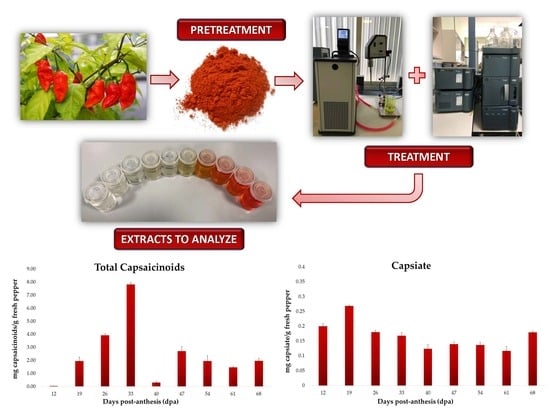
Influence of Fruit Ripening on the Total and Individual Capsaicinoids and Capsiate Content in Naga Jolokia Peppers (Capsicum chinense Jacq.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material
2.3. Ultrasound-Assisted Extraction of Capsaicinoids and Capsiate
2.4. Capsaicinoids and Capsiate Identification
2.5. Capsaicinoids and Capsiate Analysis
2.6. Statistical Analysis
3. Results and Discussion
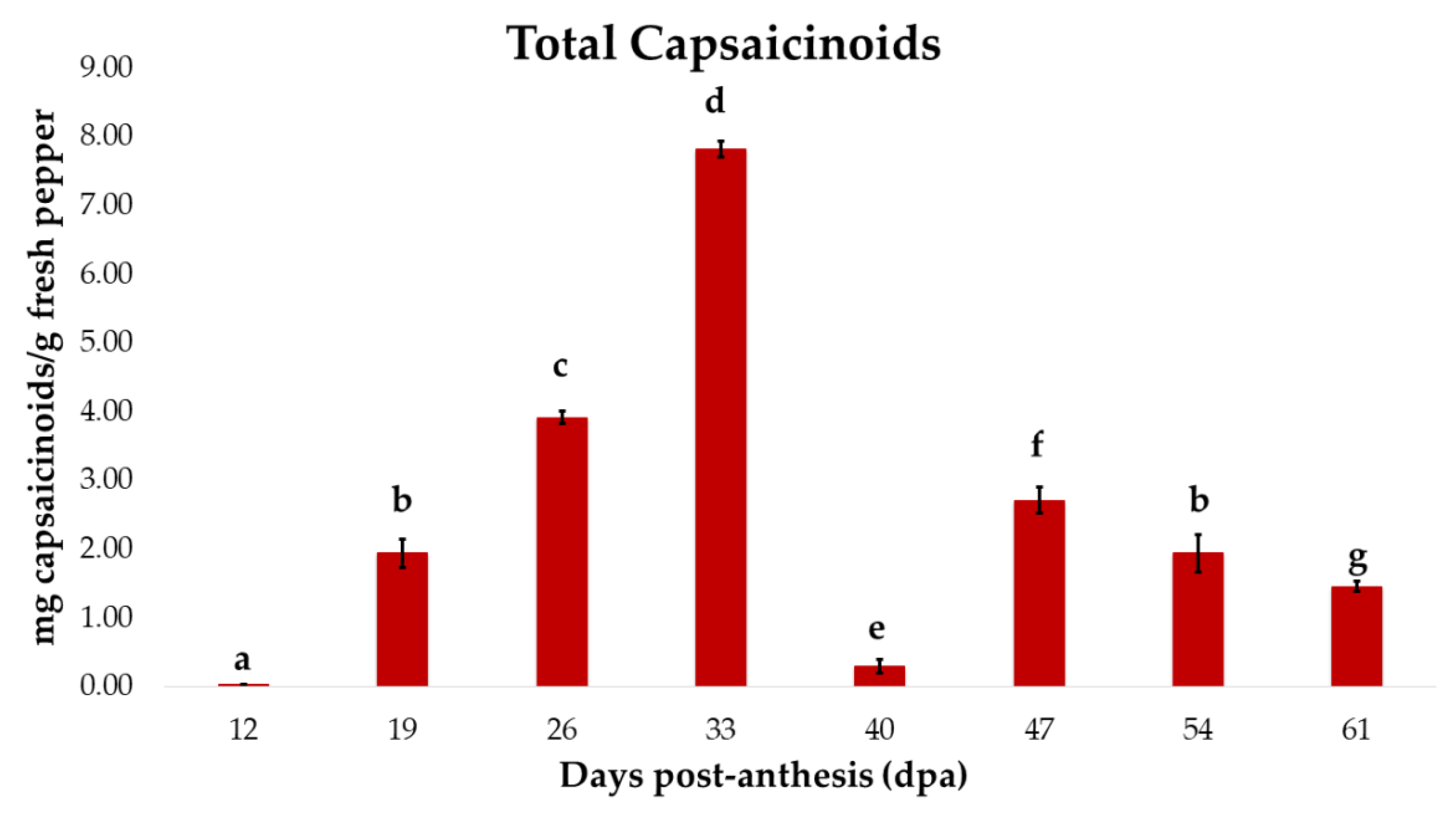
3.1. Evolution of the Total Capsaicinoid Content
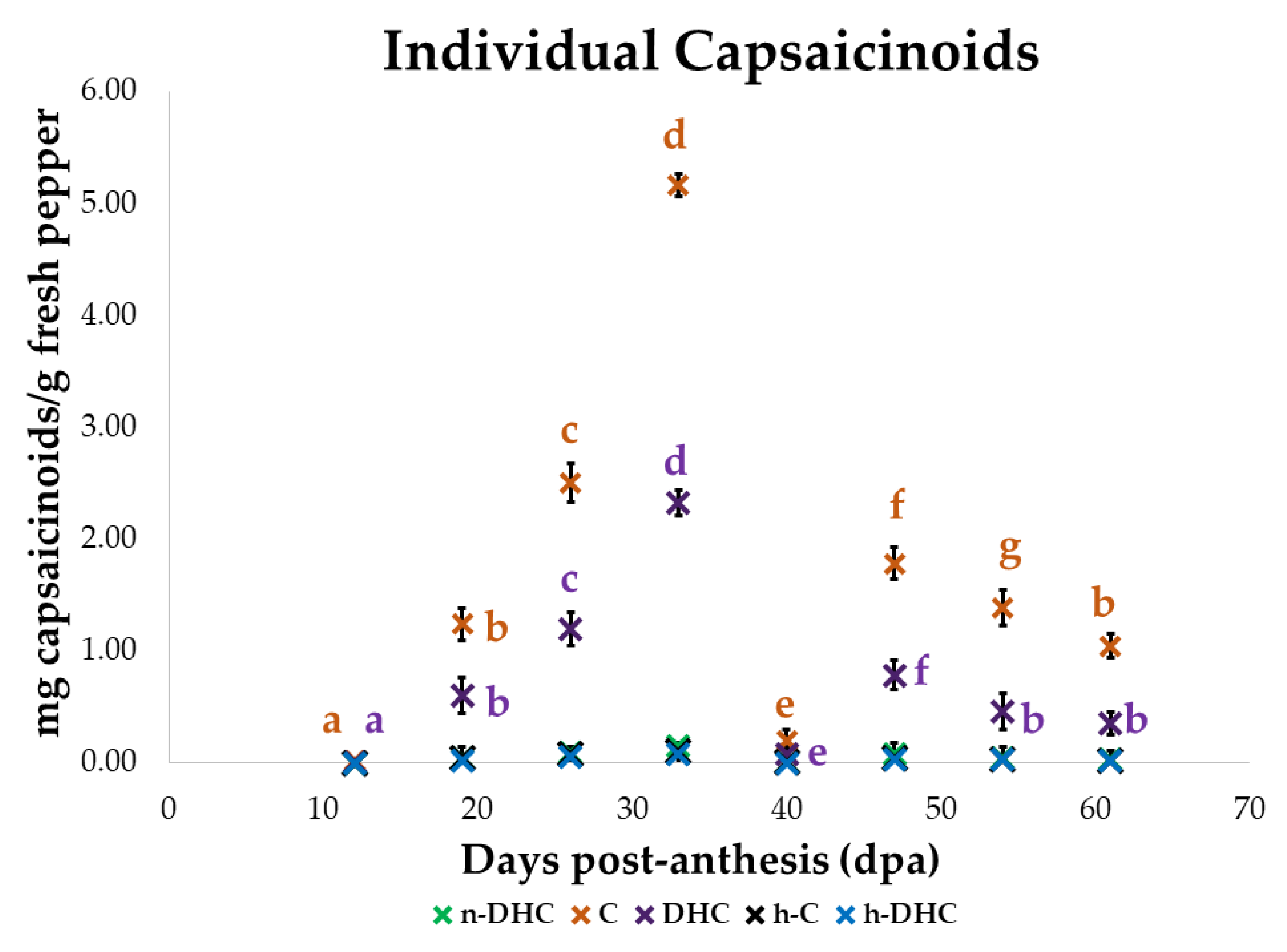
3.2. Evolution of the Individual Content of Capsaicinoids
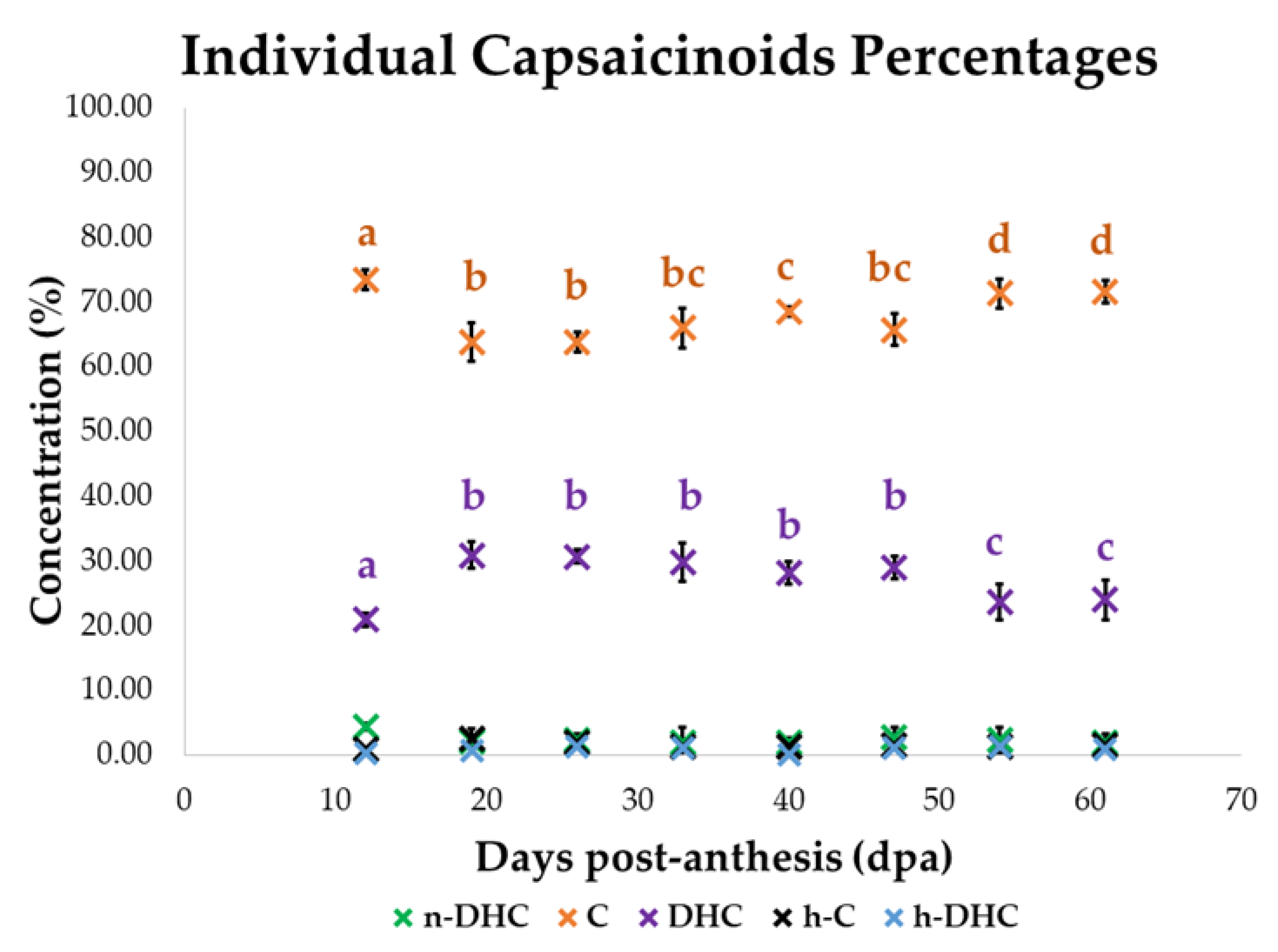
3.3. Evolution of the Standardized Values of Capsaicinoids
3.4. Evolution of Capsiate Content
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| C | Capsaicin |
| CITA | Agri-Food Research and Technology Center |
| CTE | Capsiate |
| DHC | Dihydrocapsaicin |
| DHCTE | Dihydrocapsiate |
| Dpa | Days postanthesis |
| FW | Fresh weight |
| h-C | Homocapsaicin |
| h-DHC | Homodihydrocapsaicin |
| n-DHC | Nordihydrocapsaicin |
| n-DHCTE | Nordihydrocapsiate |
| PDA | Photodiode array detector |
| QToF-MS | Quadrupole-time-of-flight mass spectrometry |
| rp-HPLC | Reverse-phase high-performance liquid chromatography |
