Mentha and Oregano Soil Amendment Induces Enhancement of Tomato Tolerance against Soilborne Diseases, Yield and Quality
Abstract
1. Introduction
2. Materials and Methods
2.1. Production of Plant Material
2.2. Soil Amendment and Decomposition of Plant Material
2.2.1. Soil Properties and C/N Ratio
2.2.2. Volatiles Constituents in Amended Soil
2.3. Production of Tomato Seedlings and Transplanting to the Amended Soil
2.4. The Production of Inoculum of Verticillium/Fusarium
2.5. Seedlings Inoculation and Cultivation
2.6. Disease Assessment
2.7. Plant Growth and Physiology Assessments
2.8. Fruit Production and Quality Evaluation
2.9. Experimental Design and Statistical Analysis
3. Results
3.1. Analysis of Soil Characteristics after Amendment with Aromatic Plants
3.1.1. C/N ratio
3.1.2. Volatiles in Soil Samples
3.2. Protective Effect of Soil Amendment against Fusarium and Verticillium Wilt
3.3. Effect of Soil Amendment on the Development and Physiology of Tomato Plants
4. Discussion
4.1. Effect of Incorporation of Aromatic Plants at Soil Environment (C/N Ratio and Volatiles)
4.2. Effect of Incorporation of Aromatic Plants at Tomato Cultivation
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Daferera, D.; Ziogas, B.; Polissiou, M. The effectiveness of plant essential oils on the growth of Botrytis cinerea, Fusarium sp. and Clavibacter michiganensis subsp. michiganensis. Crop Prot. 2003, 22, 39–44. [Google Scholar] [CrossRef]
- Kadoglidou, K.; Lagopodi, A.; Karamanoli, K.; Vokou, D.; Bardas, G.; Menexes, G.; Constantinidou, H.-I.; Constantinidou, H.-I. Inhibitory and stimulatory effects of essential oils and individual monoterpenoids on growth and sporulation of four soil-borne fungal isolates of Aspergillus terreus, Fusarium oxysporum, Penicillium expansum, and Verticillium dahliae. Eur. J. Plant Pathol. 2011, 130, 297–309. [Google Scholar] [CrossRef]
- Karamanoli, K.; Vokou, D.; Menkissoglu-Spiroudi, U.; Constantinidou, H.-I.; Constantinidou, H.-I. Bacterial colonization of the phyllosphere of Mediterranean aromatic plants. J. Chem. Ecol. 2000, 26, 2035–2048. [Google Scholar] [CrossRef]
- Gitsopoulos, T.; Kadoglidou, K.; Damalas, C. “Section 2.7: Miscellaneous Cropping Systems ”Sustainable weed control with aromatic plants and essential oils. In Weed Control: Sustainability, Hazards and Risks in Cropping Systems Worldwide; Science Publishers: Boca Raton, FL, USA, 2018; pp. 597–613. ISBN 978-1-315-15591-3. [Google Scholar]
- Dudai, N.; Poljakoff-Mayber, A.; Mayer, A.; Putievsky, E.; Lerner, H. Essential oils as allelochemicals and their potential use as bioherbicides. J. Chem. Ecol. 1999, 25, 1079–1089. [Google Scholar] [CrossRef]
- Vasilakoglou, I.; Dhima, K.; Paschalidis, K.; Ritzoulis, C. Herbicidal potential on Lolium rigidum of nineteen major essential oil components and their synergy. J. Essent. Oil Res. 2013, 25, 1–10. [Google Scholar] [CrossRef]
- Duke, S.; Cantrell, C.; Meepagala, K.; Wedge, D.; Tabanca, N.; Schrader, K. Natural toxins for use in pest management. Toxins 2010, 2, 1943–1962. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ortuño, D.; Tores, J.; Chamorro, M.; Pérez-García, A.; Vicente, A. Characterization of resistance to six chemical classes of site-specific fungicides registered for gray mold control on strawberries in Spain. Plant Dis. 2016, 100, 2234–2239. [Google Scholar] [CrossRef]
- Hahn, M. The rising threat of fungicide resistance in plant pathogenic fungi: Botrytis as a case study. J. Chem. Biol. 2014, 7, 133–141. [Google Scholar] [CrossRef]
- Hunter, P.; Reeves, D. The current status of surveillance of resistance to antimicrobial agents: Report on a meeting. J. Antimicrob. Chemother. 2002, 49, 17–23. [Google Scholar] [CrossRef][Green Version]
- Kolaei, E.A.; Cenatus, C.; Tweddell, R.J.; Avis, T. Antifungal activity of aluminium-containing salts against the development of carrot cavity spot and potato dry rot. Ann. Appl. Biol. 2013, 163, 311–317. [Google Scholar] [CrossRef]
- Soković, M.; Van Griensven, L. Antimicrobial activity of essential oils and their components against the three major pathogens of the cultivated button mushroom, Agaricus bisporus. Eur. J. Plant Pathol. 2006, 116, 211–224. [Google Scholar] [CrossRef]
- Baranauskiene, R.; Bylaite, E.; Zukauskaite, J.; Venskutonis, R. Flavor retention of peppermint (Mentha piperita L.) essential oil spray-dried in modified starches during encapsulation and storage. J. Agric. Food Chem. 2007, 55, 3027–3036. [Google Scholar] [CrossRef] [PubMed]
- Parris, N.; Cooke, P.; Hicks, K. Encapsulation of essential oils in Zein nanospherical particles. J. Agric. Food Chem. 2005, 53, 4788–4792. [Google Scholar] [CrossRef] [PubMed]
- Varona, S.; Martín, Á.; Cocero, M. Formulation of a natural biocide based on lavandin essential oil by emulsification using modified starches. Chem. Eng. Prog. 2009, 48, 1121–1128. [Google Scholar] [CrossRef]
- Symeonidou, A.; Petrotos, K.; Vasilakoglou, I.; Gkoutsidis, P.; Karkanta, F.; Lazaridou, A. Natural herbicide based on essential oils and formulated as wettable powder. European Patent Application EP 2 684 457 A1, 15 January 2014. [Google Scholar]
- Chalkos, D.; Kadoglidou, K.; Karamanoli, K.; Fotiou, C.; Pavlatou-Ve, A.; Eleftherohorinos, I.; Constantinidou, H.-I.; Vokou, D. Mentha spicata and Salvia fruticosa composts as soil amendments in tomato cultivation. Plant Soil 2010, 332, 495–509. [Google Scholar] [CrossRef]
- Chouliaras, N.; Gravanis, F.; Vasilakoglou, I.; Gougoulias, N.; Vagelas, I.; Kapotis, U.; Wogiatzi, E. The effect of basil (Ocimum basilicum L.) on soil organic matter biodegradation and other soil chemical properties. J. Sci. Food Agric. 2007, 87, 2416–2419. [Google Scholar] [CrossRef]
- Kadoglidou, K.; Chalkos, D.; Karamanoli, K.; Eleftherohorinos, I.; Constantinidou, H.-I.; Vokou, D. Aromatic plants as soil amendments: Effects of spearmint and sage on soil properties, growth and physiology of tomato seedlings. Sci. Hortic. 2014, 179, 25–35. [Google Scholar] [CrossRef]
- Dhima, K.V.; Vasilakoglou, I.; Gatsis, T.; Panou-Philotheou, E.; Eleftherohorinos, I. Effects of aromatic plants incorporated as green manure on weed and maize development. Field Crops Res. 2009, 110, 235–241. [Google Scholar] [CrossRef]
- Vasilakoglou, I.; Dhima, I.; Wogiatzi, E.; Eleftherohorinos, I.; Lithourgidis, A. Herbicidal potential of essential oils of oregano or marjoram (Origanum spp.) and basil (Ocimum basilicum) on Echinochloa crus-galli (L.) P. Beauv. and Chenopodium album L. weeds. Allelopathy J. 2007, 20, 297–306. [Google Scholar]
- Marques de Carvalho, L.; Oliveira, I.; Almeida, N.A.; Andrade, K.R. The effects of biotic interaction between tomato and companion plants on yield. Acta Hortic. 2012, 933, 347–354. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). FAO Statistical Yearbook 2012: Europe and Central Asia Food and Agriculture; FAO: Rome, Italy, 2013; Available online: http://www.fao.org/docrep/017/i3138e/i3138e00.htm (accessed on 13 March 2020).
