Abstract
Compost tea is a liquid fraction extracted from composts, and it is of great interest in sustainable agriculture because it reduces the unsustainable use of chemical-based pesticides and fertilizers. In this study, during two spinach field cycles, we evaluated the potential beneficial effect of the foliar application of a compost tea made from onion and vineyard composts either by itself (CT) or implemented with the beneficial microorganism Trichoderma harzianum T78 (CT + Th) on the “healthy quality” and yield of baby spinach. Results showed that both the CT and CT + Th treatments produced a higher spinach yield than the control, but these treatments did not result in an increase in soil dehydrogenase activity (DHA) or soil nutrient content. Furthermore, CT + Th treatment showed the highest yield, phenolic content, antioxidant capacity and flavonoid levels. Nitrate levels were below legal amounts, and they were significantly (p ≤ 0.05) lower in the CT and CT + Th treatments than in the control. Data suggest that compost tea extracts from onion waste and vineyard compost and/or enriched with T. harzianum can be used in a sustainable agriculture to increase yield and quality of baby spinach.
1. Introduction
Spinach is one of the healthiest vegetables in the human diet due to its high concentration of phytonutrients and health-promoting compounds [1]. It is a good source of vitamins and mineral elements, such as calcium, iron, phosphorus, sodium and potassium [2]. Baby spinach is characterized by its tiny and perfectly tender leaves, which are smaller (between 8 and 12 cm) and tenderer than the larger, mature spinach leaves. The production cycles are very fast, and the spinach can be harvested between 45–60 days after sowing in the Mediterranean region.
Composts teas (CTs) are oxygenated compost water extracts obtained through a suitable liquid-phase blowing process. The application of CTs benefits plant yields, mainly by improving the physiological status of the plant and/or enhancing protection from pathogens [3]. CTs have been found to stimulate root and vegetative growth [4], showing behavior close to that of biostimulants [5]. This behavior is related to the, the microorganisms within the compost tea, which lead to pest suppression and enhancement of microbial communities improving nutrient uptake or production of bioactive compounds [6]. The potential of CTs for supplementing or substituting other types of fertilizers is also promising [7]. It is well documented, however, that effects of a given CT depend on the source of the cultures and raw materials used for its production [6,7,8]. Composts from agro-industrial waste are considered more advantageous than other kinds of organic wastes [9], because they present a lower risk for pathogens, heavy metals and pharmaceuticals [8]. Furthermore, the efficiency of CTs can be increased if they are supplemented with beneficial microorganisms like Trichoderma sp., which can promote seedling establishment and enhance plant growth and plant defense reactions in some vegetable crops [10,11,12,13].
The objective of this work was to assess the effects of a compost tea made from onion and vineyard composts, both on its own (CT) and supplemented with the beneficial microorganism T. harzianum T78 (CT + Th) in comparison with untreated soil (control) on baby spinach yield in open field conditions in two crop experiments. We also tested the effects of these treatments on nutraceutics concentration in the spinach leaves, such as phenolic and flavonoid content and antioxidant capacity. We also studied the leaf nitrate concentrations as a limiting parameter for marketing and anti-nutritional compound. Finally, we determined the effect of these treatments on soil microbial activity, nutrient content and soil-borne fungal pathogens after the crop was finished, in order to assess whether any residual effect could affect soil quality at the end of the experiments.
2. Materials and Methods
2.1. Compost Tea Production
An onion waste (67.6%) and a vineyard residue (32.4%) compost was produced on a dry-weight basis at the University Miguel Hernandez (UMH) composting site. The composting process (15 Tn) was carried out using open-air piles (15 Tn) with a bio-oxidative phase of 75 days and a maturation phase of 40 days. The piles were turned periodically to ensure aeration and to control the temperature. Once the composting process was finished (120 days), the compost was milled and passed through a 2 cm sieve and stored at 4 °C until use. The compost tea (CT) was prepared weekly by mixing compost with distillate water in the ratio 1:100 w/v by a continuous forced air blowing system at 25 ± 2 °C for 24 h, just before foliar application. The mix was filtered through cheesecloth and stored at 4 °C until use. After that, the mixture was diluted (1:9; v/v) to obtain the CT used to spray the experimental spinach fields as suggested by Pane et al. [14]. An aliquot of every CT used for spraying was sampled in each experiment and freeze-dried for chemical analyses. The main compost and CT chemical characteristics are shown in Table 1.

Table 1.
Main characteristics of compost and compost tea (CT) used in the experiments (EW: early winter; LW: late winter). Values are the mean ± SD (n = 8).
2.2. Plant Material, Experimental Set-Up and Design
The field experiments were conducted in the “Campo de Cartagena” (Cartagena, Murcia, Spain) in two close plots with the following soil characteristics: TOC 36.54 ± 0.37 g kg−1; Total N 5.27 ± 0.12 g kg−1; Total P 0.39 ± 0.03 g kg−1; Total K 7.97 ± 0.47 g kg−1. A cultivar of baby spinach (Spinacia oleracea), “Maya” (Enza Zaden), was cultivated. The first experiment, “early winter”, was conducted from 7 December 2017 to 22 February 2018, with a duration of 77 days (11 weeks); the second experiment, “late winter”, was conducted from 12 February 2018 to 2 April 2018, with a shorter cropping duration of 49 days (7 weeks). The average temperature and radiation for the early winter was 11.35–10.66 °C and 125.77–121.37 w/m2, respectively. The average temperature and radiation for the late winter was 13.88–12.98 °C and 201.44–152.49 w/m2, respectively (SIAM, IMIDA).