| rp-UHPLC: | Reverse-phase ultra-high-performance liquid chromatography |
| SHUs | Scoville Heat Units |
| UAE | Ultrasound-assisted extraction |
References
- Chiaiese, P.; Corrado, G.; Minutolo, M.; Barone, A.; Errico, A. Transcriptional regulation of ascorbic acid during fruit ripening in pepper (Capsicum annuum) varieties with low and high antioxidants content. Plants 2019, 8, 206. [Google Scholar] [CrossRef] [PubMed]
- Ananthan, R.; Subhash, K.; Longvah, T. Capsaicinoids, amino acid and fatty acid profiles in different fruit components of the world hottest Naga king chilli (Capsicum chinense Jacq). Food Chem. 2018, 238, 51–57. [Google Scholar] [CrossRef]
- Penagos-Calvete, D.; Guauque-Medina, J.; Villegas-Torres, M.F.; Montoya, G. Analysis of triacylglycerides, carotenoids and capsaicinoids as disposable molecules from Capsicum agroindustry. Hortic. Environ. Biotechnol. 2019, 60, 227–238. [Google Scholar] [CrossRef]
- Dias, A.L.B.; Arroio Sergio, C.S.; Santos, P.; Barbero, G.F.; Rezende, C.A.; Martínez, J. Ultrasound-assisted extraction of bioactive compounds from dedo de moça pepper (Capsicum baccatum L.): Effects on the vegetable matrix and mathematical modeling. J. Food Eng. 2017, 198, 36–44. [Google Scholar] [CrossRef]