- Borrego-Benjumea, A.; José, B.M.; Abbasi, P.A.; Lazarovits, G.; Melero-Vara, J. Effects of incubation temperature on the organic amendment-mediated control of Fusarium wilt of tomato. Ann. Appl. Biol. 2014, 164, 453–463. [Google Scholar] [CrossRef]
- Beckman, C.H. The Nature of Wilt Diseases of Plants; American Phytopathological Society Press: Saint Paul, MN, USA, 1987; ISBN 0890540748. [Google Scholar]
- La Torre, A.; Caradonia, F.; Matere, A.; Battaglia, V. Using plant essential oils to control Fusarium wilt in tomato plants. Eur. J. Plant Pathol. 2016, 144, 487–496. [Google Scholar] [CrossRef]
- Kokkini, S.; Vokou, D. Mentha spicata (Lamiaceae) chemotypes grown wild in Greece. Econ. Bot. 1989, 43, 192–202. [Google Scholar] [CrossRef]
- Goliaris, A.; Chatzopoulou, P.; Katsiotis, S. Production of new Greek oregano clones and analysis of their essential oils. J. Herbs Spices Med. Plants 2002, 10, 29–35. [Google Scholar] [CrossRef]
- Allison, F.E. Organic carbon. In Methods of soil Analysis, Part II; American Society of Agronomy: Madison, WI, USA, 1965; pp. 1372–1376. [Google Scholar]
- Bremner, J. Determination of nitrogen in soil by the Kjeldahl method. J. Agric. Sci. 1960, 55, 11–33. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing Corporation: Carol Stream, IL, USA, 1995. [Google Scholar]
- Papadaki, A.M.; Bletsos, F.A.; Eleftherohorinos, I.G.; Menexes, G.; Lagopodi, A.L. Effectiveness of seven commercial rootstocks against Verticillium wilt and their effects on growth, yield, and fruit quality of tomato. J. Crop Prot. 2017, 102, 25–31. [Google Scholar] [CrossRef]
- Charoenporn, C.; Kanokmedhakul, S.; Lin, F.; Poeaim, S.; Soytong, K. Evaluation of bio-agent formulations to control Fusarium wilt of tomato. Afr. J. Biotechnol. 2010, 9, 5836–5844. [Google Scholar]
- Eldon, S.; Hillocks, R. The effect of reduced phytoalexin production on the resistance of upland cotton (Gossypium hirsutum) to Verticillium and Fusarium wilts. Ann. Appl. Biol. 1996, 129, 217–225. [Google Scholar] [CrossRef]
- Shanner, G.; Finney, R.E. The effect of nitrogen fertilizer on the expression of slow-mildewing resistance in knox wheat. Phytopathology 1977, 67, 1051–1056. [Google Scholar] [CrossRef]
- Sullivan, D.M.; Miller, R.O. Compost quality attributes, measurements, and variability. In Compost Utilization in Cropping Systems; Lewis Publishers: Boca Raton, FL, USA, 2001; pp. 95–120. [Google Scholar]
- Kalburtji, K.; Mamolos, A. Maize, soybean and sunflower litter dynamics in two physicochemically different soils. Nutr. Cycl. Agroecosyst. 2000, 57, 195–206. [Google Scholar] [CrossRef]
- Levy, J.S.; Taylor, B. Effects of pulp mill solids and three composts on early growth of tomatoes. Bioresour. Technol. 2003, 89, 297–305. [Google Scholar] [CrossRef]
- Vokou, D. Essential oils as allelochemicals: Research advances in Greece. In Allelopathy Update. Vol. 2. Basic and Applied Aspects; Science Publishers: New York, NY, USA, 1999; pp. 47–63. [Google Scholar]