The experimental design was a randomized complete block design with three replicates per treatment. Each replicate was carried out in 5 × 2 m plots randomly located. The spinach seeds were sown at a ratio of 700–900 seeds per m−2 (according to the company protocol for this crop). The treatments were sprayed over each spinach plot once a week, beginning one week after sowing for a total of 8 times in the early winter and 6 times in the late winter, with the following equivalents: (a) CT at 12 m3 per ha (CT); (b) CT at 12 m3 plus T. harzianum T78, (Trichosym Bio, Symborg S.L, Murcia, Spain) to 5 × 108 cfu mL−1 per ha (CT + Th); and (c) the Control, which consisted of spraying the same volume of distilled water at 12 m3 per ha (Control).
2.3. Harvesting
Harvest was carried out when the leaves reached the commercial value (8–12 cm in length). The spinach harvested from each plot was weighed for yield (g m−2) determination. Furthermore, 20 spinach leaves for each replicate were used to measure (a) the foliar area by WinRhizo (Regents Instruments Inc., Quebec City, Quebec, Canada) (cm2) and (b) the nitrate content, phenolic and flavonoid content and antioxidant capacity. The nitrate content was analyzed by ion chromatography on fresh samples water extracted (1:10) [15]. The total phenolic content was determined by the Folin-Ciocalteu colorimetric method [16]. The total flavonoid content was determined as described by Meda et al. [17]. The antioxidant capacity was evaluated in terms of the free radical scavenging capacity [18].
2.4. Soil Chemical and Biochemical Parameters
The soil was sampled at both field experiments after spinach harvesting to a depth of 10.20 cm. Five soil cores were taken in a W-pattern from each replicate and mixed thoroughly. The following parameters were measured: total organic carbon (Total C) and nitrogen (Total N) using a LECO TruSpec C/N (LECO Corporation, St Joseph, Michigan, USA), Elemental Analyzer; total P, Na, K and Ca using inductively coupled plasma-mass spectrometry (ICP-MS; ICAP 6500 DUO, Thermo Fisher Scientific, Hayward, California, USA); and dehydrogenase activity (DHA), using the reduction of 2-p-iodo-3-nitrophenyl-5-phenyl tetrazolium chloride to iodonitrophenyl formazan method [19].
2.5. Soil-Borne Pathogens
Total DNA was extracted from the soil samples (500 mg) using the DNeasy PowerSoil Kit (Qiagen, Germantown, Maryland, USA) following the modification described by Taskin et al. [20].
Fungal pathogen detection and quantification was performed using the Vegalert qPCR quantitative kit for cucurbits (Microgaia Biotech S.L, Murcia, Spain), following the PCR conditions described by Santisima-Trinidad et al. [21]. Real-time PCR was performed using a 7500 Fast Real-Time PCR system (Applied Biosystems).
T. harzianum T-78 quantification was estimated in soils by quantitative real-time PCR (qPCR) from soil DNA [13], in a total volume of 15 µL, using a 7500 Fast Real-Time PCR system (Applied Biosystems, Foster City, California, USA) with the same PCR conditions used by Santisima-Trinidad et al. [21]. The quantification was performed in triplicate.
2.6. Statistical Analysis
The parameters adjusted to a normal distribution were subjected to multivariate analysis of variance (MANOVA). Only if there was a significant difference between groups, post hoc tests (Tukey’s) were performed.
3. Results
3.1. Effects of CT on Baby-Leaf Spinach Growth and Quality
The spinach yield was significantly higher in both CT treatments (CT and CT + Th) than in the control in both experiments (early and late winter) (Figure 1a, Table 2). The CT + Th treatment showed on average 51% more yield than the control and 15% more than CT. No significant differences were observed between the experiments (Table 2). The foliar area in the CT was significantly higher than in the control and CT + Th (Figure 1, Table 2). Furthermore, the foliar area in late winter was on average 66% lower than in early winter (Figure 1b).
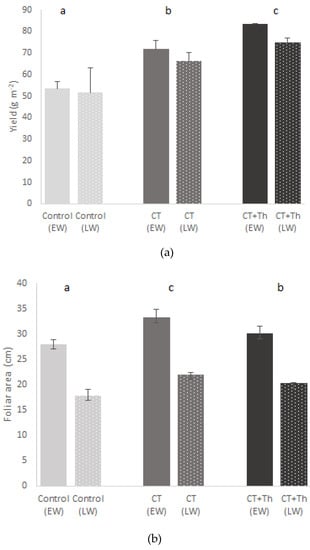
Figure 1.
