Grazing and Cutting under Different Nitrogen Rates, Application Methods and Planting Density Strongly Influence Qualitative Traits and Yield of Canola Crop
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Treatments and Methods
2.3. Field Preparation and Cultural Practices
2.4. Grazing Management

2.5. Observations
2.5.1. Quality Attributes of Canola
2.5.2. Canola Yield
2.5.3. Statistical Analysis
3. Results
3.1. Treatments Interactions
3.2. Crop Yield
3.3. Quality Parameters of Canola
3.3.1. Oil Content (%) and Protein Content (%)
3.3.2. Erucic acid Content (%)
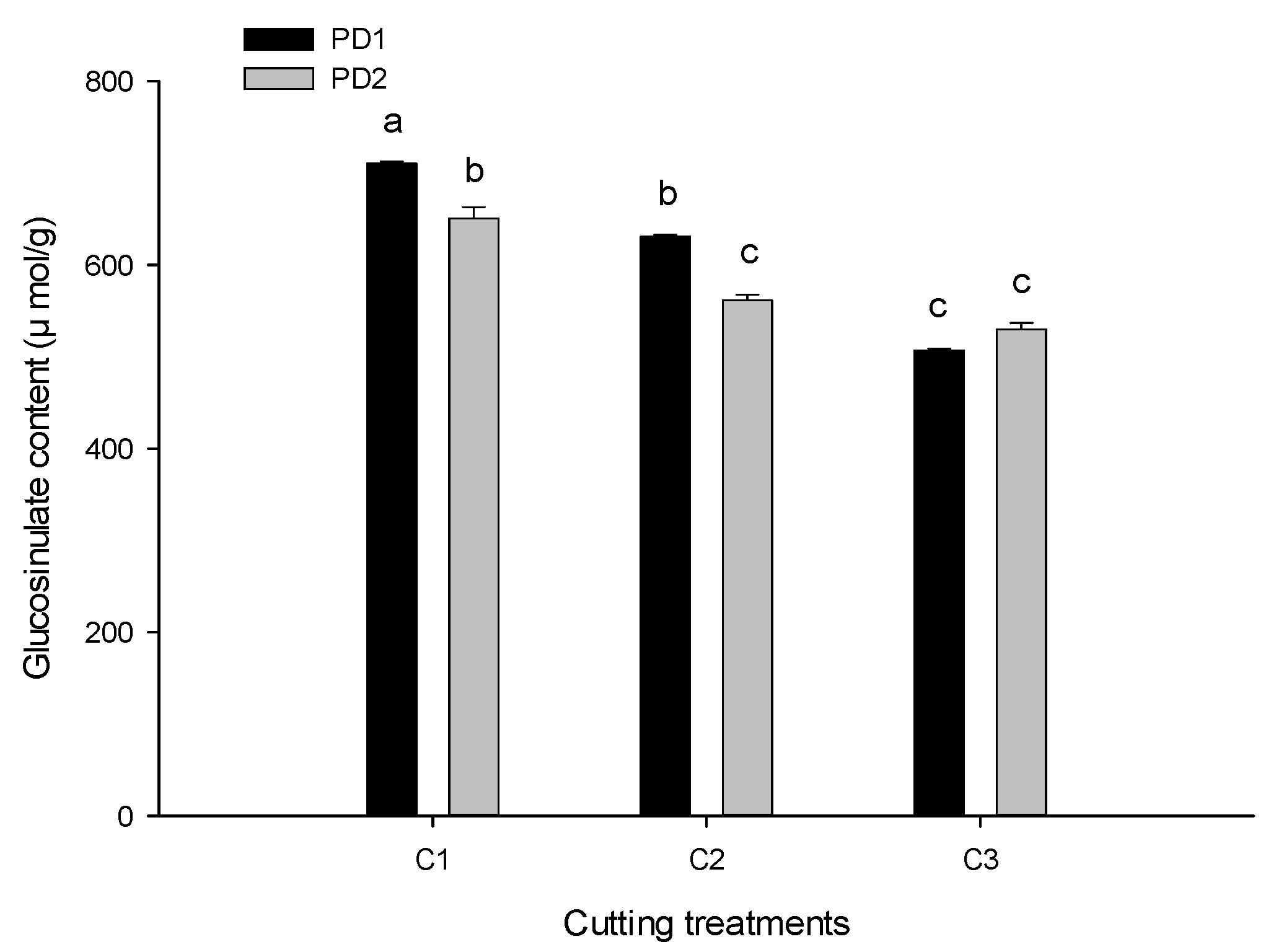
3.3.3. Glucosinolates Concentration
3.4. Economic Analysis
4. Discussion
4.1. Crop Yield
4.1.1. Effects of Grazing and Cut on Canola Yield
4.1.2. Effects of N fertilizer, Application Timings on Canola Yield
4.1.3. Effects of Planting Densities on Canola Yield
4.2. Qualitative Traits of Canola
4.2.1. Effects of Grazing and Cut on Quality Traits of Canola
4.2.2. Effects of N Fertilizer, Application Timings on Quality Traits of Canola
4.2.3. Effects of Planting Densities on Quality Traits of Canola
4.3. Interaction Effect of Factors
4.4. Economics Benefits
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Barlog, P.; Grzebisz, W. Effect of timing and nitrogen fertilizer application on winter oilseed rape (Brassica napus L.). I. Growth Dynamics and Seed Yield. J. Agron. Crop. Sci. 2004, 190, 305–310. [Google Scholar] [CrossRef]
- GOP. Economic Survey Report, 2003–2004; Govt. of Pakistan, Finance Division: Islamabad, Pakistan, 2007; pp. 3–4. [Google Scholar]
- Cheema, M.A.; Malik, M.A.; Hussain, A.; Shah, S.H.; Basra, S.M.A. Effects of time and rate of nitrogen and phosphorus application on growth and seed and oil yields of canola. J. Agron. Crop. Sci. 2001, 186, 103–110. [Google Scholar] [CrossRef]
- Grombacher, A.; Nelson, L. Canola Production. A University of Nebraska Neb Guide Publication, G92-1076-A; Institute of Agriculture and Natural Resources, University of Nebraska-Lincoln: Lincoln, NE, USA, 1992. [Google Scholar]
- Gunasekera, C.P.; Martin, L.D.; Siddique, K.H.M.; Walton, G.H. Genotype by environment interactions of indian mustard (Brassica juncea L.) and canola (Brassica napus L.) in mediterranean-type environments: Ii. Oil and protein concentrations in seed. Eur. J. Agron. 2006, 25, 13–21. [Google Scholar] [CrossRef]
- Hao, X.C.; Chang, C.; Travis, G.J. Short communication: Effect of long-term cattle manure application on relations between nitrogen and oil content in canola seed. J. Plant Nutr. Soil Sci. 2004, 167, 214–215. [Google Scholar] [CrossRef]
