Use of Fresh Scotta Whey as an Additive for Alfalfa Silage
Abstract
1. Introduction
2. Materials and Methods
2.1. Fresh Forage and Silages
2.2. Chemical Analyses of the Silages
2.3. Microbiological Analyses of the Silages
2.4. Statistical Analysis
3. Results
3.1. Characteristics of Alfalfa Yield
3.2. Nutritional Characteristics of Alfalfa Forage as Affected by Growth Stage at Harvest and Wilting
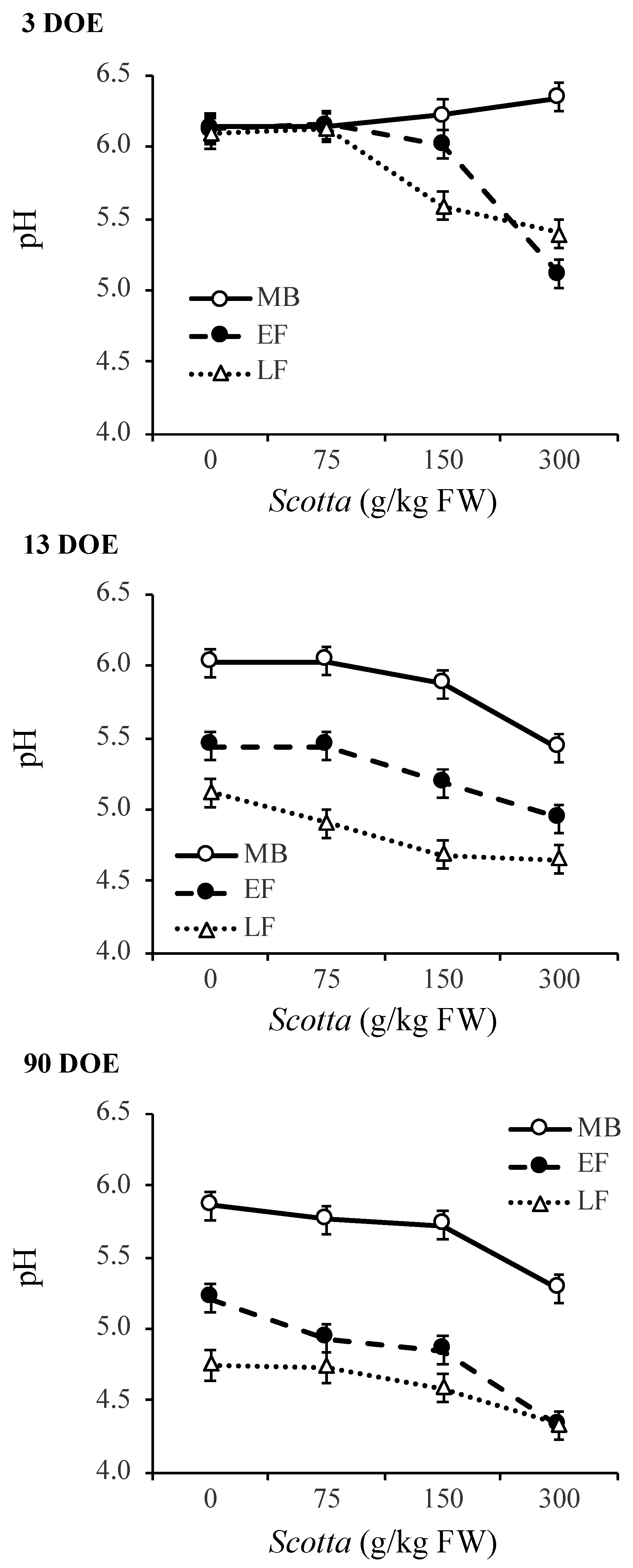
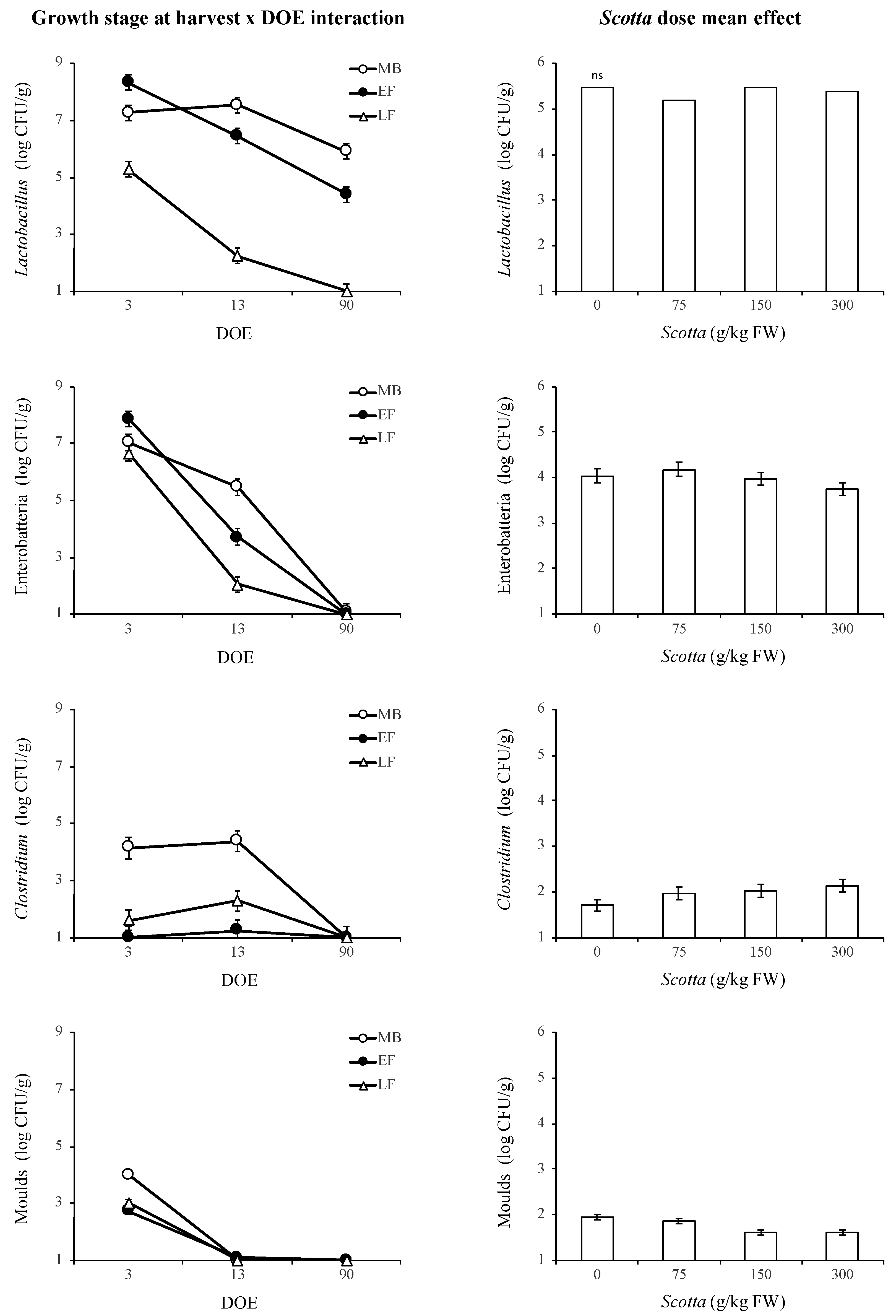
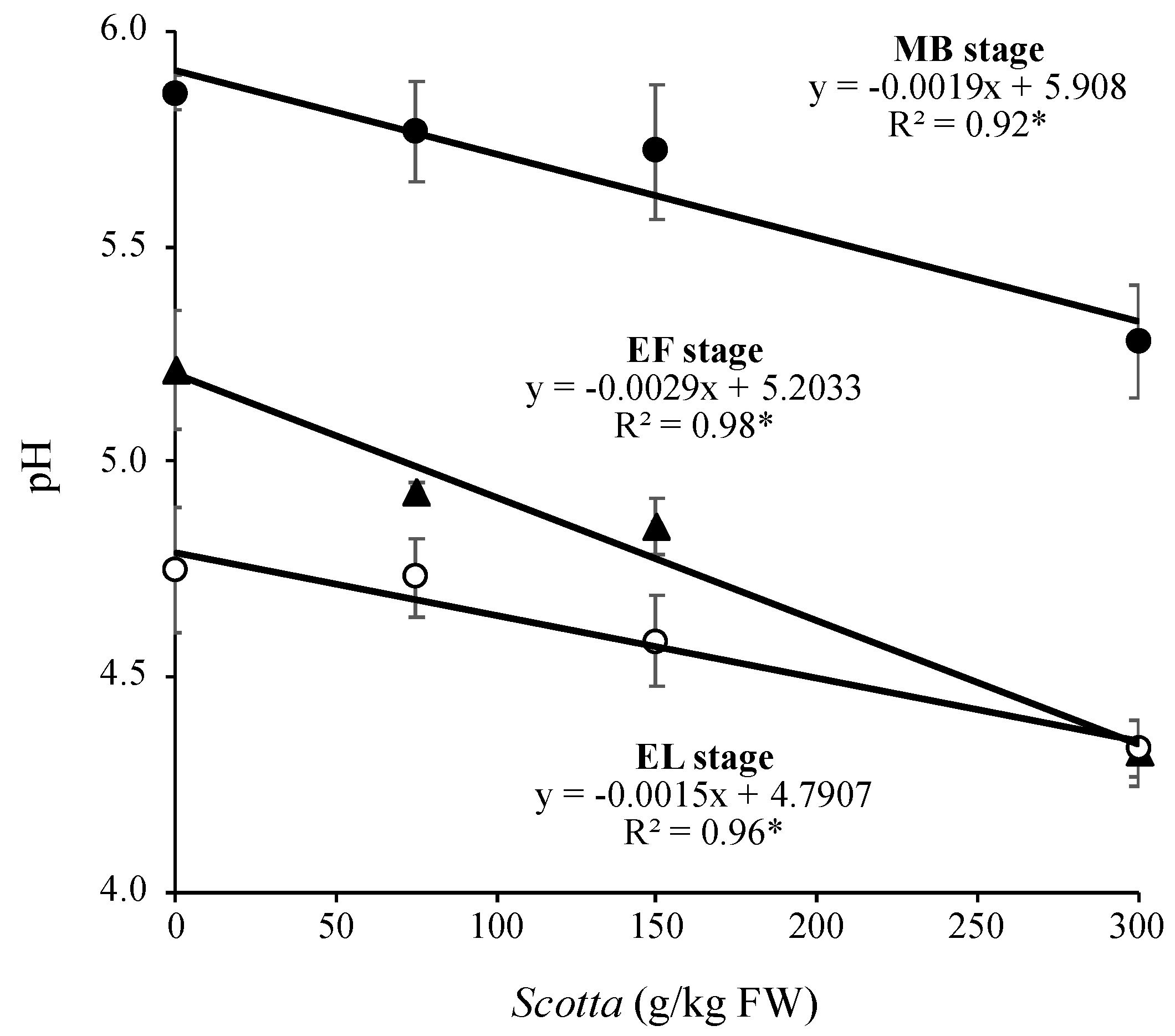
3.3. Fermentation Characteristics of Alfalfa Silage as Affected by DOE and Scotta Dose
3.4. Nutritional characteristics of Alfalfa Silage as Affected by Scotta Dose
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- McDonald, P. The Biochemistry of Silage; John Wiley and Sons: Chichester, UK, 1981. [Google Scholar]
- Jonsson, A. Growth of Clostridium tyrobutiricum during fermentation and aerobic deterioration of grass silage. J. Sci. Food Agric. 1991, 54, 557–568. [Google Scholar] [CrossRef]
- Gordon, F.J.; Dawson, L.E.R.; Ferris, C.P.; Steen, R.W.J.; Kilpatrick, D.J. The influence of wilting and forage additive type on the energy utilisation of grass silage by growing cattle. Anim. Feed Sci. Technol. 1999, 79, 15–27. [Google Scholar] [CrossRef]
- Orloff, S.; Mueller, S.C. Harvesting, curing and preservation of alfalfa. In Irrigated Alfalfa Management in Mediterranean and Desert Zones; Summers, C.G., Putnam, D.H., Eds.; University of California Agriculture and Natural Resources Publications 8300: Oakland, CA, USA, 2008; Volume 3512, pp. 1–18. [Google Scholar]
- Marsh, R. The effects of wilting on fermentation in the silo and on the nutritive value of silage. Grass For. Sci. 1979, 34, 1–10. [Google Scholar] [CrossRef]
- Hashemzadeh-Cigari, F.; Khorvash, M.; Ghorbani, G.R.; Taghizadeh, A. The effects of wilting, molasses and inoculants on the fermentation quality and nutritive value of lucerne silage. S. Afr. J. Anim. Sci. 2011, 41, 377–388. [Google Scholar] [CrossRef]
