Why Self-fertilizing Plants Still Exist in Wild Populations: Diversity Assurance through Stress-Induced Male Sterility May Promote Selective Outcrossing and Recombination
Abstract
1. Introduction
2. Abiotic Stress on Reproduction
3. Evolution of Mating Systems: From Outcrossing to Selfing
4. Mixed-Mating Systems: Having It Both Ways
5. Reproductive Assurance in Mixed-Mating by Stress-Induced Selfing
6. Reproductive Assurance in Mixed-Mating by Stress-Induced Outcrossing
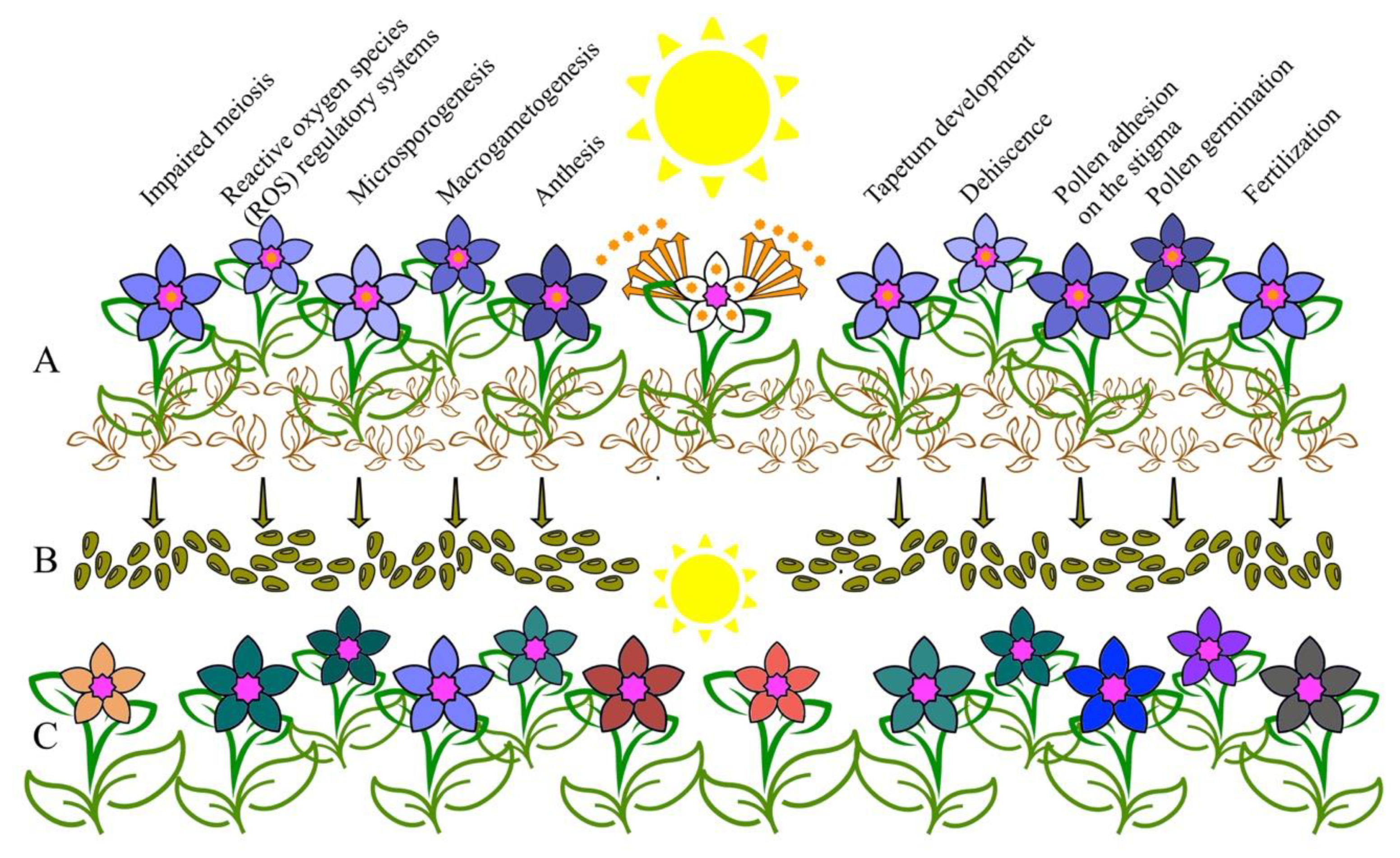
7. Diversity Assurance: Stress-induced Male Sterility Promotes Outcrossing
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Field, C.B. Climate change 2014—Impacts, Adaptation and Vulnerability: Part A: Global and Sectoral Aspects: Working group II Contribution to the IPCC Fifth Assessment Report: Volume 1: Global and Sectoral Aspects.; Cambridge University Press: New York, NY, USA, 2014; p. 1. [Google Scholar] [CrossRef]
- Hedhly, A.; Hormaza, J.I.; Herrero, M. Global warming and sexual plant reproduction. Trends Plant Sci. 2009, 14, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Bita, C.E.; Gerats, T. Plant tolerance to high temperature in a changing environment: Scientific fundamentals and production of heat stress-tolerant crops. Front. Plant Sci. 2013, 4, 273. [Google Scholar] [CrossRef] [PubMed]
- Demotes-Mainard, S.; Doussinault, S.; Meynard, J.M. Abnormalities in the male developmental programme of winter wheat induced by climatic stress at meiosis. Agronomie 1996, 6, 505–515. [Google Scholar] [CrossRef]
- Devasirvatham, V.; Gaur, P.M.; Mallikarjuna, N.; Raju, T.N.; Trethowan, R.M.; Tan, D.K.Y. Reproductive biology of chickpea response to heat stress in the field is associated with the performance in controlled environments. Field Crops Res. 2013, 142, 9–19. [Google Scholar] [CrossRef]
- Djanaguiraman, M.; Perumal, R.; Ciampitti, I.A.; Gupta, S.K.; Prasad, P.V.V. Quantifying pearl millet response to high temperature stress: Thresholds, sensitive stages, genetic variability and relative sensitivity of pollen and pistil. Plant Cell Environ. 2017, 41, 993–1007. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Nadeem, F.; Gogoi, N.; Ullah, A.; Alghamdi, S.S.; Nayyar, H.; Siddique, K.H.M. Heat stress in grain legumes during reproductive and grain-filling phases. Crop. Pasture Sci. 2017, 68, 985–1005. [Google Scholar] [CrossRef]
- Jiang, Y.; Lahlali, R.; Karunakaran, C.; Warkentin, T.D.; Davis, A.R.; Bueckert, R.A. Pollen, ovules, and pollination in pea: Success, failure, and resilience in heat. Plant Cell Environ. 2019, 42, 354–372. [Google Scholar] [CrossRef]
- Raja, M.M.; Vijayalakshmi, G.; Naik, M.L.; Basha, P.O.; Sergeant, K.; Hausman, J.F.; Khan, P.S.S.V. Pollen development and function under heat stress: From effects to responses. Acta Physiol. Plant. 2019, 41, 47. [Google Scholar] [CrossRef]
