The Effect of Salicylic Acid and 20 Substituted Molecules on Alleviating Metolachlor Herbicide Injury in Rice (Oryza sativa)
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Materials
2.2. Growth Conditions
2.3. Data Analysis
3. Results and Discussion
3.1. Safening Effects of SA and Substituted SA on Rice Growth
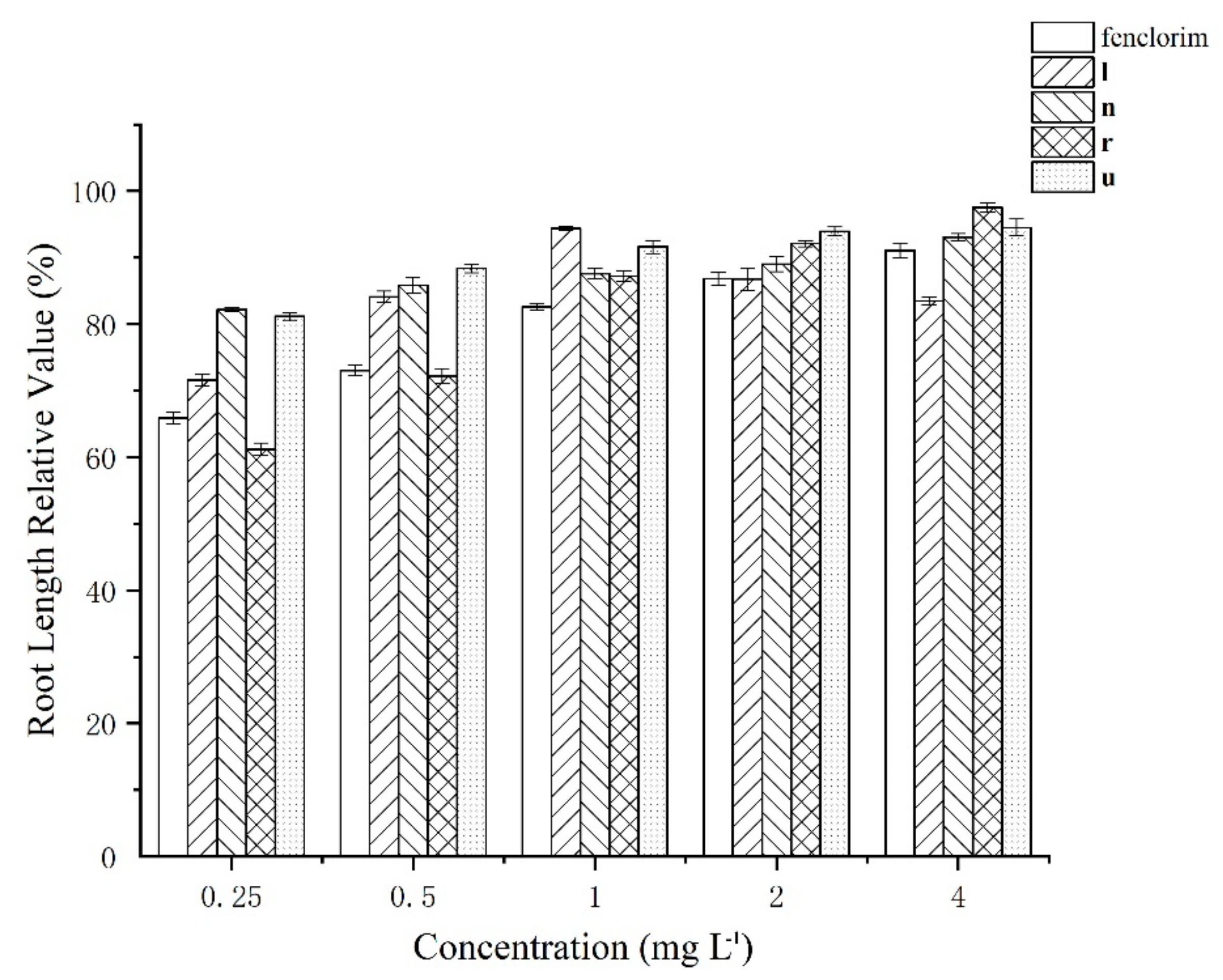
3.2. Safening Effect of Compounds l, n, r, and u in Rice at Lower Concentrations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Liu, H.; Huang, R.; Xie, F.; Zhang, S.; Shi, J. Enantioselective phytotoxicity of metolachlor against maize and rice roots. J. Hazard. Mater. 2012, 217–218, 330–337. [Google Scholar] [CrossRef]
- Coleman, J.O.D.; Frova, C.; Schroder, P.; Tissut, M. Exploiting plant metabolism for the phytoremediation of persistent herbicides. Environ. Sci. Pollut. Res. 2002, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, K.J.; Nurse, R.E.; Everman, W.J.; Sprague, C.L.; Sikkema, P.H. Tolerance of corn (Zea mays L.) to early and late glyphosate applications. Am. J. Plant Sci. 2014, 5, 2748–2754. [Google Scholar]
- Rosinger, C.; Evans, P.; Hacker, E. Crop Plant–Compatible Herbicidal Compositions Comprising Herbicides and Safeners. U.S. Patent 20070010399A1, 11 January 2007. [Google Scholar]
- Rosario–Lebron, A.; Leslie, A.W.; Chen, G.; Hooks, C.R.R. The effect of barley cover crop residue and herbicide management on the foliar arthropod community in no-till soybeans. Agronomy 2018, 8, 87. [Google Scholar] [CrossRef] [Green Version]
- Deng, X.L.; Zhou, Y.; Zheng, W.N.; Bai, L.Y.; Zhou, X.M. Dissipation dynamic and final residues of oxadiargyl in paddy fields using high-performance liquid chromatography-tandem mass spectrometry coupled with modified QuEChERS method. Int. J. Environ. Res. Public Health 2018, 15, 1680. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, S.J.P.D.; Nicolai, M.; Ferreira, R.R.; Figueira, A.V.D.O.; Christoffoleti, P.J. Herbicide selectivity by differential metabolism: Considerations for reducing crop damages. Sci. Agric. 2009, 66, 136–142. [Google Scholar] [CrossRef] [Green Version]
- Rosinger, C. Herbicide safeners: An overview. Julius Kühn Archiv 2014, 443, 516–525. [Google Scholar]
- Jablonkai, I. Herbicide safeners: Effective tools to improve herbicide selectivity. In Herbicides—Current Research and Case Studies in Use; Andrew, J.P., Jessica, A.K., Eds.; Intech: Rijeka, Coratia, 2013; pp. 589–620. [Google Scholar]