Effect of different compost tea (CT) treatments on yield (a) and foliar area (b) of baby spinach in both experiments. Bars are mean values n = 3. Error bars indicate standard deviation. Control: sprayed distilled water (12 m3 per ha); CT: sprayed compost tea (12 m3 per ha); CT + Th: sprayed compost tea (12 m3 per ha) and T. harzianum T-78 (5 × 108 cfu mL−1 per ha). EW: early winter and bars without points; LW: late winter and bars with points. Different lowercase letters about bars indicate differences between treatments (p ≤ 0.05) in EW and different uppercase letters about bars indicate differences between treatments (p ≤ 0.05) in LW.

Table 2.
Influence of different CT treatments on yield, foliar area, total phenolic, total flavonoids, antioxidant capacity and nitrate content in both experiments (early winter and late winter).
The quality of the baby-leaf spinach as measured by total flavonoids, total phenols and the antioxidant capacity did show significant interaction between treatment and crop cycle (Table 2). In terms of quality, on average, the CT + Th showed 13% higher total flavonoids and total phenolic content, and 25% higher content of antioxidant capacity than the CT and control (Figure 2). Moreover, flavonoids and antioxidant capacity values were respectively 74% higher in late winter than in early winter, while for phenols were on average 77% higher. On the other hand, we also observed a significant difference in the interaction between treatment and experiment for nitrate content (Table 2). The spinach nitrate content showed lower values in the CT and CT + Th than in the control in early winter, while in late winter it was only CT + Th. The spinach nitrate content was lower in early winter than in late winter (Figure 3).
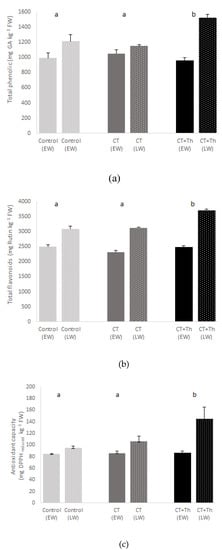
Figure 2.
Effect of different compost tea (CT) treatments on total phenolic (a) total flavonoids (b) and antioxidant capacity (c) of baby spinach in both experiments. Bars are mean values n = 3. Error bars indicate standard deviation. Control: sprayed distilled water (12 m3 per ha); CT: sprayed compost tea (12 m3 per ha); CT + Th: sprayed compost tea (12 m3 per ha) and T. harzianum T-78 (5 × 108 cfu mL−1 per ha). EW: early winter and bars without points; LW: late winter and bars with points. Different lowercase letters about bars indicate differences between treatments (p ≤ 0.05) in EW and different uppercase letters about bars indicate differences between treatments (p ≤ 0.05) in LW.
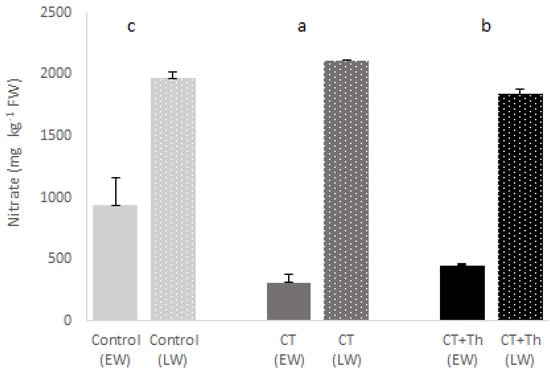
Figure 3.
Effect of different compost tea (CT) treatments on nitrate content of baby spinach in both experiments. Bars are mean values n = 3. Error bars indicate standard deviation. Control: sprayed distilled water (12 m3 per ha); CT: sprayed compost tea (12 m3 per ha); CT + Th: sprayed compost tea (12 m3 per ha) and T. harzianum T-78 (5 × 108 cfu mL−1 per ha). EW: early winter and bars without points; LW: late winter and bars with points. Different lowercase letters about bars indicate differences between treatments (p ≤ 0.05) in EW and different uppercase letters about bars indicate differences between treatments (p ≤ 0.05) in LW.
CT treatments (CT and CT + Th) significantly increased the nutrient content of the spinach leaves (P and Ca) compared to the control treatment in early winter (Table 3). Moreover, the nutrient content of the baby spinach was significantly different according to the experiment.

Table 3.
Influence of different compost tea (CT) treatments on baby spinach tissue nutrient content in both experiments. Values are the mean + SD; n = 3). Control: sprayed distilled water (12 m3 per ha); CT: sprayed compost tea (12 m3 per ha); CT + Th: sprayed compost tea (12 m3 per ha) and T. harzianum T-78 (5 × 108 cfu mL−1 per ha). EW: early winter; LW: late winter. Different lowercase letters indicate differences between treatments (p ≤ 0.05) in EW and different uppercase letters indicate differences between treatments (p ≤ 0.05) in LW.
3.2. Effects of CT on Soil Chemical Properties and Soil Microbial Activity
After harvesting, we analyzed the soil chemical parameters (Table 4). We only found significant differences between treatments in the total organic C content, which was significantly higher in CT and CT + Th than in the control (Table 4). We also observed significant differences between crop cycles: total organic C was higher in early winter, while Total N, P and K were higher in late winter (Table 3). Dehydrogenase activity (DHA) showed significant differences between both crop cycles, but not between treatments (Figure 4).

Table 4.
