Abstract
The use of grafting techniques for horticultural crops increases plant tolerance to various abiotic and biotic stresses. Tomato production under greenhouse conditions relies on plants grafted onto vigorous rootstocks because they sustain crops for longer periods. Growers under Mediterranean conditions usually grow crops in passive greenhouses during the summer and winter season, to provide fresh products throughout the year. No information is available with regard to the effect of the environment on nitrogen-use efficiency (NUE) in tomato plants grafted onto rootstocks with different vigor. In the present study, NUE, along with its components—uptake (NUpE) and utilization (NUtE) efficiencies—were evaluated in tomato plants grafted onto two interspecific rootstocks, conferring medium (“King Kong”) or high (“Kaiser”) vigor to the plants. The evaluations were carried out during the vegetative and reproductive stage in plants subjected to different environmental conditions resulting in different plant growth rates. The grafting treatments did not affect NUE, NUpE or NUtE in young plants, but at the reproductive stage, differences were found during the summer season (high N demand) where the vigorous rootstock increased NUpE from 55%, in non-grafted plants, to 94%, with the consequent differences in NUE. During the winter crop, no differences in NUE were found between the vigorous rootstock and non-grafted plants, but the less vigorous (cold-tolerant) rootstock enhanced NUpE. Significant positive relationships were found between plant growth rate and both NUE and NUpE, while NUtE decreased with increasing growth rate.
1. Introduction
Intensive vegetable production under greenhouse conditions requires high nitrogen (N) inputs [1], with the risk of polluting water sources because of large N losses to the environment [2,3]. Then, it is necessary to adapt production techniques that minimize the negative impacts on the environment. Several strategies have been proposed to reduce N pollution from intensive agricultural systems, including partial or complete replacement of conventional fertilizers by organic sources [4], recirculating drainage water in closed-loop systems [5], tightly adjusting irrigation and fertilizer inputs to crop’s needs [6,7], and selecting highly efficient varieties for N uptake [8], among others. More recently, grafting has been suggested as a practice that improves crop’s nitrogen-use efficiency (NUE) due to a combination of traits, including vigorous root systems, high water and nutrient uptake capacity, and high photosynthetic efficiency [9,10,11].
Nitrogen-use efficiency corresponds to the amount of biomass synthesized per unit of applied N. It is composed of nitrogen-uptake efficiency (NUpE) and nitrogen-utilization efficiency (NUtE). The former corresponds to the amount of N absorbed per plant divided by the amount of N applied to the root zone, while NUtE is defined as the biomass produced per unit of N absorbed. Multiplying NUpE times NUtE equals NUE [12]. These indices have been widely used as indicators for crop capacity to transform N fertilizers into biomass. Even more, it has been recently adopted as one of many indicators for the achievement in the Sustainable Development Goals established by the United Nations [13]. The calculations of these parameters are based on the accumulation of biomass in the plants; then, genetic and environmental conditions affecting the plant’s growth rate should affect NUE as well. This has not been investigated in grafted tomato crops.
In tomato crops, interspecific hybrids between Solanum lycopersicum x Solanum habrochaites are commonly used [14], providing higher rates of biomass accumulation and N uptake than intraspecific genotypes [15]. Highly vigorous interspecific rootstocks, such as ‘Multifort’ or ‘Beaufort’, promote plant growth, with an increase in nutrients use efficiency [14,16]. Similar results have been reported in studies with grafted melons and watermelons [17,18]. Plant’s growth rate is also determined by environmental parameters, such as light and air temperature, which significantly affects tomato production when comparing summer versus winter seasons under protected cultivation conditions [19]. Then, it is expected that NUE also decreases in winter in comparison to summer crops.
The demand for N drastically increases in tomato crops when switching from the vegetative to the reproductive stage [20]. Therefore, it is expected that NUE also changes through the ontogenesis of plants. Plant’s uptake efficiency is related to root’s capacity for the absorption of N from the rhizosphere, and it is coordinated with several mechanisms such as the regulation of endodermal N transporters synthesis, root xylem loading or N assimilation capacity [21]. These traits are highly dependent on plant age [22]. On the other hand, N utilization efficiency relates to the plant’s capacity to take N and synthesize various compounds that allow plants to enhance CO2 assimilation and biomass accumulation, such as chlorophyll or the enzymes involved in the Calvin-Benson cycle [23,24]. The rate of the synthesis in this compound is ultimately regulated by the plant’s growth rate [25]. Then, it is expected that NUE varies from the vegetative stage to the reproductive stage, but no information has been found in grafted tomatoes.
The aims of this study were (1) to assess NUE and its components, at the vegetative and reproductive stage in tomato plants grafted onto rootstocks conferring different vigor and (2) to evaluate the interaction between rootstocks and environmental conditions on NUE and its components. The study was conducted under the hypothesis that NUE depends on the vigor provided by the interaction between rootstocks and the environment.
2. Materials and Methods
2.1. Plant Material and Grafting Method
Tomato (Solanum lycopersicum L. var. ‘Attiya’ (AT), Rijk Zwan, De Lier, The Netherlands) plants were obtained from seeds germinated in plastic trays containing a commercial mix of peat (90%) and perlite (10%). Simultaneously, seeds of two interspecific rootstocks (S. lycopersicum × S. habrochaites, var. “King Kong” (KK) and “Kaiser” (KA), Rijk Zwan, De Lier, The Netherlands) were sown. Once plant stems reached a diameter of 2 mm, they were grafted in one of the combinations shown in Table 1. Grafting was conducted by cutting the stems at a 45° angle and joining them using silicone clips. Plants were left in a growth chamber at 25 °C, 95% relative humidity, and light intensity below 20 µmol m−2 s−1 for two days. Later, the light intensity was increased to 200 µmol m−2 s−1, and plants were maintained under these conditions for two weeks until the transplant. The percentage of successful grafted plants was higher than 95% in all combinations. This procedure was repeated before each experiment.

Table 1.
Grafting combinations used in the experiments.