- Parker, C. Herbicide protectants and antidotes—A review. Pans 1976, 22, 65–74. [Google Scholar]
- Abuqare, A.W.; Duncan, H.J. Herbicide safeners: Uses, limitations, metabolism, and mechanisms of action. Chemosphere 2002, 48, 965–974. [Google Scholar] [CrossRef]
- Deridder, B.P.; Dixon, D.P.; Beussman, D.J.; Edwards, R.; Goldsbrough, P.B. Induction of glutathione S–transferases in Arabidopsis by herbicide safeners. Plant Physiol. 2002, 130, 1497–1505. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, O.L. Herbicide antidotes: From concept to practice. In The Chemistry and Action of Herbicide Antidotes; Pallos, F.M., Casida, J.E., Eds.; Academic Press: London, UK, 1978; pp. 1–13. [Google Scholar]
- Hoffmann, O.L. 1,8-naphthalic anhydride Protective Coatings of Cereals Against Herbicidal S-ethyl N,N-dipropylthiocarbamate. DE Patent 1952910A, 25 June 1970. [Google Scholar]
- Hatzios, K.K. Herbicide antidotes: Development, chemistry, and mode of action. Adv. Agron. 1983, 36, 265–316. [Google Scholar]
- Thiessen, E.P.; Stephenson, G.R.; Anderson, G.W. Factors influencing 1,8-naphthalic anhydride activity as an antidote to barban in oats. Can. J. Plant Sci. 1980, 60, 1005–1013. [Google Scholar] [CrossRef] [Green Version]
- Dellaferrera, I.; Cortés, E.; Panigo, E.; De Prado, R.; Christoffoleti, P.; Perreta, M. First report of Amaranthus hybridus with multiple resistance to 2,4-D, dicamba, and glyphosate. Agronomy 2018, 8, 140. [Google Scholar] [CrossRef] [Green Version]
- Chang, T.S.; Merkle, M.G. Oximes as seed safeners for grain sorghum (Sorghum bicolor) to herbicides. Weed Sci. 1982, 30, 70–73. [Google Scholar] [CrossRef]
- Hatzios, K.K. Interactions of the safener flurazole with chloroacetanilide and thiocarbamate herbicides on maize (Zea mays L.). Can. J. Plant Sci. 1986, 66, 353–359. [Google Scholar] [CrossRef]
- Tsukuda, K.; Ichizen, N.; Konnai, M.; Takematsu, T. Effect of seeding depth on crop injury by pyributicarb in direct-seeded rice plants and evaluation of dymron as a safener. J. Weed Sci. Technol. 2000, 45, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Sivey, J.D.; Lehmler, H.J.; Salice, C.J.; Ricko, A.N.; Cwiertny, D.M. Environmental fate and effects of dichloroacetamide herbicide safeners: “Inert” yet biologically active agrochemical ingredients. Environ. Sci. Technol. Lett 2015, 2, 260–269. [Google Scholar] [CrossRef]