- Lloveras, J.; Ferran, J.; Alvarez, A.; Torres, L. Harvest management effects on alfalfa (Medicago sativa L.) production and quality in Mediterranean areas. Grass For. Sci. 1998, 53, 88–92. [Google Scholar] [CrossRef]
- Elizalde, J.C.; Merchen, N.R.; Faulkner, D.B. Fractionation of fiber and crude protein in fresh forages during the spring growth. J. Anim. Sci. 1999, 77, 476–484. [Google Scholar] [CrossRef]
- Yu, P.; Christensen, D.A.; McKinnon, J.J. In situ rumen degradation kinetics of timothy and alfalfa as affected by cultivar and stage of maturity. Can. J. Anim. Sci. 2004, 84, 255–263. [Google Scholar] [CrossRef]
- Yari, M.; Valizadeh, R.; Naserian, A.A.; Ghorbani, G.R.; Moghaddam, P.R.; Jonker, A.; Yu, P. Botanical traits, protein and carbohydrate fractions, ruminal degradability and energy contents of alfalfa hay harvested at three stages of maturity and in the afternoon and morning. Anim. Feed Sci. Tech. 2012, 172, 162–170. [Google Scholar] [CrossRef]
- Raguse, C.A.; Smith, D. Some nonstructural carbohydrates in forage legume herbage. J. Agric. Food Chem. 1966, 14, 423–426. [Google Scholar] [CrossRef]
- Shao, T.; Ohba, N.; Shimojo, M.; Masuda, Y. Fermentation quality of forage oat (Avena sativa L.) silages treated with pre-fermented juices, sorbic acid, glucose and encapsulated-glucose. J. Fac. Agric. Kyushu Univ. 2003, 47, 341–349. [Google Scholar]
- Ohshima, M.; Cao, L.M.; Kimura, E.; Ohshima, Y.; Yokoto, H.O. Influence of addition of previously fermented juice to alfalfa ensiled at different moisture contents. Jpn. J. Grassl. Sci. 1997, 43, 56–58. [Google Scholar]
- Wang, J.; Wang, J.Q.; Zhou, H.; Feng, T. Effects of addition of previously fermented juice prepared from alfalfa on fermentation quality and protein degradation of alfalfa silage. Anim. Feed Sci. Technol. 2009, 151, 280–290. [Google Scholar] [CrossRef]
