Effect of Exopolysaccharide-Producing Bacteria and Melatonin on Faba Bean Production in Saline and Non-Saline Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Inocula, Preparation, and Inoculation Techniques
2.2. Melatonin Application
2.3. Experimental Procedure
2.4. Data Recording
2.5. Statistical Analyses
3. Results
3.1. Vegetative Growth Parameters of Faba Bean Plants
3.2. Yield and the Components of Faba Bean Plants
3.3. Effects of Melatonin and EPS-Producing Bacteria on the Physiological Attributes of Faba Bean Plants
3.4. Effect of Melatonin and EPS-Producing Bacteria on the Nutrient Concentrations of Faba Bean Plants
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hernández, J.A. Salinity tolerance in plants: Trends and Perspectives. Int. J. Mol. Sci. 2019, 20, 2408. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S. The Critical Role of Potassium in Plant Stress Response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Abd el Latef, A.A.; Abd Allah, E.F.; Hashem, A.; Sarwat, M.; Anjum, N.A.; Gucel, S. Calcium and potassium supplementation enhanced growth, osmolyte secondary metabolite production, and enzymatic antioxidant machinery in cadmium-exposed chickpea (Cicer arietinum L.). Front. Plant Sci. 2016, 7, 513. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Yin, L.; Wang, S.; Zhang, M.; Deng, X.; Zhang, S. Enhanced root hydraulic conductance by aquaporin regulation accounts for silicon alleviated salt-induced osmotic stress in Sorghum bicolor L. Environ. Exp. Bot. 2015, 111, 42–51. [Google Scholar] [CrossRef]
- Etemadi, F.; Barkera, A.V.; Hashemi, M.; Zandvakili, O.R.; Park, Y. Nutrient accumulation in faba bean varieties. Commun. Soil Sci. Plant Anal. 2018, 49, 2064–2073. [Google Scholar] [CrossRef]
- Etemadi, F.; Hashemi, M.; Zandvakili, O.; Dolatabadian, A.; Sadeghpour, A. Nitrogen contribution from winterkilled faba bean cover crop to spring-sown sweet corn in conventional and no-till systems. Agron. J. 2018, 110, 455–462. [Google Scholar] [CrossRef]
- Fathalla, M.A. Effectiveness of exopolysaccharides and biofilm forming plant growth promoting rhizobacteria on salinity tolerance of faba bean (Vicia faba L.). Afr. J. Microbiol. Res. 2018, 12, 399–404. [Google Scholar]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 30, 3–14. [Google Scholar] [CrossRef]
- Zhan, H.; Nie, X.; Zhang, T.; Li, S.; Wang, X.; Du, X.; Tong, W.; Song, W. Melatonin: A small molecule but important for salt stress tolerance in plants. Int. J. Mol. Sci. 2019, 20, 709. [Google Scholar] [CrossRef]
- Van Oosten, M.J.; Pepe, O.; De Pascale, S.; Silletti, S.; Maggio, A. The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem. Biol. Technol. Agric. 2017, 4, 5. [Google Scholar] [CrossRef]
- Hardeland, R.; Cardinali, D.P.; Srinivasan, V.; Spence, D.W.; Brown, G.M.; Pandi-Perumal, S.R. Melatonin-A pleiotropic, orchestrating regulator molecule. Prog. Neurobiol. 2011, 93, 350–384. [Google Scholar] [CrossRef]
