Effect of Biogas Digestate and Mineral Fertilisation on the Soil Properties and Yield and Nutritional Value of Switchgrass Forage
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description and Experimental Design
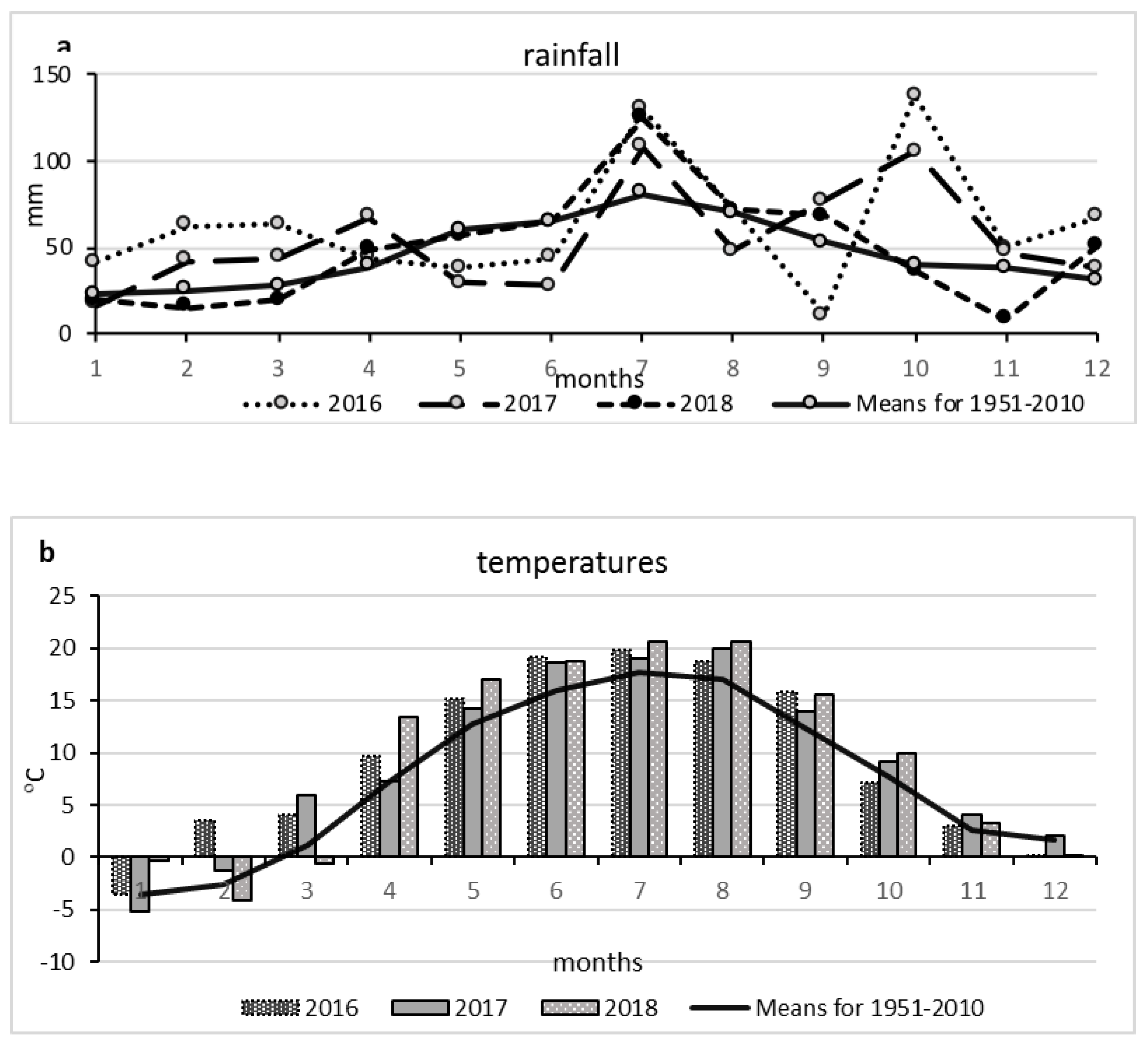
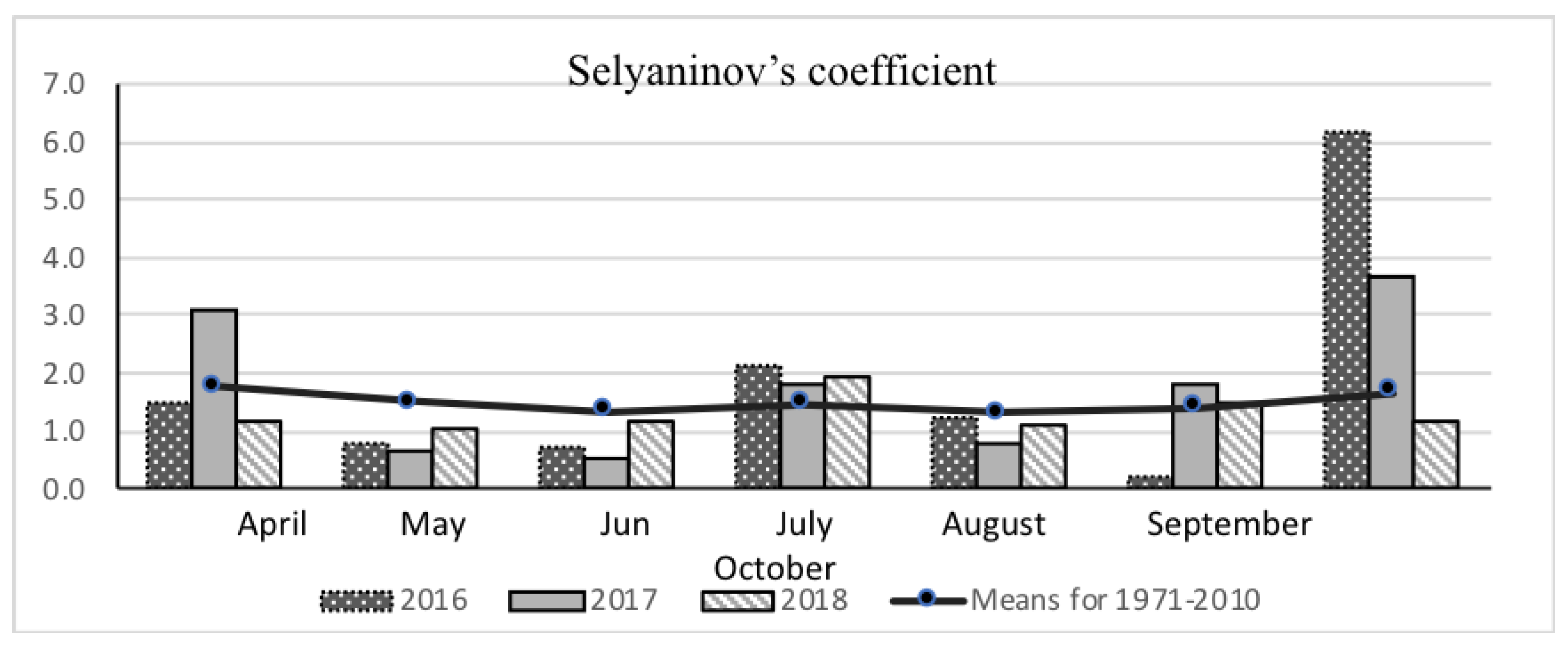
2.2. Meteorological Conditions
2.3. Measurements
2.3.1. Biomass Yield and Morphological Traits
2.3.2. Laboratory Analysis
2.4. Statistical Analysis
3. Results and Discussion
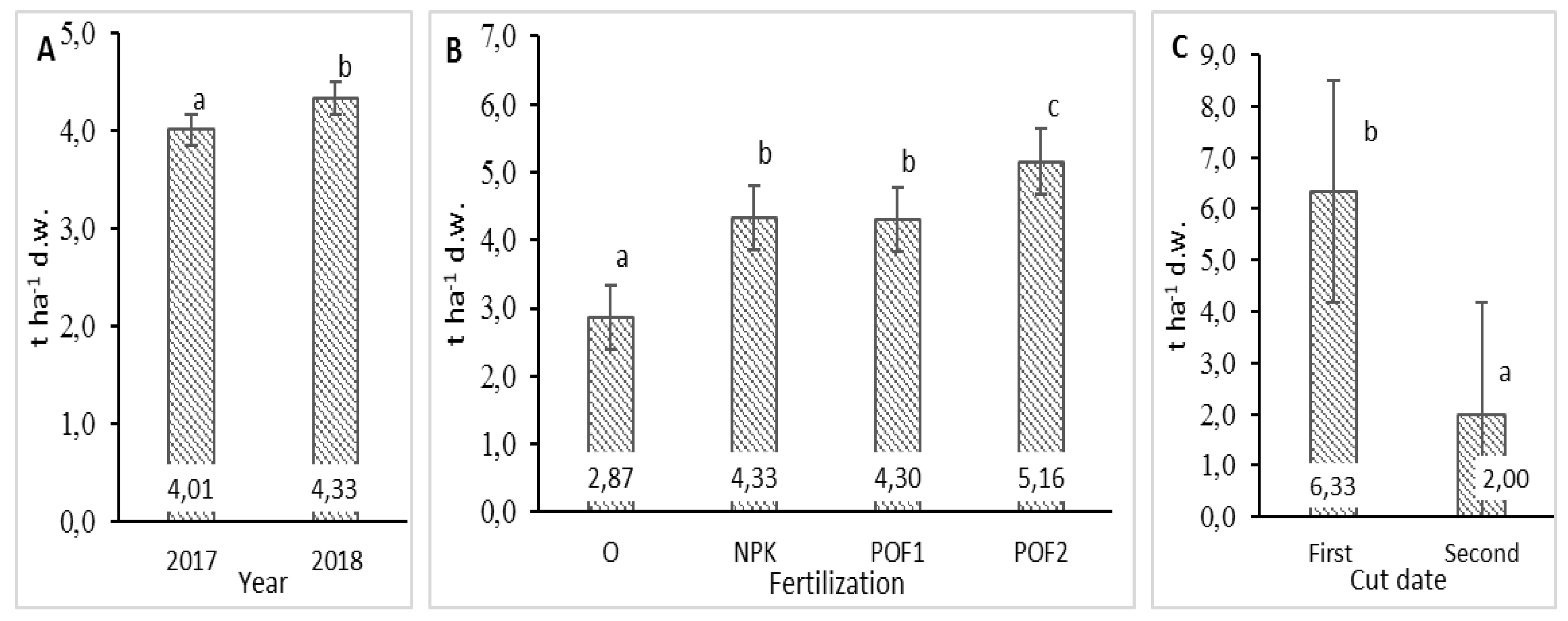
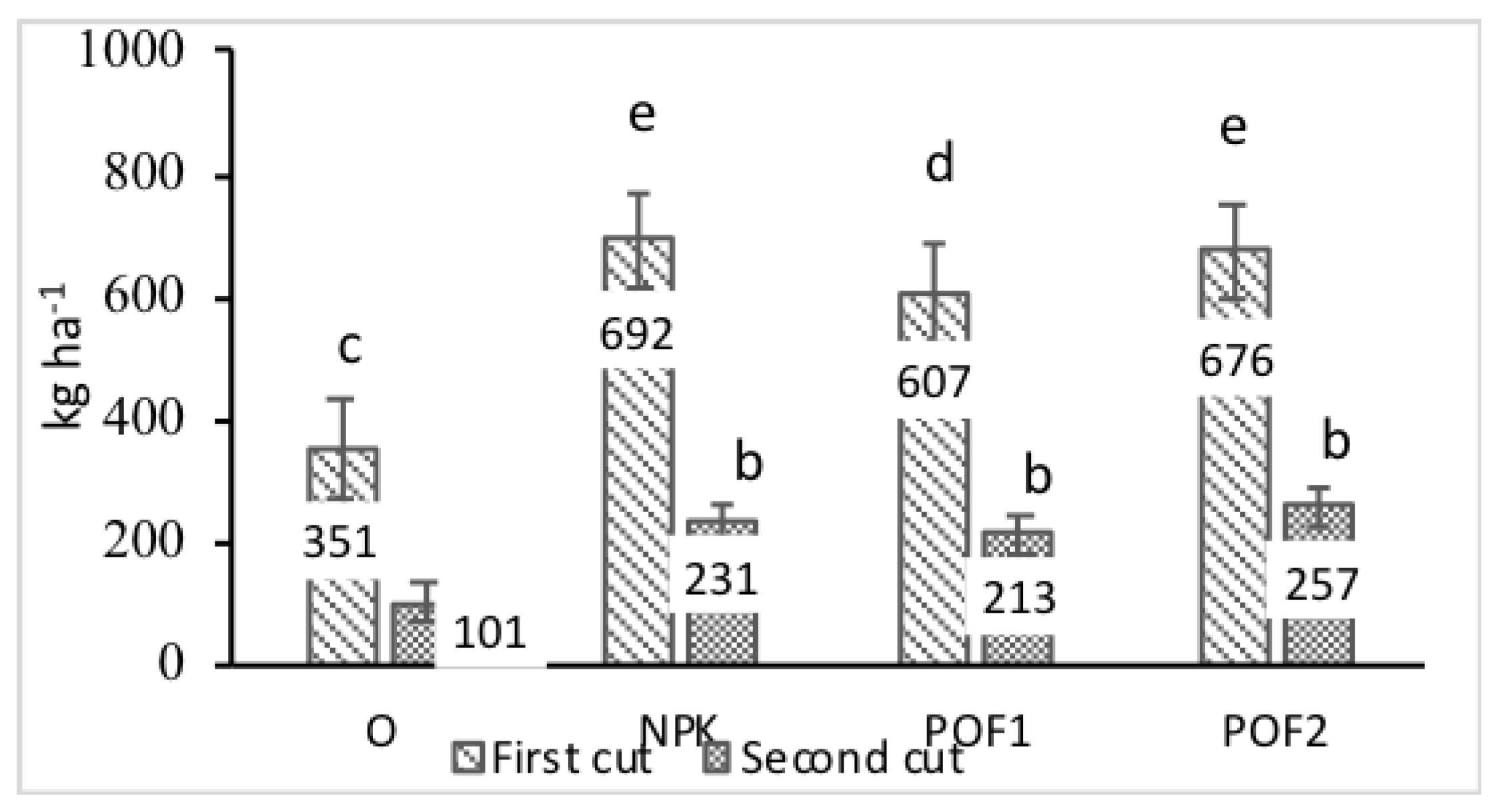
3.1. Biomass Yield and Morphological Traits
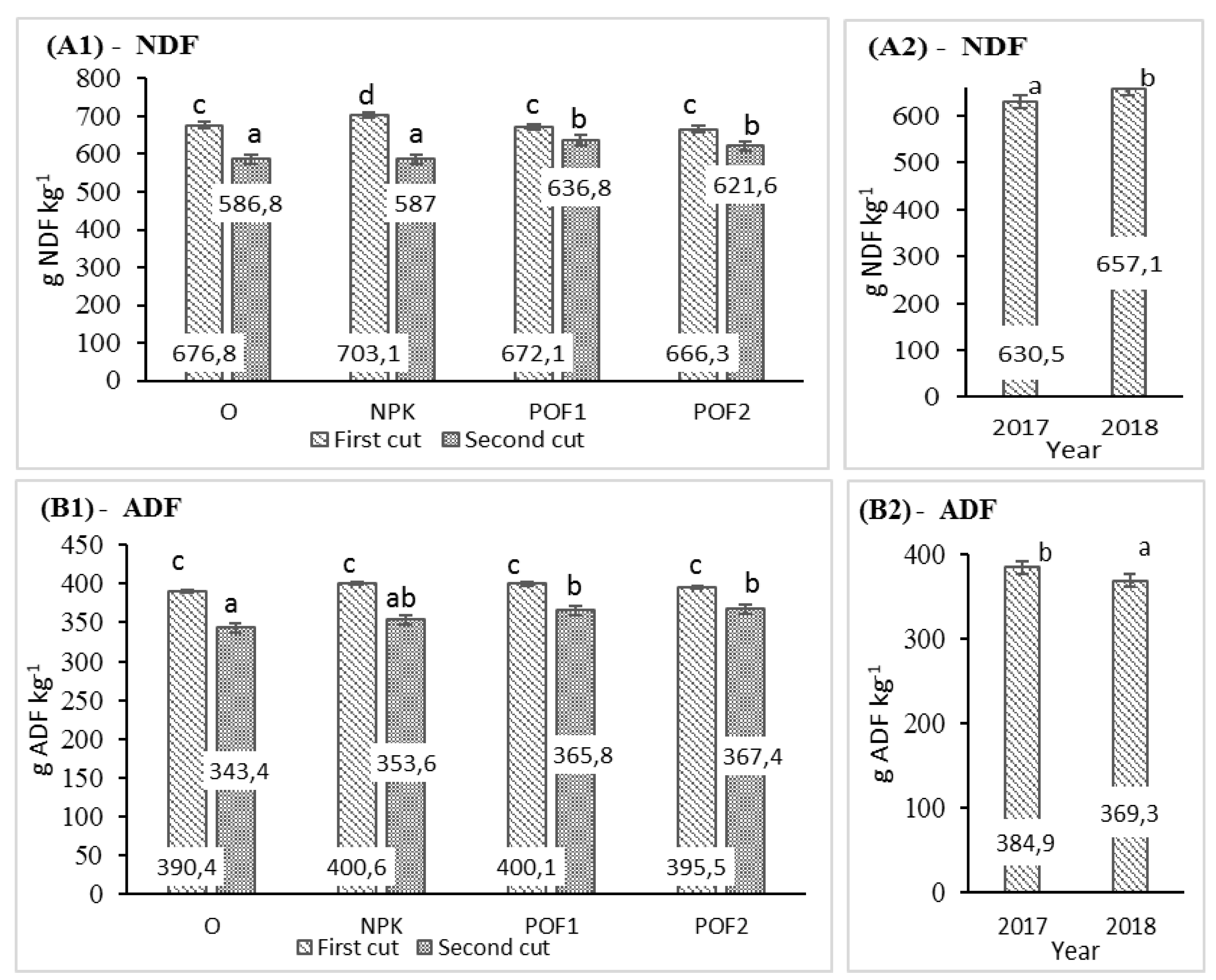
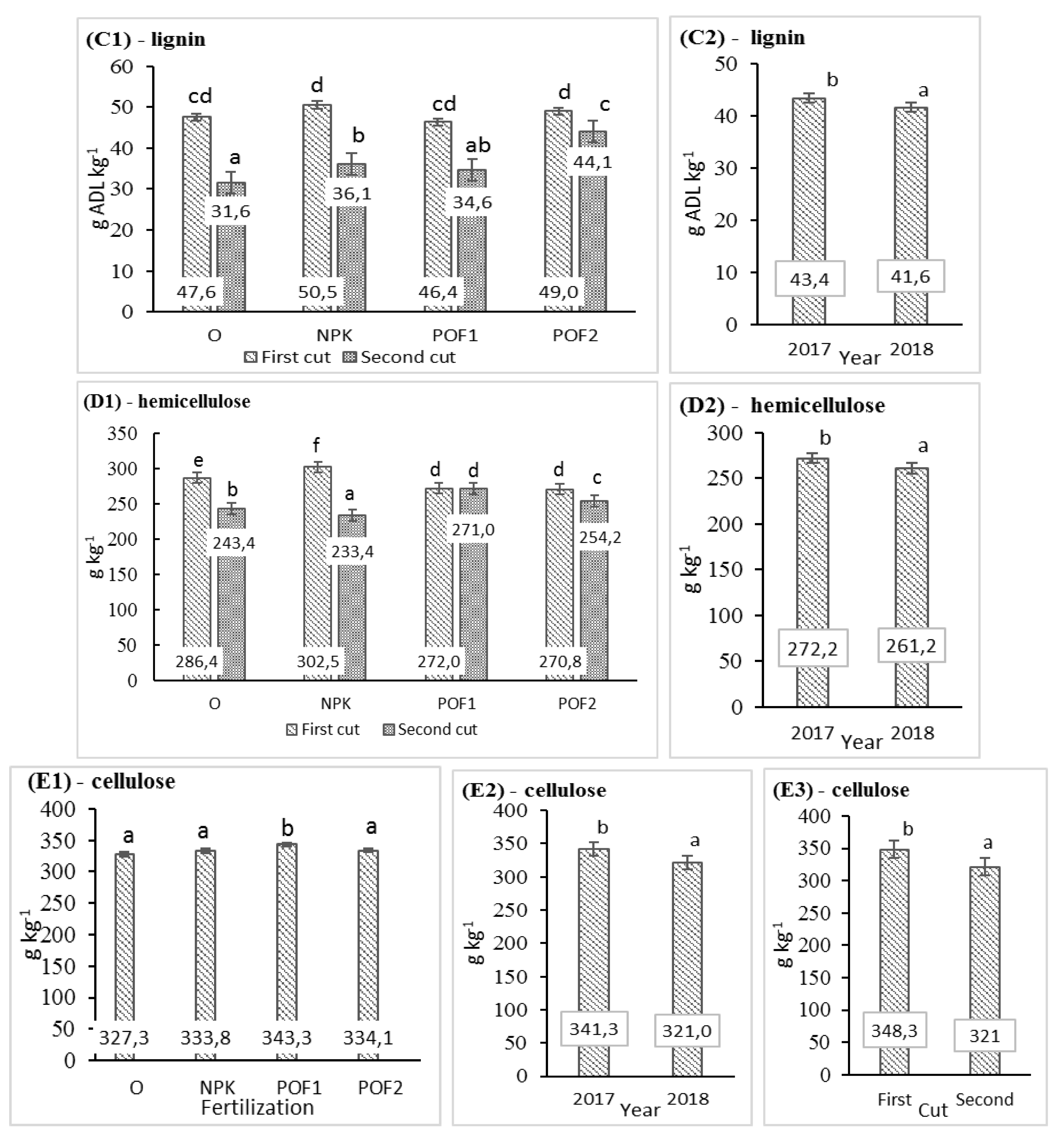
3.2. Nutritional Value
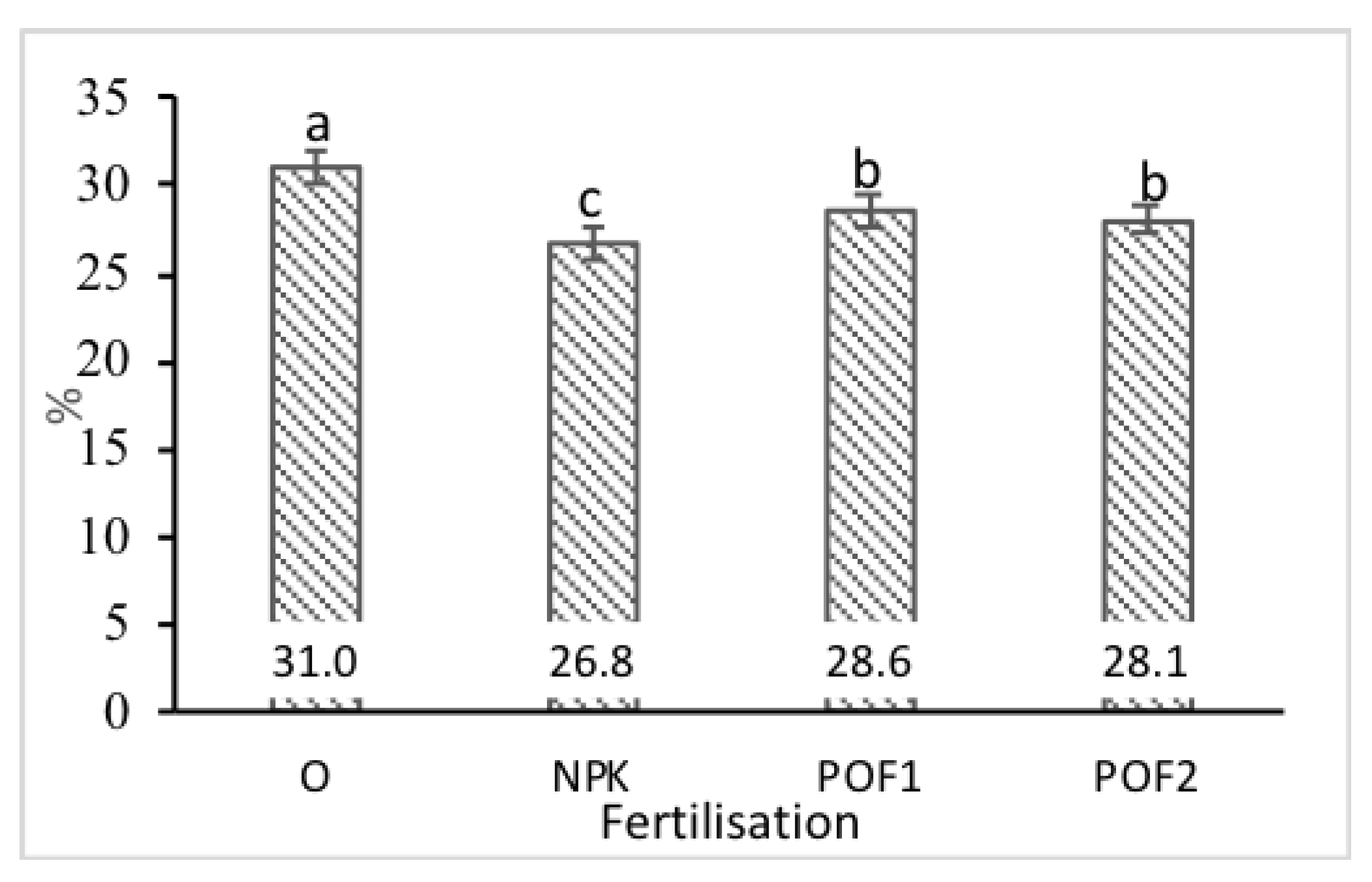
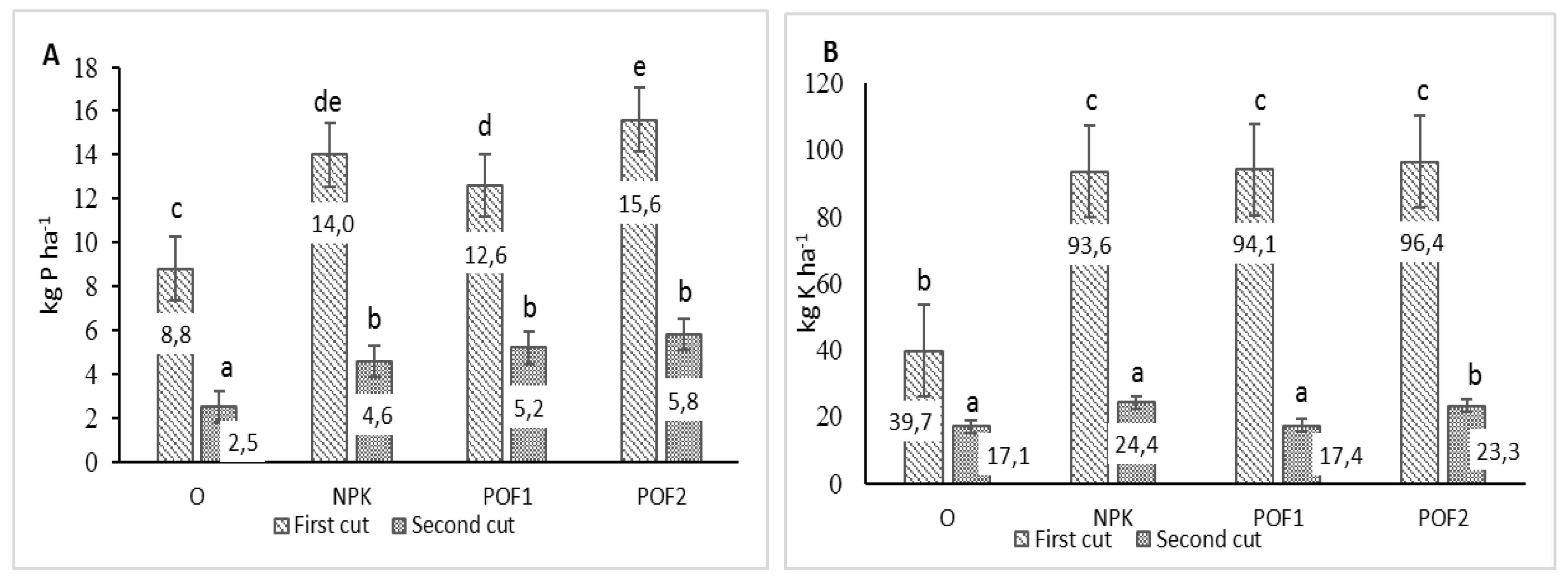
3.3. Mineral Composition
3.4. Soil Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Raport, E.E.A. Climate change, impacts and vulnerability in Europe 2012. Eur. Environ. 2012, 12. [Google Scholar] [CrossRef]
- Wullschleger, S.D.; Davis, E.B.; Borsul, M.E.; Gunderson, C.A.; Lynd, L.R. Biomass production in switchgrass across the United States: Database description and determinants of yield. Agron. J. 2010, 102, 1158–1168. [Google Scholar] [CrossRef]
- Brodowska, M.S.; Muszyński, P.; Haliniarz, M.; Brodowski, R.; Kowalczyk-Juśko, A.; Sekutowski, T.; Kurzyna-Szklarek, M. Agronomic aspects of switchgrass cultivation and use for energy purposes. Appl. Ecol. Environ. Res. 2018, 16, 5715–5743. [Google Scholar] [CrossRef]
- Vogel, K.P. Switchgrass. In Warm-Season (C4) Grasses; Moser, L.E., Sollenberger, L., Burson, B., Eds.; ASA-CSSA-SSSA: Madison, WI, USA, 2004; pp. 561–588. [Google Scholar]
- Fike, J.H.; Parrish, D.J.; Wolf, D.D.; Balasko, J.A.; Green, J.T., Jr.; Rasnake, M.; Reynolds, J.H. Switchgrass production for the upper southeastern USA: Influence of cultivar and cutting frequency on biomass yields. Biomass Bioenergy 2006, 30, 207–213. [Google Scholar] [CrossRef]