- Tyrolová, Y.; Výborná, A. Effect of the stage of maturity on the leaf percentage of lucerne and the effect of additives on silage characteristics. Czech J. Anim. Sci. 2008, 53, 330–335. [Google Scholar] [CrossRef]
- Schmidt, R.J.; Hu, W.; Mills, J.A.; Kung, L., Jr. The development of lactic acid bacteria and Lactobacillus buchneri and their effects on the fermentation of alfalfa silage. J. Dairy Sci. 2009, 92, 5005–5010. [Google Scholar] [CrossRef] [PubMed]
- Pisponen, A.; Pajumägi, S.; Mootse, H.; Karus, A.; Poikalainen, V. The lactose from Ricotta cheese whey: The effect of pH and concentration on size and morphology of lactose crystals. Dairy Sci. Technol. 2013, 93, 477–486. [Google Scholar] [CrossRef]
- Kalu, B.A.; Fick, G.W. Quantifying morphological development of alfalfa for studies of herbage quality. Crop Sci. 1981, 21, 267–271. [Google Scholar] [CrossRef]
- Darby, D.E.; Jofriet, J.C. Density of silage in horizontal silos. Can. Agric. Eng. 1993, 35, 275–280. [Google Scholar]
- Martillotti, F.; Antongiovanni, M.; Rizzi, L.; Santi, E.; Bittante, G. Metodi di Analisi per la Valutazione degli Alimenti D’impiego Zootecnico; Quaderni metodologici n. 8; CNR-IPRA: Roma, Italy, 1987. [Google Scholar]
- European Commission. Commission Regulation (EC) No. 152/2009 of 27 January 2009 laying down the methods of sampling and analysis for the official control of feed. Off. J. Eur. Union 2009, L54, 1–130. [Google Scholar]
- Canale, A.; Valente, M.E.; Ciotti, A. Determination of volatile carboxylic acids (C1–C5i) and lactic acid in aqueous acid extracts of silage by high performance liquid chromatography. J. Sci. Food Agric. 1984, 35, 1178–1182. [Google Scholar] [CrossRef]
- Rohweder, D.; Barnes, R.F.; Jorgensen, N. Proposed hay grading standards based on laboratory analyses for evaluating quality. J. Anim. Sci. 1978, 47, 747–759. [Google Scholar] [CrossRef]
- Wall, L.L.; Gehrke, C.W. Automated determination of urea and ammoniacal nitrogen (NPN) in animal feeds. J. Assoc. Off. Anal. Chem. 1981, 64, 1092–1095. [Google Scholar] [CrossRef] [PubMed]