- Nawaz, M.A.; Huang, Y.; Bie, Z.; Ahmed, W.; Reiter, R.J.; Niu, M. Melatonin: Current status and future perspectives in plant science. Front. Plant Sci. 2016, 6, 1230. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.M.; Zhang, Y.Q. Melatonin: A well-documented antioxidant with conditional pro-oxidant actions. J. Pineal Res. 2014, 57, 131–146. [Google Scholar] [CrossRef] [PubMed]
- Castanares, J.L.; Bouzo, C.A. Effect of exogenous melatonin on seed germination and seedling growth in melon (Cucumis melo L.) under salt stress. Hortic. Plant J. 2019, 5, 79–87. [Google Scholar] [CrossRef]
- Viscardi, S.; Ventorino, V.; Duran, P.; Maggio, A.; De Pascale, S.; Mora, M.L. Assessment of plant growth promoting activities and abiotic stress tolerance of Azotobacter chroococcum strains for a potential use in sustainable agriculture. J. Soil Sci. Plant Nutr. 2016, 16, 848–863. [Google Scholar] [CrossRef]
- Ali, S.; Charles, T.; Glick, B. Amelioration of high salinity stress damage by plant growth-promoting bacterial endophytes that contain ACC deaminase. Plant Physiol. Biochem. 2014, 80, 160–167. [Google Scholar] [CrossRef]
- Velmourougane, K.; Prasanna, R.; Singh, S.B.; Kumar, R.; Saha, S. Sequence of inoculation influences the nature of extracellular polymeric substances and biofilm formation in Azotobacter chroococcum and Trichoderma viride. FEMS Microbiol. Ecol. 2017, 93, fix066. [Google Scholar] [CrossRef]
- Chapman, H.D.; Pratt, P.F. Methods of Analysis for Soils, Plants and Waters; Division of Agric. Soil Sci. University of California: Riverside, CA, USA, 1961; pp. 161–175. [Google Scholar]
- Awad, M.N.; Turky, S.A.; Abdelhamid, T.M.; Attia, M. Ameliorate of environmental salt stress on the growth of Zea mays L. plants by exopolysaccharides producing bacteria. JASR 2012, 8, 2033–2044. [Google Scholar]
- Rodriguez-Navarro, D.N.; Temprano, F.; Orive, R. Survival of Rhizobium sp. (Hedysarum coronariuml.) on peat-based inoculants and inoculated seeds. Soil Biol. Biochem. 1991, 23, 75–379. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldeen, R.P.; Teare, I.D. Rapid determination of free proline for water stress studies. Plant Soil. 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Jackson, M.L. Soil Chemical Analysis; Constable: London, UK, 1973. [Google Scholar]
- Cao, Y.Y.; Yang, M.T.; Chen, S.Y.; Zhou, Z.Q.; Li, X.; Wang, X.J.; Bai, J.G. Exogenous sucrose influences antioxidant enzyme activities and reduces lipid peroxidation in water-stressed cucumber leaves. Biol. Plant. 2015, 59, 147–153. [Google Scholar] [CrossRef]
- Rady, M.M.; Bhavya Varma, C.; Howladar, S.M. Common bean (Phaseolus vulgaris L.) seedlings overcome NaCl stress as a result of presoaking in Moringa oleifera leaf extract. Sci. Hortic. 2013, 162, 63–70. [Google Scholar] [CrossRef]