- Jiang, Q.; Webb, S.L.; Yesudas, C.R.; Bhandari, H.S.; Narasimhamoorthy, B.; Bouton, J.H.; Saha, M.C. Variance components and heritability of biomass yield in switchgrass (Panicum virgatum L.) grown in the Southern Great Plains. Field Crops Res. 2014, 168, 148–155. [Google Scholar] [CrossRef]
- Piłat, J.; Majtkowski, W.; Majtkowska, G.; Żurek, G.; Mikołajczak, J. The feeding value assessment of forage from some C-4 grass species in different phases of vegetation. Part III. Panicum virgatum L. Plant Breed. Seed Sci. 2007, 55, 65–73. [Google Scholar]
- Muir, J.P.; Sanderson, M.A.; Ocumpaugh, W.R.; Jones, R.M.; Reed, R.L. Biomass Production of ‘Alamo’ Switchgrass in Response to Nitrogen, Phosphorus, and Row Spacing. Agron. J. 2001, 93, 896–901. [Google Scholar] [CrossRef]
- Brejda, J.J.; Brown, J.R.; Wyman, G.W.; Schumacher, W.K. Management of switchgrass for forage and seed production. J. Range Manag. 1994, 47, 22–27. [Google Scholar] [CrossRef]
- An, Y.; Gao, Y.; Ma, Y. Growth performance and weed control effect in response to nitrogen supply for switchgrass after establishment in the semiarid environment. Field Crops Res. 2018, 221, 175–181. [Google Scholar] [CrossRef]
- Lai, L.; Kumar, S.; Osborne, S.; Owens, V.N. Switchgrass impact on selected soil parameters, including soil organic carbon, within six years of establishment. Catena 2018, 163, 288–296. [Google Scholar] [CrossRef]
- Sanderson, M.A.; Reed, R.L.; McLaughlin, S.B.; Wullschleger, S.D.; Conger, B.V.; Parrish, D.J.; Wolf, D.D.; Taliaferro, C.; Hopkins, A.A.; Ocumpaugh, W.R.; et al. Switchgrass as a sustainable bioenergy crop. Bioresour. Technol. 1996, 56, 83–93. [Google Scholar] [CrossRef]