Influence of different compost tea (CT) treatments on soil chemical properties and soil microbial activity in both experiments after harvesting. Values are the mean + SD; n = 3). Control: sprayed distilled water (12 m3 per ha); CT: sprayed compost tea (12 m3 per ha); CT + Th: sprayed compost tea (12 m3 per ha) and T. harzianum T-78 (5 × 108 cfu mL−1 per ha). EW: early winter; LW: late winter. Different lowercase letters indicate differences between treatments (p ≤ 0.05) in EW and different uppercase letters indicate differences between treatments (p ≤ 0.05) in LW.
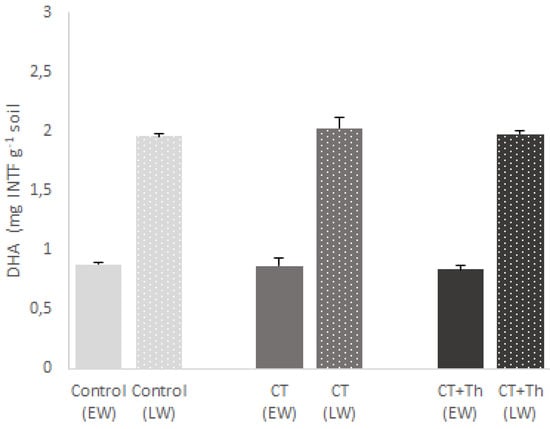
Figure 4.
Effect of different compost tea (CT) treatments on soil dehydrogenase activity (DHA) (mg INTF g−1 soil) Bars are mean values n = 3. Error bars indicate standard deviation. Control: sprayed distilled water (12 m3 per ha); CT: sprayed compost tea (12 m3 per ha); CT + Th: sprayed compost tea (12 m3 per ha) and T. harzianum T-78 (5 × 108 cfu mL−1 per ha). EW: early winter and bars without points; LW: late winter and bars with points. (Treatment: F = 2.145; p = 0.160; Cycle: F = 5604.522; p = 0.000; Interaction: F = 2.167; p = 0.157). Different lowercase letters indicate differences between treatments (p ≤ 0.05) in EW and different uppercase letters indicate differences between treatments (p ≤ 0.05) in LW.
3.3. Effects of CT on Soil-Borne Fungal Pathogen Abundance and Disease Incidence
Among the different fungal pathogens analyzed in soils by Vegalert qPCR, only Alternaria sp., Fusarium oxysporum, Fusarium solani and Stemphylium botryosum were detected in both experiments (Table 5). Furthermore, significant differences were only observed between crop cycles: the abundance of fungal pathogens was significantly higher in late winter than in early winter (Table 5). In spite of the presence of the above-mentioned pathogens in the soils studied, the baby spinach did not show any disease incidence.

Table 5.
Influence of different compost tea (CT) treatments on abundance (log copies gen g−1 soil) of fungal pathogen in soil after harvesting in both experiments. Values are the mean + SD; n = 3). Control: sprayed distilled water (12 m3 per ha); CT: sprayed compost tea (12 m3 per ha); CT + Th: sprayed compost tea (12 m3 per ha) and T. harzianum T-78 (5 × 108 cfu mL−1 per ha). EW: early winter; LW: late winter.
4. Discussion
The compost showed similar nutrient contents, pH and EC values to those of other agro-industrial composts [22,23]. The germination index of the compost (88%) demonstrated no negative germination effect [24] considering that compost reached the stable and mature stage validating its suitability for using in compost tea preparation [25,26,27].
4.1. Effects of CT on Baby Spinach Growth and Quality
Applying CT produced a higher spinach yield and foliar area than the control treatment. This improvement corroborates previous studies, such as those conducted by Hargreaves et al. [28], Marin et al. [29] and Bernal-Vicente et al. [26]. This fact could be attributed to a combination of factors, including beneficial plant microorganisms like biostimulants, biofertilisers, biopesticide microorganisms and/or growth promoter compounds like phytohormones that can be found in compost teas [30,31]. In our experiment, in general, no nutrient differences were observed in the spinach tissue in the CT treatments, except in the case of Ca and P. The amount of nutrients applied through the CT treatment should be minimal in comparison to the amount of nutrients applied via chemical fertilizers in commercial crops. However, Micheal [32] pointed out that nutrients from compost tea could be also responsible for yield increase, demonstrating that as soon as one hour after application, some nutrients were in the plant rhizosphere available to be taken in. From our results, it therefore seems more plausible that a kind of biostimulation could be responsible for the yield increase. Furthermore, Morales-Corts et al. [7] indicated that CT from green waste materials usually shows the presence of indole 3-acetic-acid (IAA) and salicylic acid-like compounds [33] that could positively affect spinach growth.
The incorporation of T. harzianum into the compost tea (CT + Th) also increased the spinach yield. It could be due to the secondary metabolites released by T. harzianum that might influence plant growth, mainly through signaling hormone-like compounds, most notably auxins [33]. The positive effect of T. on plant growth is equally remarkable and has been recognized as an ability independent to its antifungal ability [34,35,36], because an increase in growth has been observed in the absence of any detectable diseases and in sterile soil [37].