- Radi, A.A.; Farghaly, F.A.L.; Hamada, A.M. Physiological and biochemical responses of salt-tolerant and salt-sensitive wheat and bean cultivars to salinity. J. Biol. Earth Sci. 2013, 3, 72–88. [Google Scholar]
- Hasanuzzaman, M.; Nahar, K.; Fujita, M. Plant response to salt stress and role of exogenous protectants to mitigate salt-induced damages. In Ecophysiology and Responses of Plants under Salt Stress; Ahmad, P., Azooz, M.M., Prasad, M.N.V., Eds.; Springer: New York, NY, USA, 2013; pp. 25–87. [Google Scholar]
- Allen, G.J.; Wyn Jones, R.G.; Leigh, R.A. Sodium transport measured in plasma membrane vesicles isolated from wheat genotypes with differing K+/Na+ discrimination traits. Plant Cell Environ. 1995, 18, 105–115. [Google Scholar] [CrossRef]
- Nabil, M.; Coudret, A. Effects of sodium chloride on growth, tissue elasticity and solute adjustment in two Acacia nilotica subspecies. Physiol. Plant. 1995, 93, 217–224. [Google Scholar] [CrossRef]
- Arora, M.; Kaushik, A.; Rani, N.; Kaushik, C.P. Effect of cyanobacterial exopolysaccharides on salt stress alleviation and seed germination. J. Environ. Biol. 2010, 31, 701–704. [Google Scholar]
- Egamberdiyeva, D.H.; Flich, G. Influence of growth-promoting bacteria on the growth of wheat in different soils and temperatures. Soil Biol. Biochem. 2003, 35, 973–978. [Google Scholar] [CrossRef]
- Egamberdiyeva, D.H.; Flich, G. Effect of plant growth-promoting bacteria on growth and nutrient uptake of cotton and pea in a semi-arid region of Uzbekistan. J. Arid Environ. 2004, 56, 293–301. [Google Scholar] [CrossRef]
- Van Oosten, M.J.; Stasio, E.D.; Cirillo, V.; Silletti, S.; Ventorino, V.; Pepe, O.; Raimondi, G.; Maggio, A. Root inoculation with Azotobacter chroococcum 76A enhances tomato plants adaptation to salt stress under low N conditions. BMC Plant Biol. 2018, 18, 205. [Google Scholar] [CrossRef]
- Chaudhary, D.; Narula, N.; Sindhu, S.S.; Behl, R.K. Plant growth stimulation of wheat (Triticum aestivum L.) by inoculation of salinity tolerant Azotobacter strains. Physiol. Mol. Biol. Plants 2013, 19, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Hasnain, S.; Berge, O. Effect of exo-polysaccharides producing bacterial inoculation on growth of roots of wheat (Triticum aestivum L.) plants grown in a salt-affected soil. Int. J. Environ. Sci. Tech. 2006, 3, 43–51. [Google Scholar] [CrossRef]
- Qurashi, A.W.; Sabri, A.N. Bacterial exopolysaccharide and biofilm formation stimulate chickpea growth and soil aggregation under salt stress. BJM 2012, 43, 1183–1191. [Google Scholar] [CrossRef]
- Wei, W.; Li, Q.T.; Chu, Y.N.; Reiter, R.J.; Yu, X.M.; Zhu, D.H.; Zhang, W.K.; Ma, B.; Lin, Q.; Zhang, J.S.; et al. Melatonin enhances plant growth and abiotic stress tolerance in soybean plants. J. Exp. Bot. 2015, 66, 695–707. [Google Scholar] [CrossRef] [PubMed]