- Karamanoli, K.; Kadoglidou, K.; Tananaki, C.; Thrasyvoulou, A.; Constantinidou, H.-I.; Vokou, D. Transformations of Mentha spicata essential oil in the soil environment. Planta Med. 2008, 74, P149. [Google Scholar] [CrossRef]
- Karamanoli, K.; Ainalidou, A.; Bouzoukla, F.; Vokou, D. Decomposition profiles of leaf essential oils in the soil environment. Ind. Crops Prod. 2018, 124, 394–401. [Google Scholar] [CrossRef]
- Cox, S.D.; Gustafson, J.; Mann, J.; Markham, J.; Liew, Y.C.; Hartland, R.P.; Bell, H.C.; Warmington, J.R.; Wyllie, S.G. Tea tree oil causes K+ leakage and inhibits respiration in Escherichia coli. Lett. Appl. Microbiol. 1998, 26, 355–358. [Google Scholar] [CrossRef]
- Trombetta, D.; Castelli, F.; Sarpietro, M.G.; Venuti, V.; Cristani, M.; Daniele, C.; Saija, A.; Mazzanti, G.; Bisignano, G. Mechanisms of antibacterial action of three monoterpenes. Antimicrob. Agents Chemother. 2005, 49, 2474–2478. [Google Scholar] [CrossRef]
- Cox, S.D.; Mann, J.; Markham, J.; Bell, H.C.; Gustafson, J.; Warmington, J.R.; Wyllie, S.G. The mode of antimicrobial action of the essential oil of Melaleuca alternifolia (tea tree oil). J. Appl. Microbiol. 2000, 88, 170–175. [Google Scholar] [CrossRef]
- Wang, R.; Peng, S.; Zeng, R.; Ding, L.-W.; Xu, Z.-F. Cloning, expression and wounding induction of β-caryophyllene synthase gene from Mikania micrantha HBK and allelopathic potential of β-caryophyllene. Allelopath. J. 2009, 24, 35–44. [Google Scholar]
- Degenhardt, J.; Hiltpold, I.; Köllner, T.; Frey, M.; Gierl, A.; Gershenzon, J.; Hibbard, B.; Ellersieck, M.; Turlings, T. Restoring a maize root signal that attracts insect-killing nematodes to control a major pest. Proc. Natl. Acad. Sci. USA 2009, 106, 13213–13218. [Google Scholar] [CrossRef]
- Rasmann, S.; Köllner, T.; Degenhardt, J.; Hiltpold, I.; Toepfer, S.; Kuhlmann, U.; Gershenzon, J.; Turlings, T. Recruitment of entomopathogenic nematodes by insect-damaged maize roots. Nature 2005, 434, 732–737. [Google Scholar] [CrossRef]
- Cheng, A.-X.; Xiang, C.-Y.; Li, J.; Yang, C.; Hu, W.-L.; Wang, L.-J.; Lou, Y.-G.; Chen, X.-Y. The rice (E)-β-Caryophyllene synthase (OsTPS3) accounts for the major inducible volatile sesquiterpenes. Phytochemistry 2007, 68, 1632–1641. [Google Scholar] [CrossRef]
- Kashiwabara, M.; Kamo, T.; Makabe, H.; Shibata, H.; Hirota, M. Repraesentins D, E and F, New plant growth promoters from Lactarius repraesentaneus. Biosci. Biotechnol. Biochem. 2006, 70, 1502–1505. [Google Scholar] [CrossRef] [PubMed]
- Kamo, T.; Matsue, M.; Kashiwabara, M.; Hirota, M. 1,2-Dehydrolactarolide A, A new plant growth regulatory lactarane sesquiterpene from Lactarius vellereus. Biosci. Biotech. Bioch. 2006, 70, 2307–2309. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ribeiro, H.; Romero, A.M.; Pereira, H.; Maracaja, P.; Cabral, F.; Vasconcelos, E. Evaluation of a compost obtained from forestry wastes and solid phase of pig slurry as a substrate for seedlings production. Bioresour. Technol. 2007, 98, 3294–3297. [Google Scholar] [CrossRef] [PubMed]