- Sage, T.L.; Bagha, S.; Lundsgaard-Nielsen, V.; Branch, H.A.; Sultmanis, S.; Sage, R.F. The effect of high temperature stress on male and female reproduction in plants. Field Crops Res. 2015, 182, 30–42. [Google Scholar] [CrossRef]
- Smith, A.R.; Zhao, D. Sterility caused by floral organ degeneration and abiotic stresses in Arabidopsis and cereal grains. Front. Plant Sci. 2016, 7, 1503. [Google Scholar] [CrossRef]
- Thuzar, M.; Putch, A.B.; Abdullah, N.A.P.; Lassim, M.B.M.; Kamaruzaman, J. The effects of temperature stress on the quality and yield of soya bean (Glycine max L.) Merrill. J. Agric. Sci. 2010, 2, 172–179. [Google Scholar]
- Sharma, K.D.; Nayyar, H. Regulatory networks in pollen development under cold stress. Front. Plant Sci. 2016, 7, 402. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.D.; Jang, S.; Soh, M.-S.; Lee, J.; Yun, S.D.; Oh, S.A.; Park, S.K. High daytime temperature induces male sterility with developmental defects in male reproductive organs of Arabidopsis. Plant Biotechnol. Rep. 2019, 13, 635–643. [Google Scholar] [CrossRef]
- De Storme, N.; Geelen, D. The impact of environmental stress on male reproductive development in plants: Biological processes and molecular mechanisms. Plant Cell Environ. 2013, 37, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Gross, Y.; Kigel, J. Differential sensitivity to high temperature of stages in the reproductive development of common bean (Phaseolus vulgaris L.). Field Crops Res. 1994, 36, 201–212. [Google Scholar] [CrossRef]
- Rieu, I.; Twell, D.; Firon, N. Pollen development at high temperature: From acclimation to collapse. Plant. Physiol. 2017, 173, 1967. [Google Scholar] [CrossRef]
- Sita, K.; Sehgal, A.; Hanumantha Rao, B.; Nair, R.M.; Vara Prasad, P.V.; Kumar, S.; Gaur, P.M.; Farooq, M.; Siddique, K.H.M.; Varshney, R.K.; et al. Food legumes and rising temperatures: Effects, adaptive functional mechanisms specific to reproductive growth stage and strategies to improve heat tolerance. Front. Plant Sci. 2017, 8, 1658. [Google Scholar] [CrossRef]
- Suzuki, N.; Katano, K. Coordination between ROS regulatory systems and other pathways under heat stress and pathogen attack. Front. Plant Sci. 2018, 9, 490. [Google Scholar] [CrossRef]
- Barton, D.A.; Cantrill, L.C.; Law, A.M.K.; Phillips, C.G.; Sutton, B.G.; Overall, R.L. Chilling to zero degrees disrupts pollen formation but not meiotic microtubule arrays in Triticum aestivum L. Plant Cell Environ. 2014, 37, 2781–2794. [Google Scholar] [CrossRef]
- Hedhly, A. Sensitivity of flowering plant gametophytes to temperature fluctuations. Environ. Exp. Bot. 2011, 74, 9–16. [Google Scholar] [CrossRef]