- Sarropoulou, V.N.; Therios, I.N.; Dimassi-Theriou, K.N. Melatonin promotes adventitious root regeneration in vitro shoot tip explants of the commercial sweet cherry rootstocks CAB-6P (Prunus cerasus L.), Gisela 6 (P. cerasus x P. canescens), and MxM 60 (P. avium x P. mahaleb). J. Pineal Res. 2012, 52, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Hardeland, R.; Manchester, L.C.; Korkmaz, A.; Ma, S.; Rosales-Corral, S.; Reiter, R.J. Functional roles of melatonin in plants, and perspectives in nutritional and agricultural science. J. Exp. Bot. 2012, 63, 577–597. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.J.; Zhang, N.; Yang, R.C.; Wang, L.; Sun, Q.Q.; Li, D.B.; Cao, Y.Y.; Weeda, S.; Zhao, B.; Ren, S.; et al. Melatonin promotes seed germination under high salinity by regulating antioxidant systems, ABA and GA4 interaction in cucumber (Cucumis sativus L.). J. Pineal Res. 2014, 57, 269–279. [Google Scholar] [CrossRef]
- Mahmood, S.; Daur, I.; Al-Solaimani, S.G.; Ahmad, S.; Madkour, M.H.; Yasir, M.; Hirt, H.; Ali, S.; Ali, Z. Plant growth promoting rhizobacteria and silicon synergistically enhance salinity tolerance of mung bean. Front. Plant Sci. 2016, 7, 876. [Google Scholar] [CrossRef]
- Li, H.; Chang, J.; Chen, H.; Wang, Z.; Gu, X.; Wei, C.; Zhang, Y.; Ma, J.; Yang, J.; Zhang, X. Exogenous melatonin confers salt stress tolerance to watermelon by improving photosynthesis and redox homeostasis. Front. Plant Sci. 2017, 8, 295. [Google Scholar] [CrossRef]
- Hahm, M.S.; Son, J.S.; Hwang, Y.J.; Kwon, D.K.; Ghim, S.Y. Alleviation of salt stress in pepper (Capsicum annum L.) plants by plant growth-promoting rhizobacteria. J. Microbiol. Biotechnol. 2017, 27, 1790–1797. [Google Scholar] [CrossRef]
- Richter, J.A.; Behr, J.H.; Erban, A.; Kopka, J.; Zörb, C. Ion-dependent metabolic responses of Vicia faba L. to salt stress. Plant Cell Environ. 2019, 42, 295–309. [Google Scholar] [CrossRef]
- Hasegawa, P.M.; Bressan, R.A.; Zhu, J.K.; Bohnert, H.J. Plant cellular and molecular responses to height salinity. Annu. Rev. Plant Physiol. 2000, 51, 463–499. [Google Scholar] [CrossRef]
- Afridi, M.S.; Mahmood, T.; Salam, A.; Mukhtar, T.; Mehmood, S.; Ali, J.; Khatoon, Z.; Bibi, M.; Javed, M.T.; Sultan, T.; et al. Induction of tolerance to salinity in wheat genotypes by plant growth promoting endophytes: Involvement of ACC deaminase and antioxidant enzymes. Plant Physiol. Biochem. 2019, 139, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.M.; Khan, A.L.; Waqas, M.; You, Y.H.; Kim, J.H.; Kim, J.G. Plant growth-promoting rhizobacteria reduce adverse effects of salinity and osmotic stress by regulating phytohormones and antioxidantsin Cucumis sativus. J. Plant Interact. 2014, 9, 673–682. [Google Scholar] [CrossRef]
