeumannomyces graminis var. tritici in wheat. Biol. Control. 2004, 29, 359–366. [Google Scholar] [CrossRef]
- Newitt, J.; Prudence, S.; Samuel, J.N.; Hutchings, M.I.; Worsley, S. Biocontrol of Cereal Crop Diseases Using Streptomycetes. Pathogens 2019, 8, 78. [Google Scholar] [CrossRef]
- Pirgozliev, S.R.; Edwards, S.G.; Hare, M.C.; Jenkinson, P. Strategies for the control of Fusarium head blight in cereals. Eur. J. Plant. Pathol. 2003, 109, 731–742. [Google Scholar] [CrossRef]
- Jennings, P.; Turner, J.A.; Nicholson, P. Overview of Fusarium Ear Blight in the UK-Effect of Fungicide Treatment on Disease Control and Mycotoxin Production; International Conference on Pests and Diseases: Brighton, UK, 2000; pp. 707–712. [Google Scholar]
- Haidukowski, M.; Pascale, M.; Perrone, G.; Pancaldi, D.; Campagna, C.; Visconti, A. Effect of fungicides on the development of Fusarium head blight, yield and deoxynivalenol accumulation in wheat inoculated under field conditions with Fusarium graminearum and Fusarium culmorum. J. Sci. Food Agric. 2005, 85, 191–198. [Google Scholar] [CrossRef]
- Wegulo, S.N.; Bockus, W.W.; Nopsa, J.H.; Wolf, E.D.; Eskridge, K.M.; Peiris, K.H.S.; Dowell, F.E. Effects of integrating cultivar resistance and fungicide application on Fusarium Head Blight and deoxynivalenol in winter wheat. Plant. Dis. 2011, 95, 554–560. [Google Scholar] [CrossRef]
- Willyerd, K.T.; Li, C.; Madden, L.V.; Bradley, C.A.; Bergstrom, G.C.; Sweets, L.E.; McMullen, M.; Ransom, J.K.; Grybauskas, A.; Osborne, L.; et al. Efficacy and stability of integrating fungicide and cultivar resistance to manage Fusarium head blight and deoxynivalenol in wheat. Plant. Dis. 2012, 96, 957–967. [Google Scholar] [CrossRef]
- Damalas, C.A.; Eleftherohorinos, I.G. Pesticide exposure, safety issues, and risk assessment indicators. Int. J. Environ. Res. Public. Health 2011, 8, 1402–1419. [Google Scholar] [CrossRef]
- Magan, N.; Hope, R.; Colleate, A.; Baxter, E.S. Relationship Between Growth and Mycotoxin Production by Fusarium species, Biocides and Environment. Eur. J. Plant. Pathol. 2002, 108, 685–690. [Google Scholar] [CrossRef]
- Gwiazdowska, D. Poprawa Jakości i Bezpieczeństwa Zdrowotnego Żywności i Pasz Poprzez Mikrobiologiczną Eliminację Mikotoksyn Fuzaryjnych; Wyd. Uniwersytetu Ekonomicznego: Poznań, Poland, 2014; pp. 1–26. [Google Scholar]
- Simpson, D.R.; Weston, G.E.; Turner, J.A.; Jennings, P.; Nicholson, P. Differential control of head blight pathogens of wheat by fungicides and consequences for mycotoxin contamination of grain. Eur. J. Plant. Pathol. 2001, 107, 421–431. [Google Scholar] [CrossRef]
- Wild, C.P.; Miller, J.D.; Groopman, J.D. Mycotoxin control in low-and middle-income countries. Lyon France Int. Agency Res. Cancer Work. Group Rep. 2015, 9, 31–42. [Google Scholar]
- Mutiga, S.K.; Mushongi, A.A.; Kangéthe, E.K. Enhancing food safety through adoption of long-term technical advisory, financial, and storage support services in maize growing areas of East Africa. Sustainability 2019, 11, 2827. [Google Scholar] [CrossRef]
- Omotayo, O.P.; Omotayo, A.O.; Mwanza, M.; Babalola, O.O. Prevalence of mycotoxins and their consequences on human health. Toxicol Res. 2019, 35, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chulze, S.N. Strategies to reduce mycotoxin levels in maize during storage: A review. Food Addit Contam Part. A Chem Anal. Control. Expo. Risk Assess. 2010, 27, 651–657. [Google Scholar] [CrossRef]