2.2. Experimental Conditions
Two sets of experiments were conducted using the plants obtained after grafting. The first experiments were set under growth chamber conditions to evaluate N uptake in young plants, and two experiments, one during the summer season and a second during winter, were conducted under greenhouse conditions to allow plants to grow and produce fruit. Details of each experiment are described below.
2.2.1. Vegetative Stage
Thirty-two plants from each grafting treatment were placed in 6-L plastic containers and kept in a growth chamber set at 25 °C day/night air temperature and relative humidity of 50 ± 6%. One set of containers was subjected to medium light conditions (400 µmol PAR m−2 s−1), and a second set was assigned to high radiation conditions (800 µmol PAR m−2 s−1), with a photoperiod of 10 h d−1 (daily light integral of 3.13 and 6.26 MJ PAR m−2, respectively). The light levels were achieved using dimmable LED lamps (model LightDNA, Valoya, Helsinki, Finland). Each container held four plants, with a total of four replicates (containers) per Grafting x Light combination. In each light treatment, the containers were arranged in a completely randomized design. Plants were fed with a nutrient solution containing the following nutrients: 3.0 mM N, 0.5 mM P, 3.0 mM K, 1.0 mM Ca, 1.0 mM Mg, 1.0 mM S, 45 µM Fe-EDDHA, 40 µM B, 1.0 µM Mn, 0.2 µM Zn, 0.1 µM Cu, and, 0.3 µM Mo. All N was applied as potassium nitrate (KNO3, 5 atom % 15N, Sigma-Aldrich, Darmstadt, Germany) to discriminate between N absorbed after grafting from N that could potentially be remobilized. The electrical conductivity and pH of the solution were 1.2 dS m−1 and 5.8, respectively. The solution was continuously aerated by a pump injecting air at a 200 mL min−1 rate. The nutrient solution was completely replaced twice per week.
2.2.2. Reproductive Stage
Two greenhouse experiments were conducted to evaluate NUE in plants at the reproductive stage. The greenhouse was located in Santiago, Chile (33°29′46.90″S, 70°36′30.92″W) at the School of Agriculture and Forestry, Pontificia Universidad Católica de Chile. The first experiment was established on 1 December 2017 for the summer season, and the second experiment was established on 2 May 2018 for the winter season. For both experiments, twelve plants of each treatment were established in a passive greenhouse using a completely randomized block design. Each block held three replicates of each grafting treatment.
For the summer experiment, the daily average temperature and average minimum and maximum in the greenhouse were 26.3 ± 3.6 °C, 15.2 ± 2.8 °C, and 32.7 ± 4.3 °C, respectively. The average daily light integral was 15.52 ± 0.19 MJ PAR m−2. During the winter crop, the daily average temperature, average minimum, and average maximum were 15.4 ± 1.2 °C, 5.1 ± 2.3 °C, and 18.8 ± 3.5 °C, respectively. The average daily light integral was 6.19 ± 0.23 MJ PAR m−2.
Individual plants were transplanted into 10 L containers filled with peat. Each container was connected to two 2 L h−1 drippers supplying 0.3 L of a nutrient solution in each irrigation. The irrigation frequency was adjusted accordingly to the plant’s demand, evaluated as the amount of drainage after each irrigation. Additionally, the electrical conductivity (EC) in the drainage was monitored daily, and once the value reached a 20% higher value than that in the nutrient solution, each container received a 600 mL irrigation with tap water only. Macronutrients were supplied accordingly to the plant’s requirements (Table 2). Micronutrients were supplied at the following concentration throughout the experiment: 45 µM Fe-EDDHA, 40 µM B, 1.0 µM Mn, 0.1 µM Cu, 0.3 µM Mo, and 0.2 µM Zn. The pH of the solution was adjusted at 5.8, using phosphoric acid. Plants were trained in a single stem. The development of each cluster in each plant was monitored daily. The fruits were harvested once all fruits in the cluster reached full color.

Table 2.
Composition of the nutrient solution used in the greenhouse experiments at each developmental stage.
2.3. Analytical Determinations
2.3.1. Chlorophyll Content
In the growth chamber experiment, before plant harvest, chlorophyll a (chl a) and chlorophyll b (chl b) content were determined following the methodology presented by Parry et al. [26]. Five 63.6 mm2 disks were collected from the youngest fully expanded leaf of plants in each treatment. Each disk was individually placed in 30 mL glass tubes, and 10 mL of dimethylsulfoxide was added. The tubes were incubated in an oven at 65 °C for 30 min prior to determination in a spectrophotometer (model BioTek Power Wave HT, Shimadzu, Tokyo, Japan). Chlorophyll a and b content was then determined using the absorbance (A) at 649 and 665 nm as in the following relations:
Chl a (µg mL−1) = 12.47·A665 − 3.62·A649
Chl b (µg mL−1) = 25.06·A649 − 6.5·A665
Total chlorophyll (chl) content was calculated as the sum of chl a plus chl b. The results were scaled to µg of chlorophyll m−2 using the area of the disks.
In the greenhouse experiments, leaf chlorophyll content was measured using a portable chlorophyll concentration meter (MC-100, Apogee Instruments, Logan, UT, USA). This method allowed for a fast and accurate determination of chlorophyll content [26]. Ten leaves per plant (total of 480 measurements per day) were measured 64, 78, and 94 days after transplant.
2.3.2. Biomass and Tissue N Determinations
In the growth chamber experiment, plants were harvested after three weeks of growth; split into roots, stems, and leaves; fresh weighed; and dried in an oven at 55 °C for 48 h. After plant dry weight (DW) was recorded, total N in each tissue sample was determined by Kjeldahl distillation, while 15N was determined by optical emission spectrometry (model NOI 7, Fischer Analysen Instrumente, Leipzig, Germany) [27].
In the greenhouse experiments, fruit fresh weight was recorded for each fruit individually in the same truss. Following, the DW of each fruit was measured after being oven-dried at 40 °C for 72 h. In the summer experiment, whole plants were harvested after the fifth truss (115 days after transplant), while in the winter experiment, plants were harvested after the third truss (156 days after transplant). At harvest, plants were split into roots, stems, and leaves for fresh weight measurements. Later, the DW was recorded in each organ after being oven-dried at 55 °C for 48 h. The dry samples were ground to pass through a 40 mesh sieve, and the total N content was determined in composite samples (one per treatment per block) by Kjeldahl distillation.