- Kästner, J.; Knorre, D.V.; Himanshu, H.; Erb, M.; Baldwin, I.T.; Meldau, S. Salicylic acid, a plant defense hormone, is specifically secreted by a molluscan herbivore. PLoS ONE 2014, 9, e86500. [Google Scholar] [CrossRef] [Green Version]
- Larsen, R.J.; Falk, D.E. Effects of a seed treatment with a neonicotinoid insecticide on germination and freezing tolerance of spring wheat seedlings. Can. J. Plant Sci. 2013, 93, 535–540. [Google Scholar] [CrossRef] [Green Version]
- Khan, W.; Prithiviraj, B.; Smith, D.L. Photosynthetic responses of corn and soybean to foliar application of salicylates. J. Plant Physiol. 2003, 160, 485–492. [Google Scholar] [CrossRef]
- Hayat, Q.; Hayat, S.; Irfan, M.; Ahmad, A. Effect of exogenous salicylic acid under changing environment: A review. Environ. Exp. Bot. 2010, 68, 14–25. [Google Scholar] [CrossRef]
- Shimakawa, A.; Shiraya, T.; Ishizuka, Y.; Wada, K.C.; Mitsui, T.; Takeno, K. Salicylic acid is involved in the regulation of starvation stress-induced flowering in Lemna paucicostata. J. Plant Physiol. 2012, 169, 987–991. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Qin, G.; Li, B.; Wang, Q.; Meng, X. Effects of salicylic acid on disease resistance and postharvest decay control of fruits. Stewart Postharvest Rev. 2007, 3, 1–7. [Google Scholar]
- Alaey, M.; Babalar, M.; Naderi, R.; Kafi, M. Effect of pre- and postharvest salicylic acid treatment on physio-chemical attributes in relation to vase-life of rose cut flowers. Postharvest Biol. Technol. 2011, 61, 91–94. [Google Scholar] [CrossRef]
- Shi, H.; Wang, Y.; Li, Z.; Zhang, D.; Zhang, Y.; Xiang, D.; Li, Y.; Zhang, Y. Pear IAA1 gene encoding an auxin-responsive Aux/IAA protein is involved in fruit development and response to salicylic acid. Can. J. Plant Sci. 2014, 94, 263–271. [Google Scholar] [CrossRef]
- Malamy, J.; Carr, J.P.; Klessig, D.F.; Raskin, I. Salicylic acid: A likely endogenous signal in the resistance response of tobacco to viral infection. Science 1990, 250, 1002–1004. [Google Scholar] [CrossRef] [Green Version]
- Métraux, J.P.; Signer, H.; Ryals, J.; Ward, E.; Wyss-Benz, M.; Gaudin, J.; Raschdorf, K.; Schmid, E.; Blum, W.; Inverardi, B. Increase in salicylic acid at the onset of systemic acquired resistance in cucumber. Science 1990, 250, 1004–1006. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Ruiz, J.; Arnao, M. Relationship of melatonin and salicylic acid in biotic/abiotic plant stress responses. Agronomy 2018, 8, 33. [Google Scholar] [CrossRef] [Green Version]