- Hocking, P.J.; Strapper, M. Effect of sowing time and nitrogen fertilizer on canola and wheat, and nitrogen fertilizer on Indian mustard. II. Nitrogen concentrations, N accumulation, and N use efficiency. Aust. J. Agric. Res. 2001, 52, 635–644. [Google Scholar] [CrossRef]
- Faraji, A.; Sadeghi, S.; Asadi, M. Evaluation of nitrogen and irrigationeffects on yield and yield components of canola varieties in Gonbad. J. Agric. Res. Nat. Resour. 2005, 12, 63–72. [Google Scholar]
- Wright, G.C.; Smith, C.J.; Woodroffe, M.R. The effect of irrigation and nitrogen fertilizer on rapeseed (Brassica napus) production in South- Eastern Australia, I. Growth and seed yield. Irrig. Sci. 1988, 9, 1–13. [Google Scholar] [CrossRef]
- Sommer, S.G.; Schjoerring, J.K.; Denmead, O.T. Ammonia emission from mineral fertilizers and fertilized crops. Adv. Agron. 2004, 82, 557–622. [Google Scholar]
- Kirkegaard, J.A.; Sprague, S.J.; Hamblin, P.J.; Graham, J.M.; Lilley, J.M. Refining crop and livestock management for dual-purpose spring canola (Brassicanapus). Crop Pasture Sci. 2006, 63, 429–443. [Google Scholar] [CrossRef]
- Daun, J.K.; Clear, K.M.; Williams, P. Comparison of three whole seed near infrared analyzers for measuring quality components of canola seed. J. Am. Oil Chem. Soc. 1994, 71, 1063–1068. [Google Scholar] [CrossRef]
- Canola Council of Canada. The Grower’s Manual (Online); Canola Council of Canada: Winnipeg, MB, Canada, 2005. [Google Scholar]
- Jan, M.; Shah, P.; Hollington, P.; Khan, M.; Sohail, Q. Agriculture research: Design and analysis, a monograph. NWFP Agric. Univ. Pesh. Pak. 2009, 1, 232–250. [Google Scholar]
- Kirkegaard, J.; Sprague, S.J.; Marcroft, S.; Potter, T.D.; Graham, J.; Virgona, J. Identifying canola varieties for dual-purpose use. In Global Issues—Paddock Action, Proceedings of the 14th Australian Agronomy Conference, Adelaide South, Australia, 21–25 September 2008; Australian Society of Agronomy: Gosford, Australia, 2008. [Google Scholar]
- Khan, A.H.; Khalil, I.A.; Shah, H. Nutritional yield and oil quality of canola cultivars grown in NWFP. Sarhad J. Agric. 2004, 20, 287–290. [Google Scholar]
- Susko, D.J.; Superfisky, B. A comparison of artificial defoliation techniques using canola (Brassica napus). Plant Ecol. 2009, 202, 169–175. [Google Scholar] [CrossRef]