2.4. NUE, NUpE, and NUtE Calculations
In order to compare between growth stages, NUE was calculated as the ratio between total biomass, expressed as DW, per unit of N supplied to the crop [28] as in the following equation:
NUE = (g Total Biomass)/(g N supplied)
In the greenhouse, supplied N was calculated as the sum of each irrigation volume times the corresponding N concentration. The uptake efficiency (NUpE) was obtained from the amount of N absorbed per plant divided by the amount of N supplied, as in Equation (4).
NUpE = (g N absorbed)/(g N supplied)
Absorbed N was determined as the sum of each organ’s N content by multiplying each organ’s dry biomass by its corresponding N content. N-utilization efficiency (NUtE) was calculated as the ratio between the plant’s total biomass per unit of N absorbed (Equation (5)).
NUtE = (g Total Biomass)/(g N absorbed)
2.5. Relative Growth Rate (RGR) Calculations
Using plant’s total DW, the relative growth rate (RGR) was calculated as the difference between transplant (DW1) and harvest (DW2) for each plant divided by the number of days until the harvest of the whole plant (t) [29], as in the following equation:
RGR = (ln(DW2) − ln(DW1))/t
2.6. Statistical Analysis
Differences in plant biomass, chlorophyll content, NUE, NUpE, and NUtE between treatments were assessed by ANOVA and mean separation by the Least Significant Difference (LSD) test. In the growth chamber experiment, the effects evaluated were grafting and light treatments. In the greenhouse experiments, grafting and season effects were evaluated. The relationship between growth rate and NUE, NUpE, or NUtE were evaluated by linear regression analysis. All analyses were carried out in ‘R’ software through the InfoStat console [30].
3. Results
3.1. Vegetative Stage
No differences in plant biomass accumulation were found between grafting treatments (p < 0.4799). Under high radiation, plants produced more biomass (3.89 ± 0.22 g DW plant−1) than under medium radiation (2.49 ± 0.15 g DW plant−1) (p < 0.0001). This differences in biomass accumulation led to a higher N uptake per plant when exposed to higher radiation levels (p < 0.0031) (Table 3). However, no differences in N uptake among grafting treatments were found under any of the light levels (Table 3).

Table 3.
Nitrogen uptake rate under 400 µmol PAR m−2 s−1 or 800 µmol PAR m−2 s−1 for each grafting treatment.
The grafting treatments or the light level had no effect on the content of total chl, chl a, or chl b, with average values of 294.89 ± 9.41, 236.86 ± 5.94, and 58.04 ± 4.55 µmol m−2, respectively. The chl a/b ratio decreased (p < 0.0106) from 5.17 ± 0.34 under medium light conditions to 4.15 ± 0.22 under high radiation.
The allocation of absorbed N to the leaves (p < 0.6355), stems (p < 0.6981), or roots (p < 0.5583) was similar between treatments, with a 69.0%, 22.8%, and 8.2% allocation to each organ, respectively. The amount of N in the stems was not affected by the light conditions (p < 0.2723). However, the allocation of N to the leaves decreased from 70.4% to 67.6% (p < 0.0098) when the light level was increased. The opposite occurred in the roots, with an increase from 7.4% to 9.1% (p < 0.0049).
No differences in NUE (p < 0.3865), NUpE (p < 0.7660), or NUtE (p < 0.5719) were found between the grafting treatments (Figure 1). However, the light level had a significant effect on NUE (p < 0.0017), which increased from 17.87 ± 1.13 to 24.24 ± 1.37 g g−1 with a light increase from 400 to 800 µmol m−2 s−1 (Figure 1). The uptake efficiency remained unaffected by the light level (p < 0.1988), with an average value of 0.68 ± 0.03 g g−1 (Figure 1). On the other hand, NUtE increased from 28.39 ± 0.85 to 33.80 ± 0.98 g g−1 (p < 0.0005) with the increase in radiation (Figure 1). Despite the increase in NUtE from medium to high radiation in all treatments, AT-KA was the only treatment that showed a significant increase (p < 0.0384) under higher light conditions.
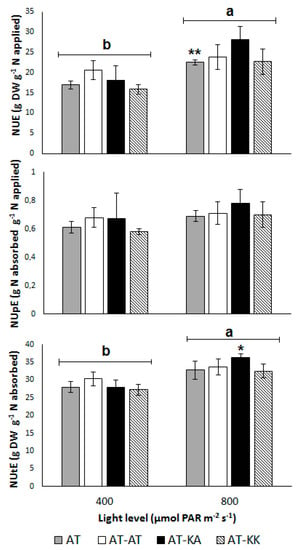
Figure 1.
Nitrogen-use (NUE; top), -uptake (NUpE; middle), and -utilization efficiency (NUtE; bottom) for each treatment under medium or high light levels. Bars represent mean ± s.e. of four replicates. Asterisks denote the level of significance (*: p < 0.05; **: p < 0.01) for the difference in the same treatment across light levels. (a, b) Letters above light groups denote significant differences (p < 0.05) between light levels.
No significant relation between growth rate, represented by the relative growth rate, and NUE (p < 0.1041) or NUtE (p < 0.4277) was found (Figure 2). Increases in RGR significantly enhanced NUpE (p < 0.0093) at this developmental stage.
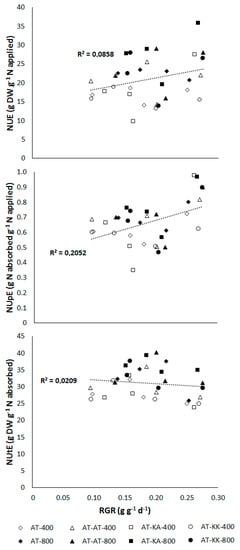
Figure 2.
Relationship between nitrogen-use (NUE; top), -uptake (NUpE; middle), and, -utilization efficiency (NUtE; bottom) to relative growth rate (RGR) in plants at the vegetative stage. Open symbols denote measurements under medium (400 µmol m−2 s−1) light intensity, solid symbols represent measurements under high (800 µmol m−2 s−1) light intensity.