- Keshwani, D.R.; Cheng, J.J. Switchgrass for bioethanol and other value-added applications: A review. Bioresour. Technol. 2009, 100, 1515–1523. [Google Scholar] [CrossRef] [PubMed]
- Lemus, R.; Brummer, E.C.; Moore, K.J.; Molstad, N.E.; Burras, C.L.; Barker, M.F. Biomass yield and quality of 20 switchgrass populations in southern Iowa, USA. Biomass Bioenergy 2002, 23, 433–442. [Google Scholar] [CrossRef]
- Somerville, C.; Youngs, H.; Taylor, C.; Davis, S.C.; Long, S.P. Feedstocks for lignocellulosic biofuels. Science 2010, 329, 790–792. [Google Scholar] [CrossRef]
- Vogel, K.P.; Brejda, J.J.; Walters, D.T.; Buxton, D.R. Switchgrass biomass production in the Midwest USA: Harvest and nitrogen management. Agron. J. 2002, 94, 413–420. [Google Scholar] [CrossRef]
- Vogel, K.P.; Gautam, S.; Saathoff, A.J.; Mitchell, R.B. Agronomy & Horticulture—Faculty Publications. 2011. Available online: http://digitalcommons.unl.edu/agronomyfacpub/1028 (accessed on 21 December 2019).
- Vogel, K.P.; Sarath, G.; Saathoff, A.J.; Mitchell, R.B. Switchgrass. In Energy Crops; Halford, N.G., Karp, A., Eds.; RSC Energy and Environment Series; Royal Society of Chemistry: London, UK, 2011; Volume 3, pp. 341–380. [Google Scholar]
- Ashworth, A.J.; Weiss, S.A.; Keyser, P.D.; Allen, F.L.; Tyler, D.D.; Taylor, A.; Beamer, K.P.; West, C.P.; Pote, D.H. Switchgrass composition and yield response to alternative soil amendments under intensified heat and drought conditions. Agric. Ecosyst. Environ. 2016, 233, 415–424. [Google Scholar] [CrossRef]
- Ma, Y.; An, Y.; Shui, J.; Sun, Z. Adaptability evaluation of switchgrass (Panicum virgatum L.) cultivars on the Loess Plateau of China. Plant Sci. 2011, 181, 638–643. [Google Scholar] [CrossRef]
- Haquea, M.; Epplin, F.M.; Taliaferro, C.M. Nitrogen and Harvest Frequency Effect on Yield and Cost for Four Perennial Grasses. Agron. J. 2009, 101, 1463–1469. [Google Scholar] [CrossRef]