- Faize, L.; Faize, M. Functional analogues of salicylic acid and their use in crop protection. Agronomy 2018, 8, 5. [Google Scholar] [CrossRef] [Green Version]
- Eichhorn, H.; Klinghammer, M.; Becht, P.; Tenhaken, R. Isolation of a novel ABC-transporter gene from soybean induced by salicylic acid. J. Exp. Bot. 2006, 57, 2193–2201. [Google Scholar] [CrossRef] [Green Version]
- Vlot, A.C.; Liu, P.P.; Cameron, R.K.; Park, S.W.; Yang, Y.; Kumar, D.; Zhou, F.; Padukkavidana, T.; Gustafsson, C.; Pichersky, E. Identification of likely orthologs of tobacco salicylic acid-binding protein 2 and their role in systemic acquired resistance in Arabidopsis thaliana. Plant J. 2008, 56, 445–456. [Google Scholar] [CrossRef]
- Yildirim, E.; Turan, M.; Guvenc, I. Effect of foliar salicylic acid applications on growth, chlorophyll, and mineral content of cucumber grown under salt stress. J. Plant Nutr. 2008, 31, 593–612. [Google Scholar] [CrossRef]
- Bertoncelli, J.D.; Mazaro, S.M.; Rocha, R.D.C.D.S.; Dalacosta, N.L.; Lewandowski, A.; Wagner, J.A. Salicylic acid in the induction of resistance to beet seedling damping-off and antifungal activity against Fusarium sp., in vitro. Semin. Cienc. Agrar. 2016, 37, 67–75. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Mun, B.G.; Khan, A.L.; Waqas, M.; Kim, H.H.; Shahzad, R.; Imran, M.; Yun, B.W.; Lee, I.J. Regulation of reactive oxygen and nitrogen species by salicylic acid in rice plants under salinity stress conditions. PLoS ONE 2018, 13, e0192650. [Google Scholar] [CrossRef] [Green Version]
- Lenk, M.; Wenig, M.; Mengel, F.; Häußler, F.; Vlot, A. Arabidopsis thaliana immunity–related compounds modulate disease susceptibility in barley. Agronomy 2018, 8, 142. [Google Scholar] [CrossRef] [Green Version]
- Bickers, U.; Willms, L.; Rosinger, C. Use of Aromatic Compounds as Safeners. EP Patent 20040741232, 23 July 2004. [Google Scholar]
- Tang, X.K.; Zhou, X.M.; Wu, J.; Li, J.B.; Bai, L.Y. A novel function of sanshools: The alleviation of injury from metolachlor in rice seedlings. Pestic. Biochem. Physiol. 2014, 110, 44–49. [Google Scholar] [CrossRef]
- Zheng, W.N.; Zhu, Z.Y.; Deng, Y.N.; Wu, Z.C.; Zhou, Y.; Zhou, X.M.; Bai, L.Y.; Deng, X.L. Synthesis, crystal structure, herbicide safening, and antifungal activity of N-(4,6-dichloropyrimidine-2-yl)benzamide. Crystals 2018, 8, 75. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Shen, X.F.; Fang, Y. Fenclorim effects on rice germination and yield. Can. J. Plant Sci. 2013, 93, 237–241. [Google Scholar] [CrossRef]