- Upadhyay, S.K.; Singh, D.P.; Saikia, R. Genetic diversity of plant growth promoting rhizobacteria from rhizospheric soil of wheat under saline conditions. Curr. Microbiol. 2009, 59, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Sandhya, V.; Ali, A.S.; Grover, M.; Reddy, G.; Venkateswarlu, B. Alleviation of drought stress effects in sunflower seedlings by the exopolysaccharides producing Pseudomonas putida strain GAP-P45. Biol. Fertil. Soils 2009, 46, 17–26. [Google Scholar] [CrossRef]
- Han, Q.H.; Huang, B.; Ding, C.B.; Zhang, Z.W.; Chen, Y.E.; Hu, C.; Zhou, L.J.; Huang, Y.; Liao, J.Q.; Yuan, S.; et al. Effects of melatonin on anti-oxidative systems and photosystem II in cold-stressed rice seedlings. Front. Plant Sci. 2017, 8, 1–14. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Alamri, S.; Al-Khaishany, M.Y.; Khan, M.N.; Al-Amri, A.; Ali, H.M.; Ibrahim, A.; Alaraidh, I.A.; Alsahli, A.A. Exogenous Melatonin Counteracts NaCl-Induced damage by regulating the antioxidant system, proline and carbohydrates metabolism in tomato seedlings. Int. J. Mol. Sci. 2019, 20, 353. [Google Scholar] [CrossRef]
- XD, X.; Sun, Y.; Sun, B.; Zhang, J.; Guo, X.Q. Effects of exogenous melatonin on active oxygen metabolism of cucumber seedlings under high temperature stress. Ying Yong Sheng Tai Xue Bao 2010, 21, 1295–1300. [Google Scholar]
- Sarropoulou, V.; Dimassi-Theriou, K.; Therios, I.; Koukourikou-Petridou, M. Melatonin enhances root regeneration, photosynthetic pigments, biomass, total carbohydrates and proline content in the cherry rootstock PHL-C (Prunus avium × Prunus cerasus). Plant Physiol. Biochem. 2012, 61, 162–168. [Google Scholar] [CrossRef]
- Garg, B.K. Nutrient uptake and management under drought: Nutrient–moisture interaction. Curr. Agric. 2003, 27, 1–8. [Google Scholar]
- Gomes, M.A.C.; Pestana, I.A.; Santa-Catarina, C.; Hauser-Davis, R.A.; Suzuki, M.S. Salinity effects on photosynthetic pigments, proline, biomass and nitric oxide in Salvinia auriculata Aubl. Acta Limnol. Bras. 2017, 29, e9. [Google Scholar] [CrossRef]
- Abdallah, S.B.; Aung, B.; Amyot, L.; Lalin, I.; Lachâal, M.; Karray-Bouraoui, N. Salt stress (NaCl) affects plant growth and branch pathways of carotenoid and flavonoid biosyntheses in Solanum nigrum. Acta Physiol. Plant. 2016, 38, 1–13. [Google Scholar] [CrossRef]
- Iqbal, N.; Umar, S.; Khan, N.A. Nitrogen availability regulates proline and ethylene production and alleviates salinity stress in mustard (Brassica juncea). J. Plant Physiol. 2015, 178, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Stoeva, N.; Kaymakanova, M. Effect of salt stress on the growth and photosynthesis rate of bean plants (Phaseolus vulgaris L.). JCEA 2008, 9, 385–392. [Google Scholar]
- Parida, A.; Das, A.B.; Das, P. NaCl stress causes changes in photosynthetic pigments, proteins and other metabolic components in the leaves of a true Mangrove, Bruguiera parviflora, in hydroponics cultures. J. Plant Biol. 2002, 45, 28–36. [Google Scholar] [CrossRef]
- Maathuis, F.J.M.; Amtmann, A. K+ Nutrition and Na+ toxicity: The basis of cellular k+/Na+ ratios. Ann. Bot. 1999, 84, 123–133. [Google Scholar] [CrossRef]