- Kostov, O.; Tzvetkov, Y.; Kaloianova, N.; Cleemput, O. Production of tomato seedlings on composts of vine branches and grape prunings, husks and seeds. Compost Sci. Util. 1996, 4, 55–61. [Google Scholar] [CrossRef]
- Arancon, N.; Edwards, C.; Bierman, P.; Metzger, J.; Lee, S.; Welch, C. Effects of vermicomposts on growth and marketable fruits of field-grown tomatoes, peppers and strawberries: The 7th international symposium on earthworm ecology Cardiff Wales 2002. Pedobiologia 2003, 47, 731–735. [Google Scholar]
- Manios, T. The composting potential of different organic solid wastes: Experience from the island of Crete. Environ. Int. 2004, 29, 1079–1089. [Google Scholar] [CrossRef]
- Ismail, H.; Mohd Saud, H. Effect of rice straw compost under normal and stressful condition on quality of tomatoes grown in two different media. Ecol. Environ. Conserv. 2003, 9, 533–536. [Google Scholar]
- Kostov, O.; Petkova, G.; Tzvetkov, Y.; Lynch, J. Aerobic composting of plant wastes and their effect on the yield of ryegrass and tomatoes. Biol. Fertil. Soils 1996, 23, 20–25. [Google Scholar] [CrossRef]
- Toumpeli, A.; Pavlatou-Ve, A.; Kostopoulou, S.; Mamolos, A.; Siomos, A.; Kalburtji, K. Composting Phragmites australis Cav. plant material and compost effects on soil and tomato (Lycopersicon esculentum Mill.) growth. J. Environ. Manag. 2013, 128, 243–251. [Google Scholar] [CrossRef]
- Vokou, D.; Margaris, N. Decomposition of terpenes by soil microorganisms. Pedobiologia 1988, 31, 413–419. [Google Scholar]
- Atiyeh, R.; Lee, S.; Edwards, C.; Arancon, N.; Metzger, J. The influence of humic acids derived from earthworm-processed organic wastes on plant growth. Bioresour. Technol. 2002, 84, 7–14. [Google Scholar] [CrossRef]
- Canellas, L.; Olivares, F.; Okorokova-Façanha, A.; Façanha, A. Humic acids isolated from earthworm compost enhance root elongation, lateral root emergence, and plasma membrane H-ATPase activity in maize roots. Plant Physiol. 2002, 130, 1951–1957. [Google Scholar] [CrossRef]
- Scaglia, B.; Nunes, R.; Rezende, M.; Tambone, F.; Adani, F. Investigating organic molecules responsible of auxin-like activity of humic acid fraction extracted from vermicompost. Sci. Total Environ. 2016, 562, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Hoitink, H.; Boehm, M. Biocontrol within the context of soil microbial Communities: A substrate-dependent phenomenon. Annu. Rev. Phytopathol. 1999, 37, 427–446. [Google Scholar] [CrossRef] [PubMed]
- Pascual, J.; Hernández, T.; Garcia, C.; Leij, F.A.A.M.; Lynch, J. Long-term suppression of Pythium ultimum in arid soil using fresh and composted municipal wastes. Biol. Fertil. Soils. 2000, 30, 478–484. [Google Scholar] [CrossRef]
- Tuitert, G.; Szczech, M.; Bollen, G. Suppression of Rhizoctonia soclani in potting mixtures amended with compost made from organic household waste. Phytopathology 1998, 88, 764–773. [Google Scholar] [CrossRef][Green Version]