- Meteo Pisa. Available online: http://www.meteopisa.net/stazioni/PisaSG.php (accessed on 21 February 2020).
- Masoni, A.; Mariotti, M.; Arduini, I.; Pampana, S.; Ercoli, L. Nitrate leaching from forage legume crops and residual effect on Italian ryegrass. Agrochimica 2015, 59, 75–91. [Google Scholar]
- Yu, P.; Christensen, D.A.; McKinnon, J.J.; Markert, J.D. Effect of variety and maturity stage on chemical composition, carbohydrate and protein subfractions, in vitro rumen degradability and energy values of timothy and alfalfa. Can. J. Anim. Sci. 2003, 83, 279–290. [Google Scholar] [CrossRef]
- Canbolat, O.; Kamalak, A.; Ozkan, C.O.; Erol, A.; Sahin, M.; Karakas, E.; Ozkose, E. Prediction of relative feed value of alfalfa hays harvested at different maturity stages using in vitro gas production. Livest. Res. Rural Dev. 2006, 18, 27. [Google Scholar]
- Pop, I.M.; Radu-Rusu, C.G.; Simeanu, D.; Albu, A.; Popa, V. Characterization of the nutritional value of alfalfa harvested at different stages of vegetation using cell walls content based methods. Lucrări Ştiinţifice-Seria Zootehnie 2010, 53, 350–354. [Google Scholar]
- Karayilanli, E.; Ayhan, V. Investigation of feed value of alfalfa (Medicago sativa L.) harvested at different maturity stages. Legume Res. 2016, 39, 237–247. [Google Scholar] [CrossRef]
- Castle, M.E.; Watson, J.N. The relationship between the DM content of herbage for silage making and effluent production. Grass For. Sci. 1973, 28, 135–138. [Google Scholar] [CrossRef]
- Repetto, J.L.; Echarri, V.; Aguerre, M.; Cajarville, C. Use of fresh cheese whey as an additive for Lucerne silages: Effects on chemical composition, conservation quality and ruminal degradation of cell walls. Anim. Feed Sci. Technol. 2011, 170, 160–164. [Google Scholar] [CrossRef]
- Hartinger, T.; Gresner, N.; Südekum, K.H. Effect of wilting intensity, dry matter content and sugar addition on nitrogen fractions in lucerne silages. Agriculture 2019, 9, 11. [Google Scholar] [CrossRef]