3.2. Reproductive Stage
During the summer, plants grafted onto the vigorous rootstock (AT-KA) accumulated more biomass in the leaves (p < 0.0128), stems (p < 0.0002) and roots (p < 0.0155) than the other treatments. No differences were found in fruit biomass (p < 0.1025) among grafting treatments. The increase in stem biomass is accompanied by an enhancement of the relative amount of N allocated to this organ (p < 0.0030), where AT-KA placed 22.95% of total plant’s N in the stem versus an 18.17% in the other treatments. This resulted in a significant reduction of N allocated to the fruits in AT-KA, which reached 26.52% versus 36.97% and 33.56% in the non-grafted plants and AT-KK, respectively. However, no differences in fruit yield were observed. Roots received 14.87% of plant’s N, while 31.54% was allocated to the leaves. AT-KA absorbed 44% more N per plant than the other grafting treatments (p < 0.0001) (Table 4).

Table 4.
Nitrogen uptake per plant during the summer or winter season. Different letters in the same column denote significant differences (p < 0.05) between grafting treatments.
In the winter season, no differences in leaves (p < 0.2464), stem (p < 0.1446), root (p < 0.2746), or fruit (p < 0.2114) biomass accumulation were found between the treatments. Similarly, the allocation of N to the various organs was similar among grafting treatments, with 17.30%, 28.25%, 43.79%, and 10.67% allocated to the fruits, leaves, stems, and roots, respectively. No differences in N uptake per plant were observed during the winter crop (p < 0.0567) (Table 4).
Leaf chlorophyll content declined slowly after plants were topped. In the summer season, chlorophyll concentration decreased from 535.39 ± 7.41 to 383.92 ± 39.48 µmol m−2 between 64 and 94 days after transplant. In the winter crop, chlorophyll contents were slightly higher, but a similar trend than in the summer crop was observed, with values declining from 546.51 ± 23.78 to 498.65 ± 5.11 µmol m−2 between 64 and 94 days after transplant. In any of these measurements, significant differences between grafting treatments were found.
In the summer crop, both rootstocks improved NUE (p < 0.0130) in comparison to the non-grafted plants (Figure 3). The vigorous rootstock (AT-KA) has a remarkably high uptake efficiency in comparison to the other treatments (p < 0.0001), with values of 0.94 ± 0.06 versus 0.65 ± 0.05 g of absorbed N per unit of applied N (Figure 3). This represents a total of 19.08 g of absorbed N per plant in the vigorous grafting treatment, while the average uptake in the other treatments reached 13.22 g of absorbed N plant−1. However, this higher uptake capacity did not affect largely the utilization efficiency in comparison to the other treatments. During the winter, no differences in NUE (p < 0.1427) or NUtE (p < 0.4232) were observed between grafting treatments, but the less vigorous rootstock significantly increased NUpE (p < 0.0496) from 0.48 ± 0.04 to 0.62 ± 0.06 g g−1 (Figure 3).
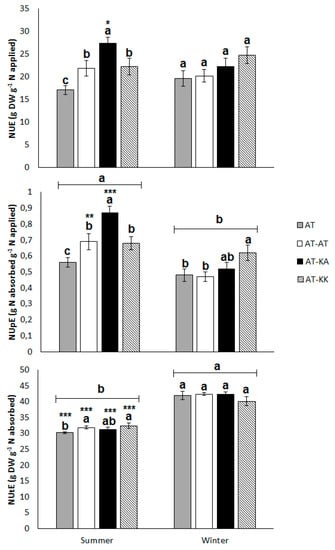
Figure 3.
Nitrogen-use (NUE; top), -uptake (NUpE; middle), and, -utilization efficiency (NUtE; bottom) for each treatment during summer or winter season. Bars represent means ± s.e. of twelve replicates. Different letters on top of the bars denote significant differences (p < 0.05) among treatments under the same growing season. Asterisks denote significant differences (*: p < 0.05; **: p < 0.01; and, ***: p < 0.0001) in the same treatment between seasons. (a, b) Letters above light groups denote significant differences (p < 0.05) between light levels.
The aggregated analysis of both seasons indicates that both rootstocks significantly increase NUpE (p < 0.0001) with values of 0.70 ± 0.05, 0.65 ± 0.03, and 0.53 ± 0.02 g g−1 for AT-KA, AT-KK, and the non-grafted treatment, respectively. The uptake efficiency is also affected by the season (p < 0.0001), with higher values during the summer than winter. On the contrary, NUtE is not affected by the grafting treatments (p < 0.1746), but it is influenced by the season (p < 0.0001), where the utilization efficiency reduces from 41.38 ± 0.45 in the winter to 31.54 ± 0.34 g g−1 in the summer. Across the year, NUE is significantly affected only by the rootstocks (p < 0.0009) and not by the season (p < 0.6499). AT-KA, as well as AT-KK, significantly improves NUE in comparison to the ungrafted plants, with values of 24.82 ± 1.26, 23.52 ± 1.31, and, 19.08 ± 0.82 g g−1, respectively.
A highly significant positive relation between NUE (p < 0.0001) and NUpE (p < 0.0001) to the growth rate of the plants was found at the reproductive stage (Figure 4). On the contrary, the utilization efficiency declines (p < 0.0001) with higher RGR (Figure 4).
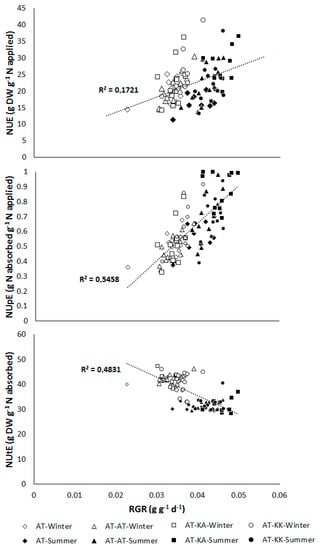
Figure 4.