- Ioneva, Z.S. Effect of potassium ion Na+ uptake by plants in conditions of chloride salinity. Fiziolo. Rasten. 1988, 14, 42–47. [Google Scholar]
- Greenway, H.; Munns, R. Mechanism of salt tolerance in halophytes. Annu. Rev. Plant Physiol. 1980, 31, 149–190. [Google Scholar] [CrossRef]
- Rojas-Tapias, D.; Moreno-Galv, A.; Pardo-Daz, S.; Obando, M.; Rivera, D.; Bonilla, R. Effect of inoculation with plant growth-promoting bacteria (PGPB) on amelioration of saline stress in maize (Zea mays). Appl. Soil Ecol. 2012, 61, 264–272. [Google Scholar] [CrossRef]
- Li, C.; Wang, P.; Wei, Z.; Liang, D.; Liu, C.; Yin, L. The mitigation effects of exogenous melatonin on salinity-induced stress in Malus hupehensis. J. Pineal Res. 2012, 53, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, E.M.; Gillespie, J.L.; Morina, J.C.; Franklin, R.B. Salinity affects microbial activity and soil organic matter content in tidal wetlands. Glob. Chang. Biol. 2014, 20, 1351–1362. [Google Scholar] [CrossRef] [PubMed]
- Yan, N.; Marschner, P.; Cao, W.; Zuo, C.; Qin, W. Influence of salinity and water content on soil microorganisms. ISWCR 2015, 3, 316–323. [Google Scholar] [CrossRef]
- Liu, T.; Zhao, F.; Liu, Z.; Zuo, Y.; Hou, J.; Wang, Y. Identification of melatonin in Trichoderma spp. and detection of melatonin content under controlled-stress growth conditions from T. asperellum. JBM 2016, 56, 838–843. [Google Scholar]
- Rodriguez-Naranjo, M.I.; Torija, M.J.; Mas, A.; Cantos-Villar, E.; Garcia-Parrilla, M.D.C. Production of melatonin by Saccharomyces strains under growth and fermentation conditions. J. Pineal Res. 2012, 53, 219–224. [Google Scholar] [CrossRef]
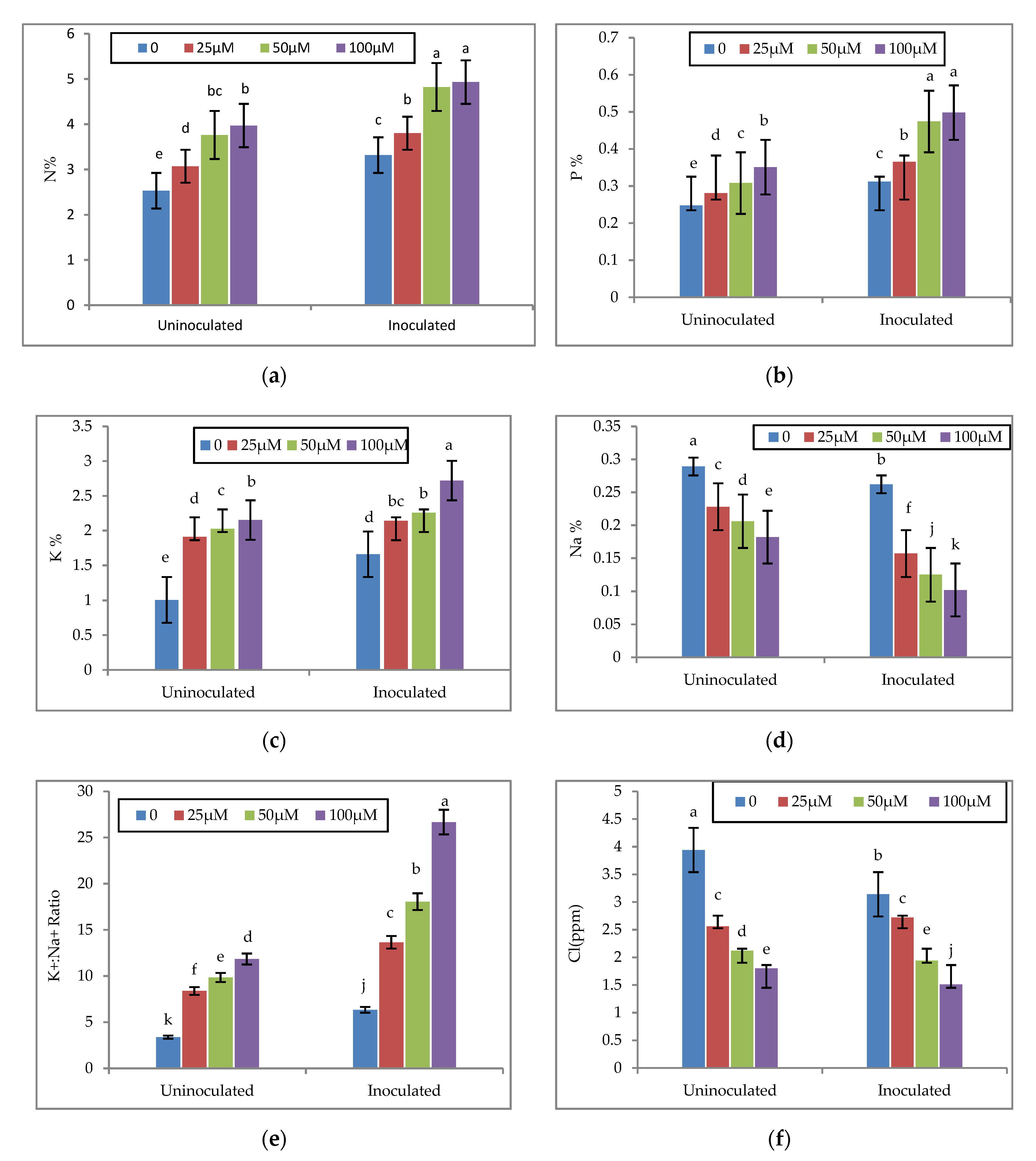
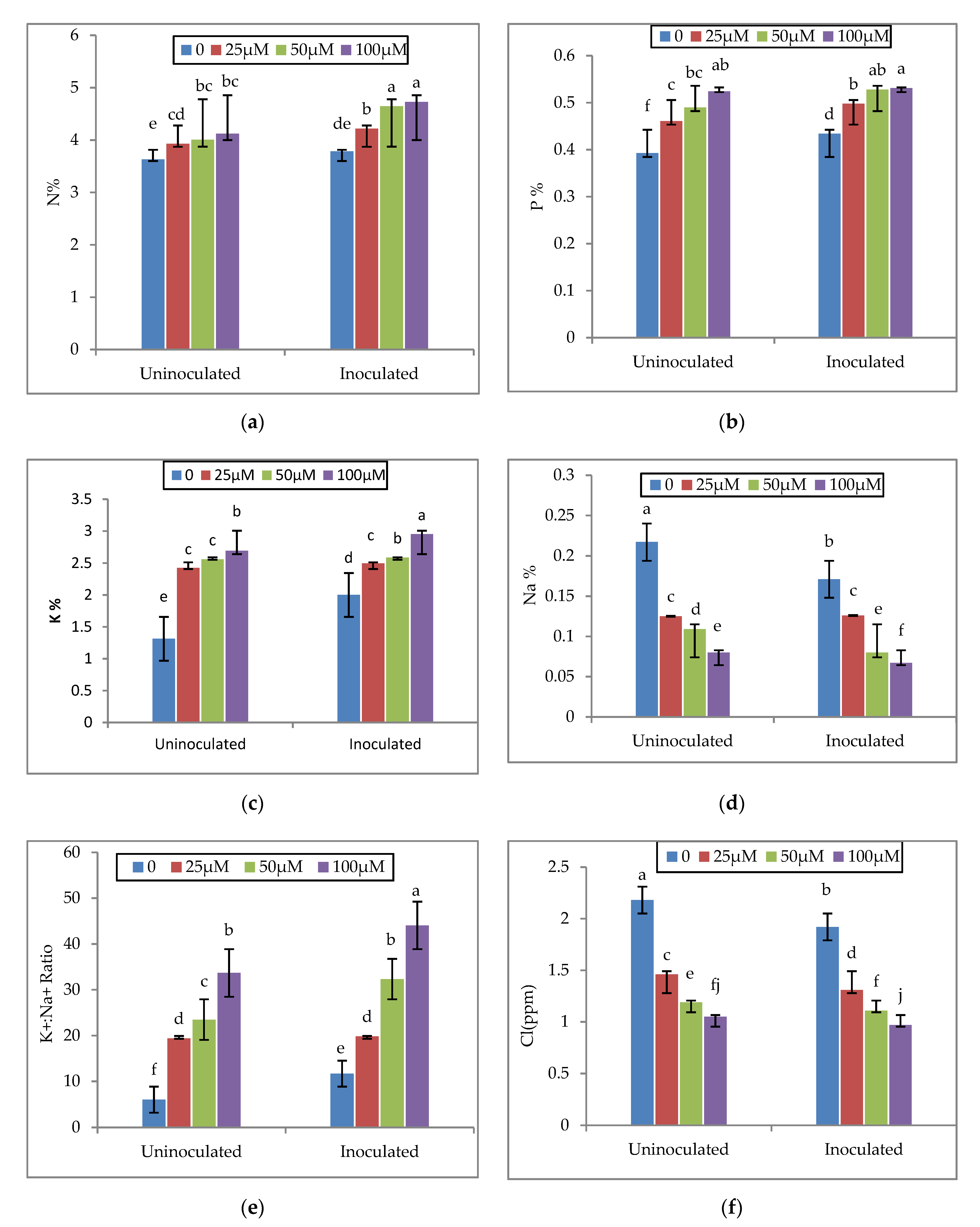
| Properties | Particle Size Distribution | Soil Texture | pH 1:2.5 | ECe dSm−1 | Soluble Cations (meq l−1) | Soluble Anions (meql−1) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sand (%) | Silt (%) | Clay (%) | Na+ | K+ | Ca++ | Mg++ | HCO3− | Cl− | SO4− | ||||
| Location 1 | 54.7 | 26.8 | 18.5 | Sandy loam | 7.76 | 6.5 | 38.5 | 5.2 | 15.4 | 6.3 | 5.9 | 37.1 | 22.4 |
| Location 2 | 28.9 | 55.5 | 15.6 | Silty loam | 7.98 | 1.47 | 6.4 | 1.5 | 5.2 | 1.8 | 1.2 | 8.5 | 5.2 |
| Treatments | Melatonin (µM) | Shoot Length (cm) | No of Leaves Plant−1 | No of Branches Plant−1 | Shoot FW (g Plant−1) | Shoot DW (g Plant−1) |
|---|---|---|---|---|---|---|
| Uninoculated | 0 | * 39.70 ± 0.6e | 12.77 ± 0.70f | 1.33 ± 0.21e | 35.85 ± 0.9e | 7.65 ± 0.06e |
| 25 | 42.00 ± 1.0e | 17.50 ± 0.29e | 1.73 ± 0.61d | 42.28 ± 0.45d | 9.46 ± 0.04d | |
| 50 | 49.20 ± 1.7cd | 19.95 ± 0.37d | 1.98 ± 0.03cd | 50.41 ± 0.66c | 10.43 ± 0.06d | |
| 100 | 56.00 ± 2.5b | 24.65 ± 0.39bc | 2.28 ± 0.41bc | 61.47 ± 0.83b | 13.65 ± 0.05b | |
| Means | ** 46.68B | 18.72B | 1.83B | 47.50B | 10.30B | |
| Inoculated | 0 | 46.70 ± 0.8d | 18.24 ± 0.15de | 1.75 ± 0.63d | 44.90 ± 0.64d | 10.37 ± 0.18d |