- Barbero, G.F.; Liazid, A.; Azaroual, L.; Palma, M.; Barroso, C.G. Capsaicinoid contents in peppers and pepper-related spicy foods. Int. J. Food Prop. 2016, 19, 485–493. [Google Scholar] [CrossRef]
- De Aguiar, A.C.; Sales Silva, L.P.; de Rezende, C.A.; Barbero, G.F.; Martínez, J. Encapsulation of pepper oleoresin by supercritical fluid extraction of emulsions. J. Supercrit. Fluids 2016, 112, 37–43. [Google Scholar] [CrossRef]
- Hervert-Hernández, D.; Sáyago-Ayerdi, S.G.; Goñi, I. Bioactive compounds of four hot pepper varieties (Capsicum annuum L.), antioxidant capacity, and intestinal bioaccessibility. J. Agric. Food Chem. 2010, 58, 3399–3406. [Google Scholar] [CrossRef]
- Thán, M.; Németh, J.; Szilvássy, Z.; Pintér, E.; Helyes, Z.; Szolcsányi, J. Systemic anti-inflammatory effect of somatostatin released from capsaicin-sensitive vagal and sciatic sensory fibres of the rat and guinea-pig. Eur. J. Pharmacol. 2000, 399, 251–258. [Google Scholar] [CrossRef]
- Smith, H.; Brooks, J.R. Capsaicin-based therapies for pain control. Prog. Drug Res. 2014, 68, 129–146. [Google Scholar]
- Molina-Torres, J.; Garcia-Chavez, A.; Ramirez-Chavez, E. Antimicrobial properties of alkamides present in flavouring plants traditionally used in Mesoamerica: Affinin and capsaicin. J. Ethnopharmacol. 1999, 64, 241–248. [Google Scholar] [CrossRef]