- Cotxarrera, L.; Trillas, M.I.; Steinberg, C.; Alabouvette, C. Use of sewage sludge compost and Trichoderma asperellum isolates to suppress Fusarium wilt of tomato. Soil Biol. Biochem. 2002, 34, 467–476. [Google Scholar] [CrossRef]
- Raviv, M. Compost as a tool to suppress plant diseases: Established and putative mechanisms. Acta Hortic. 2016, 1146, 11–24. [Google Scholar] [CrossRef]
- Hjeljord, L.; Tronsmo, A. Trichoderma and Gliocladium in biological control: An overview. In Trichoderma and Gliocladium. Enzymes, Biological Control and Commercial Applications; Taylor and Francis: London, UK, 1998; Volume 2, pp. 131–151. [Google Scholar]
- Cheuk, W.; Lo, K.; Copeman, R.; Joliffe, P.; Fraser, B. Disease suppression on greenhouse tomatoes using plant waste compost. J. Environ. Sci. Health Part B 2005, 40, 449–461. [Google Scholar] [CrossRef]
- Castaño, R.; Aviles, M. Factors that affect the capacity of growing media to suppress verticillium wilt. Acta Hortic. 2013, 1013, 465–471. [Google Scholar] [CrossRef]
- Giotis, C.; Markellou, E.; Theodoropoulou, A.; Toufexi, E.; Hodson, R.; Shotton, P.; Shiel, R.; Cooper, J.; Leifert, C. Effect of soil amendments and biological control agents (BCAs) on soil-borne root diseases caused by Pyrenochaeta lycopersici and Verticillium albo-atrum in organic greenhouse tomato production systems. Eur. J. Plant Pathol. 2009, 123, 387–400. [Google Scholar] [CrossRef]
- Davis, J.; Huisman, O.; Everson, D.; Nolte, P.; Sorenson, L.; Schneider, A. The suppression of Verticillium wilt of potato using corn as a green manure crop. Am. J. Potato Res. 2010, 87, 195–208. [Google Scholar] [CrossRef]


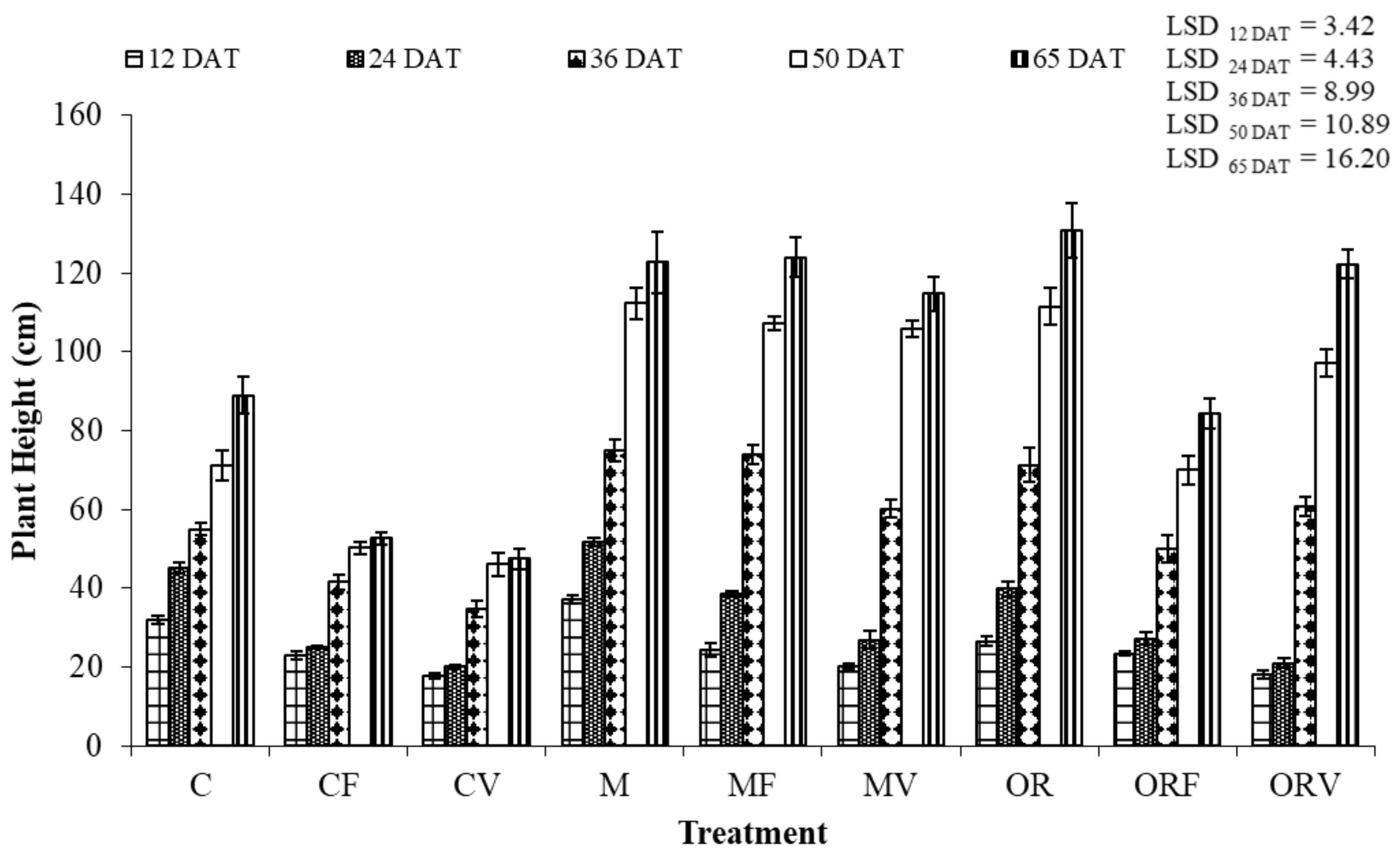
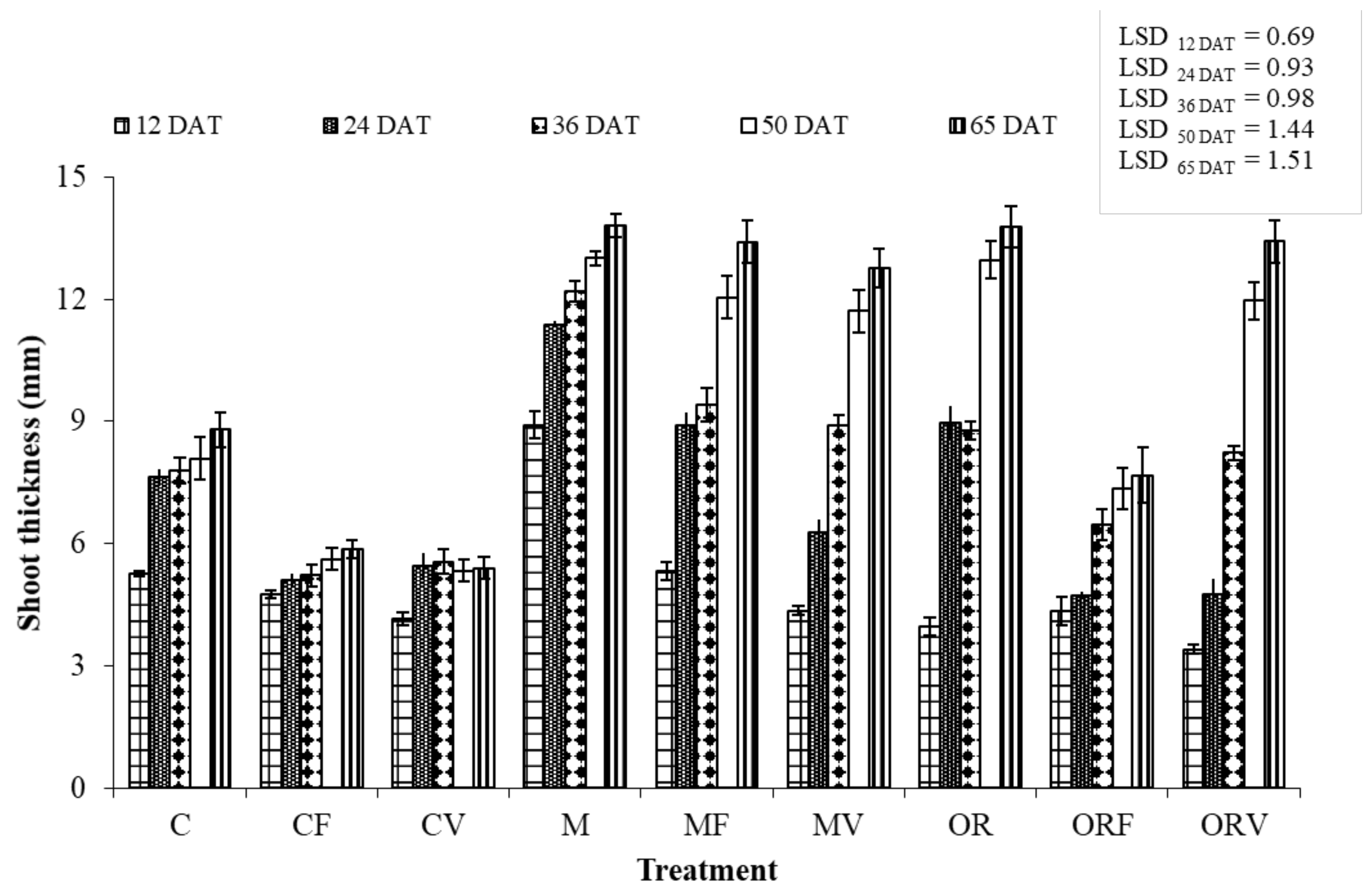
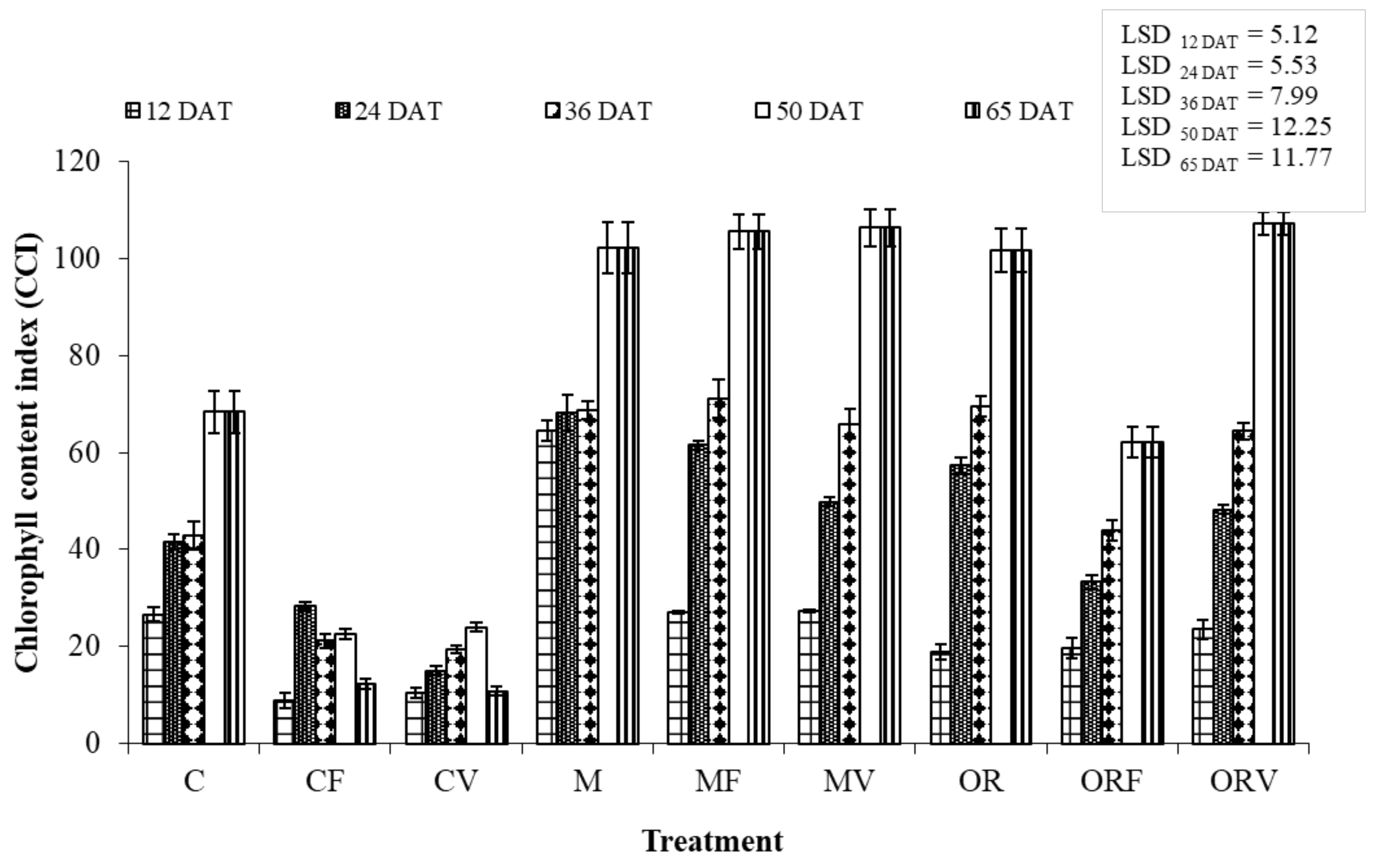
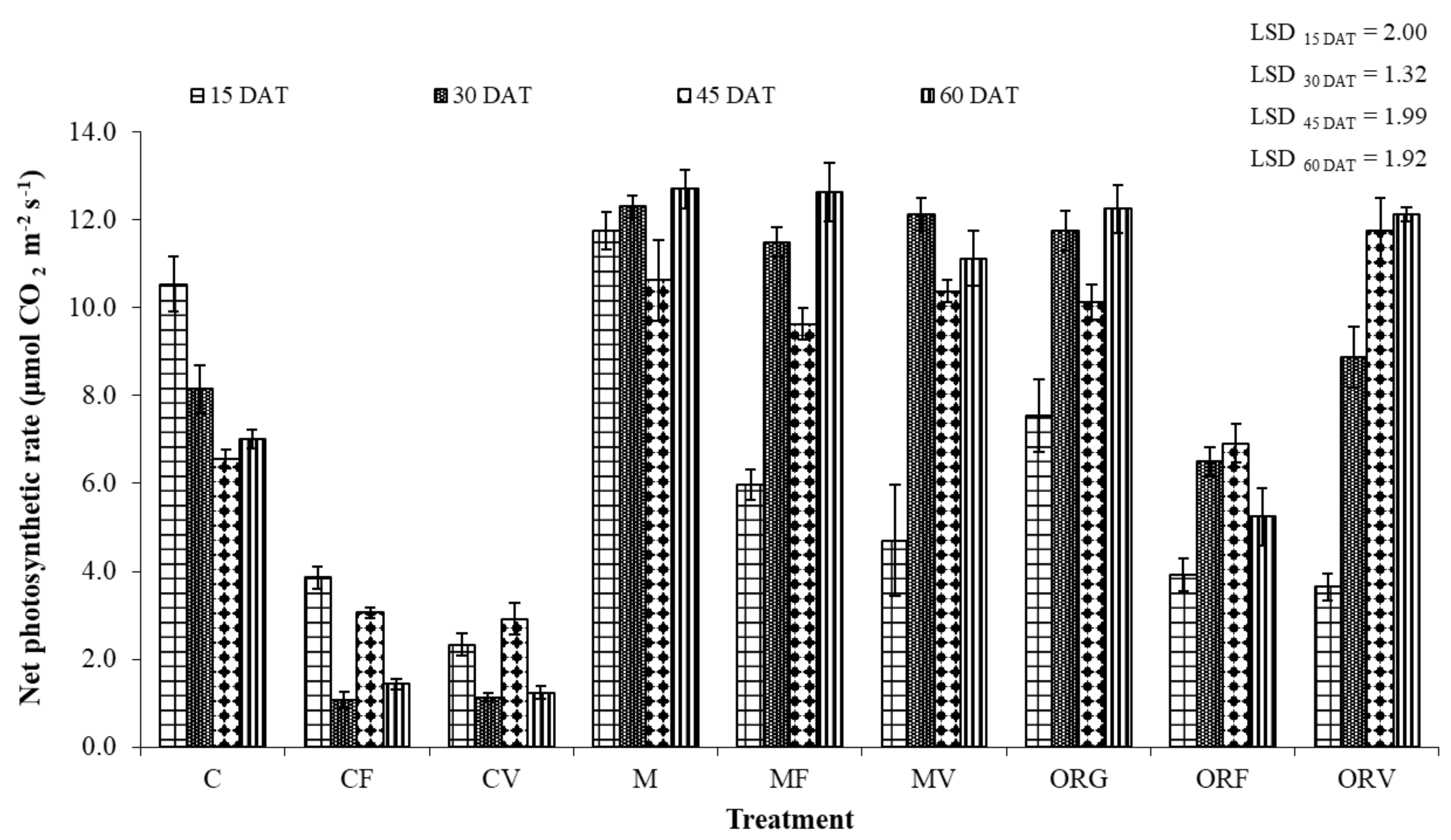
| Treatment Abbreviations | Treatments |
|---|---|
| C | Control non-amended soil + non inoculated seedlings |
| CF | Control non-amended soil + seedling inoculation with Fol |
| CV | Control non-amended soil + seedling inoculation with Vd |
| M | Soil + 4% (w/w) M. spicata plant material+non inoculated seedlings |
| MF | Soil + 4% (w/w) M. spicata plant material+seedling inoculation with Fol |
| MV | Soil + 4% (w/w) M.spicata plant material+seedling inoculation with Vd |
| OR | Soil + 4% (w/w) O. vulgare subsp. hirtum plant material+non inoculated seedlings |
| ORF | Soil + 4% (w/w) O. vulgare subsp. hirtum plant material+seedling inoculation with Fol |
| ORV | Soil + 4% (w/w) O. vulgare subsp. hirtum plant material+seedling inoculation with Vd |
| Days After Incorporation (DAI) | Total Yield of Essential Oil (μL/100 g of Soil) | |
|---|---|---|
| Spearmint | Oregano | |
| 0 | 129.10 | 270.30 |
| 15 | 24.00 | 35.50 |
| 30 | 4.16 | 14.50 |
| 60 | 2.37 | 6.75 |
| 90 | 0.50 | 1.80 |
| Identified Compounds | RRIa | LRIb | Concentration (%)c | ||||
|---|---|---|---|---|---|---|---|
| (i) Spearmint | (ii) Soil Amended with Spearmint | ||||||
| Days After Soil Amendment | |||||||