| 25 | 51.70 ± 1.4c | 22.88 ± 013c | 1.93 ± 0.48cd | 53.56 ± 0.52c | 11.92 ± 0.03c | |
| 50 | 67.30 ± 2.8a | 25.19 ± 0.18b | 2.60 ± 0.07b | 60.37 ± 0.16b | 12.87 ± 0.06bc | |
| 100 | 69.00 ± 1.9a | 27.82 ± 0.10a | 2.98 ± 0.40a | 70.80 ± 0.71a | 15.57 ± 0.23a | |
| Means | 58.68A | 23.54A | 2.32A | 57.41A | 12.66A |
| Treatments | Melatonin (µM) | Shoot Length (cm) | No of Leaves Plant−1 | No of Branches Plant−1 | Shoot FW (g Plant−1) | Shoot DW (g Plant−1) |
|---|---|---|---|---|---|---|
| Uninoculated | 0 | * 50.03 ± 0.99j | 19.30 ± 0.30e | 1.75 ± 0.1f | 48.42 ± 3.1e | 9.68 ± 0.2e |
| 25 | 53.10 ± 0.50f | 22.00 ± 0.50d | 2.05 ± 0.2e | 53.18 ± 6.2d | 11.72 ± 0.6d | |
| 50 | 56.23 ± 0.38e | 26.00 ± 0.50c | 2.62 ± 0.1d | 59.45 ± 8.1c | 12.72 ± 0.9d | |
| 100 | 57.83 ± 0.71e | 28.70 ± 0.80b | 3.05 ± 0.2bc | 66.80 ± 7.5b | 16.32 ± 0.8b | |
| Means | ** 54.30B | 24.00B | 2.37B | 56.96B | 12.61B | |
| Inoculated | 0 | 60.06 ± 0.78d | 24.70 ± 0.40c | 2.20 ± 0.1e | 55.16 ± 3.3d | 12.28 ± 0.3d |
| 25 | 64.50 ± 0.38c | 28.70 ± 0.60b | 2.80 ± 0.2cd | 59.90 ± 6.1c | 14.08 ± 0.6c | |
| 50 | 72.56 ± 0.67b | 30.00 ± 0.80b | 3.10 ± 0.1b | 64.37 ± 6.8b | 15.24 ± 0.7bc | |
| 100 | 74.76 ± 0.57a | 34.00 ± 0.50a | 3.40 ± 0.2a | 73.71 ± 8.4a | 18.08 ± 0.6a | |
| Means | 67.97A | 29.35A | 2.88A | 63.29A | 14.92A |
| Treatments | Melatonin (µM) | Average 100-Seed Weight (g) | Seed Weight (g Plant−1) | Seed Yield (Ton ha−1) |
|---|---|---|---|---|
| Uninoculated | 0 | * 41.6 ± 0.05j | 26.19 ± 1.9e | 2.20 ± 0.03j |
| 25 | 54.63 ± 0.05e | 34.80 ± 1.3d | 2.69 ± 0.05f | |
| 50 | 60.63 ± 0.06d | 39.20 ± 1.7c | 3.10 ± 0.06e | |
| 100 | 62.97 ± 0.07c | 45.10 ± 2.3b | 3.47 ± 0.03cd | |
| Means | ** 54.96B | 36.32B | 2.87B | |
| Inoculated | 0 | 48.69 ± 0.06f | 35.17 ± 1.5d | 3.26 ± 0.06de |
| 25 | 73.83 ± 0.07b | 40.70 ± 1.2c | 3.75 ± 0.05bc | |
| 50 | 77.73 ± 0.07a | 46.60 ± 1.3b | 4.01 ± 0.06b | |
| 100 | 78.30 ± 0.04a | 50.62 ± 1.5a | 4.30 ± 0.07a | |
| Means | 69.64A | 43.27A | 3.83A |
| Treatments | Melatonin (µM) | Average 100-Seed Weight (g) | Seed Weight (g Plant−1) | Seed Yield (Ton ha−1) |
|---|---|---|---|---|
| Uninoculated | 0 | * 53.43 ± 0.5j | 33.49 ± 1.5f | 3.70 ± 0.05f |
| 25 | 68.53 ± 0.5e | 42.21 ± 1.9e | 4.37 ± 0.05e | |
| 50 | 76.37 ± 0.7d | 50.07±1.4d | 4.77 ± 0.06c | |
| 100 | 81.27 ± 0.6c | 55.57 ± 1.3c | 4.87 ± 0.05c | |
| Means | ** 69.90B | 45.30B | 4.31B | |
| Inoculated | 0 | 64.37 ± 0.04f | 41.70 ± 1.7e | 4.32 ± 0.06e |
| 25 | 78.87 ± 0.05c | 55.42 ± 1.5c | 4.57 ± 0.09d | |
| 50 | 83.73 ± 0.06b | 57.70 ± 2.3b | 5.10 ± 0.08b | |
| 100 | 88.73 ± 0.05a | 59.15 ± 1.4a | 5.38 ± 0.09a | |
| Means | 78.925A | 53.49A | 4.84A |
| Treatments | Melatonin (µM) | Photosynthetic Pigments (mg g−1 FW) | RWC (%) | Proline (mg g−1 FW) | ||
|---|---|---|---|---|---|---|
| Chlorophyll a | Chlorophyll b | Carotenoid | ||||