- Miesel, J.R.; Jach-Smith, L.C.; Renz, M.J.; Jackson, R.D. Distribution of switchgrass (Panicum virgatum L.) aboveground biomass in response to nitrogen addition and across harvest dates. Biomass Bioenergy 2017, 100, 74–83. [Google Scholar] [CrossRef]
- Stroup, J.A.; Sanderson, M.A.; Muir, J.P.; McFarland, M.J.; Reed, R.L. Comparison of growth and performance in upland and lowland switchgrass types to water and nitrogen stress. Bioresour. Technol. 2003, 86, 65–72. [Google Scholar] [CrossRef]
- Guretzky, J.A.; Ball, J.; Cook, B.J. Nitrogen fertiliser rate and weather dictate nutritive value of fall stockpiled bermudagrass. Forage Grassl. 2008, 6. [Google Scholar] [CrossRef]
- Jung, G.A.; Shaffer, J.A.; Stout, W.L. Switchgrass and Big Bluestem Responses to Amendments on Strongly Acid Soil. Agron. J. 1988, 80, 669–676. [Google Scholar] [CrossRef]
- Staley, T.E.; Stout, W.L.; Jung, G.A. Nitrogen Use by Tall Fescue and Switchgrass on Acidic Soils of Varying Water Holding Capacity. Agron. J. 1991, 83, 732–738. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, L.; Feng, L.; Sun, D.; Dang, Y. Comparison of varying operating parameters on heavy metals ecological risk during anaerobic co-digestion of chicken manure and corn stover. Bioresour. Technol. 2008, 247, 660–668. [Google Scholar] [CrossRef] [PubMed]
- Gissen, C.; Prade, T.; Kreuger, E.; Nges, I.A.; Rosenqvist, H.; Svensson, S.E.; Lantz, M.; Mattsson, J.E.; Borjesson, P.; Bjornsson, L. Comparing energy crops for biogas production-yields, energy input and costs in cultivation using digestate and mineral fertilisation. Biomass Bioenergy 2014, 64, 199–210. [Google Scholar] [CrossRef]
- WRAP. DC-Agri, Field Experiments for Quality Digestate and Compost in Agriculture—WP1 Report, Prepared by Bhogal et al. 2015. Available online: www.wrapni.org.uk (accessed on 29 January 2020).