Bioactive compounds (flavonoids, phenolic acids, and tannins, among others) are extra nutritional constituents that naturally occur in small quantities in plant and food products, and they are considered human health-promoters [38,39]. The incorporation of T. harzianum into the compost tea (CT + Th) also increased the antioxidant capacity and phenolic compounds by 42% and 29%, respectively, compared to the control and CT treatments in the late winter but not in early winter, probably due to the higher amount of T. harzianum in the soil. Hua-Bin et al. [40] found a strong correlation between antioxidant activity and the total phenolic and flavonoid content in plants suggesting that phenolic compounds could be the major contributor to antioxidant capacity, thus activating or priming induced systemic resistance mechanisms [41] Similar results were observed by Yedidia et al. [42] in cucumber plants treated with Trichoderma asperellum and by Pascale et al. [41] in grapes treated with two Trichoderma strains.
Nitrate concentrations accumulated in the edible parts of the spinach leaves must be within the legal EU limits (<3500 mg kg−1 FW) (Commission regulation No1258/2011). The nitrate values of both commercial trials in this study were under the maximum allowed. The most notable result in terms of nitrate content in our assay is the fact that the spinach tissue in the CT treatment showed lower nitrate levels than in the other treatments in early winter. The reduced nitrate level in different vegetables like lettuce when is treated with compost and compost tea is something to take into account for human health and has been previously investigated [39,43] The most plausible explanation for this decrease is related to the potential inhibition of nitrifying bacteria due the potential organic mineralization rate from the incorporated CT, which would produce nitrates reducing nitrification processes [44,45,46]. The obligate chemolitho autotrophs Nitrosomonas spp. and Nitrospira spp. are particularly inhibited by the presence of organic compounds [47,48,49,50]. The increase in nitrates in the CT + Th treatment, on the other hand, is probably due to the fact that T. harzianum can act as a root nitrate uptake helper [51,52].
It is notable that the nitrate accumulation in late winter showed values similar to those found by Manojlovic et al. [53], in the range of 1000–2300 mg kg−1 FW, while the early winter values were below 500 mg kg−1 FW, similar to the pattern observed by Kapoulas et al. [54]. This is because low light conditions influence nitrate reductase activity and decrease the conversion of nitrate into amino acids, leading to a higher concentration of nitrates [55].
4.2. Effects of CT on Soil Chemical Properties and Soil Microbial Activity
The application of different CTs did not increase the soil microbial activity, although we did observe improvements in baby spinach yield and quality. It is possible that the CT dose was not enough to enhance soil microbial activity after cropping [6,7] but it was sufficient to contribute supplementary beneficial substances for plant growth that were produced by microorganisms during tea production process or contained within the original material. We likely observed an increase in total carbon in CT treatments due to the higher root exudates and plant remains directly related to the higher yield observed [56,57].
The qPCR analysis indicated an abundance of different soil fungal pathogens (Alternaria sp., Fusarium oxysporum, Fusarium solani and Stemphylium botryosum) in all treatments after harvesting, although no symptoms were observed in the spinach crop. It is possible that the amount and type of pathogens in conjunction with the environmental factors were not enough to affect spinach crops [21], or that the spinach plants are “asymptomatic hosts”.
5. Conclusions
Our results indicate that the foliar application of compost tea, from mature onion-vineyard compost, increases the yield and improves the quality of the baby spinach, which is richer in phenolic content, antioxidant capacity and flavonoids and has lower nitrate content. The efficiency of compost tea is increased when it is supplemented with Trichoderma harzianum, a biological control agent. We believe that this is the first work that shows the effectiveness of the application of this combination. However, further studies are needed on the mechanisms by which these benefits are obtained, particularly those relate to plant metabolomic and resistance induction. In addition, further research into this aspect could help to develop sustainable agricultural techniques based on the reduced use of fertilizers and pesticides. Overall, our findings suggest that the application of compost tea plus T. harzianum can be a sustainable practice in intensive cropping systems to enhance crop productivity and quality.
Author Contributions
Conceptualization, M.R. and J.A.P.; data curation, M.H.-N., A.G. and P.L.-P.; formal analysis, M.R., M.H.-N. and A.G.; funding acquisition, J.A.F. and J.A.P.; investigation, M.R., C.E.-G. and P.L.-P.; project administration J.A.F. and J.A.P.; supervision, M.R.; visualization, C.E.-G.; writing—original draft, M.R.; writing—review and editing, J.A.F., C.E.-G. and J.A.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Ministry of Economy and Competitiveness: AGL-2014-52732-C2–1-R and AGL-2014-52732-C2-2-R.