- Ji, X.; Shiran, B.; Wan, J.; Lewis, D.C.; Jenkins, C.L.D.; Condon, A.G.; Richards, R.A.; Dolferus, R. Importance of pre-anthesis anther sink strength for maintenance of grain number during reproductive stage water stress in wheat. Plant Cell Environ. 2010, 33, 926–942. [Google Scholar] [CrossRef]
- Müller, F.; Rieu, I. Acclimation to high temperature during pollen development. Plant Reprod. 2016, 29, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Baker, H.G. Self-compatibility and establishment after “long-distance” dispersal. Evolution 1955, 9, 347–349. [Google Scholar]
- Kamran-Disfani, A.; Agrawal, A.F. Selfing, adaptation and background selection in finite populations. J. Evol. Biol. 2014, 27, 1360–1371. [Google Scholar] [CrossRef]
- Stebbins, G.L. Self fertilization and population variability in the higher plants. Am. Nat. 1957, 91, 337–354. [Google Scholar] [CrossRef]
- Arunkumar, R.; Ness, R.W.; Wright, S.I.; Barrett, S.C.H. The evolution of selfing is accompanied by reduced efficacy of selection and purging of deleterious mutations. Genetics 2015, 199, 817–829. [Google Scholar] [CrossRef]
- Goodwillie, C.; Kalisz, S.; Eckert, C.G. The evolutionary enigma of mixed mating systems in plants: Occurrence, theoretical explanations, and empirical evidence. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 47–79. [Google Scholar] [CrossRef]
- Wright, S.; Ness, R.; Foxe, J.; Barrett, S. Genomic consequences of outcrossing and selfing in plants. Int. J. Plant Sci. 2008, 169, 105–118. [Google Scholar] [CrossRef]
- Igic, B.; Busch, J.W. Is self-fertilization an evolutionary dead end? New Phytol. 2013, 198, 386–397. [Google Scholar] [CrossRef]
- Spigler, R.B.; Kalisz, S. Persistent pollinators and the evolution of complete selfing. Am. J. Bot. 2017, 104, 1783–1786. [Google Scholar] [CrossRef]
- Whitehead, M.R.; Lanfear, R.; Mitchell, R.J.; Karron, J.D. Plant mating systems often vary widely among populations. Front. Ecol. Evol. 2018, 6, 38. [Google Scholar] [CrossRef]
- Speroni, G.; Izaguirre, P.; Bernardello, G.; Franco, J. Reproductive versatility in legumes: The case of amphicarpy in Trifolium polymorphum. Plant Biol. 2013, 16, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Fenster, C.B.; Martén-Rodríguez, S. Reproductive assurance and the evolution of pollination specialization. Int. J. Plant Sci. 2007, 168, 215–228. [Google Scholar] [CrossRef]
- Opedal, Ø.H.; Bolstad, G.H.; Hansen, T.F.; Armbruster, W.S.; Pélabon, C. The evolvability of herkogamy: Quantifying the evolutionary potential of a composite trait. Evolution 2017, 71, 1572–1586. [Google Scholar] [CrossRef] [PubMed]
- Kissling, J.; Barrett, S.C.H. Diplostigmaty in plants: A novel mechanism that provides reproductive assurance. Biol. Lett. 2013, 9, 20130495. [Google Scholar] [CrossRef] [PubMed]