- McCormick, J.I.; Virgona, J.M.; Kirkegaard, J.A. Growth and yield of dual-purpose canola (Brassica napus) under drier inland seasonal conditions of south-eastern Australia. Crop Pasture Sci. 2012, 63, 635–646. [Google Scholar] [CrossRef]
- Salehian, H.; Rafiey, M.; Fathiand, G.; Siadat, S.A. Effect of plant density on growth and seed yield of colza varieties under Andimeshk conditions. In Proceedings of the 7th Iranian Crop Sciences Congress, Karaj, Iran, 15 March 2007. [Google Scholar]
- Sprague, S.J.; Kirkegaard, J.A.; Graham, J.M.; Dove, H.; Kelman, W.M. Crop and livestock production for dual-purpose winter canola (Brassica napus) in the high-rainfall zone of south-eastern Australia. Field Crop. Res. 2014, 156, 30–39. [Google Scholar] [CrossRef]
- Al-Jaloud, A.A.; Hussain, G.; Karimulla, S.; Alhamdi, A.H. Effect of irrigation and nitrogen on yield and yield components of two rapeseed cultivars. Agric. Water Manag. 1996, 30, 57–68. [Google Scholar] [CrossRef]
- Balint, T.; Rengel, Z.; Allen, D. Australia canola germplasm differs in nitrogen and sulphur defficiency. Aust. J. Agric. Res. 2008, 59, 1164–1174. [Google Scholar] [CrossRef]
- Buttar, G.S.; Thind, H.S.; Aujla, M.S. Methods of planting and irrigation at various levels of nitrogen affect the seed yield and water use efficiency in transplanted oil seed rape. Agric. Water Manag. 2005, 85, 253–260. [Google Scholar] [CrossRef]
- Cheema, M.A. Production Efficiency of Canola (Brassica napus L.) cv. Shiralee under Different Agro-Management Practices. Ph.D. Thesis, Department of Agronomy, University of Agriculture, Faisalabad, Pakistan, 1999. [Google Scholar]
- Zaman, M. Effect of Rate and Time of Nitrogen Application on Growth, Seed Yield and Oil Contents of Canola (Brassica napus L.). Bachelor’s Thesis, Department of Agronomy, University of Agriculture, Faisalabad, Pakistan, 2003. [Google Scholar]
- Dahmardeh, M.; Mahmood, R.; Valizadeh, J. Effect of plant density and cultivars on growth, yield and yield components of faba bean (Viciafaba L.). Afr. J. Biotechnol. 2010, 9, 8643–8647. [Google Scholar]
- Shengwu, H.; Ovesna, J.; Kueera, L.; Kueera, V.; Vyvadilova, M. Evaluation of genetic diversity of Brassica napusgermplasm from China and Europe assessed by RAPD markers. Plant Soil Environ. 2003, 49, 106–113. [Google Scholar]
- Kirkegaard, J.A.; Sprague, S.J.; Lilley, J.M.; McCormick, J.I.; Virgona, J.M.; Morrison, M.J. Physiological response of spring canola (Brassica napus) to defoliation in diverse environments. Field Crop. Res. 2012, 125, 61–68. [Google Scholar] [CrossRef]