Acknowledgments
This work was supported by projects AGL-2014-52732-C2–1-R and AGL-2014-52732-C2-2-R from the Ministry of Economy and Competitiveness of Spain.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Morelock, T.E.; Correll, J.C. Spinach, in Vegetables I: Asteraceae, Brassicaceae, Chenopodiaceae, and Cucurbitaceae; Prohens, J., Nuez, F., Eds.; Springer: New York, NY, USA, 2008; pp. 189–218. [Google Scholar]
- Avsar, B. Genetic Diversity of Turkish Spinach Cultivars (Spinacia oleracea L.). Master’s Thesis, Izmir Institute of Technology, İzmir, Turkey, 2011. [Google Scholar]
- Siddiqui, Y.; Meon, S.; Ismail, R.; Rahmani, M.; Ali, A. Bio-efficiency of compost extracts on the wet rot incidence, morphological and physiological growth of okra (Abelmoschus esculentus [(L.) Moench]). Sci. Hortic. 2008, 117, 9–14. [Google Scholar] [CrossRef]
- Shaheen, M.; Rizkf, A.; Sawano, M.; Bakrym, O. Sustaining the quality and quantity of onion productivity throughout complementarity treatments between compost tea and amino acids. Middle East J. Agric. 2013, 2, 108–115. [Google Scholar]
- Piccolo, A.; Nardi, S.; Concheri, G. Structural characteristics of humic substances as related to nitrate uptake and growth regulation in plant systems. Soil Biol. Biochem. 1992, 24, 373–380. [Google Scholar] [CrossRef]
- Parr, J.F.; Hornick, S.B.; Papendick, R.I. Transition from Conventional Agriculture to Natural Farming Systems: The Role of Microbial Inoculants and Biofertilizer [Online]. International Nature Farming Research Center 2002. Available online: http://www.infrc.or.jp/knf/PDF%20KNF%20Conf%20Data/C4-4-120.pdf (accessed on 16 September 2019).
- Morales-Corts, M.R.; Pérez-Sanchez, R.; Gómez- Sánchez, M. Efficiency of garden waste compost teas on tomato growth and its suppressiveness against soilborne pathogens. Sci. Agric. 2018, 75, 400–409. [Google Scholar] [CrossRef]
- Moretti, S.M.L.; Bertoncini, I.B.; Abreu-Junior, C.H. Composting sewage sludge with green waste from tree pruning. Sci. Agric. 2015, 72, 432–439. [Google Scholar] [CrossRef]
- Ros, M.; Hernández, M.T.; García, C.; Bernal, A.; Pascual, J.A. Biopesticide effect on Green compost against fusarium wilt on melon plants. J. Appl. Microbiol. 2005, 98, 845–854. [Google Scholar] [CrossRef]
- Celar, F.; Valič, N. Effects of Trichoderma spp. and Gliocladium roseum culture filtrates on seed germination of vegetables and maize. J. Plant Dis. Prot. 2005, 112, 343–350. [Google Scholar]
- Rabeendran, N.; Moot, D.J.; Jones, E.E.; Stewart, A. Inconsistent growth promotion of cabbage and lettuce from Trichoderma isolates. N. Z. Plant Prot. 2000, 53, 143–146. [Google Scholar] [CrossRef]
- Hoyos-Carvajal, L.; Ordua, S.; Bissett, J. Growth stimulation in bean (Phaseolus vulgaris L.) by Trichoderma. Biol. Control 2009, 51, 409–416. [Google Scholar] [CrossRef]
- López-Mondéjar, R.; Bernal-Vicente, A.; Ros, M.; Tittarelli, F.; Canali, S.; Intrigliolo, F.; Pascual, J.A. Utilisation of citrus compost-based growing media implemented with Trichoderma harzianum T-78 in Cucumis melo L. seedling production. Bioresour. Technol. 2010, 101, 3718–3723. [Google Scholar] [CrossRef]
- Pane, C.; Celano, G.; Villecco, D.; Zaccardelli, M. Control of Botrytis cinerea, Alternaria alternata and Pyrenochaeta lycopersici on tomato with whey compost-tea applications. Crop Prot. 2012, 38, 80–86. [Google Scholar] [CrossRef]
- Lara, L.J.; Egea-Gilabert, C.; Niñirola, D.; Conesa, E.; Fernández, J.A. Effect of aeration of the nutrient solution on the growth and quality of purslane (Portulaca oleracea). J. Hortic. Sci. Biotechnol. 2011, 86, 603–610. [Google Scholar] [CrossRef]
- Everette, J.D.; Bryant, Q.M.; Green, A.M.; Abbey, Y.A.; Wangila, G.M.; Walker, R.B. Through study of reactivity of various compound classes toward the Folin-Ciocalteu reagent. J. Agric. Food Chem. 2010, 58, 8139–8144. [Google Scholar] [CrossRef] [PubMed]
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005, 91, 571–577. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of free radical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- García, C.; Hernández, T.; Costa, F.; Ceccanti, B.; Masciandaro, G. The Dehydrogenase Activity of Soil as an Ecological Marker in Processes of Perturbed System Regeneration. In Proceedings of the XI International Symposium of Environmental Biochemistry, CSIC, Salamanca, Spain, September 1993; pp. 89–100. [Google Scholar]