Relationship between nitrogen-use (NUE; top), -uptake (NUpE; middle), and, -utilization efficiency (NUtE; bottom) to the relative growth rate (RGR) in plants at the reproductive stage grown under greenhouse during summer or winter seasons. Open symbols represent measurements from the winter experiment, and solid symbols denote measurements from the summer experiment for each grafting treatment.
4. Discussion
4.1. NUE and Developmental Stage
At the vegetative stage, no differences in NUE were found among grafting treatments. However, the stimulation of growth by different light levels increased NUE. This effect is related to an increase in NUtE, which denotes a higher capacity of plants to synthesize more biomass per unit of N absorbed. To do this, plants require to enhance their capacity to assimilate inorganic N, which was verified in a previous study where the activity of nitrate reductase was shown to increase in tomato leaves exposed to higher light intensities [31]. Several studies in cereal crops [32,33,34] and muskmelon [35] report a positive correlation between chlorophyll content and NUtE. Our results show no changes in chlorophyll content under different NUE values, in agreement with some reports in grafted Solanaceae crops, such as tomato or bell pepper [36,37]. However, the ratio between chl a/b increased under lower light intensities, implying a switch in the allocation of N towards the light-harvesting complex instead of other protein complexes in the photosystem II [38,39].
In adult plants under greenhouse conditions, grafting onto rootstocks of different vigor increase NUE, as the result of an enhancement in N uptake. In tomato crops, only 25% of the total N demand is absorbed during the vegetative stage [20]. After fruit onset, an important increase in plant’s N demand occurs, as the result of the high strength of fruits as sinks for N and N-containing compounds, which are utilized to synthesize amino acids, proteins, enzymes, and secondary metabolites in the fruits [40,41,42,43]. Plants adapt to these conditions of higher demand by stimulating the activity of enzymes involved in carbon assimilation [44], and with this, to supply more carbohydrates to the roots to support higher N uptake rates. This is accompanied by increasing the stem hydraulic conductance [45], as the result of higher nitrate concentrations in the xylem sap [46,47].
It is also possible to assume that at the reproductive stage, roots in the grafted treatments have developed a more robust mechanism to supply N to the shoot. Other authors have reported increases in the root volume in plants grafted onto vigorous rootstocks [48,49]. In our experiments, the vigorous rootstock (AT-KA) presented 70% more root biomass than the ungrafted plants but only during the summer season.
4.2. Rootstock Effects on NUE
The grafting treatments used in our experiments presented different rates of biomass accumulation, especially during the summer season, under similar N availability conditions. Rootstock genotypes can affect the whole plant vigor by mechanisms involving root synthesis and xylem transport of phytohormones [50,51]. For instance, rootstocks with enhanced cytokinin and abscisic acid synthesis have been identified to promote growth in tomato plants under salt stress [50,52]. Studies in grafted fruit trees attribute differences in plant growth rate to the capacity of water transport to the shoot [53,54]. In grafted tomato plants, a higher number of vessels with larger diameter was reported in a rootstock genotype originally from Honduras [55]. Higher growth rates require that roots adapt in order to supply higher amounts of nutrients, including N, to the shoots. In a previous study, we showed that nitrate uptake in roots from “Kaiser” relies mostly on the activity of a dual-affinity root plasma membrane transporter, LeNRT1.1, as opposed to non-grafted plants which absorb nitrate mainly through the activity of a low-affinity transporter, LeNRT1.2 [31]. Then, the synthesis of more efficient root plasma membrane transporters (LeNRT1.1) plus a larger volume of roots, confers higher uptake efficiency to the rootstocks than non-grafted plants.
Under similar environmental conditions, it is relevant that plants present a high uptake capacity, so as to assure the applied N is intended for plant production, but also to reduce N losses from the greenhouse. NUE reductions from 27 to 13 g fruit DW g-1 N applied have been reported in greenhouse tomato grown directly in the soil with N doses of 150 and 350 kg N ha−1 [56]. This implies high N losses by leaching under high N fertilization doses. Nitrogen accumulation in the solution in recirculating systems can easily double the originally supplied concentration [57]; then, it is worth noting that the vigorous rootstock presented an uptake efficiency above 90% during the summer, in contrast to the non-grafted plants, which were below 60%. This is a significant difference that can contribute either to reduce N inputs into the growing systems or to decrease the amount of N lost through the drainage, both actions significantly limiting the pollution to the environment, especially in open substrate cultivation systems [58]. Similar results in the uptake efficiency have been reported by recirculating the nutrient solution until it reached electrical conductivities values of 10 dS m−1 [59]. However, this increased the proportion of low diameter berries, which was not observed in our study (data not shown). Then, based on our results, the use of vigorous rootstocks is advised as an effective tool to comply with the regulations enforcing the amount of N discarded from production fields.
4.3. Rootstock x Environment Interaction
A significant Rootstock x Season interaction was found for NUE and NUpE. During the summer season, the highest NUpE values were achieved using “Kaiser” as the rootstock, but during the winter, the highest values were obtained with “King Kong”. The demand for N reduces during the winter season because of a decrease in the plant’s growth rate due to the lower temperatures and light availability. This is accompanied by anatomical and physiological adaptations, which reduce N content in the leaves [60]. Our results show a reduction in leaf N content from 36.02 to 25.05 mg N g−1 DW (data not shown) from summer to winter seasons, with the consequent increase in NUtE during winter. Then, during winter, the growth stimulation by the vigorous rootstocks is lost, leading to an accumulation of N in the stems (44% during winter versus 23% in summer in AT-KA). In the winter season, the rootstock tolerant to colder temperatures (“King Kong”) provides better NUE to the crops. Rootstock genotypes tolerant to cold temperatures enhance the synthesis of antioxidants in the roots and shoots [61], protecting the photosynthetic apparatus under these conditions [62]. In Arabidopsis thaliana, the expression of a gene encoding for a high-affinity nitrate transporter, NRT2.6, has been related to the regulation of reactive oxygen species, providing more tolerance to stress conditions [63]. Differences in the expression of genes encoding for root nitrate transporters have been reported in a previous study [31], but this specific gene has not been described in tomato.