- Różyło, K.; Oleszczuk, P.; Jośko, I.; Kraska, P.; Kwiecińska-Poppe, E.; Andruszczak, S. An ecotoxicological evaluation of soil fertilised with biogas residues or mining waste. Environ. Sci. Pollut. Res. 2015, 22, 7833–7842. [Google Scholar] [CrossRef]
- Stefaniuk, M.; Bartminski, P.; Różyło, K.; Dębicki, R.; Oleszczuk, P. Ecotoxicological assessment of residues from different biogas production plants used as fertiliser for soil. J. Hazard. Mater. 2015, 298, 195–202. [Google Scholar] [CrossRef]
- Pivato, A.; Vanin, S.; Raga, R.; Lavagnolo, M.C.; Barausse, A.; Rieple, A.; Laurent, A.; Cossu, R. Use of digestate from a decentralized on-farm biogas plant as fertiliser in soils: An ecotoxicological study for future indicators in risk and life cycle assessment. Waste Manag. 2016, 49, 78–89. [Google Scholar] [CrossRef]
- Risberg, K.; Cederlund, H.; Pell, M.; Arthurson, V.; Schnürer, A. Comparative characterization of digestate versus pig slurry and cow manure—Chemical composition and effects on soil microbial activity. Waste Manag. 2017, 61, 529–538. [Google Scholar] [CrossRef]
- Pranagal, J.; Tomaszewska-Krojańska, D.; Small, H.; Ligęza, S. Impact of selected waste applications on soil compaction. Agron. Sci. 2019, 74, 19–32. [Google Scholar] [CrossRef]
- Galvez, A.; Sinicco, T.; Cayuela, M.L.; Mingorance, M.D.; Fornasier, F.; Mondini, C. Short term effects of bioenergy by-products on soil C and N dynamics, nutrient availability and biochemical properties. Agric. Ecosys. Environ. 2012, 160, 3–14. [Google Scholar] [CrossRef]
- Smith, J.; Abegaz, A.; Matthews, R.B.; Subedi, M.; Orskov, E.R.; Tumwesige, V.; Smith, P. What is the potential for biogas digesters to improve soil carbon sequestration in Sub-Saharan Africa? Comparison with other uses of organic residues. Biomass Bioenergy 2014, 70, 73–86. [Google Scholar] [CrossRef]
- Marcato, C.E.; Mohtar, R.; Revel, J.C.; Pouech, P.; Hafidi, M.; Guiresse, M. Impact of anaerobic digestion on organic matter quality in pig slurry. Int. Biodeterior. Biodegrad. 2009, 63, 260–266. [Google Scholar] [CrossRef]
- Garg, R.N.; Pathak, H.; Das, D.K.; Tomar, R.K. Use of fly ash and biogas slurry for improving wheat yield and physical properties of the soil. Environ. Monit. Assess. 2005, 107, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Odlare, M.; Pell, M.; Svensson, K. Changes in soil chemical and microbiological properties during 4 years of application of various organic residues. Waste Manag. 2008, 28, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- Insam, H.; Gomez-Brandon, M.; Ascher, J. Manure-based biogas fermentation residues e Friend or foe of soil fertility? Soil Biol. Biochem. 2015, 84, 1–14. [Google Scholar] [CrossRef]
- Różyło, K.; Świeca, M.; Gawlik-Dziki, U.; Andruszczak, S.; Kwiecińska-Poppe, P.; Kraska, P. Phytochemical properties and heavy metal accumulation in wheat grain after three years’ fertilisation with biogas digestate and mineral waste. Agric. Food Sci. 2017, 26, 148–159. [Google Scholar] [CrossRef]
- Makadi, M.; Tomocsik, A.; Eichler-Loebermann, B.; Schiemenz, K. Nutrient cycling by using residues of bioenergy production—Effects of biogas-digestate on plant and soil parameters. Cereal Res. Commun. 2008, 36, 1807–1810. [Google Scholar] [CrossRef]
- Andruschkewitsch, M.; Wachendorf, C.; Wachendorf, M. Effects of digestates from different biogas production systems on above and belowground grass growth and the nitrogen status of the plant-soil-system. Grassl. Sci. 2013, 59, 183–195. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Selim, S.; Jaakkola, S.; Makela, P.S.A. Chemical composition and in vitro digestibility of whole-crop maize fertilised with synthetic fertiliser or digestate and harvested at two maturity stages in Boreal growing conditions. Agric. Food Sci. 2017, 26, 47–55. [Google Scholar] [CrossRef]
- Abubaker, J.; Risberg, K.; Pell, M. Biogas residues as fertilisers – Effects on wheat growth and soil microbial activities. Appl. Energy 2012, 99, 126–134. [Google Scholar] [CrossRef]
- Walsh, J.J.; Jones, D.L.; Chadwick, D.R.; Williams, A.P. Repeated application of anaerobic digestate, undigested cattle slurry and inorganic fertiliser N: Impacts on pasture yield and quality. Grass Forage Sci. 2018, 73, 758–763. [Google Scholar] [CrossRef]
- Tilvikienė, V.; Šlepetienė, A.; Kadžiulienė, Ž. Effects of 5 years of digestate application on biomass production and quality of cocksfoot (Dactylis glomerata L.). Grass Forage Sci. 2017, 73, 206–217. [Google Scholar] [CrossRef]
- Rancane, S.; Karklins, A.; Lazdina, D.; Berzins, P.; Bardule, A.; Butlers, A.; Lazdins, A. The evaluation of biomass yield and quality of Phalaris arundinacea and Festulolium fertilised with bio-energy waste products. Agron. Res. 2016, 14, 198–210. [Google Scholar]
- Nkoa, R. Agricultural benefits and environmental risks of soil fertilisation with anaerobic digestates: A review. Agron. Sustain. Dev. 2013, 34, 473–492. [Google Scholar] [CrossRef]
- Czekała, W.; Lewicki, A.; Pochwatka, P.; Czekała, A.; Wojcieszak, D.; Jóźwiakowski, K.; Waliszewska, H. Digestate management in polish farms as an element of the nutrient cycle. J. Clean. Prod. 2020, 242, 118454. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014, Update 2015 International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports No. 106; FAO: Rome, Italy, 2015. [Google Scholar]
- Act on Fertilisers and Fertilisation. Available online: http://isap.sejm.gov.pl/isap.nsf/download.xsp/WDU20180001259/O/D20181259.pdf (accessed on 29 December 2019).
- Skowera, B.; Jędrszczyk, E.; Kopcińska, J.; Ambroszczyk, A.M.; Kołtun, A. The effects of hydrothermal conditions during vegetation period on fruit quality of processing tomatoes. Poll. J. Environ. Stud. 2014, 23, 195–202. [Google Scholar]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 2005; ISBN 0-935584-77-3. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fibre, neutral detergent fibre and non-starch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- StatSoft. Electronic Statistic Texbook 2006, Krakow. Available online: http://www.statsoft.pl/textbook/stathome.html (accessed on 10 December 2019).