- Lancaster, R.J.; Brunswick, L.F.C.; Wilson, R.K. Evaluation of formic acid as an additive for lucerne silage. N. Z. J. Exp. Agric. 1977, 5, 107–111. [Google Scholar] [CrossRef]
- Weissbach, F. New developments in crop conservation. In Proceedings of the 11th International Silage Conference, Aberystwyth, UK, 8–11 September 1996; Jones, D.H.I., Ed.; IGER: Aberystwyth, UK, 1996; pp. 11–25. [Google Scholar]
- Ottaviani, F. L’analisi Microbiologica dei Prodotti Lattiero Caseari, 1st ed.; Tecniche Nuove: Milano, Italy, 1991; pp. 351–374. [Google Scholar]
- Dash, S.K.; Voelker, H.H.; Muller, L.D.; Schingoethe, D.J. Comparison between whey and lactose as alfalfa haylage additives. J. Anim. Sci. 1974, 39, 115–123. [Google Scholar] [CrossRef]
- Kung, L., Jr.; Shaver, R.D.; Grant, R.J.; Schmidt, R.J. Silage review: Interpretation of chemical, microbial, and organoleptic components of silages. J. Dairy Sci. 2018, 101, 4020–4033. [Google Scholar] [CrossRef] [PubMed]
- Truchado, P.; Gil, M.I.; Reboleiro, P.; Rodelas, B.; Allende, A. Impact of solar radiation exposure on phyllosphere bacterial community of red-pigmented baby leaf lettuce. Food Microbiol. 2017, 66, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Wieringa, G.W. The effect of wilting on butyric acid fermentation in silage. Neth. J. Agric. Sci. 1958, 6, 204–210. [Google Scholar]
- Bastiman, B. Factors affecting silage effluent production. Exp. Husb. 1976, 31, 40–46. [Google Scholar]
- Hashemzadeh-Cigari, F.; Khorvash, M.; Ghorbani, G.R.; Ghasemi, E.; Taghizadeh, A.; Kargar, S.; Yang, W.Z. Interactive effects of molasses by homofermentative and heterofermentative inoculants on fermentation quality, nitrogen fractionation, nutritive value and aerobic stability of wilted alfalfa (Medicago sativa L.) silage. J. Anim. Physiol. Anim. Nutr. 2014, 98, 290–299. [Google Scholar] [CrossRef]