- Funk, V.A.; Fragman-Sapir, O. Gymnarrheneae (Gymnarrhenoideae). In Systematics, Evolution, and Biogeography of Compositae; Funk, V.A., Susanna, A., Stuessy, T., Bayer, R., Eds.; International Association for Plant Taxonomy: Vienna, Austria, 2009; pp. 327–332. [Google Scholar]
- Arista, M.; Berjano, R.; Viruel, J.; Ortiz, M.Á.; Talavera, M.; Ortiz, P.L. Uncertain pollination environment promotes the evolution of a stable mixed reproductive system in the self-incompatible Hypochaeris salzmanniana (Asteraceae). Ann. Bot. 2017, 120, 447–456. [Google Scholar] [CrossRef]
- Ashman, T.-L. The evolution of separate sexes: A focus on the ecological context. In The Ecology and Evolution of Flowers; Harder, L.D., Barrett, S.C.H., Eds.; Oxford University Press: Oxford, UK, 2006; pp. 204–222. [Google Scholar]
- Cheplick, G.P. Plasticity of chasmogamous and cleistogamous reproductive allocation in grasses. Aliso J. Syst. Evol. Bot. 2007, 23, 286–294. [Google Scholar] [CrossRef]
- Campbell, C.S.; Quinn, J.A.; Cheplick, G.P.; Bell, T.J. Cleistogamy in grasses. Annu. Rev. Ecol. Syst. 1983, 14, 411–441. [Google Scholar] [CrossRef]
- Turuspekov, Y.; Mano, Y.; Honda, I.; Kawada, N.; Watanabe, Y.; Komatsuda, T. Identification and mapping of cleistogamy genes in barley. Theor. Appl. Genet. 2004, 109, 480–487. [Google Scholar] [CrossRef]
- Culley, T.M.; Klooster, M.R. The cleistogamous breeding system: A review of its frequency, evolution, and ecology in angiosperms. Bot. Rev. 2007, 73, 1–30. [Google Scholar] [CrossRef]
- Jarne, P.; Charlesworth, D. The evolution of the selfing rate in functionally hermaphrodite plants and animals. Annu. Rev. Ecol. Syst. 1993, 24, 441–466. [Google Scholar] [CrossRef]
- Bishop, J.; Jones, H.E.; Lukac, M.; Potts, S.G. Insect pollination reduces yield loss following heat stress in faba bean (Vicia faba L.). Agric. Ecosyst. Environ. 2016, 220, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Bishop, J.; Jones, H.E.; O’Sullivan, D.M.; Potts, S.G. Elevated temperature drives a shift from selfing to outcrossing in the insect-pollinated legume, faba bean (Vicia faba). J. Exp. Bot. 2017, 68, 2055–2063. [Google Scholar] [CrossRef] [PubMed]
- Folke, S.H.; Delph, L.F. Environmental and physiological effects on pistillate flower production in Silene noctiflora L. (Caryophyllaceae). Int. J. Plant Sci. 1997, 158, 501–509. [Google Scholar] [CrossRef]
- Davis, S.L.; Delph, L.F. Prior selfing and gynomonoecy in Silene noctiflora L. (Caryophyllaceae): Opportunities for enhanced outcrossing and reproductive assurance. Int. J. Plant Sci. 2005, 166, 475–480. [Google Scholar] [CrossRef]
- Kay, K.M.; Picklum, D.A. Drought alters the expression of mating system traits in two species of Clarkia. Evol. Ecol. 2013, 27, 899–910. [Google Scholar] [CrossRef]