- Mutiga, S.K.; Were, V.; Hoffmann, V.; Harvey, H.W.; Milgroom, M.G.; Nelson, R.J. Extent and drivers of mycotoxin contamination: Inferences from a survey of Kenyan maize mills. Phytopathology 2014, 104, 1221–1231. [Google Scholar] [CrossRef]
- Grenier, B.; Bracarense, A.P.; Leslie, J.F.; Oswald, I.P. Physical and chemical methods for mycotoxin decontamination in maize. In Mycotoxin Reduction in Grain Chains; Leslie, J.F., Logrieco, A.F., Eds.; Wiley Blackwell: New Delhi, IA, USA, 2014; pp. 116–129. [Google Scholar]
- Karlovsky, P.; Suman, M.; Berthiller, F.; Meester, J.; Eisenbrand, G.; Perrin, I.; Oswald, I.P.; Speijers, G.; Chiodini, A.; Recker, T.; et al. Impact of food processing and detoxification treatments on mycotoxin contamination. Mycotoxin Res. 2016, 32, 179–205. [Google Scholar] [CrossRef]
- Munkvold, G.P.; Desjardins, A.E. Fumonisins in maize: Can we reduce their occurrence? Plant. Dis. 1988, 81, 556–565. [Google Scholar] [CrossRef]
- Afolabi, C.G.; Ojiambo, P.S.; Ekpo, E.J.A.; Menkir, M.; Bandyopadhyay, R. Evaluation of maize inbred lines for resistance to Fusarium ear rot and fumonisin accumulation in grain in tropical Africa. Plant. Dis. 2007, 91, 279–286. [Google Scholar] [CrossRef]
- Cheli, F.; Pinotti, L.; Rossi, L.; Dell’Orto, V. Effect of milling procedures on mycotoxin distribution in wheat fractions: A review. LWT-Food Sci. Technol. 2013, 54, 307–314. [Google Scholar] [CrossRef]
- Tanaka, K.; Sago, Y.; Zheng, Y.; Nakagawa, H.; Kushiro, M. Mycotoxins in rice. Int. J. Food Microbiol. 2007, 20, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Huwig, A.; Freimund, S.; Käppeli, O.; Dutler, H. Mycotoxin detoxication of animal feed by different adsorbents. Toxicol. Lett. 2001, 122, 179–188. [Google Scholar] [CrossRef]
- Galvano, F.; Pietri, A.; Bertuzzi, T.; Bognanno, M.; Chies, L.; Angelis, A.; Galvano, M. Activeted carbons: In vitro affinity for fumonisin B1 andrelation of adsorption ability to physicochemical parameter. J. Food Prot. 1997, 60, 985–991. [Google Scholar] [CrossRef]
- Camilo, S.B.; Ono, C.J.; Ueno, Y.; Hirooka, E.Y. Anti-Fusarium moniliforme activity and fumonisin biodegradation by corn and silage microflora. Braz. Arch. Biol. Technol. 2000, 43, 2. [Google Scholar] [CrossRef]
- Kang, M.H.; Pengkit, A.; Choi, K.; Jeon, S.S.; Choi, H.W.; Shin, D.B.; Choi, E.H.; Uhm, H.S.; Park, G. Differential inactivation of fungal spores in water and on seeds by ozone and arc discharge plasma. PLoS ONE 2015, 10, e0139263. [Google Scholar] [CrossRef]
- Waskow, A.; Betschart, J.; Butscher, D.; Oberbossel, G.; Klöti, D.; Büttner-Mainik, A.; Adamcik, J.; Rohr, P.R.; Schuppler, M. Characterization of efficiency and mechanisms of cold atmospheric pressure plasma decontamination of seeds for sprout production. Front. Microbiol. 2018, 9, 3164. [Google Scholar] [CrossRef]
- Agriopoulou, S.; Stamatelopoulou, E.; Varzakas, T. Advances in occurrence, importance, and mycotoxin control strategies: Prevention and detoxification in foods. Foods 2020, 9, 137. [Google Scholar] [CrossRef]