- Liu, C.; Lai, Y.J.; Lu, X.N.; Guo, P.T.; Luo, H.L. Effect of lactic acid bacteria inoculants on alfalfa (Medicago sativa L.) silage quality: Assessment of degradation (in situ) and gas production (in vitro). J. Int. Agric. 2016, 15, 2834–2841. [Google Scholar] [CrossRef]
- Touqir, N.A.; Khan, M.A.; Sarwar, M.; Nisa, M.; Lee, W.S.; Lee, H.J.; Kim, H.S. Influence of varying dry matter and molasses levels on berseem and lucerne silage characteristics and their in situ digestion kinetics in Nili buffalo bulls. Asian-Aust. J. Anim. Sci. 2007, 20, 887–893. [Google Scholar] [CrossRef]



| Harvest | Forage | Crude Protein | NDF | TDN |
|---|---|---|---|---|
| Mid Bud | 1548 c | 329.6 b | 538.2 c | 991.8 c |
| Early Flower | 2426 b | 440.1 ab | 945.4 b | 1393.7 b |
| Late Flower | 3374 a | 540.7 a | 1443.7 a | 1993.1 a |
| Harvest | CP | EE | Ash | NDF | ADF | ADL | RFV | TDN | WSC |
|---|---|---|---|---|---|---|---|---|---|
| MB | 21.3 a | 1.6 a | 8.8 a | 34.7 b | 24.1 c | 5.6 c | 190.5 a | 64.0 a | 6.3 a |
| EF | 18.1 b | 1.7 a | 8.2 b | 39.1 a | 29.0 b | 6.8 b | 160.4 b | 57.6 b | 4.1 b |
| LF | 16.1 c | 1.7 a | 7.4 c | 42.8 a | 31.6 a | 8.2 a | 140.1 c | 59.0 b | 2.9 c |
| Wilting | EE | NDF | ADF | RFV |
|---|---|---|---|---|
| Unwilted | 1.8 a | 41.2 a | 29.4 a | 150.1 b |
| Wilted | 1.5 b | 36.4 b | 27.1 b | 177.3 a |
| Treatment | WSC | Lactic Acid | Acetic Acid | Butyric Acid | Propionic Acid | Ethanol | NH3 | Lactic/Acetic |
|---|---|---|---|---|---|---|---|---|
| Harvest | ||||||||
| MB | 2.61 a | 0.54 b | 0.27 c | 0.11 a | <0.01 | 0.008 a | 4.9 a | 2.0 c |
| EF | 1.09 b | 1.56 a | 0.57 a | 0.11 a | <0.01 | 0.011 a | 5.5 a | 2.7 b |
| LF | 1.36 b | 1.62 a | 0.47 b | 0.13 a | <0.01 | 0.007 a | 5.3 a | 3.5 a |
| DOE | ||||||||
| 3 | 2.60 a | 0.47 c | 0.30 c | 0.10 a | <0.01 | 0.007 b | 5.4 a | 1.6 b |
| 13 | 1.58 b | 1.47 b | 0.43 b | 0.13 a | <0.01 | 0.005 b | 5.6 a | 3.4 a |
| 90 | 0.88 c | 1.77 a | 0.57 a | 0.13 a | <0.01 | 0.014 a | 4.7 a | 3.1 a |
| Scotta (g kg−1 Fresh Weight) | ||||||||
| 0 | 2.00 a | 0.94 c | 0.40 a | 0.11 a | <0.01 | 0.007 a | 5.4 a | 2.4 c |