- Söchtling, H.P.; Verret, J.A. Effects of different cultivation systems (soil management and nitrogen fertilization) on the epidemics of fungal diseases in oilseed rape (Brassica napus L. var. napus). J. Plant Dis. Prot. 2004, 111, 1–29. [Google Scholar] [CrossRef]
- Rathke, G.W.; Christen, O.; Diepenbrock, W. Effects of nitrogen source and rate on productivity and quality of winter oilseed rape (Brassica napus L.) grown in different crop rotations. Field Crop. Res. 2005, 94, 103–113. [Google Scholar] [CrossRef]
- Akhtar, K.; Wang, W.; Khan, A.; Ren, G.; Afridi, M.Z.; Feng, Y.; Yang, G. Wheat straw mulching offset soil moisture deficient for improving physiological and growth performance of summer sown soybean. Agric. Water Manag. 2019, 211, 16–25. [Google Scholar] [CrossRef]
- Saleem, M.; Cheema, A.M.; Malik, A.M. Agro-EconomicAssessment of Canola Planted under Different Levels of Nitrogenand Row Spacing. Int. J. Agric. Biol. 2001, 3, 27–30. [Google Scholar]
- Asare, E.; Scarisbrick, D.H. Rate of nitrogen and sulphur fertilizers on yield, yield components and seed quality of oilseed rape (Brassica napus L.). Field Crop. Res. 1995, 44, 41–46. [Google Scholar] [CrossRef]
- Omirou, M.D.; Papadopoulou, K.K.; Papastylianou, I.; Constantinou, M.; Karpouzas, D.G.; Asimakopoulos, I.; Ehaliotis, C. Impact on nitrogen and sulfur fertilization on the composition of glucosinolates in relation to sulfur assimilation in different plant organs of broccoli. J. Agric. Food Chem. 2009, 57, 9408–9417. [Google Scholar] [CrossRef]
- Holmes, M.R.J. Nitrogen. In Nutrition of the Oilseed Rape Crop; Applied Science Publishers: Barking, UK, 1980; pp. 21–67. [Google Scholar]
- Kutcher, H.R.; Malhi, S.S.; Gill, K.S. Topography and management of nitrogen and fungicide affects diseases and productivity of canola. Agron. J. 2005, 97, 533–541. [Google Scholar] [CrossRef]
- Brennan, R.F.; Mason, M.G.; Walton, G.H. Effect of nitrogen fertilizer on the concentrations of oil and protein in canola (Brassica napus) seed. J. Plant Nutr. 2000, 23, 339–348. [Google Scholar] [CrossRef]
- Ahmad, G.; Jan, A.; Arif, M.; Jan, M.T.; Khattak, R.A. Influence of nitrogen and sulfur fertilization on quality of canola (Brassica napus L.) under rain fed conditions. J. Zhejiang Univ. Sci. B 2007, 8, 731–737. [Google Scholar] [CrossRef]
- Narits, L. Effect of nitrogen rate and application time to yield and quality of winter oilseed rape (Brassica napus L. var. oleiferasubvar. biennis). Agron. Res. 2010, 8, 671–686. [Google Scholar]