- Bley, K.; Boorman, G.; Mohammad, B.; McKenzie, D.; Babbar, S. A comprehensive review of the carcinogenic and anticarcinogenic potential of capsaicin. Toxicol. Pathol. 2012, 40, 847–873. [Google Scholar] [CrossRef] [PubMed]
- Joo, J.I.; Kim, D.H.; Choi, J.W.; Yun, J.W. Proteomic analysis for antiobesity potential of capsaicin on white adipose tissue in rats fed with a high fat diet. J. Proteome Res. 2010, 9, 2977–2987. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.J.; Peng, J.; Li, Y.J. Recent advances in the study on capsaicinoids and capsinoids. Eur. J. Pharmacol. 2011, 650, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yazawa, S.; Suetome, N.; Okamoto, K.; Namiki, T. Content of capsaicinoids and capsaicinoid-like substances in fruit of pepper (Capsicum annuum L.) hybrids made with ‘CH-19 Sweet’ as a parent. J. Jpn. Soc. Hortic. Sci. 1989, 58, 601–607. [Google Scholar] [CrossRef]
- Fayos, O.; Savirón, M.; Orduna, J.; Barbero, G.F.; Mallor, C.; Garcés-Claver, A. Quantitation of capsiate and dihydrocapsiate and tentative identification of minor capsinoids in pepper fruits (Capsicum spp.) by HPLC-ESI-MS/MS (QTOF). Food Chem. 2019, 270, 264–272. [Google Scholar] [CrossRef]