| 15 | 30 | 60 | 90 | ||||
| α-pinene | 937 | 937 | 0.96 | 1.92 | 2.68 | 1.48 | |
| sabinene | 976 | 976 | 0.64 | 1.30 | 0.82 | 0.38 | |
| β-pinene | 979 | 980 | 1.23 | 2.47 | 3.26 | 1.83 | |
| β-myrcene | 993 | 991 | 0.81 | 1.28 | 0.49 | ||
| α-terpinene | 1018 | 1018 | 0.48 | ||||
| limonene/β-phellendrene | 1030 | 1031 | 10.80 | 18.49 | 9.61 | 3.95 | |
| 1,8-cineole/eucalyptol | 1034 | 1033 | 5.58 | 6.84 | 1.39 | 0.52 | |
| cis-β-ocimene | 1042 | 1040 | 0.36 | 0.53 | |||
| γ-terpinene | 1061 | 1062 | 0.79 | ||||
| cis-sabinene hydrate | 1069 | 1065 | 0.51 | 2.46 | |||
| terpinen-4-ol | 1178 | 1177 | 2.04 | 0.51 | |||
| α-terpineol | 1188 | 1189 | 0.49 | 0.33 | |||
| dihydrocarveol | 1193 | 1193 | 8.60 | 7.07 | 0.80 | 1.17 | |
| dihydrocarvone | 1196 | 1194 | 1.91 | 0.30 | |||
| trans-carveol | 1219 | 1217 | 0.37 | 0.43 | 0.58 | ||
| cis-carveol | 1229 | 1229 | 1.10 | 0.45 | |||
| carvone | 1243 | 1242 | 53.06 | 26.44 | 0.71 | 0.90 | 0.43 |
| dihydrocarveol acetate | 1328 | 1326 | 3.72 | 8.03 | 12.96 | 7.80 | 0.58 |
| cis-carvyl acetate | 1367 | 1365 | 0.71 | 1.32 | 1.65 | 0.83 | |
| α-copaene | 1375 | 1376 | 0.42 | 0.83 | 1.25 | ||
| β-bourbonene | 1384 | 1384 | 1.00 | 3.92 | 18.12 | 27.43 | 35.57 |
| β-cubebene | 1390 | 1390 | 0.37 | 0.24 | 0.32 | ||
| β-elemene | 1391 | 1391 | 1.67 | 2.10 | 2.00 | ||
| β-caryophyllene | 1415 | 1418 | 1.07 | 4.77 | 6.11 | 7.21 | |
| β-gurjunene | 1426 | 1431 | 0.40 | 1.83 | 2.70 | 3.15 | |
| aromadendrene | 1442 | 1439 | 0.39 | 1.98 | 3.08 | 4.29 | |
| α-humulene | 1451 | 1454 | 0.39 | 0.49 | 0.49 | ||
| allo-aromadendrene | 1458 | 1458 | 1.42 | 0.35 | 0.66 | 0.50 | |
| cis-muurola-4-(14),-5-diene | 1462 | 1465 | 0.42 | 0.33 | 4.67 | 4.39 | 4.29 |
| γ-muurolene | 1477 | 1477 | 1.55 | 2.19 | 2.82 | ||
| germacrene-D | 1480 | 1480 | 1.43 | 4.34 | 13.41 | 9.75 | 10.33 |
| bicyclogermacrene | 1492 | 1494 | 0.40 | 1.90 | 1.58 | 1.73 | |
| γ-cadinene | 1511 | 1513 | 0.48 | 0.79 | 3.41 | ||
| cis-calamenene | 1522 | 1521 | 0.49 | 2.03 | 3.52 | 4.03 | |
| α-cadinene | 1539 | 1537 | 0.54 | 0.83 | 0.91 | ||
| viridiflorol | 1589 | 1590 | 0.49 | 1.32 | 4.01 | 3.80 | 2.61 |
| 1,10-di-epi-cubenol | 1614 | 1618 | 0.88 | 1.02 | 1.37 | ||
| t-cadinol | 1644 | 1645 | 0.45 | 0.70 | |||
| α-cadinol | 1657 | 1652 | 0.30 | 0.65 | 1.04 | 0.50 | |
| α-bisabolol | 1689 | 1685 | 0.50 | 0.31 | |||
| mintsulfide | 1743 | 1744 | 0.30 | 0.69 | 0.76 | ||
| kaurene | 2029 | 2034 | 0.40 | ||||
| Total identified compounds % | 95.59 | 96.53 | 94.56 | 94.01 | 89.64 | ||
| Spearmint Essential oil yield (%) | 3.26% | ||||||
| Identified Compounds | RRIa | LRIb | Concentration (%)c | ||||
|---|---|---|---|---|---|---|---|
| (i) Oregano | (ii) Soil Amended with Oregano | ||||||
| Days after Soil Amendment | |||||||
| 15 | 30 | 60 | 90 | ||||
| a-thujene | 929 | 931 | 0.52 | 0.61 | 1.65 | 0.68 | |
| a-pinene | 936 | 937 | 0.85 | 0.65 | 1.75 | 1.19 | |
| α-camphene | 949 | 953 | 0.46 | 0.34 | |||
| β-pinene | 976 | 980 | 0.31 | 0.39 | |||