- Busch, J.W.; Neiman, M.; Koslow, J.M.; Kalisz, S. Evidence for maintenance of sex by pathogens in plants. Evolution 2004, 58, 2584–2590. [Google Scholar] [CrossRef]
- Levin, D.A. Pest pressure and recombination systems in plants. Am. Nat. 1975, 109, 437–451. [Google Scholar] [CrossRef]
- Benitez, E.R.; Khan, N.A.; Matsumura, H.; Abe, J.; Takahashi, R. Varietal differences and morphology of cleistogamy in soybean. Crop. Sci. 2010, 50, 185–190. [Google Scholar] [CrossRef]
- Ray, J.D.; Kilen, T.C.; Abel, C.A.; Paris, R.L. Soybean natural cross-pollination rates under field conditions. Environ. Biosaf. Res. 2003, 2, 133–138. [Google Scholar] [CrossRef]
- Okada, T.; Jayasinghe, J.E.A.R.M.; Nansamba, M.; Baes, M.; Warner, P.; Kouidri, A.; Correia, D.; Nguyen, V.; Whitford, R.; Baumann, U. Unfertilized ovary pushes wheat flower open for cross-pollination. J. Exp. Bot. 2017, 69, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Branch, H.A.; Sage, R.F. Reproductive heat tolerance in a Mojave desert annual plant, Trianthema portulacastrum. Am. J. Bot. 2018, 105, 2018–2024. [Google Scholar] [CrossRef] [PubMed]
- Dolferus, R.; Powell, N.; Ji, X.; Ravash, R.; Edlington, J.; Oliver, S.; Van Dongen, J.; Shiran, B. The Physiology of Reproductive-Stage Abiotic Stress Tolerance in Cereals. In Molecular Stress Physiology of Plants; Rout, G.R., Das, A.B., Eds.; Springer: New Delhi, India, 2013; pp. 193–216. [Google Scholar]
- Briggs, K.G.; Kiplagat, O.K.; Johnson-Flanagan, A.M. Floret sterility and outcrossing in two spring wheat cultivars. Can. J. Plant Sci. 1999, 79, 321–328. [Google Scholar] [CrossRef]
- Gaines, T.A.; Byrne, P.F.; Westra, P.; Nissen, S.J.; Henry, W.B.; Shaner, D.L.; Chapman, P.L. An empirically derived model of field-scale gene flow in winter wheat. Crop. Sci. 2007, 47, 2308–2316. [Google Scholar] [CrossRef]
- Weerakoon, W.; Abeywickrama, T.; De Costa, J.; Maruyama, A. Out-crossing of Heat Stress Affected Spikelets of Lowland Rice in the Sub-humid Zone of Sri Lanka and Its Long-term Implications. In Proceedings of the 3rd Annual MARCO Symposium, Tsukuba, Japan, 5–9 October 2009; pp. 32–37. [Google Scholar]
- Li, R.; Wang, S.; Duan, L.; Li, Z.; Christoffers, M.J.; Mengistu, L.W. Genetic diversity of wild oat (Avena fatua) populations from China and the United States. Weed Sci. 2007, 55, 95–101. [Google Scholar] [CrossRef]
- Yu, Q.; Liu, Q.; Xiong, Y.; Xiong, Y.; Dong, Z.; Yang, J.; Liu, W.; Ma, X.; Bai, S. Genetic diversity and population divergence of a rare, endemic grass (Elymus breviaristatus) in the southeastern Qinghai-Tibetan plateau. Sustainability 2019, 11, 5863. [Google Scholar] [CrossRef]
- Zhang, Z.; Xie, W.; Zhang, J.; Zhao, X.; Zhao, Y.; Wang, Y. Phenotype- and SSR-based estimates of genetic variation between and within two important Elymus species in western and northern China. Genes 2018, 9, 147. [Google Scholar] [CrossRef]