- Taskin, B.; Gozen, A.G.; Duran, M. Selective quantification of viable Escherichia coli bacteria in biosolids by quantitative PCR with propidium monoazide modification. Appl. Environ. Microbiol. 2011, 77, 4329–4335. [Google Scholar] [CrossRef]
- Santísima-Trinidad, A.B.; Montiel-Rozas, M.M.; Diéz-Rojo, M.A.; Pascual, J.A.; Ros, M. Impact of foliar fungicides on target and non-target soil microbial communities in cucumber crops. Ecotoxicol. Environ. Safe 2018, 166, 78–85. [Google Scholar] [CrossRef]
- Blaya, J.; Lloret, E.; Ros, M.; Pascual, J.A. Identification of predictor parameters to determine agro-industrial compost suppressiveness against Fusarium oxysporum and Phytophthora capsici disease in muskmelon and pepper seedlings. J. Sci. Food Agric. 2015, 95, 1482–1490. [Google Scholar] [CrossRef]
- Morales, A.B.; Ros, M.; Ayuso, L.M.; Bustamante, M.A.; Moral, R.; Pascual, J.A. Agroindustrial Composts to reduce the use of peat and fungicides in the cultivation of muskmelon seedlings. J. Sci. Food Agric. 2017, 97, 875–881. [Google Scholar] [CrossRef]
- Luo, Y.; Liang, J.; Zeng, G.; Chen, M.; Mo, D.; Li, G. Seed germination test for toxicity evaluation of compost: Its roles, problems and prospects. Waste Manag. 2018, 71, 109–114. [Google Scholar] [CrossRef]
- Bernal-Vicente, A.; Ros, M.; Tittarelli, F.; Intrigliolo, F.; Pascual, J.A. Citrus compost and its water extract for cultivation of melon plants in greenhouse nurseries: Evaluation of nutriactive and biocontrol effects. Bioresour. Technol. 2008, 99, 8722–8728. [Google Scholar] [CrossRef] [PubMed]
- Shaban, H.; Fazeli-nasab, B. An Overview of the Benefits of Compost tea on Plant and Soil Structure. Adv. Biomed. Res. 2015, 6, 154–158. [Google Scholar]
- Arancon, N.; Edwards, C.; Dick, R.; Dick, L. Vermicompost tea production and plant growth impacts. BioCycle 2007, 48, 51–52. [Google Scholar]
- Hargreaves, J.C.; Adl, M.; Warman, P.R. Are compost teas an effective nutrient amendment in the cultivation of strawberries? Soil and plant tissue effects. J. Sci. Food Agric. 2009, 89, 390–397. [Google Scholar] [CrossRef]
- Marin, F.; Diánez, F.; Santos, M.; Carretero, F.; Gea, F.J.; Castañeda, C.; Navarro, M.J.; Yau, J.A. Control of Phytophthora capsici and Phytophthora parasitica on pepper (capsicum annum L.) with compost teas form different sources, and their effects on plant growth promotion. Phytopathol. Mediterr. 2014, 53, 216–228. [Google Scholar]
- Edwards, C.C.; Arancon, N.Q.; Greytak, S. Effects of vermicompost teas on plant growth and disease. Biocycle 2006, 47, 28–31. [Google Scholar]
- Zamora-Nahum, S.; Danon, M.; Hadar, Y.; Chen, Y. Chemical properties of compost extracts inhibitory to germination of Sclerotium rolfsii. Soil Biol. Biochem. 2008, 40, 2523–2529. [Google Scholar] [CrossRef]
- Michael, G. The control of root hair formation: Suggested mechanisms. J. Plant Nutr. Soil Sci. 2001, 164, 111–119. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.; Macías-Rodríguez, L.; del-Val, E.; Larsen, J. Ecological functions of Trichoderma spp. and their secondary metabolites in the rhizosphere: Interactions with plants. FEMS Microbiol. Ecol. 2016, 92, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Harman, G.E.; Petzoldt, R.; Comis, A.; Chen, J. Interactions between Trichoderma harzianum strain T22 and maize inbred line Mo17 and effects of these interactions on disease caused by Pythium ultimum and Colletotrichum graminicola. Phytopathology 2004, 94, 147–153. [Google Scholar] [CrossRef]
- Haggag, W.M.; Abo-Sedera, S.A. Characteristics of three Trichoderma species in peanut haulms compost involved in biocontrol of cumin wilt disease. Int. J. Agric. Biol. 2005, 7, 222–229. [Google Scholar]
- Nahar, M.S.; Rahman, M.A.; Kibria, M.G.; Rezaul-Karim, A.N.M.; Miller, S.A. Use of Tricho-compost and Tricho-leachate for management of soil-borne pathogens and production on healthy cabbage seedlings. Bangladesh J. Agric. Res. 2012, 37, 653–664. [Google Scholar] [CrossRef]
- Topolovec-Pintarić, S. Trichoderma: Invisible Partner for Visible Impact on Agriculture. In Trichoderma—The Most Widely Used Fungicide; Manjur Shah, M., Sharif, U., Buhari, T.R., Eds.; IntechOpen: London, UK, 2019; pp. 1–21. [Google Scholar]