- Mohapatra, D.; Kumar, S.; Kotwaliwale, N.; Singh, K.K. Critical factors responsible for fungi growth in stored food grains and non-Chemical approaches for their control. Ind. Crop. Prod. 2017, 108, 162–182. [Google Scholar] [CrossRef]
- Trombete, F.M.; Porto, Y.D.; Freitas-Silva, O.; Pereira, R.V.; Direito, G.M.; Saldanha, T.; Fraga, M.E. Efficacy of ozone treatment on mycotoxins and fungal reduction in artificially contaminated soft wheat grains: Efficacy of O3 on mycotoxins and fungi. J. Food Process. Preserv. 2016, 41, e12927. [Google Scholar] [CrossRef]
- Li, M.M.; Guan, E.Q.; Bian, K. Effect of ozone treatment on deoxynivalenol and quality evaluation of ozonised wheat. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2015, 32, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, H.P.S.; Manthey, F.A.; Simsek, S. Quality of bread made from ozonated wheat (Triticum aestivum L.) flour. J. Sci. Food Agric. 2011, 91, 1576–1584. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Shao, H.; Luo, X.; Wang, R.; Li, Y.; Li, Y.; Luo, Y.; Chen, Z. Effect of ozone treatment on deoxynivalenol and wheat quality. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Solanki, M.K.; Abdelfattah, A.; Britzi, M.; Zakin, V.; Wisniewski, M.; Droby, S.; Sionov, E. Shifts in the composition of the microbiota of stored wheat grains in response to fumigation. Front. Microbiol. 2019, 10, 1098. [Google Scholar] [CrossRef]
- Ji, C.; Fan, Y.; Zhao, L. Review on biological degradation of mycotoxins. Anim. Nutr. 2016, 2, 127–133. [Google Scholar] [CrossRef]
- Kagot, V.; Okoth, S.; De Boevre, M.; De Saeger, S. Biocontrol of Aspergillus and Fusarium mycotoxins in Africa: Benefits and limitations. Toxins 2019, 11, 109. [Google Scholar] [CrossRef]
- Vanhoutte, I.; Audenaert, K.; Gelder, L.D. Biodegradation of mycotoxins: Tales from known and unexplored worlds. Front. Microbiol. 2016, 7, 561. [Google Scholar] [CrossRef]
- El-Nezami, H.; Kankaanpää, P.; Salminen, S.; Ahokas, J. Ability of dairy strains of latic acid bacteria to bind a common food carcinogen, aflatoxin B1. Food Chem. Toxicol. 1998, 36, 321–326. [Google Scholar] [CrossRef]
- El-Nezami, H.; Polychronaki, N.; Salminen, S.; Mykkänen, H. Binding rather than metabolism may explain the interaction of two food-grade Lactobacillus strains with zearalenone and its derivative ά-zearalenol. Appl. Environ. Microbiol. 2002, 68, 3545–3549. [Google Scholar] [CrossRef]
- Said, L.B.; Gaudreau, H.; Dallaire, L.; Tessier, M.; Fliss, I. Bioprotective culture: A new generation of food additives for the preservation of food quality and safety. Ind. Biotechnol. 2019, 15, 3–doi:10. [Google Scholar] [CrossRef]
- Juodeikiene, G.; Bartkiene, E.; Cernauskas, D.; Cizeikiene, D.; Zadeike, D.; Lele, V.; Bartkevics, V. Antifungal activity of lactic acid bacteria and their application for Fusarium mycotoxinreduction in malting wheat grains. LWT-Food Sci. Technol. 2018, 89, 307–314. [Google Scholar] [CrossRef]
- Čvek, D.; Markov, K.; Frece, J.; Friganović, M.; Duraković, L.; Delaš, F. Adhesion of zearalenone to the surface of lactic acid bacteria cells. Croat. J. Food Technol. Biotechnol. Nutr. 2012, 7, 49–52. Available online: https://pdfs.semanticscholar.org/9ea1/c5dbf545e9f2e7c7b4a203ff56b3606d5d5d.pdf (accessed on 19 February 2020).