- Koshi, P.T.; Stubbendieck, J.; Eck, H.V.; Mccully, W.G. Switchgrasses: Forage Yield, Forage Quality and Water-use Efficiency. J. Range Manag. 1982, 35, 623–627. Available online: https://pdfs.semanticscholar.org (accessed on 30 January 2020). [CrossRef]
- Guretzky, J.A.; Biermacher, J.T.; Cook, B.J.; Kering, M.K.; Mosali, J. Switchgrass for forage and bioenergy: Harvest and nitrogen rate effects on biomass yields and nutrient composition. Plant Soil 2011, 339, 69–81. [Google Scholar] [CrossRef]
- Rehm, G.W. Yield and quality of a warm-season grass mixture treated with N, P, and atrazine. Agron. J. 1984, 76, 731–734. [Google Scholar] [CrossRef]
- Mohammed, T.A.; Raun, W.; Kakani, G.; Zhang, H.; Taylor, R.; Desta, K.G.; Jared, C.; Mullock, J.; Bushong, J.; Sutradhar, A.; et al. Nutrient sources and harvesting frequency on quality biomass production of switchgrass (Panicum virgatum L.) for biofuel. Biomass Bioenergy 2015, 81, 242–248. [Google Scholar] [CrossRef]
- Crolla, A.; Kinsley, C.; Pattey, E. Land Application of Digestate; Woodhead Publishing Limited: Philadelphia, PA, USA, 2013; pp. 302–325. [Google Scholar]
- George, J.R.; Obermann, D. Spring defoliation to improve summer supply and quality of switchgrass. Agron. J. 1989, 81, 47–52. [Google Scholar] [CrossRef]
- Cherney, J.H.; Cherney, D.J.R.; Fox, D.G.; Chase, L.E.; Van Soest, P.J. Evaluating forages for dairy cattle. In Proceedings of the American Forage and Grassland Council, Lancaster, PA, USA, 6–10 March 1994; p. 207. [Google Scholar]
- Richner, J.M.; Kallenbach, R.L.; Roberts, C.A. Dual Use Switchgrass: Managing Switchgrass for Biomass Production and Summer Forage. Agron. J. 2014, 106, 1438–1444. [Google Scholar] [CrossRef]
- Kering, M.K.; Guretzky, J.A.; Interrante, S.M.; Butler, T.J.; Biermacher, J.T.; Mosali, J. Harvest Timing Affects Switchgrass Production, Forage Nutritive Value, and Nutrient Removal. Crop Sci. 2013, 53, 1809–1817. [Google Scholar] [CrossRef]
- Waramit, N.; Moore, K.J.; Fales, S.L. Forage quality of native warm season grasses in response to nitrogen fertilisation and harvest date. Anim. Feed Sci. Technol. 2012, 174, 46–59. [Google Scholar] [CrossRef]
- Giannoulis, K.; Bartzialis, D.; Skoufogianni, E.; Danalatos, N. Nutrients Use Efficiency and Uptake Characteristics of Panicum virgatum for Fodder Production. J. Agric. Sci. 2017, 9, 233–234. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Beef Cattle: Seventh Revised Edition: Update 2000; The National Academies Press: Washington, DC, USA, 2000. [Google Scholar] [CrossRef]
- Marschner, P. Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: London, UK, 2011; pp. 135–178. [Google Scholar]
- Piątek, M.; Bartkowiak, A. Assessment of selected physicochemical properties of soil fertilised with digestate. Water-Environ.-Rural Areas 2019, 19, 55–66. [Google Scholar]
- Skowrońska, M.; Filipek, T. Life cycle assessment of fertilisers: A review. Int. Agrophys. 2013, 28, 101–110. [Google Scholar] [CrossRef]
- Khalil, M.I.; Hossain, M.B.; Schmidhalter, U. Carbon and nitrogen mineralization in different upland soils of the subtropics treated with organic materials. Soil Biol. Biochem. 2005, 37, 1507–1518. [Google Scholar] [CrossRef]
- Bengtsson, G.; Bengtson, P.; Mansson, K.F. Gross nitrogen mineralization-, immobilization-, and nitrification rates as a function of soil C/N ratio and microbial activity. Soil Biol. Biochem. 2013, 35, 143–154. [Google Scholar] [CrossRef]
- Brodowski, S.; Amelung, W.; Haumaier, L.; Abetz, Z.; Zech, W. Morphological and chemical properties of black carbon in physical soil fractions as revealed by scanning electron microscopy and energy dispersive x-ray spectroscopy. Geoderma 2005, 128, 116. [Google Scholar] [CrossRef]
- Cheng, C.H.; Lehmann, J.; Thies, J.E.; Burton, S.D.; Engelhard, M.H. Oxidation of black carbon by biotic and abiotic processes. Org. Geochem. 2006, 37, 477. [Google Scholar] [CrossRef]
- Łabętowicz, J.M.; Kuszelewski, L.; Korc, M.; Szulc, W. The importance of organic fertilisation for crop stability and ionic balance of light soil. Zesz. Prob. Post. Nauk Rol. 1999, 465, 123–1334. [Google Scholar]
- Sanik, J., Jr.; Perhins, A.T.; Schrenk, W.G. The effect of the calcium -magnesium ratio on the solubility and availability of plant nutrients. Soil Sci. Soc. Am. Proc. 1952, 16, 263–267. [Google Scholar] [CrossRef]
- Möller, K.; Müller, T. Effects of anaerobic digestion on digestate nutrient availability and crop growth: A review. Eng. Life Sci. 2012, 12, 242–257. [Google Scholar] [CrossRef]