- Kris-Etherton, P.M.; Hecker, K.D.; Bonanome, A.; Coval, S.M.; Binkoski, A.E.; Hilpert, K.F.; Griel, A.E.; Etherton, T.D. Bioactive compounds in foods: Their role in the prevention of cardiovascular disease and cancer. Am. J. Med. 2002, 113 (Suppl. 9B), 71S–88S. [Google Scholar] [CrossRef]
- Giménez, A.; Fernández, J.A.; Pascual, J.A.; Ros, M.; López-Serrano, M.; Egea-Gilabert, C. An agroindustrial compost as alternative to peat for production of baby leaf red lettuce in a floating system. Sci. Hortic. 2019, 46, 907–915. [Google Scholar] [CrossRef]
- Hua-Bin, L.; Chi-Chun, W.; Ka-Wing, C.; Feng, C. Antioxidant properties in vitro and total phenolic contents in methanol extracts from medicinal plants. LWT—Food Sci. Technol. 2008, 41, 385–390. [Google Scholar]
- Pascale, A.; Vinale, F.; Manganiello, G.; Nigro, M.; Lanzuise, S.; Ruocco, M.; Marra, R.; Lombardi, N.; Woo, S.L.; Lorito, M. Trichoderma and its secondary metabolites improve yield and quality. Crop Prot. 2017, 92, 176–181. [Google Scholar] [CrossRef]
- Yedidia, I.; Shoresh, M.; Kerem, Z.; Benhamou, N.; Kapulnik, Y.; Chet, I. Concomitant induction of systemic resistance to Pseudomonas syringae pv. lachrymans in cucumber by Trichoderma asperellum (T-203) and accumulation of phytoalexins. Appl. Environ. Microbiol. 2003, 69, 7343–7353. [Google Scholar] [CrossRef]
- Hassan, S.K.; El–Abssawy, A.A.; Khoder, M.I. Characteristics of gas–phase nitric acid and ammonium–nitrate–sulfate aerosol, and their gas–phase precursors in a suburban area in Cairo, Egypt. Atmos. Pollut. Res. 2013, 4, 117–129. [Google Scholar] [CrossRef]
- Jensen, H.L. Effect of organic compounds on Nitrosomonas. Nature 1950, 165, 974. [Google Scholar] [CrossRef]
- Rittenberg, S.C. The roles of exogenous organic matter in the physiology of chemolithotrophic bacteria. Adv. Microb. Physiol. 1969, 3, 159–196. [Google Scholar]
- Smith, A.J.; Hoare, D.S. Specialist phototrophs, lithotrophs, and methylotrophs: A unity among a diversity of prokaryotes? Bacteriol. Rev. 1977, 41, 419–448. [Google Scholar] [CrossRef] [PubMed]
- Krummel, A.; Harms, H. Effect of organic matter on growth and cell yield of ammonia-oxidizing bacteria. Arch. Microbiol. 1982, 133, 50–54. [Google Scholar] [CrossRef]
- Takahashi, R.; Kondo, N.; Usui, K.; Kanehira, T.; Shinohara, M.; Tokuyama, T. Pure isolation of a new chemoautotrophic ammonia-oxidizing bacterium on gellan gum plate. J. Ferment. Bioeng. 1992, 74, 52–54. [Google Scholar] [CrossRef]
- Stutte, G.W. Nitrogen dynamics in the CELSS breadboard facility at Kennedy Space Center. Life Support Biosph. Sci. Int. J. Earth Space 1996, 3, 67–74. [Google Scholar]
- Xu, Z.; Zheng, S.; Yang, G.; Zhang, Q.; Wang, L. Nitrification inhibition by naphthalene derivatives and its relationship with copper. Bull. Environ. Contam. Toxicol. 2000, 64, 542–549. [Google Scholar] [CrossRef]
- Harman, G.E. Myths and Dogmas of Biocontrol. Changes in perceptions derived from research on Trichoderma harzianum T-22. Plant Dis. 2000, 84, 377–393. [Google Scholar] [CrossRef]
- Lynch, J.M.; Wilson, K.L.; Ousley, M.A.; Whipps, J.M. Response of lettuce to Trichoderma treatment. Lett. Appl. Microbiol. 1991, 12, 59–61. [Google Scholar] [CrossRef]
- Manojlovic, M.; Cabilovski, R.; Nikolic, L.J.; Dzigurski, D.; Seremesic, S.; Baveec, M. Ground cover management and farmyard manure effects on soil nitrogen dynamics, productivity and economics of organically grown lettuce (Lactuca sativa L. subsp. secalina). J. Integr. Agric. 2017, 16, 947–958. [Google Scholar]
- Kapoulas, N.; Koukounaras, A.; Ilic, Z.S. Nutritional quality of lettuce and onion as companion plants from organic and conventional production in north Greece. Sci. Hortic. 2017, 219, 310–318. [Google Scholar] [CrossRef]
- Burns, I.G.; Zhang, K.; Turner, M.K.; Edmondson, R. Iso-osmotic regulation of nitrate accumulation in lettuce. J. Plant Nutr. 2010, 34, 283–313. [Google Scholar] [CrossRef]
- Pascual, J.A.; García, C.; Hernández, T.; Moreno, J.; Ros, M. Soil microbial activity as a biomarker of degradation and remediation processes. Soil Biol. Biochem. 2000, 32, 1877–1883. [Google Scholar] [CrossRef]
- Ros, M.; García, C.; Hernández, T. Soil microbial Activity after restoration of a semiarid soil by organic amendments. Soil Biol. Biochem. 2003, 35, 463–469. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).