- Caminada, D.; Escher, C.; Fent, K. Cytotoxicity of pharmaceuticals found in aquatic systems: Comparison of PLHC-1 and RTG-2 fish cell lines. Aquat. Toxical. 2006, 79, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Lammer, E.; Carr, G.J.; Wendler, K.; Rawlings, J.M.; Belanger, S.E.; Braunbeck, T. Is the fish embryo toxicity test (FET) with the zebrafish (Danio rerio) a potential alternative for the fish acute toxicity test? Comp. Biochem. Physiol. C 2009, 149, 196–209. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.L.; Wang, X.Y.; Mao, Z.H.; Lv, Y.; Pan, J.H.; Yu, P.Z. Design, synthesis and evaluation of the molluscicidal activity of novel hydrocarbyl substituted salicylic acid derivatives. Afr. J. Pharm. Pharmacol. 2014, 8, 644–650. [Google Scholar]
- Coleman, S.; Linderman, R.; Hodgson, E.; Rose, R.L. Comparative metabolism of chloroacetamide herbicides and selected metabolites in human and rat liver microsomes. Environ. Health Persp. 2000, 108, 1151–1157. [Google Scholar]
- Harris, R.M.; Hawker, R.J.; Langman, M.J.; Singh, S.; Waring, R.H. Inhibition of phenolsulphotransferase by salicylic acid: A possible mechanism by which aspirin may reduce carcinogenesis. Gut 1998, 42, 272–275. [Google Scholar] [CrossRef] [Green Version]



| Compound | Safening Effect (% of Non-Treated Control) | ||
|---|---|---|---|
| Plant Height | Root Length | Fresh Weight | |
| M | 44.87 ± 0.35 | 46.35 ± 0.71 | 68.20 ± 0.78 |
| a | 97.28 ± 0.48 | 88.40 ± 1.52 | 95.90 ± 0.53 |
| b | 90.45 ± 0.98 | 80.94 ± 1.23 | 91.80 ± 1.62 |
| c | 95.14 ± 0.37 | 91.79 ± 0.72 | 90.91 ± 0.81 |
| d | 91.01 ± 0.65 | 90.79 ± 0.83 | 96.03 ± 0.42 |
| e | 91.77 ± 0.60 | 92.13 ± 0.98 | 92.12 ± 1.06 |
| f | 92.75 ± 0.51 | 90.98 ± 1.26 | 91.21 ± 1.16 |
| g | 93.29 ± 0.45 | 92.67 ± 1.73 | 93.20 ± 0.45 |
| h | 96.19 ± 0.39 | 90.69 ± 0.64 | 93.72 ± 0.85 |
| i | 93.53 ± 0.63 | 91.92 ± 0.76 | 98.40 ± 0.56 |
| j | 95.39 ± 1.22 | 86.98 ± 0.82 | 92.91 ± 0.41 |
| k | 97.49 ± 0.96 | 93.70 ± 0.72 | 96.61 ± 0.95 |
| l | 98.15 ± 0.43 | 95.48 ± 0.10 | 90.50 ± 0.31 |
| m | 90.44 ± 0.05 | 94.65 ± 0.55 | 87.32 ± 2.19 |
| n | 94.00 ± 0.12 | 81.31 ± 0.78 | 90.54 ± 0.70 |
| o | 96.22 ± 0.46 | 88.69 ± 1.22 | 87.31 ± 0.73 |
| p | 92.78 ± 0.45 | 98.17 ± 1.21 | 91.51 ± 0.65 |
| q | 95.01 ± 0.80 | 88.22 ± 0.72 | 89.88 ± 0.55 |
| r | 94.27 ± 0.68 | 84.76 ± 0.55 | 88.03 ± 0.83 |
| s | 88.59 ± 0.50 | 94.56 ± 0.72 | 97.45 ± 1.56 |
| t | 92.87 ± 1.17 | 87.65 ± 1.85 | 94.15 ± 1.18 |