- Chen, X.J.; Zhu, Z.J.; Ni, X.L.; Qian, Q.Q. Effect of Nitrogen and Sulfur Supply on Glucosinolates in Brassica campestris ssp. chiensis. Agric. Sci. China 2006, 5, 603–608. [Google Scholar] [CrossRef]
- Zhao, F.; Evans, E.J.; Bilsborrow, P.E.; Syers, J.K. Influence of nitrogen and sulphur on the glucosinolates profile of rapeseed (Brassica napus L.). J. Sci. Food Agric. 2006, 64, 295–304. [Google Scholar] [CrossRef]
- Falk, K.L.; Tokuhisa, J.G.; Gershenzon, J. The effect of sulfur nutrition on plant glucosinolates content: Physiology and molecular mechanisms. Plant Biol. 2007, 9, 573–581. [Google Scholar] [CrossRef]
- Saeedi, F.H.; Bagheri, H.; Rasoli, S.A.; Ghasemi, J. The effect of planting density on morphological traits of spring canola cultivars in Chaloos region of Iran. Int. J. Agron. Plant Prod. 2013, 4, 911–914. [Google Scholar]
- Khan, A.; Zheng, J.; Tan, D.K.Y.; Khan, A.; Akhtar, K.; Kong, X.; Fahad, S. Changes in Leaf Structural and Functional Characteristics when Changing Planting Density at Different Growth Stages Alters Cotton Lint Yield under a New Planting Model. Agronomy 2019, 9, 859. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.; Kong, X.; Najeeb, U.; Zheng, J.; Tan, D.K.Y.; Akhtar, K.; Zhou, R. Planting density induced changes in cotton biomass yield, fiber quality, and phosphorus distribution under beta growth model. Agronomy 2019, 9, 500. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.; Wang, L.; Ali, S.; Tung, S.A.; Hafeez, A.; Yang, G. Optimal planting density and sowing date can improve cotton yield by maintaining reproductive organ biomass and enhancing potassium uptake. Field Crop. Res. 2017, 214, 164–174. [Google Scholar] [CrossRef]
- Gan, Y.; Malhi, S.S.; Brandt, S.; Katepa-Mupondwa, F.; Stevenson, C. Nitrogen use efficiency and nitrogen uptake of juncea Canola under diverse environments. Agron. J. 2007, 100, 285–295. [Google Scholar] [CrossRef]
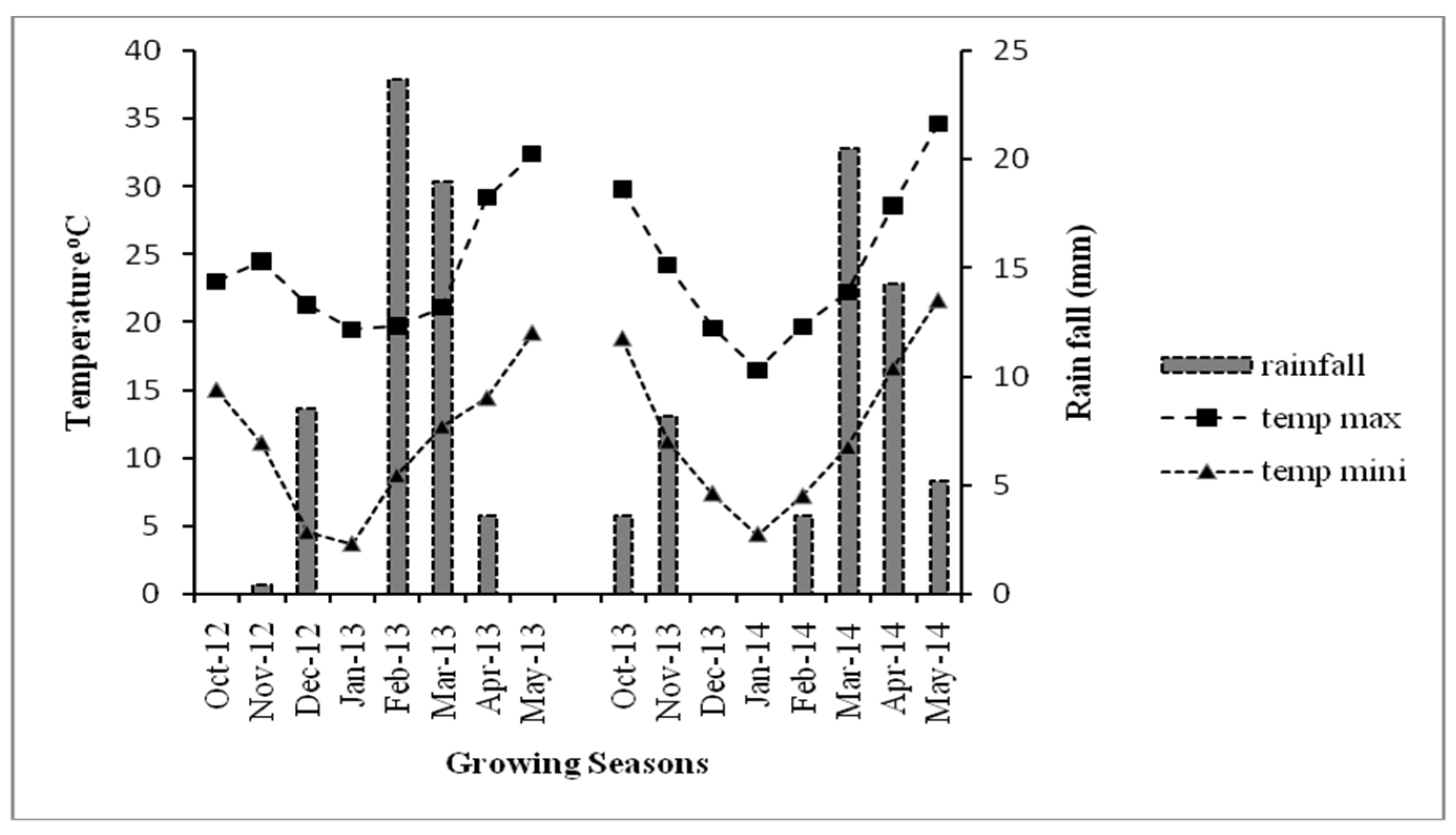


| Main Plot Factors | Treatment Levels | Sub Plot Factors | Treatment Levels |
|---|---|---|---|
| Cuttings (C) | No-cut (C1) | Nitrogen application timings (NT) | (1) full application at sowing (NT1) |
| Cut (C2) | (2) half dose of N both at sowing and start of rosette stage (NT2) | ||
| Grazing (C3) | (3) one third dose of N each at sowing, start of rosette stage and soon after cut at late rosette stage (60 days after sowing (NT3) | ||
| Nitrogen Levels (NL) | 80 kg ha−1 (NL1) | Planting density (PD) | 20 plants m−2 (PD1) |