- Juś, K.; Gwiazdowska, D. Wykorzystanie bakterii glebowych z rodzaju Brevibacillus do dekontaminacji zearalenonu. The use of the soil bacteria of the genus Brevibacillus for zearalenone decontamination. Studia Oeconomica Posnaniensia 2016, 4, 27–39. [Google Scholar] [CrossRef]
- Hsu, T.-C.; Yi, P.-J.; Lee, T.-Y.; Liu, J.-R. Probiotic characteristics and zearalenone-removal ability of a Bacillus licheniformis strain. PLoS ONE 2018, 13, e0194866. [Google Scholar] [CrossRef]
- Kamimura, H. Conversion of zearalenone to zearalenone glycoside by Rhizopus sp. Appl. Environ. Microbiol. 1986, 52, 515–519. [Google Scholar] [CrossRef]
- Plasencia, J.; Mirocha, C.J. Isolation and characterization of zearalenone sulfate produced by Fusarium spp. Appl. Environ. Microbiol. 1991, 57, 146–150. [Google Scholar] [CrossRef]
- Jard, G.; Liboz, T.; Mathieu, F.; Guyonvarc’, H.A.; Andre, F.; Delaforge, M.; Lebrihi, A. Transformation of zearalenone to zearalenone-sulfate by Aspergillus spp. World Mycotoxin J. 2010, 3, 183–191. [Google Scholar] [CrossRef]
- King, R.R.; McQeen, R.E.; Levesque, D.; Greenhalgh, R. Transformation of deoxynivalenol (vomitoxin) by rumen microorganisms. J. Agric. Food Chem. 1984, 32, 1181–1183. [Google Scholar] [CrossRef]
- Westlake, K.; Mackie, R.I.; Dutton, M.F. In vitro metabolism of mycotoxins by bacterial, protozoal and bovin ruminal fluid preparations. Anim. Feed Sci. Technol. 1989, 25, 169–178. [Google Scholar] [CrossRef]
- Kollarczik, B.; Gareis, M.; Hanelt, M. In vitro transformation of the Fusarium mycotoxins deoxynivalenol and zearalenone by the normal gut microflora of pigs. Nat. Toxins 1994, 2, 105–110. [Google Scholar] [CrossRef]
- Young, J.C.; Ting, Z.; Hai, Y.; Honghui, Z.; Jianhua, G. Degradation of the trichothecene mycotoxins by chicken intestinal microbes. Food Chem. Toxicol. 2007, 45, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Halász, A.; Lásztity, R.; Abonyi, T.; Bata, Á. Decontamination of mycotoxin-containing food and feed by biodegradation. Food Rev. Int. 2009, 25, 284–298. [Google Scholar] [CrossRef]
- Ueno, Y.; Nakayama, K.; Ishii, K.; Tashiro, F.; Minoda, Y.; Omori, T.; Komagata, K. Metabolism of T-2 toxin in Curtobacterium sp. strain 114-2. Appl. Env. Microbiol. 1983, 46, 120–127. [Google Scholar] [CrossRef]
- Shima, J.; Takase, S.; Takahashi, Y.; Iwai, Y.; Fujimoto, H.; Yamazaki, M.; Ochi, K. Novel detoxification of the trichothecene mycotoxin deoxynivalenol by a soil bacterium isolated by enrichment culture. Appl. Environ. Microbiol. 1997, 63, 3825–3830. [Google Scholar] [CrossRef]
- Völkl, A.; Vogler, B.; Schollenberger, M.; Karlovsky, P. Microbial detoxification of mycotoxin deoxynivalenol. J. Basic Microbiol. 2004, 44, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Erikson, G.S.; Petterson, T.; Lundh, T. Comparative cytotoxicity of deoxynivalenol, nivalenol, their acylated derivatives and deepoxy metabolites. Food Chem. Toxicol. 2004, 42, 614–619. [Google Scholar] [CrossRef]
- He, J.; Bondy, G.S.; Zhou, T.; Caldwell, D.; Boland, G.J.; Scott, P.M. Toxicology of 3-epi-deoxynivalenol, a deoxynivalenol-transformation product by Devosia mutans 17-2-E-8. Food Chem. Toxicol. 2015, 84, 250–259. [Google Scholar] [CrossRef]
- Motomura, M.; Lourenço, C.E.; Venturini, D.; Ueno, Y.; Hirooka, E.Y. Screening and isolation of anti-Fusarium moniliforme compounds producing microorganisms from soil and corn. Rev. Microbiol. 1996, 27, 213–217. [Google Scholar]
- Kabak, B.; Dobson, A.; Var, I. Strategies to prevent mycotoxin contamination of food and animal feed: A review. Crit. Rev. Food Sci. Nutr 2006, 46, 593–619. [Google Scholar] [CrossRef]
- Humer, E.; Lucke, A.; Harder, H.; Metzler-Zebeli, B.U.; Böhm, J.; Zebeli, O. Effects of citric and lactic acid on the reduction of deoxynivalenol and its derivatives in feeds. Toxins 2016, 8, 285. [Google Scholar] [CrossRef]