| Uninoculated | 0 | * 0.809 ± 0.430e | 0.283 ± 0.025j | 0.171 ± 0.014k | 45.32 ± 0.19j | 0.251 ± 0.07f |
| 25 | 0.936 ± 0.025d | 0.315 ± 0.020e | 0.187 ± 0.015j | 55.79 ± 0.15f | 0.293 ± 0.06ef | |
| 50 | 1.049 ± 0.025cd | 0.356 ± 0.024d | 0.202 ± 0.014f | 60.37 ± 0.19d | 0.327 ± 0.06de | |
| 100 | 1.162 ± 0.028bc | 0.509 ± 0.008b | 0.230 ± 0.021d | 65.79 ± 0.23c | 0.348 ± 0.07cd | |
| Means | ** 0.989B | 0.366B | 0.198B | 56.82B | 0.305B | |
| Inoculated | 0 | 1.056 ± 0.023cd | 0.383 ± 0.022d | 0.219 ± 0.022e | 58.00 ± 0.17e | 0.362 ± 0.07cd |
| 25 | 1.147 ± 0.017bc | 0.451 ± 0.026c | 0.245 ± 0.020c | 65.00 ± 0.23c | 0.395 ± 0.07c | |
| 50 | 1.258 ± 0.066b | 0.585 ± 0.023a | 0.284 ± 0.018b | 70.23 ± 0.18b | 0.474 ± 0.08b | |
| 100 | 1.385 ± 0.020a | 0.616 ± 0.022a | 0.292 ± 0.015a | 75.80 ± 0.24a | 0.526 ± 0.10a | |
| Means | 1.212A | 0.509A | 0.260A | 67.26A | 0.439A | |
| Treatments | Melatonin (µM) | Photosynthetic Pigments (mg g−1 FW) | RWC (%) | Proline (mg g−1 FW) | ||
|---|---|---|---|---|---|---|
| Chlorophyll a | Chlorophyll b | Carotenoid | ||||
| Uninoculated | 0 | * 1.155 ± 0.014f | 0.405 ± 0.014e | 0.235 ± 0.007e | 59.35 ± 0.94f | 0.110 ± 0.01d |
| 25 | 1.253 ± 0.046e | 0.445 ± 0.006d | 0.245 ± 0.014e | 67.75 ± 0.44e | 0.115 ± 0.07d | |
| 50 | 1.286 ± 0.020e | 0.503 ± 0.006c | 0.284 ± 0.023d | 70.76 ± 0.48d | 0.122 ± 0.05d | |
| 100 | 1.340 ± 0.009d | 0.544 ± 0.020b | 0.292 ± 0.031cd | 74.77 ± 0.45c | 0.159 ± 0.07c | |
| Means | ** 1.259B | 0.474B | 0.264B | 68.160B | 0.127B | |
| Inoculated | 0 | 1.345 ± 0.028d | 0.504 ± 0.029c | 0.284 ± 0.029d | 66.6 ± 0.12e | 0.150 ± 0.08c |
| 25 | 1.418 ± 0.051c | 0.525 ± 0.023bc | 0.305 ± 0.040bc | 74.01 ± 0.12c | 0.213 ± 0.06b | |
| 50 | 1.456 ± 0.014b | 0.574 ± 0.009a | 0.312 ± 0.011b | 80.82 ± 0.039b | 0.231 ± 0.09b | |
| 100 | 1.499 ± 0.028a | 0.603 ± 0.011a | 0.329 ± 0.008a | 84.46 ± 0.31a | 0.263 ± 0.07a | |
| Means | 1.430A | 0.552A | 0.308A | 76.47A | 0.214A | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd El-Ghany, M.F.; Attia, M. Effect of Exopolysaccharide-Producing Bacteria and Melatonin on Faba Bean Production in Saline and Non-Saline Soil. Agronomy 2020, 10, 316. https://doi.org/10.3390/agronomy10030316
Abd El-Ghany MF, Attia M. Effect of Exopolysaccharide-Producing Bacteria and Melatonin on Faba Bean Production in Saline and Non-Saline Soil. Agronomy. 2020; 10(3):316. https://doi.org/10.3390/agronomy10030316
Chicago/Turabian StyleAbd El-Ghany, Mona F., and Magdy Attia. 2020. "Effect of Exopolysaccharide-Producing Bacteria and Melatonin on Faba Bean Production in Saline and Non-Saline Soil" Agronomy 10, no. 3: 316. https://doi.org/10.3390/agronomy10030316
APA StyleAbd El-Ghany, M. F., & Attia, M. (2020). Effect of Exopolysaccharide-Producing Bacteria and Melatonin on Faba Bean Production in Saline and Non-Saline Soil. Agronomy, 10(3), 316. https://doi.org/10.3390/agronomy10030316