| 75 | 1.69 b | 1.05 bc | 0.46 a | 0.12 a | <0.01 | 0.006 a | 5.2 a | 2.3 c |
| 150 | 1.74 b | 1.19 b | 0.42 a | 0.12 a | <0.01 | 0.013 a | 5.1 a | 2.8 b |
| 300 | 1.33 c | 1.77 a | 0.47 a | 0.13 a | <0.01 | 0.008 a | 5.3 a | 3.8 a |
| Harvest | CP | EE | Ash | NDF | ADF | ADL | RFV | TDN |
|---|---|---|---|---|---|---|---|---|
| MB | 20.8 a | 1.5 b | 9.3 a | 30.1 b | 24.3 b | 5.3 c | 216.9 a | 64.4 a |
| EF | 17.4 b | 2.1 a | 8.7 b | 36.8 a | 30.5 a | 6.6 b | 165.6 b | 61.8 b |
| LF | 16.7 c | 1.9 a | 8.2 c | 39.1 a | 31.6 a | 7.4 a | 155.5 b | 60.4 c |
| Scotta | DM | CP | Ash | NDF | ADF | ADL | RFV | TDN |
|---|---|---|---|---|---|---|---|---|
| 0 | 37.0 a | 18.5 a | 8.5 c | 37.0 a | 30.3 a | 6.8 a | 167.9 b | 61.5 a |
| 75 | 34.6 b | 18.7 a | 8.7 b | 35.1 b | 29.5 a | 6.7 a | 178.1 ab | 62.3 a |
| 150 | 33.1 c | 18.3 a | 8.9 a | 34.5 b | 27.9 b | 6.1 b | 183.2 a | 62.6 a |
| 300 | 29.6 d | 17.7 a | 9.0 a | 34.6 b | 27.5 b | 6.2 b | 188.1 a | 62.6 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mariotti, M.; Fratini, F.; Cerri, D.; Andreuccetti, V.; Giglio, R.; Angeletti, F.G.S.; Turchi, B. Use of Fresh Scotta Whey as an Additive for Alfalfa Silage. Agronomy 2020, 10, 365. https://doi.org/10.3390/agronomy10030365
Mariotti M, Fratini F, Cerri D, Andreuccetti V, Giglio R, Angeletti FGS, Turchi B. Use of Fresh Scotta Whey as an Additive for Alfalfa Silage. Agronomy. 2020; 10(3):365. https://doi.org/10.3390/agronomy10030365
Chicago/Turabian StyleMariotti, Marco, Filippo Fratini, Domenico Cerri, Victoria Andreuccetti, Roberta Giglio, Francesco G. S. Angeletti, and Barbara Turchi. 2020. "Use of Fresh Scotta Whey as an Additive for Alfalfa Silage" Agronomy 10, no. 3: 365. https://doi.org/10.3390/agronomy10030365
APA StyleMariotti, M., Fratini, F., Cerri, D., Andreuccetti, V., Giglio, R., Angeletti, F. G. S., & Turchi, B. (2020). Use of Fresh Scotta Whey as an Additive for Alfalfa Silage. Agronomy, 10(3), 365. https://doi.org/10.3390/agronomy10030365