- Zirkler, D.; Peters, A.; Kaupenjohann, M. Elemental composition of biogas residues: Variability and alteration during anaerobic digestion. Biomass Bioenergy 2014, 67, 89–98. [Google Scholar] [CrossRef]
| Activity | Year of the Experiment | ||
|---|---|---|---|
| 2016 | 2017 | 2018 | |
| Mineral fertilisation | 24 April | 13 May | 10 May |
| Planting | 29 April | - | - |
| Biogas digestate application | 25 May | 15 May | 12 May |
| Mineral nitrogen application | 27 May | 30 May | 28 May |
| Weed control | 14 June | - | - |
| First cut | Only One on 16 November | 13 July | 10 July |
| Second cut | 10 October | 14 October | |
| Parameters | Years | ||
|---|---|---|---|
| 2016 | 2017 | 2018 | |
| Dry matter (g kg−1) | 42.9 ± 1.72 | 62.4 ± 3.12 | 65.6 ± 3.94 |
| pH (in 1 mol−1 KCl) | 7.8 ± 0.39 | 8.2 ± 0.35 | 8.5 ± 0.39 |
| Corg (g kg−1) | 343.8 ± 15.5 | 339.0 ± 11.9 | 357.0 ± 15.0 |
| Ntot. (g kg−1) | 2.0 ± 0.07 | 3.0 ± 0.12 | 3.1 ± 0.15 |
| C:N | 17.19 ± 0.62 | 11.30 ± 0.46 | 11.52 ± 0.05 |
| P (mg kg−1) | 460 ± 18.8 | 1090 ± 39.2 | 1040 ± 55.1 |
| K (mg kg−1) | 1167 ± 61.8 | 1155 ± 54.3 | 1225 ± 53.0 |
| Ca (mg kg−1) | 552.5 ± 23.8 | 1140 ± 58.1 | 1900 ± 100.7 |
| Mg (mg kg−1) | 366.5 ± 18.7 | 131.5 ± 6.4 | 272 ± 14.1 |
| Na (mg kg−1) | 498.3 ± 16.9 | 536.2 ± 23.1 | 597.5 ± 23.3 |
| Cu (mg kg−1) | 7.00 ± 0.27 | 18.7 ± 0.94 | 12.5 ± 0.54 |
| Zn (mg kg−1) | 44.2 ± 2.25 | 71.8 ± 3.50 | 93.9 ± 4.40 |
| Fe (mg kg−1) | 371 ± 21.9 | 688 ± 1.72 | 699.5 ± 37.9 |
| Mn (mg kg−1) | 31.5 ± 1.92 | 72.2 ± 37.8 | 96.5 ± 6.8 |
| Pb (mg kg−1) | <0.1 ± 0.00 | <0.1 ± 0.00 | <0.1 ± 0.00 |
| Ni (mg kg−1) | <0.1 ± 0.00 | <0.1 ± 0.00 | <0.1 ± 0.00 |
| Cr (mg kg−1) | <0.1 ± 0.00 | <0.1 ± 0.00 | <0.1 ± 0.00 |
| Cd (mg kg−1) | 0.20 ± 0.01 | 0.27 ± 0.02 | 0.18 ± 0.01 |
| Biomass | Number of Panicles per Plant | Panicles Height | Leaf Percentage | |||||
|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | |
| Year (Y) | 12.59 | ** | 13.9 | ** | 23.0 | *** | 0.84 | n.s. |
| Cut (C) | 2437.1 | *** | 7420.5 | *** | 1845.0 | *** | 20.86 | *** |
| Fertilisation (F) | 116.3 | *** | 1.0 | n.s. | 131.0 | *** | 0.79 | n.s. |
| Y × C | 0.22 | n.s. | 1.3 | n.s. | 23.0 | *** | 0.99 | n.s. |
| Y × F | 0.35 | n.s. | 0.3 | n.s. | 0.0 | n.s. | 1.02 | n.s. |
| C × F | 0.46 | n.s. | 40.2 | *** | 8.0 | ** | 0.96 | n.s. |
| Cut Date | Fertili-sation | Number of Tillers per Plant | Height (cm) | Content in Switchgrass Biomass (g kg−1 d.w.) | |||
|---|---|---|---|---|---|---|---|
| Crude Ash | Crude Protein | Crude Fat | Crude Fibre | ||||
| First | O | 110b ± 3.81 | 92.9d ± 1.32 | 55.5a ± 0.40 | 80.3ab ± 0.37 | 25.8d ± 1.40 | 351.6c ± 2.44 |
| NPK | 152d ± 2.49 | 107.2e ± 1.98 | 61.2b ± 0.43 | 106.2e ± 0.59 | 23.7cd ± 1.06 | 352.5c ± 3.52 | |
| POF1 | 126c ± 6.12 | 111.0ef ± 2.00 | 65.9c ± 0.38 | 86.2bc ± 0.87 | 23.8c ± 0.75 | 351.1c ± 3.81 | |
| POF2 | 157d ± 6.17 | 113.8f ± 2.08 | 65.5c ± 0.38 | 92.0cd ± 1.11 | 20.6b ± 0.61 | 346.6c ± 4.30 | |
| Second | O | 20a ± 0.98 | 71.1a ± 0.97 | 122.6g ± 1.30 | 73.3a ± 0.64 | 13.0a ± 0.73 | 321.3b ± 7.50 |
| NPK | 15a ± 0.76 | 77.7b ± 0.82 | 92.8d ± 1.17 | 108.1e ± 5.86 | 14.3a ± 0.36 | 303.3a ± 2.83 | |
| POF1 | 18a ± 0.43 | 81.5c ± 0.72 | 117.4f ± 1.87 | 106.5e ± 1.35 | 14.8a ± 0.51 | 325.8b ± 3.58 | |
| POF2 | 16a ± 0.67 | 84.1c ± 0.87 | 106.2e ± 1.16 | 103.5de ± 1.58 | 12.6a ± 0.23 | 317.3ab ± 4.27 | |
| Years | 2017 | 74.0A ± 12.4 | 90.6A ± 2.86 | 86.50A ± 5.60 | 94.25A ± 3.00 | 17.6A ± 1.07 | 340.6B ± 4.11 |
| 2018 | 79.2B ± 13.1 | 94.2B ± 3.58 | 85.20A ± 5.00 | 94.80A ± 2.70 | 18.1A ± 1.06 | 326.8A ± 3.84 | |
| Dry Matter | Crude Ash | Crude Protein | Crude Fat | Crude Fibre | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | F | p | |
| Year (Y) | 0.70 | n.s. | 0.70 | n.s. | 0.10 | n.s. | 0.853 | n.s. | 32.7 | *** |
| Cut (C) | 0.10 | n.s. | 9426.2 | *** | 14.2 | ** | 143.6 | *** | 195.6 | *** |
| Fertilisation (F) | 12.8 | *** | 119.6 | *** | 52.7 | *** | 6.3 | ** | 4.2 | * |
| Y × C | 0.00 | n.s. | 25.0 | *** | 0.50 | n.s. | 0.05 | n.s. | 0.0 | n.s. |
| Y × F | 1.2 | n.s. | 0.50 | n.s. | 0.04 | n.s. | 0.01 | n.s. | 0.2 | n.s. |
| C × F | 0.90 | n.s. | 196.3 | ** | 10.1 | *** | 58.4 | *** | 5.4 | ** |
| NDF | ADF | ADL | Hemicelulose | Celulose | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | F | p | |
| Year (Y) | 120.0 | *** | 48.3 | *** | 5.32 | * | 68.0 | *** | 56.0 | *** |
| Cut (C) | 873.0 | *** | 304.2 | *** | 258.6 | *** | 592.0 | *** | 220.0 | *** |