- Wu, W.-D.; Liu, W.-H.; Sun, M.; Zhou, J.-Q.; Liu, W.; Zhang, C.-L.; Zhang, X.-Q.; Peng, Y.; Huang, L.-K.; Ma, X. Genetic diversity and structure of Elymus tangutorum accessions from western China as unraveled by AFLP markers. Hereditas 2019, 156, 8. [Google Scholar] [CrossRef]
- Vuorinen, L.A.; Kalendar, R.; Fahima, T.; Korpelainen, H.; Nevo, E.; Schulman, H.A. Retrotransposon-based genetic diversity assessment in wild emmer wheat (Triticum turgidum ssp. dicoccoides). Agronomy 2018, 8, 107. [Google Scholar] [CrossRef]
- Nieto-López, R.M.; Soler, C.; Garcia, P. Genetic diversity in wild Spanish populations of Thinopyrum junceum and Thinopyrum junceiforme using endosperm proteins and PCR-based markers. Hereditas 2003, 139, 18–27. [Google Scholar] [CrossRef]
- Sandro, P.; Gutiérrez, L.; Speranza, P. Distribution of genetic and phenotypic diversity in the autogamous perennial Paspalum dilatatum subsp. flavescens Roseng., Arrill. & Izag. (Poaceae). Genet. Resour. Crop. Evol. 2019, 66, 1205–1216. [Google Scholar] [CrossRef]
- Frels, K.; Chopra, R.; Dorn, M.K.; Wyse, L.D.; Marks, D.M.; Anderson, A.J. Genetic diversity of field pennycress (Thlaspi arvense) reveals untapped variability and paths toward selection for domestication. Agronomy 2019, 9, 302. [Google Scholar] [CrossRef]
- Dolferus, R.; Ji, X.; Richards, R.A. Abiotic stress and control of grain number in cereals. Plant Sci. 2011, 181, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Darwin, C. On the Origin of Species by Natural Selection; Penguin Books Ltd.: Harmondsworth, UK, 1987. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Ginkel, M.; Flipphi, R.C.H. Why Self-fertilizing Plants Still Exist in Wild Populations: Diversity Assurance through Stress-Induced Male Sterility May Promote Selective Outcrossing and Recombination. Agronomy 2020, 10, 349. https://doi.org/10.3390/agronomy10030349
van Ginkel M, Flipphi RCH. Why Self-fertilizing Plants Still Exist in Wild Populations: Diversity Assurance through Stress-Induced Male Sterility May Promote Selective Outcrossing and Recombination. Agronomy. 2020; 10(3):349. https://doi.org/10.3390/agronomy10030349
Chicago/Turabian Stylevan Ginkel, Maarten, and Ronald C. H. Flipphi. 2020. "Why Self-fertilizing Plants Still Exist in Wild Populations: Diversity Assurance through Stress-Induced Male Sterility May Promote Selective Outcrossing and Recombination" Agronomy 10, no. 3: 349. https://doi.org/10.3390/agronomy10030349
APA Stylevan Ginkel, M., & Flipphi, R. C. H. (2020). Why Self-fertilizing Plants Still Exist in Wild Populations: Diversity Assurance through Stress-Induced Male Sterility May Promote Selective Outcrossing and Recombination. Agronomy, 10(3), 349. https://doi.org/10.3390/agronomy10030349