| 120 kg ha−1 (NL2) | 40 plants m−2 (PD2) |
| SOV | Grain Yield | Biological Yield | Oil Content | Protein Content | Glucosinulates Content | Eurcic Acid |
|---|---|---|---|---|---|---|
| Year | 134 ns | 7.397 * | 1598.49 * | 10,560.2 | 799.26 * | 346.56 * |
| Cuttings (C) | 1,482,015 * | 1.383 * | 18.93 * | 27.7 ns | 330.24 * | 115.59 * |
| N-levels (NL) | 204,857 * | 5,140,844 ns | 243.63 * | 135.9 | 1547.22 * | 22.556 ns |
| Planting densities (PD) | 45,182 ns | 4,630,281 * | 18.84 * | 1.2 ns | 1.23 ns | 6.476 ns |
| Nitrogen timings (NT) | 30,938 ns | 6,378,573 * | 23.12 * | 1.9 ns | 6.18 ns | 14.529 ns |
| C × NL | ns | * | * | ns | * | ns |
| C × NT | * | ns | * | ns | ns | * |
| C × PD | ns | ns | ns | ns | ns | ns |
| NL × PD | ns | ns | ns | ns | ns | ns |
| NT × NL | ns | * | ns | ns | ns | ns |
| NT × PD | * | ns | * | ns | ns | * |
| C × NT × NL | ns | ns | ns | ns | ns | ns |
| C × NT × PD | ns | ns | ns | ns | ns | ns |
| NT × NL × PD | ns | ns | ns | ns | ns | ns |
| C × NL × NT × PD | ns | ns | ns | ns | ns | ns |
| Variables | 2012–2013 | 2013–2014 | Two Years Average |
|---|---|---|---|
| Cutting treatments (C) | |||
| C1 | 9695 a | 9353 a | 9524a |
| C2 | 7272 b | 9774 a | 8523 b |
| C3 | 6080 c | 8432 b | 7256 c |
| LSD (0.05) | 598 | 631 | 407 |
| Nitrogen levels (NL) | |||
| NL1 | 8099 | 8794 b | 8446 |
| NL2 | 7932 | 9578 a | 8755 |
| Significance level | ns | * | ns |
| N application timings (NT) | |||
| NT1 | 7974 ab | 9023 | 8496 |
| NT2 | 7700 b | 9428 | 8564 |
| NT3 | 8372 a | 9107 | 8739 |
| LSD (0.05) | 500 | 506 | 352 |
| Planting density (PD) | |||
| PD1 | 7785 b | 8584 b | 8454 b |
| PD2 | 8264 a | 9787 a | 8747 a |
| Significance level | * | * | * |
| Year (*) | 8016 b | 9186 a | |
| Variables | 2012–2013 | 2013–2014 | Two Years Average |
|---|---|---|---|
| Cutting treatments (C) | |||
| C1 | 1291 a | 1128 a | 1210 a |
| C2 | 983 b | 1068 b | 1025 b |
| C3 | 886 c | 968 c | 927 c |
| LSD (0.05) | 72.0 | 54.1 | 42.2 |
| Nitrogen levels (NL) | |||
| NL1 | 1022 b | 1024 b | 1023 b |
| NL2 | 1084 a | 1086 a | 1085 a |
| Significance level | * | * | * |
| N application timings (NT) | |||
| NT1 | 1021 b | 1054 a | 1045 ab |
| NT2 | 1029 b | 1058 a | 1078 a |
| NT3 | 1109 a | 1052 a | 1039 b |
| LSD (0.05) | 42.5 | 64.6 | 38.3 |
| Planting density (PD) | |||
| PD1 | 1066 | 1057 | 1039 |
| PD2 | 1044 | 1053 | 1068 |
| Significance level | ns | ns | ns |
| Year (ns) | 1053 | 1055 | |
| Variables | 2012–2013 | 2013–2014 | Two Years Average |