5. Conclusions
Interspecific rootstocks of Solanum lycopersicum x Solanum habrochaites with different vigor enhance NUE in tomato crops cultivated under greenhouse conditions during summer and winter in Mediterranean conditions. The main effect of rootstocks is on the uptake efficiency, which reaches values of 94% in plants grafted onto vigorous rootstocks under summer conditions, reducing N losses to the environment. At the vegetative stage, NUE is not affected by the rootstock. Nitrogen-use and -uptake efficiency increase linearly with the plant’s growth rate, while N-utilization efficiency decreases linearly.
Author Contributions
Conceptualization, F.A.; methodology, F.A., A.N., and X.V.; formal analysis, F.A. and M.S.; data curation, F.A.; writing—Original draft preparation, F.A.; writing—Review and editing, A.N.; project administration, F.A.; funding acquisition, F.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Council for Scientific and Technological Research (CONICYT, Chile) through the project FONDECYT 11160026.
Acknowledgments
The authors acknowledge the contribution of Sofía Villar in data collection during the winter experiment.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Min, J.; Zhao, X.; Shi, W.; Xing, G.; Zhu, Z. Nitrogen balance and loss in a greenhouse vegetable system in southeastern China. Pedosphere 2011, 21, 464–472. [Google Scholar] [CrossRef]
- Zhu, J.H.; Li, X.L.; Christie, P.; Li, J.L. Environmental implications of low nitrogen use efficiency in excessively fertilized hot pepper (Capsicum frutescens L.) cropping systems. Agric. Ecosyst. Environ. 2005, 111, 70–80. [Google Scholar] [CrossRef]
- He, F.; Chen, Q.; Jiang, R.; Chen, X.; Zhang, F. Yield and nitrogen balance of greenhouse tomato (Lycopersicum esculentum Mill.) with conventional and site-specific nitrogen management in northern China. Nutr. Cycl. Agroecosyst. 2007, 77, 1–14. [Google Scholar] [CrossRef]
- Yang, S.; Wang, Y.; Liu, R.; Xing, L.; Yang, Z. Improved crop yield and reduced nitrate nitrogen leaching with straw return in a rice-wheat rotation of Ningxia irrigation district. Sci. Rep. 2018, 8, 9458. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. The influence of drip irrigation or subirrigation on zucchini squash grown in closed-loop substrate culture with high and low nutrient solution concentrations. Hortscience 2009, 44, 306–311. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, K.; Zhang, K.; Jiang, L.; Xu, Y. Simulation of nitrogen fate for greenhouse cucumber grown under different water and fertilizer management using the Rotate_N model. Agric. Water Manag. 2012, 112, 21–32. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Gao, L.; Tian, Y. Irrigation has more influence than fertilization on leaching water quality and the potential environmental risk in excessively fertilized vegetable soils. PLoS ONE 2018, 13, e0204570. [Google Scholar] [CrossRef]
- Benincasa, P.; Guiducci, M.; Tei, F. The nitrogen use efficiency: Meaning and sources of variation—Case studies on three vegetable crops in central Italy. Horttechnology 2011, 21, 266–273. [Google Scholar] [CrossRef]
- Borgognone, D.; Colla, G.; Rouphael, Y.; Cardarelli, M.; Rea, E.; Schwarz, D. Effect of nitrogen form and nutrient solution pH on growth and mineral composition of self-grafted and grafted tomatoes. Sci. Hortic. 2013, 149, 61–69. [Google Scholar] [CrossRef]
- De Pascale, S.; Rouphael, Y.; Gallardo, M.; Thompson, R.B. Water and fertilization management of vegetables: State of art and future challenges. Eur. J. Hortic. Sci. 2018, 83, 306–318. [Google Scholar] [CrossRef]
- Rouphael, Y.; Kyriacou, M.C.; Colla, G. Vegetable grafting: A toolbox for securing yield stability under multiple stress conditions. Front. Plant Sci. 2018, 8, 2255. [Google Scholar] [CrossRef]
- Moll, R.H.; Kamprath, E.J.; Jackson, W.A. Analysis and interpretation of factors which contribute to the efficiency of nitrogen utilization. Agron. J. 1982, 74, 562–564. [Google Scholar] [CrossRef]
- Zhang, X.; Davidson, E.A.; Mauzerall, D.L.; Searchinger, T.D.; Dumas, P.; Shen, Y. Managing nitrogen for sustainable development. Nature 2015, 528, 51–59. [Google Scholar] [CrossRef]
- King, S.R.; Davis, A.R.; Zhang, X.; Crosby, K. Genetic breeding and selection of rootstocks for Solanaceae and Cucurbitaceae. Sci. Hortic. 2010, 127, 106–111. [Google Scholar] [CrossRef]
- Leonardi, C.; Giuffrida, F. Variation of plant growth and macronutrient uptake in grafted tomatoes and eggplants on three different rootstocks. Eur. J. Hort. Sci. 2006, 71, 97–101. [Google Scholar]
- Djidonou, D.; Zhao, X.; Simonne, E.H.; Koch, K.E. Yield, water-, and nitrogen-use efficiency in field-grown, grafted tomatoes. Hortscience 2013, 48, 485–492. [Google Scholar] [CrossRef]
- Colla, G.; Cardona, C.M.; Cardarelli, M.; Rouphael, Y. Improving nitrogen use efficiency in melon by grafting. Hortscience 2010, 45, 559–565. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Mirabelli, C.; Cardarelli, M. Nitrogen-use efficiency traits of mini-watermelon in response to grafting and nitrogen-fertilization doses. J. Plant Nutr. Soil Sci. 2011, 174, 933–941. [Google Scholar] [CrossRef]