| β-myrcene | 990 | 991 | 1.76 | 0.67 | 0.34 | ||
| α-terpinene | 1015 | 1018 | 1.40 | 0.70 | 0.65 | 0.67 | |
| p-cymene | 1024 | 1026 | 6.27 | 5.56 | 15.11 | 13.54 | |
| limonene/β-phellendrene | 1029 | 1031 | 0.48 | 1.69 | 0.76 | 0.59 | |
| 1,8-cineole/eucalyptol | 1032 | 1033 | 0.56 | ||||
| γ-terpinene | 1059 | 1062 | 4.26 | 0.86 | 0.30 | 0.55 | |
| cis-sabinene hydrate | 1066 | 1065 | 0.55 | 1.78 | 2.01 | ||
| trans-sabinene hydrate | 1094 | 1098 | 0.30 | 0.99 | 1.31 | ||
| borneol | 1163 | 1165 | 0.52 | 0.48 | 1.50 | 2.37 | |
| terpinen-4-ol | 1175 | 1177 | 0.89 | 1.05 | 1.75 | 2.57 | |
| dihydrocarveol | 1191 | 1193 | 0.37 | 0.31 | 0.98 | ||
| dihydrocarvone | 1195 | 1194 | 0.45 | ||||
| trans-carveol | 1215 | 1217 | 0.60 | ||||
| carvone | 1241 | 1242 | 0.79 | 0.91 | |||
| thymoquinone | 1248 | 1250 | 0.31 | 3.55 | |||
| thymol | 1293 | 1290 | 0.84 | 0.36 | 0.30 | 1.13 | |
| carvacrol | 1307 | 1298 | 78.31 | 78.52 | 58.86 | 48.22 | |
| dihydrocarveol acetate | 1331 | 1326 | 0.46 | 0.90 | |||
| α-copaene | 1376 | 1376 | 0.93 | ||||
| β-bourbonene | 1384 | 1384 | 38.15 | ||||
| β-elemene | 1390 | 1391 | 2.45 | ||||
| cis-caryophyllene | 1406 | 1408 | 0.46 | ||||
| a-gurjunene | 1410 | 1409 | 0.49 | ||||
| β-caryophyllene | 1416 | 1418 | 1.52 | 1.90 | 4.79 | 11.42 | 7.72 |
| β-gurjunene | 1425 | 1431 | 3.42 | ||||
| aromadendrene | 1441 | 1439 | 4.62 | ||||
| α-humulene | 1452 | 1454 | 0.55 | 1.27 | 0.52 | ||
| allo-aromadendrene | 1456 | 1458 | 0.79 | ||||
| cis-muurola-4(14),5-diene | 1462 | 1465 | 4.97 | ||||
| β-acoradiene | 1467 | 1467 | 3.06 | ||||
| germacrene-D | 1476 | 1480 | 9.97 | ||||
| bicyclogermacrene | 1492 | 1494 | 1.77 | ||||
| γ-cadinene | 1513 | 1513 | 0.30 | 0.32 | 0.35 | 1.89 | 1.08 |
| cis-calamenene | 1520 | 1521 | 0.39 | 3.61 | |||
| α-cadinene | 1538 | 1537 | 0.94 | ||||
| caryophyllene oxide | 1582 | 1581 | 0.31 | 0.53 | 2.27 | ||
| viridiflorol | 1589 | 1590 | 2.14 | ||||
| 1,10-di-epi-cubenol | 1612 | 1618 | 0.41 | ||||
| α-cadinol | 1656 | 1652 | 0.39 | ||||
| mintsulfide | 1742 | 1744 | 0.33 | 0.61 | |||
| Total identified compounds % | 97.92 | 97.33 | 96.67 | 92.74 | 92.34 | ||
| Oregano Essential oil yield (%) | 6.90% | ||||||
| Variation Source | Significance of F-Ratio | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| df | Shoot Length | Shoot Thickness | CCIa | A neta | ADIa | df | Yield Per Plant | pH | oBrixa | Dry Matter | |
| ET | 1 | NS | NS | NS | NS | NS | 1 | NS | NS | NS | NS |
| IAP | 2 | *** | *** | *** | *** | *** | 2 | *** | *** | *** | *** |
| SF | 2 | *** | *** | *** | *** | *** | 2 | *** | *** | *** | *** |
| IAP×SF | 4 | *** | *** | *** | *** | *** | 4 | *** | *** | *** | *** |
| ET×IAP | 2 | NS | NS | NS | NS | NS | 2 | NS | NS | NS | NS |
| ET×SF | 2 | NS | NS | NS | NS | NS | 2 | NS | NS | NS | NS |
| ET×IAP×SF | 4 | NS | NS | NS | NS | NS | 4 | NS | NS | NS | NS |
| DAT | 4 | *** | *** | *** | *** | NS | |||||
| DAT×ET | 4 | NS | NS | NS | NS | NS | |||||