|---|---|---|---|
| Cutting treatments (C) | |||
| C1 | 40.78 a | 45.95 a | 43.37 a |
| C2 | 40.03 b | 45.51 ab | 42.77 b |
| C3 | 39.51 b | 45.18 b | 42.35 c |
| LSD (0.05) | 0.67 | 0.59 | 0.42 |
| Nitrogen levels (NL) | |||
| NL1 | 41.13 a | 46.66 a | 43.89 a |
| NL2 | 39.09 b | 44.44 b | 41.77 b |
| Significance level | * | * | * |
| N application timings (NT) | |||
| NT1 | 39.81 b | 45.33 ab | 42.26 b |
| NT2 | 39.77 b | 45.09 b | 43.39 a |
| NT3 | 40.74 a | 46.22 a | 42.83 ab |
| LSD (0.05) | 0.75 | 0.90 | 0.58 |
| Planting density (PD) | |||
| PD1 | 40.00 | 45.35 | 42.53 b |
| PD2 | 40.21 | 45.76 | 43.12 a |
| Significance level | * | ns | * |
| Year (*) | 40.11 b | 45.55 a | |
| Variables | 2012–2013 | 2013–2014 | Two Years Average |
|---|---|---|---|
| Cutting treatments (C) | |||
| C1 | 36.26 | 21.56 b | 28.91 b |
| C2 | 37.25 | 23.00 a | 30.14 a |
| C3 | 36.19 | 23.21 a | 29.70 ab |
| LSD (0.05) | 2.01 | 1.07 | 1.06 |
| Nitrogen levels (NL) | |||
| NL1 | 35.90 | 21.69 b | 28.79 b |
| NL2 | 37.26 | 23.50 a | 30.38 a |
| Significance level | ns | * | * |
| N application timings (NT) | |||
| NT1 | 35.90 b | 22.93 | 29.44 |
| NT2 | 36.45 ab | 22.57 | 29.76 |
| NT3 | 37.37 a | 22.58 | 29.55 |
| LSD (0.05) | 1.42 | 0.83 | 0.81 |
| Planting density (PD) | |||
| PD1 | 36.24 | 23.03 a | 29.5 |
| PD2 | 36.92 | 22.16 b | 29.66 |
| Significance level | * | * | ns |
| Year (*) | 22.59 b | 36.58 a | |
| Variables | 2012–2013 | 2013–2014 | Two Years Average |
|---|---|---|---|
| Cutting treatments (C) | |||
| C1 | 32.3 b | 31.2 b | 31.0 b |
| C2 | 34.1 ab | 33.1 ab | 32.9 a |
| C3 | 34.7 a | 33.6 a | 33.4 a |
| LSD (0.05) | 1.94 | 1.91 | 1.26 |
| Nitrogen levels (NL) | |||
| NL1 | 33.4 | 32.3 | 32.1 |
| NL2 | 34.1 | 32.9 | 32.8 |
| Significance level | ns | ns | ns |
| N application timings (NT) | |||
| NT1 | 33.9 | 32.8 | 32.9 a |
| NT2 | 33.9 | 32.8 | 32.0 b |
| NT3 | 33.3 | 32.1 | 32.4 ab |
| LSD (0.05) | ns | ns | 0.805 |
| Planting density (PD) | |||
| PD1 | 33.7 | 32.6 | 32.3 |
| PD2 | 33.7 | 32.5 | 32.6 |
| Significance level | ns | ns | ns |
| Year (*) | 33.72 a | 31.2 b | |
| Variables. | 2012–2013 | 2013–2014 | Two Years Average |
|---|---|---|---|
| Cutting treatments (C) | |||
| C1 | 59.97 b | 66.18 a | 64.48 a |
| C2 | 62.78 a | 63.67 b | 61.82 b |
| C3 | 58.03 c | 62.46 b | 60.25 c |
| LSD (0.05) | 1.88 | 1.91 | 1.26 |
| Nitrogen levels (NL) | |||
| NL1 | 57.59 b | 61.42 a | 59.61 b |
| NL2 | 62.93 a | 66.79 b | 64.86 a |