- Tewolde, F.T.; Lu, N.; Shiina, K.; Maruo, T.; Takagaki, M.; Kozai, T.; Yamori, W. Nighttime supplemental LED inter-lighting improves growth and yield of single-truss tomatoes by enhancing photosynthesis in both winter and summer. Front. Plant Sci. 2016, 7, 448. [Google Scholar] [CrossRef]
- Tapia, M.L.; Gutiérrez, V. Distribution pattern of dry weight, nitrogen, phosphorus, and potassium through tomato ontogenesis. J. Plant Nutr. 1997, 20, 783–791. [Google Scholar] [CrossRef]
- Marschner, P. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: San Diego, CA, USA, 2012. [Google Scholar]
- Wells, C.; Eissenstat, D. Beyond the roots of young seedlings: The influence of age and order on fine root physiology. J. Plant Growth Regul. 2002, 21, 324–334. [Google Scholar] [CrossRef]
- Mae, T. Physiological nitrogen efficiency in rice: Nitrogen utilization, photosynthesis, and yield potential. Plant Soil 1997, 196, 201–210. [Google Scholar] [CrossRef]
- Baligar, V.C.; Fageria, N.K.; He, Z.L. Nutrient use efficiency in plants. Commun. Soil Sci. Plan. 2001, 32, 921–950. [Google Scholar] [CrossRef]
- Glass, A.D.M. Nitrogen use efficiency of crop plants: Physiological constraints upon nitrogen absorption. Crit. Rev. Plant Sci. 2003, 22, 453–470. [Google Scholar] [CrossRef]
- Parry, C.; Blonquist, J.M.; Bugbee, B. In situ measurement of leaf chlorophyll concentration: Analysis of the optical/absolute relationship. Plant Cell Environ. 2014, 37, 2508–2520. [Google Scholar] [CrossRef]
- Nario, A.; Pino, I.; Zapata, F.; Albornoz, M.P.; Baherle, P. Nitrogen (15N) fertiliser use efficiency in peach (Prunus persica L.) cv. Goldencrest trees in Chile. Sci. Hortic. 2003, 97, 279–287. [Google Scholar] [CrossRef]
- Fageria, N.K.; Baligar, V.C. Enhancing nitrogen use efficiency in crop plants. Adv. Agron. 2005, 88, 97–185. [Google Scholar]
- Albornoz, F.; Lieth, J.H. N, P, K and S uptake response to various levels of CO2 assimilation and growth rate in lettuce. J. Plant Nutr. 2017, 40, 773–783. [Google Scholar] [CrossRef]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Gonzalez, L.; Tablada, M.; Robledo, C.W. InfoStat Version 2014, Grupo InfoStat, 2014; Universidad Nacional de Córdoba: Córdoba, Argentina, 2014. [Google Scholar]
- Albornoz, F.; Gebauer, M.; Ponce, C.; Cabeza, R. LeNRT1.1 improves nitrate uptake in grafted tomato plants under high nitrogen demand. Int. J. Mol. Sci. 2018, 19, 3921. [Google Scholar] [CrossRef]
- Wu, P.; Tao, Q.N. Genotypic response and selection pressure on nitrogen-use efficiency in rice under different nitrogen regimes. J. Plant Nutr. 1995, 18, 487–500. [Google Scholar] [CrossRef]
- Gallais, A.; Coque, M. Genetic variation and selection for nitrogen use efficiency in maize: A synthesis. Maydica 2005, 50, 531. [Google Scholar]
- Hassan, M.S.; Khair, A.; Haque, M.M.; Azad, A.K.; Hamid, A. Genotypic variation in traditional rice varieties for chlorophyll content, SPAD value and nitrogen use efficiency. Bangladesh J. Agric. Res. 2009, 34, 505–515. [Google Scholar] [CrossRef]
- Liu, Y.; Qi, H.; Bai, C.; Qi, M.; Xu, C.; Hao, J.; Li, Y.; Li, T. Grafting helps improve photosynthesis and carbohydrate metabolism in leaves of muskmelon. Int. J. Biol. Sci. 2011, 7, 1161–1170. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Goto, R.; De Miguel, A.; Marsal, J.; Gorbe, E.; Calatayud, A. Effect of different rootstocks on growth, chlorophyll a fluorescence and mineral composition of two grafted scions of tomato. J. Plant Nutr. 2013, 36, 825–835. [Google Scholar] [CrossRef]
- García-Bañuelos, M.; Sanchez, E.; Gardea-Bejar, A.; Muñoz-Marquez, E.; Soto-Parra, J.; Ojeda-Barrios, D. Nitrogen use efficiency and yield in response graft bell pepper cultivars. Emir. J. Food Agric. 2017, 29, 420–428. [Google Scholar] [CrossRef]
- Evans, J.R.; Poorter, H. Photosynthetic acclimation of plants to growth irradiance: The relative importance of specific leaf area and nitrogen partitioning in maximizing carbon gain. Plant Cell Environ. 2001, 24, 755–767. [Google Scholar] [CrossRef]
- Kitajima, K.; Hogan, K.P. Increases of chlorophyll a/b ratios during acclimation of tropical woody seedlings to nitrogen limitation and high light. Plant Cell Environ. 2003, 26, 857–865. [Google Scholar] [CrossRef]
- Richards, D.; Goubran, F.H.; Collins, K.E. Root-shoot equilibria in fruiting tomato plants. Ann. Bot. 1979, 43, 401–404. [Google Scholar] [CrossRef]
- Robinson, N.L.; Hewitt, J.D.; Bennett, A.B. Sink metabolism in tomato fruit. I. Developmental changes in carbohydrate metabolizing enzymes. Plant Physiol. 1988, 87, 727–730. [Google Scholar] [CrossRef]
- Yelle, S.; Chetelat, R.T.; Dorais, M.; DeVerna, J.W.; Bennett, A.B. Sink metabolism in tomato fruit. IV. Genetic and biochemical analysis of sucrose accumulation. Plant Physiol. 1991, 95, 1026–1035. [Google Scholar] [CrossRef]
- Moco, S.; Bino, R.J.; Vorst, O.; Verhoeven, H.A.; De Groot, J.; Van Beek, T.A.; Vervoort, J.; De Vos, C.H.R. A liquid chromatography-mass spectrometry-based metabolome database for tomato. Plant Physiol. 2006, 141, 1205–1218. [Google Scholar] [CrossRef] [PubMed]