| Fertilisation (F) | 16.0 | *** | 10.7 | *** | 11.51 | *** | 6.0 | * | 13.0 | *** |
| Y × C | 0.0 | n.s. | n.s. | n.s. | 0.00 | n.s. | 0.0 | n.s. | 0.0 | n.s. |
| Y × F | 1.0 | n.s. | n.s. | n.s. | 0.04 | n.s. | 0.0 | n.s. | 0.0 | n.s. |
| C × F | 63.0 | *** | 4.2 | * | 13.09 | *** | 124.0 | *** | 2.0 | n.s. |
| P | K | Ca | Mg | Na | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | F | p | |
| Year (Y) | 0.02 | n.s. | 22.2 | *** | 4.73 | * | 14.61 | *** | 1.90 | n.s. |
| Cut (C) | 2.33 | n.s. | 77.06 | *** | 302.0 | *** | 306.1 | *** | 44.5 | *** |
| Fertilisation (F) | 3.17 | * | 17.78 | *** | 0.865 | n.s. | 27.4 | *** | 4.95 | ** |
| Y x C | 0.09 | n.s. | 9.73 | ** | 1.85 | n.s. | 4.73 | * | 0.01 | n.s. |
| Y x F | 0.19 | n.s. | 9.67 | *** | 0.99 | n.s. | 2.81 | n.s. | 1.34 | n.s. |
| C x F | 4.92 | ** | 74.31 | *** | 9.13 | *** | 41.28 | *** | 10.4 | *** |
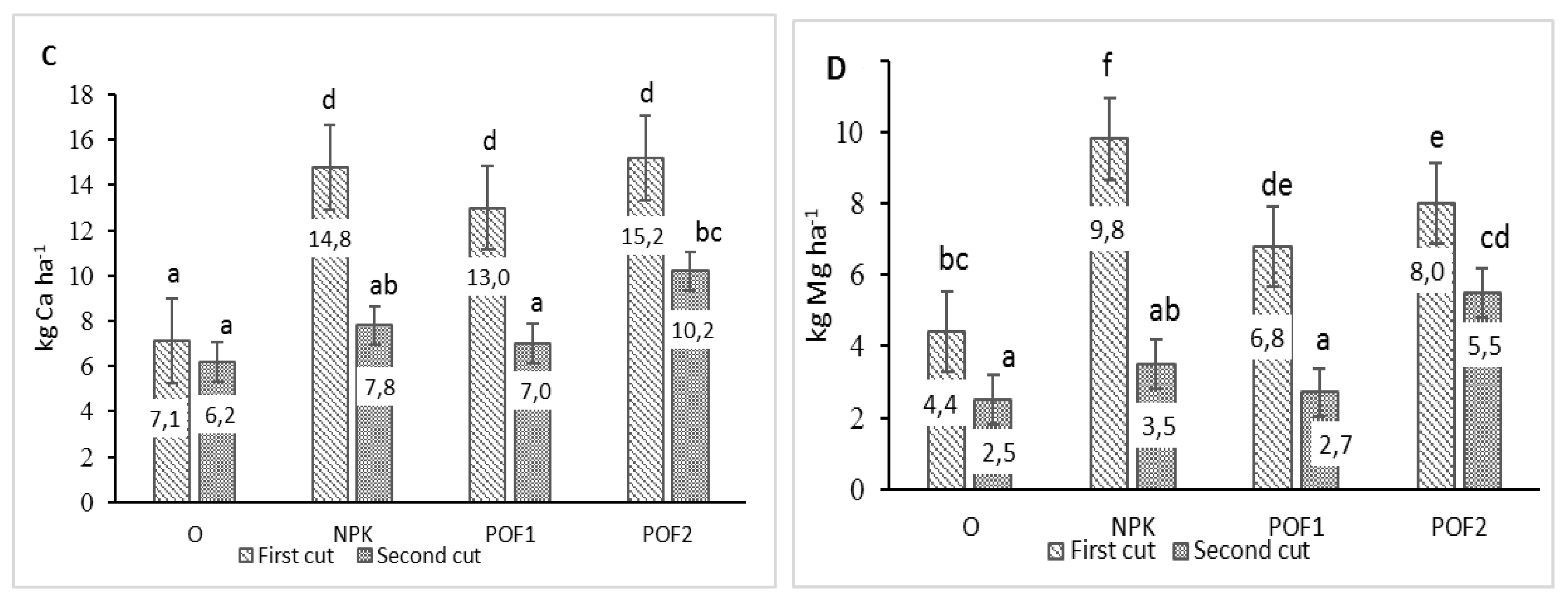
| Cut Date | Fertilisation | Macronutrient Content (g kg−1 d.w.) | ||||
|---|---|---|---|---|---|---|
| P | K | Ca | Mg | Na | ||
| First | O | 1.92ab ± 0.05 | 9.15a ± 0.43 | 1.65a ± 0.14 | 1.00a ± 0.03 | 0.35ab ± 0.01 |
| NPK | 2.15abc ± 0.03 | 14.33d ± 0.27 | 2.26b ± 0.07 | 1.50bc ± 0.05 | 0.48c ± 0.01 | |
| POF1 | 2.02abc ± 0.35 | 12.30b ± 0.30 | 1.98ab ± 0.05 | 1.03a ± 0.02 | 0.43bc ± 0.02 | |
| POF2 | 1.99abc ± 0.35 | 14.26cd ± 0.20 | 1.95ab ± 0.15 | 1.02a ± 0.04 | 0.41bc ± 0.02 | |
| Second | O | 1.80a ± 0.24 | 12.54bc ± 0.33 | 4.51d ± 0.23 | 1.34b ± 0.07 | 0.27a ± 0.03 |
| NPK | 2.13abc ± 0.02 | 11.40b ± 0.48 | 3.62cd ± 0.19 | 1.62cd ± 0.05 | 0.39b ± 0.02 | |
| POF1 | 2.61bc ± 0.99 | 8.67a ± 0.58 | 3.47c ± 0.15 | 1.83d ± 0.07 | 0.34ab ± 0.02 | |
| POF2 | 2.32c ± 0.08 | 9.31a ± 0.80 | 4.06cd ± 0.27 | 2.18e ± 0.16 | 0.28a ± 0.03 | |
| Years | 2017 | 2.11A ± 0.07 | 11.01A ± 0.52 | 2.79A ± 0.20 | 1.36A ± 0.07 | 0.36A ± 0.02 |
| 2018 | 2.03A ± 0.07 | 11.98B ± 0.43 | 3.10B ± 0.25 | 1.52B ± 0.10 | 0.38A ± 0.01 | |
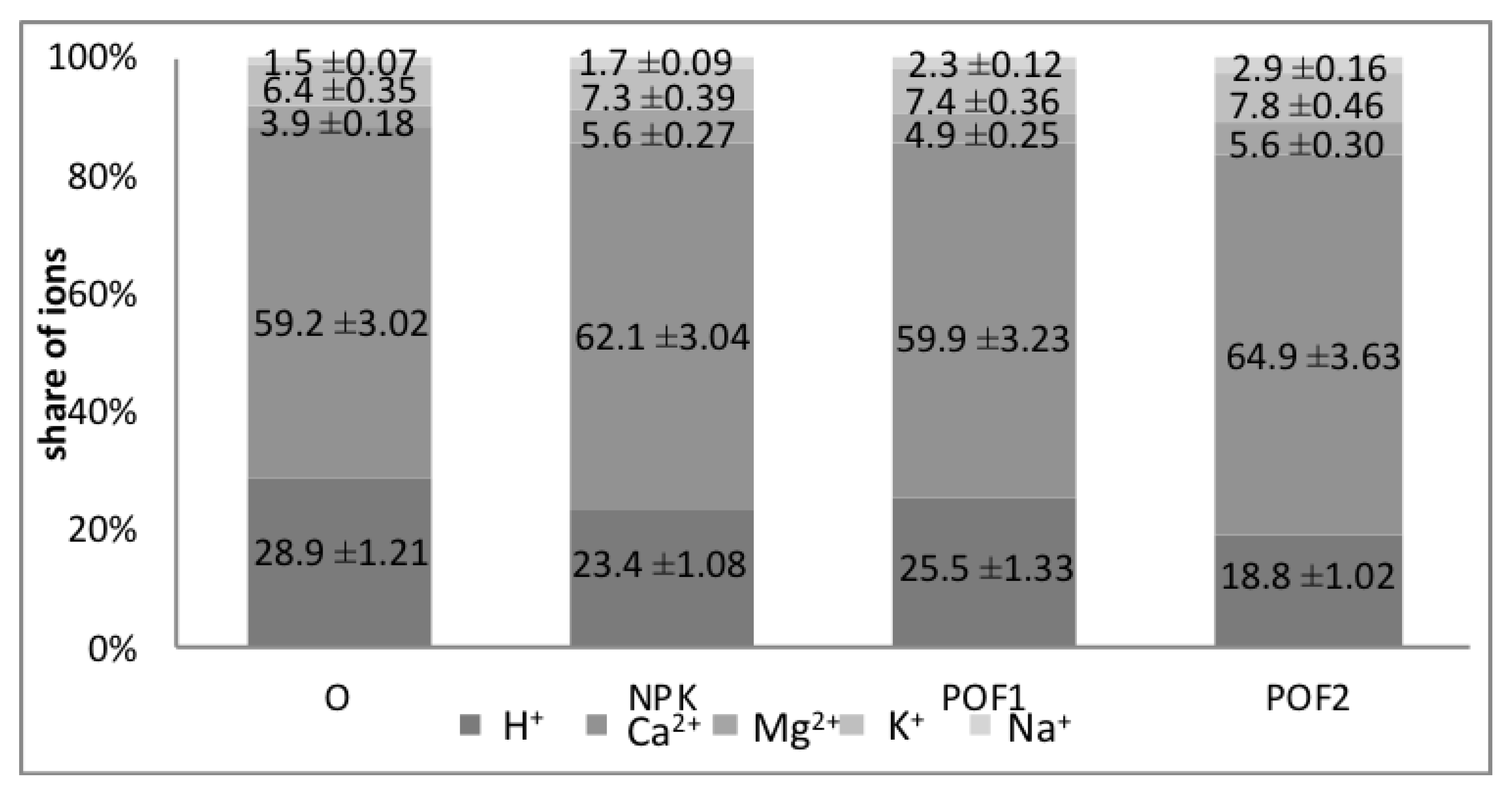
| Parameters | Autumn 2018 | |||
|---|---|---|---|---|
| O | NPK | POF1 | POF2 | |
| pHKCL | 4.5a ± 0.20 | 4.6a ± 0.22 | 4.6a ± 0.21 | 4.9b ± 0.23 |
| Corg (g kg−1) | 5.7a ± 0.24 | 5.9ab ± 0.28 | 6.2bc ± 0.27 | 6.4c ± 0.28 |
| Nog. (g kg−1) | 0.64a ± 0.02 | 0.77b± 0.11 | 0.73b ± 0.10 | 0.76b ± 0.11 |
| C:N | 9.06c ± 0.38 | 7.92a ± 0.34 | 8.08ab ± 0.31 | 8.16b ± 0.34 |
| Hydrolytic acidity (Hh) (mmol H+ kg−1) | 31.5b ± 1.74 | 30.2b ± 1.58 | 30.1b ± 1.36 | 25.1a ± 1.31 |
| Cation exchange capacity (CEC) (mmol (+) kg−1) | 104.1a ± 5.83 | 129.2bc ± 6.98 | 123.5b ± 6.05 | 133.2c ± 6.93 |
| The sum of exchangeable cations (EAC) (mmol (+) kg−1) | 74.0a ± 3.48 | 99.0c ± 5.05 | 92.0b ± 4.78 | 108.1d ± 5.73 |
| Sorption complex saturation with exchangeable cations (BS) (%) | 71.1a ± 3.25 | 76.6b ± 3.68 | 74.4b ± 3.30 | 81.2c ± 4.22 |
| Content of available nutrients | ||||
| P (mg kg−1) | 34.3a ± 1.77 | 51.1b ± 2.30 | 51.5b ± 2.58 | 52.8b ± 2.75 |
| K (mg kg−1) | 40.7a ± 2.36 | 55.6b ± 3.00 | 56.4b ± 3.22 | 70.6c ± 4.20 |
| Mg (mg kg−1) | 43.0a ± 2.19 | 70.0c ± 4.13 | 63.0b ± 3.28 | 66.0bc ± 3.37 |
| Cu (mg kg−1) | 1.4a ± 0.06 | 1.9b ± 0.07 | 1.3a ± 0.05 | 1.9b ± 0.08 |
| Zn (mg kg−1) | 5.6a ± 0.33 | 6.0b ± 0.29 | 5.8ab ± 0.31 | 7.4c ± 0.34 |
| Fe (mg kg−1) | 903ab ± 31.5 | 933c ± 34.5 | 877a ± 29.7 | 914b ± 34.6 |
| Mn (mg kg−1) | 189.0c ± 9.07 | 179.0b ± 8.06 | 171.0ab ± 8.04 | 166.0a ± 7.99 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Głowacka, A.; Szostak, B.; Klebaniuk, R. Effect of Biogas Digestate and Mineral Fertilisation on the Soil Properties and Yield and Nutritional Value of Switchgrass Forage. Agronomy 2020, 10, 490. https://doi.org/10.3390/agronomy10040490
Głowacka A, Szostak B, Klebaniuk R. Effect of Biogas Digestate and Mineral Fertilisation on the Soil Properties and Yield and Nutritional Value of Switchgrass Forage. Agronomy. 2020; 10(4):490. https://doi.org/10.3390/agronomy10040490
Chicago/Turabian StyleGłowacka, Aleksandra, Bogdan Szostak, and Renata Klebaniuk. 2020. "Effect of Biogas Digestate and Mineral Fertilisation on the Soil Properties and Yield and Nutritional Value of Switchgrass Forage" Agronomy 10, no. 4: 490. https://doi.org/10.3390/agronomy10040490
APA StyleGłowacka, A., Szostak, B., & Klebaniuk, R. (2020). Effect of Biogas Digestate and Mineral Fertilisation on the Soil Properties and Yield and Nutritional Value of Switchgrass Forage. Agronomy, 10(4), 490. https://doi.org/10.3390/agronomy10040490