| Significance level | * | * | * |
| N application timings (NT) | |||
| NT1 | 60.07 | 63.74 | 62.45 |
| NT2 | 60.27 | 64.26 | 62.23 |
| NT3 | 60.45 | 64.32 | 61.87 |
| LSD (0.05) | 1.07 | 1.51 | 0.99 |
| Planting density (PD) | |||
| PD1 | 60.76 | 64.29 | 62.11 |
| PD2 | 59.76 | 63.93 | 62.26 |
| Significance level | ns | ns | ns |
| Year (*) | 60.26 b | 64.11 a | |
| Yield, Value or Cost | C1 | C2 | C3 |
|---|---|---|---|
| Forage yield (kg ha−1) | 0.0 | 13.9 | 14.1 |
| Grain yield (kg ha−1) | 12.4 | 10.5 | 9.5 |
| Straw yield (kg ha−1) | 92.1 | 74.3 | 63.8 |
| Forage value (USD) | 0.0 | 69.5 | 70.3 |
| Grain value (USD) | 884.5 | 1067.1 | 863.4 |
| Straw value (USD) | 552.4 | 446.1 | 383.1 |
| Gross income (USD) | 1436.9 | 1582.8 | 1316.9 |
| Net income over control (USD) | 1057.2 | 1202.1 | 936.2 |
| Value cost ratio (VCR %) | 2.78 | 3.1 | 2.4 |
| Plant density m−2 | Cutting | N rate | NT1 | NT2 | NT3 |
|---|---|---|---|---|---|
| PD1 | C1 | NL1 | 2.95 | 2.57 | 2.82 |
| C1 | NL2 | 2.59 | 2.21 | 2.71 | |
| C2 | NL1 | 3.00 | 3.35 | 3.00 | |
| C2 | NL2 | 2.71 | 3.36 | 3.17 | |
| C3 | NL1 | 3.09 | 3.35 | 2.92 | |
| C3 | NL2 | 2.85 | 2.22 | 1.58 | |
| PD2 | C1 | NL1 | 2.60 | 3.30 | 3.10 |
| C1 | NL2 | 3.02 | 2.69 | 2.80 | |
| C2 | NL1 | 3.52 | 4.16 | 2.57 | |
| C2 | NL2 | 3.22 | 3.06 | 2.83 | |
| C3 | NL1 | 2.18 | 2.63 | 2.07 | |
| C3 | NL2 | 2.59 | 2.23 | 1.96 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaheer, S.; Arif, M.; Akhtar, K.; Khan, A.; Khan, A.; Bibi, S.; Muhammad Aslam, M.; Ali, S.; Munsif, F.; Jalal, F.; et al. Grazing and Cutting under Different Nitrogen Rates, Application Methods and Planting Density Strongly Influence Qualitative Traits and Yield of Canola Crop. Agronomy 2020, 10, 404. https://doi.org/10.3390/agronomy10030404
Zaheer S, Arif M, Akhtar K, Khan A, Khan A, Bibi S, Muhammad Aslam M, Ali S, Munsif F, Jalal F, et al. Grazing and Cutting under Different Nitrogen Rates, Application Methods and Planting Density Strongly Influence Qualitative Traits and Yield of Canola Crop. Agronomy. 2020; 10(3):404. https://doi.org/10.3390/agronomy10030404
Chicago/Turabian StyleZaheer, Sajjad, Muhammad Arif, Kashif Akhtar, Ahmad Khan, Aziz Khan, Shahida Bibi, Mehtab Muhammad Aslam, Salman Ali, Fazal Munsif, Fazal Jalal, and et al. 2020. "Grazing and Cutting under Different Nitrogen Rates, Application Methods and Planting Density Strongly Influence Qualitative Traits and Yield of Canola Crop" Agronomy 10, no. 3: 404. https://doi.org/10.3390/agronomy10030404