- Varga, J.; Kocsubé, S.; Péteri, Z.; Vágvölgyi, C.; Tóth, B. Chemical, physical and biological approaches to prevent ochratoxin induced toxicoses in humans and animals. Toxins 2010, 2, 1718–1750. [Google Scholar] [CrossRef] [PubMed]
- Norred, W.P.; Voss, K.A.; Bacon, C.W.; Riley, R.T. Effectiveness of ammonia treatment in detoxification of fumonisin contaminated corn. Food Chem. Toxicol. 1991, 29, 815–819. [Google Scholar] [CrossRef]
- Tsedaley, B.; Adugna, G. Detection of fungi infecting maize (Zea mays L.) seeds in different storages around Jimma, Southwestern Ethiopia. J. Plant. Pathol. Microbiol. 2016, 7, 3. [Google Scholar] [CrossRef]
- Méndez-Albores, A.; Del Río-García, J.C.; Moreno-Martínez, E. Decontamination of aflatoxin duckling feed with aqueous citric acid treatment. Anim. Feed Sci. Tech. 2007, 135, 249–262. [Google Scholar] [CrossRef]
| Cereals | Country | Mycotoxins | Content (Range or Average) [µg kg−1] | References |
|---|---|---|---|---|
| Wheat | Slovakia | DON | 119–3119 | [123] |
| Canada | 100–14300 | [124] | ||
| Poland | 5–1671 | [125] | ||
| 1600–38900 | [126] | |||
| 25–2975 | [9] | |||
| Norway | 86 | [19] | ||
| Poland | T-2 | 1–22 | [9] | |
| Poland | HT-2 | 2–55 | [9] | |
| Norway | n.d. | [19] | ||
| Brazil | FB1 | 958–4906 | [127] | |
| Serbia | 750–5400 | [128] | ||
| United States | 5–2210 | [129] | ||
| United States | FB2 | 2–249 | [129] | |
| Triticale | Poland | DON | 196–1326 | [9] |
| T-2 | 2–3 | [9] | ||
| FB1 | 342 | [9] | ||
| ZEA | 4–86 | [9] | ||
| Rye | Poland | DON | 0-6000 | [126] |
| Barley | Poland | DON | 76–222 (spring barley) | [9] |
| 54–1602 (winter barley) | ||||
| Norway | 44 | [19] | ||
| Lithuania | trace-198 | [130] | ||
| Poland | T-2 | 2–11(spring barley) | [9] | |
| 1–5 (winter barley) | ||||
| Poland | HT-2 | 8–74 (spring barley) | [9] | |
| 3–69 (winter barley) | ||||
| Norway | <20–21 | [19] | ||
| Argentina | NIV | 0.0002–0.0161 | [34] | |
| Poland | FB1 | 101 (spring barley) | [9] | |
| Poland | ZEA | 2–31 (spring barley) | [9] | |
| 2–19 (winter barley) | ||||
| Oat | Germany | DON | 52–302 | [131] |
| Poland | 220–2150 | [132] | ||
| Poland | 67–149 | [9] | ||
| Norway | 426 | [19] | ||
| Finland | 870–5600 | [62] | ||
| Germany | NIV | 11–192 | [131] | |
| Poland | 13–1031 | [132] | ||
| Finland | n.d.–87 | [62] | ||
| Finland | T-2 | n.d.–150 | [62] | |
| Germany | 20–244 | [131] | ||
| Poland | 32–311 | [132] | ||
| Poland | 9–29 | [9] | ||
| Poland | HT-2 | 30–651 | [132] | |
| Poland | 46–93 | [9] | ||
| Norway | 117 | [19] | ||
| Germany | 205–296 | [131] | ||
| Finland | n.d.–550 | [62] | ||
| Poland | DAS | 21–980 | [62] | |
| Poland | ZEA | 5–15 | [9] | |
| Maize | Poland | DON | 6150 (2013) | [133] |
| Germany | 170 | [134] | ||
| Poland | NIV | 300 (2013) | [133] | |
| UK | FB1 | 200–6000 | [135] | |
| Ghana | FB2 | 10–770 | [136] | |
| Thailand | ZEA | 900 | [137] | |
| Croatia | 2–511 | [93] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mielniczuk, E.; Skwaryło-Bednarz, B. Fusarium Head Blight, Mycotoxins and Strategies for Their Reduction. Agronomy 2020, 10, 509. https://doi.org/10.3390/agronomy10040509
Mielniczuk E, Skwaryło-Bednarz B. Fusarium Head Blight, Mycotoxins and Strategies for Their Reduction. Agronomy. 2020; 10(4):509. https://doi.org/10.3390/agronomy10040509
Chicago/Turabian StyleMielniczuk, Elżbieta, and Barbara Skwaryło-Bednarz. 2020. "Fusarium Head Blight, Mycotoxins and Strategies for Their Reduction" Agronomy 10, no. 4: 509. https://doi.org/10.3390/agronomy10040509
APA StyleMielniczuk, E., & Skwaryło-Bednarz, B. (2020). Fusarium Head Blight, Mycotoxins and Strategies for Their Reduction. Agronomy, 10(4), 509. https://doi.org/10.3390/agronomy10040509