| u | 95.76 ± 1.15 | 91.34 ± 0.52 | 92.74 ± 0.82 |
| Comd. | Safening Effect (% of Non-Treated Control) | ||
|---|---|---|---|
| Plant Height | Root Length | Fresh Weight | |
| M1 | 44.87 ± 0.35 | 46.35 ± 0.71 | 68.20 ± 0.78 |
| F + M | 88.42 ± 0.28 | 73.93 ± 0.42 | 91.45 ± 0.79 |
| a + M | 47.98 ± 0.61 | 81.80 ± 0.36 | 82.44 ± 0.97 |
| b + M | 72.90 ± 0.81 | 86.59 ± 0.93 | 90.56 ± 0.55 |
| c + M | 69.37 ± 0.73 | 84.59 ± 0.89 | 87.30 ± 0.80 |
| d + M | 70.51 ± 0.12 | 80.97 ± 0.99 | 92.16 ± 0.77 |
| e + M | 53.24 ± 0.56 | 67.76 ± 0.68 | 85.55 ± 0.51 |
| f + M | 63.01 ± 0.35 | 83.74 ± 0.72 | 88.21 ± 0.79 |
| g + M | 60.80 ± 0.39 | 89.34 ± 0.76 | 87.90 ± 0.38 |
| h + M | 38.44 ± 0.45 | 51.53 ± 0.95 | 81.00 ± 0.36 |
| i + M | 46.55 ± 0.35 | 80.72 ± 0.65 | 77.14 ± 0.90 |
| j + M | 47.06 ± 0.98 | 82.58 ± 0.85 | 86.82 ± 0.62 |
| k + M | 36.55 ± 0.32 | 61.12 ± 0.92 | 85.15 ± 0.08 |
| l + M | 88.45 ± 0.12 | 71.11 ± 0.91 | 93.41 ± 0.95 |
| m + M | 81.25 ± 0.49 | 83.31 ± 0.22 | 93.09 ± 0.69 |
| n + M | 91.10 ± 0.37 | 71.17 ± 0.54 | 89.42 ± 0.58 |
| o + M | 40.66 ± 0.28 | 60.54 ± 0.56 | 71.41 ± 0.77 |
| p + M | 64.60 ± 0.54 | 83.00 ± 0.73 | 82.14 ± 0.64 |
| q + M | 53.82 ± 0.46 | 63.27 ± 0.36 | 65.92 ± 0.45 |
| r + M | 84.57 ± 0.28 | 90.23 ± 0.67 | 93.46 ± 0.21 |
| s + M | 47.95 ± 0.31 | 71.65 ± 0.42 | 78.69 ± 0.95 |
| t + M | 45.79 ± 0.23 | 71.77 ± 0.92 | 81.81 ± 0.41 |
| u + M | 87.45 ± 0.28 | 89.01 ± 0.31 | 89.47 ± 0.10 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, X.; Zheng, W.; Zhou, X.; Bai, L. The Effect of Salicylic Acid and 20 Substituted Molecules on Alleviating Metolachlor Herbicide Injury in Rice (Oryza sativa). Agronomy 2020, 10, 317. https://doi.org/10.3390/agronomy10030317
Deng X, Zheng W, Zhou X, Bai L. The Effect of Salicylic Acid and 20 Substituted Molecules on Alleviating Metolachlor Herbicide Injury in Rice (Oryza sativa). Agronomy. 2020; 10(3):317. https://doi.org/10.3390/agronomy10030317
Chicago/Turabian StyleDeng, Xile, Wenna Zheng, Xiaomao Zhou, and Lianyang Bai. 2020. "The Effect of Salicylic Acid and 20 Substituted Molecules on Alleviating Metolachlor Herbicide Injury in Rice (Oryza sativa)" Agronomy 10, no. 3: 317. https://doi.org/10.3390/agronomy10030317
APA StyleDeng, X., Zheng, W., Zhou, X., & Bai, L. (2020). The Effect of Salicylic Acid and 20 Substituted Molecules on Alleviating Metolachlor Herbicide Injury in Rice (Oryza sativa). Agronomy, 10(3), 317. https://doi.org/10.3390/agronomy10030317