- Greef, J.M. Productivity of maize (Zea mays L.) in relation to morphological and physiological characteristics under varying amounts of nitrogen supply. J. Agron. Crop Sci. 1994, 172, 317–326. [Google Scholar] [CrossRef]
- Zhang, H.; Li, W.; Adams, H.D.; Wang, A.; Wu, J.; Jin, C.; Guan, D.; Yuan, F. Responses of woody plant functional traits to nitrogen addition: A meta-analysis of leaf economics, gas exchange, and hydraulic traits. Front. Plant Sci. 2018, 9, 683. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa-Sakurai, J.; Hayashi, H.; Murai-Hatano, M. Nitrogen availability affects hydraulic conductivity of rice roots, possibly through changes in aquaporin gene expression. Plant Soil 2014, 379, 289–300. [Google Scholar] [CrossRef]
- Orieux, C.; Demarest, G.; Decau, M.; Beauclair, P.; Bataille, M.; Le Deunff, E. Changes in 15NO3- availability and transpiration rate are associated with a rapid diurnal adjustment of anion contents as well as 15N and water fluxes between the roots and shoots. Front. Plant Sci. 2018, 9, 1751. [Google Scholar] [CrossRef] [PubMed]
- Djidonou, D.; Zhao, X.; Brecht, J.K.; Cordasco, K.M. Influence of interspecific hybrid rootstocks on tomato growth, nutrient accumulation, yield, and fruit composition under greenhouse conditions. Horttechonology 2017, 27, 868–877. [Google Scholar] [CrossRef]
- Nawaz, M.A.; Wang, L.; Jiao, Y.; Chen, C.; Zhao, L.; Mei, M.; Yu, Y.; Bie, Z.; Huang, Y. Pumpkin rootstock improves nitrogen use efficiency of watermelon scion by enhancing nutrient uptake, cytokinin content, and expression of nitrate reductase genes. Plant Growth Regul. 2017, 82, 233–246. [Google Scholar] [CrossRef]
- Martínez-Ballestero, M.; Alcaraz-López, C.; Muries, B.; Mota-Cadenas, C.; Carvajal, M. Physiological aspects of rootstock-scion interactions. Sci. Hortic. 2010, 127, 112–118. [Google Scholar] [CrossRef]
- Albacete, A.; Martínez-Andujar, C.; Martínez-Pérez, A.; Thompson, A.J.; Dodd, I.C.; Pérez-Alfocea, F. Unraveling rootstock x scion interactions to improve food security. J. Exp. Bot. 2015, 66, 2211–2226. [Google Scholar] [CrossRef]
- Albacete, A.; Cantero-Navarro, E.; Balibrea, M.E.; Großkinsky, D.K.; De la Cruz González, M.; Martínez-Andújar, C.; Smigocki, A.C.; Roitsch, T.; Pérez-Alfocea, F. Hormonal and metabolic regulation of tomato fruit sink activity and yield under salinity. J. Exp. Bot. 2014, 65, 6081–6095. [Google Scholar] [CrossRef]
- Cohen, S.; Nair, A. The effect of three rootstocks on water use, canopy conductance and hydraulic parameters of apple trees and predicting canopy from hydraulic conductance. Plant Cell Environ. 2002, 25, 17–28. [Google Scholar] [CrossRef]
- Tombesi, S.; Johnson, R.S.; Day, K.R.; DeJong, T.M. Relationship between xylem vessel characteristics, calculated axial hydraulic conductance and size-controlling capacity of peach rootstocks. Ann. Bot. 2010, 105, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Sory, A.; Nieto-Angel, R.; Rodríguez-Pérez, J.E.; Barrientos-Priego, A.F.; Ibañez-Castillo, L.A.; Romabchik, E.; Núñez-Colín, C.A. Variación anatómica del xilema en tallo de cultivares de tomate injertados en un tipo criollo. Rev. Chapingo Ser. Hortic. 2010, 16, 67–76. [Google Scholar]
- Du, Y.; Cao, H.; Liu, S.; Gu, X.; Cao, Y. Response of yield, quality, water and nitrogen use efficiency of tomato to different levels of water and nitrogen under drip irrigation in northwestern China. J. Integr. Agric. 2017, 16, 1153–1161. [Google Scholar] [CrossRef]
- Gent, M.P.N.; Short, M.R. Effect on yield and quality of a simple system to recycle nutrient solution to greenhouse tomato. Hortscience 2012, 47, 1641–1645. [Google Scholar] [CrossRef]
- Massa, D.; Incrocci, L.; Maggini, R.; Carmassi, G.; Campiotti, C.A.; Pardossi, A. Strategies to decrease water drainage and nitrate emission from soilless culture of greenhouse tomato. Agric. Water Manag. 2010, 97, 971–980. [Google Scholar] [CrossRef]
- Signore, A.; Serio, F.; Santamaria, P. A Targeted management of the nutrient solution in a soilless tomato crop according to plant needs. Front. Plant Sci. 2016, 7, 391. [Google Scholar] [CrossRef]
- Lambers, H.; Chapin, F.S.; Pons, T.L. Plant Physiological Ecology, 2nd ed.; Springer: New York, NY, USA, 2008. [Google Scholar]
- Ntatsi, G.; Savvas, D.; Klaring, H.; Schwarz, D. Growth, yield, and metabolic responses of temperature-stressed tomato to grafting onto rootstocks differing in cold tolerance. J. Am. Soc. Hort. Sci. 2014, 139, 230–243. [Google Scholar] [CrossRef]
- Sui, N.; Li, M.; Liu, X.Y.; Wang, N.; Fang, W.; Meng, Q.W. Response of xanthophyll cycle and chloroplastic antioxidant enzymes to chilling stress in tomato over-expressing glycerol-3-phosphate acyltransferase gene. Photosynthetica 2007, 45, 447–454. [Google Scholar] [CrossRef]
- Wu, K.; Dechorgnat, J.; Patrit, O.; Krapp, A.; Fagard, M.; Daniel-Vedele, F. Characterization of the nrt2.6 gene in Arabidopsis thaliana: A link with plant response to biotic and abiotic stress. PLoS ONE 2012, 7, e42491. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).