| DAT×IAP | 8 | *** | *** | *** | *** | *** | |||||
| DAT×SF | 8 | *** | *** | *** | NS | *** | |||||
| DAT×ET×IAP | 8 | NS | NS | NS | NS | NS | |||||
| DAT×ET×SF | 8 | NS | NS | NS | NS | NS | |||||
| DAT×IAP×SF | 16 | *** | *** | *** | *** | *** | |||||
| DAT×ET×IAP×SF | 16 | NS | NS | NS | NS | NS | |||||
| Average Degree of Infection (ADI)a | AUDPC | |||
|---|---|---|---|---|
| Days after Inoculation | ||||
| Treatment | 15 | 30 | 50 | |
| C | - | - | - | 0 |
| CF | 3.5 ± 0.10 ab | 4.9 ± 0.11 a | 4.8 ± 0.13 b | 160 |
| CV | 3.6 ± 0.13 a | 5.0 ± 0.12 a | 5.1 ± 0.09 a | 160.5 |
| M | - | - | - | 0 |
| MF | 2.2 ± 0.17 b | 1.3±0.09 cd | 1.2 ± 0.08 d | 51.25 |
| MV | 2.0 ± 0.19 b | 1.1±0.06 d | 1.1 ± 0.06 d | 45.25 |
| OR | - | - | - | 0 |
| ORF | 3.3 ± 0.16 a | 4.0 ± 0.13 b | 4.1 ± 0.16 c | 135.75 |
| ORV | 3.2 ± 0.13 a | 1.5 ± 0.10 c | 1.0 ± 0.04 d | 60.25 |
| Yield Per Plant (kg) | Physicochemical Properties of Tomato Fruits | |||||
|---|---|---|---|---|---|---|
| Treatment | pH | Soluble Solids Content (°brix) | Dry Matter (%) | |||
| C | 1130 a ± 107 c b | 3.92 ± 0.03 bc | 3.13 ± 0.04 c | 94.51 ± 0.12 bc | ||
| CF | 138 ± 31 e | 4.00 ± 0.02 abc | 2.30 ± 0.02 e | 94.79 ± 0.05 b | ||
| CV | 179 ± 38 e | 3.82 ± 0.03 c | 2.70 ± 0.05 de | 90.31 ± 0.07 e | ||
| M | 2290 ± 172 ab | 3.82 ± 0.02 c | 4.20 ± 0.05 b | 93.98 ± 0.12 cd | ||
| MF | 2144 ± 171 ab | 3.94 ± 0.02 bc | 4.40 ± 0.02 b | 93.39 ± 0.10 d | ||
| MV | 2202 ± 161 ab | 4.04 ± 0.00 abc | 5.00 ± 0.05 a | 94.50 ± 0.12 bc | ||
| OR | 2437 ± 167 a | 3.95 ± 0.05 abc | 3.20 ± 0.02 c | 94.61 ± 0.05 bc | ||
| ORF | 636 ± 85 d | 4.08 ± 0.02 ab | 3.10 ± 0.02 cd | 95.61 ± 0.05 a | ||
| ORV | 2004 ± 213 b | 4.17 ± 0.01 a | 4.10 ± 0.05 b | 94.31 ± 0.07 bc | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kadoglidou, K.; Chatzopoulou, P.; Maloupa, E.; Kalaitzidis, A.; Ghoghoberidze, S.; Katsantonis, D. Mentha and Oregano Soil Amendment Induces Enhancement of Tomato Tolerance against Soilborne Diseases, Yield and Quality. Agronomy 2020, 10, 406. https://doi.org/10.3390/agronomy10030406
Kadoglidou K, Chatzopoulou P, Maloupa E, Kalaitzidis A, Ghoghoberidze S, Katsantonis D. Mentha and Oregano Soil Amendment Induces Enhancement of Tomato Tolerance against Soilborne Diseases, Yield and Quality. Agronomy. 2020; 10(3):406. https://doi.org/10.3390/agronomy10030406
Chicago/Turabian StyleKadoglidou, Kalliopi, Paschalina Chatzopoulou, Eleni Maloupa, Argyrios Kalaitzidis, Sopio Ghoghoberidze, and Dimitrios Katsantonis. 2020. "Mentha and Oregano Soil Amendment Induces Enhancement of Tomato Tolerance against Soilborne Diseases, Yield and Quality" Agronomy 10, no. 3: 406. https://doi.org/10.3390/agronomy10030406
APA StyleKadoglidou, K., Chatzopoulou, P., Maloupa, E., Kalaitzidis, A., Ghoghoberidze, S., & Katsantonis, D. (2020). Mentha and Oregano Soil Amendment Induces Enhancement of Tomato Tolerance against Soilborne Diseases, Yield and Quality. Agronomy, 10(3), 406. https://doi.org/10.3390/agronomy10030406