- Swint, J.M.; Beining, K.M.; Bryant, J.A.; Tucker, R.M.; Ludy, M.J. Comparison of Capsaicin and Capsiate’s Effects at a Meal. Chemosens. Percept. 2015, 8, 174–182. [Google Scholar] [CrossRef]
- Sutoh, K.; Kobata, K.; Yazawa, S.; Watanabe, T. Capsinoid is biosynthesized from phenylalanine and valine in a non-pungent pepper, Capsicum annuum L. cv. CH-19 Sweet. Biosci. Biotechnol. Biochem. 2006, 70, 1513–1516. [Google Scholar] [CrossRef]
- Islam, M.A.; Sharma, S.S.; Sinha, P.; Negi, M.S.; Neog, B.; Tripathi, S.B. Variability in capsaicinoid content in different landraces of Capsicum cultivated in north-eastern India. Sci. Hortic. 2015, 183, 66–71. [Google Scholar] [CrossRef]
- Barbero, G.F.; Molinillo, J.M.G.; Varela, R.M.; Palma, M.; Macías, F.A.; Barroso, C.G. Application of Hansch’s Model to capsaicinoids and capsinoids: A study using the quantitative structure-Activity relationship. A novel method for the synthesis of capsinoids. J. Agric. Food Chem. 2010, 58, 3342–3349. [Google Scholar] [CrossRef]
- Barbero, G.F.; Molinillo, J.M.G.; Varela, R.M.; Palma, M.; Macías, F.A.; Barroso, C.G. Method for the Chemical Synthesis of Capsinoids. U.S. Patent 20100256413A1, 2010. [Google Scholar]
- De Aguiar, A.C.; Coutinho, J.P.; Barbero, G.F.; Godoy, H.T.; Martínez, J. Comparative study of capsaicinoid composition in Capsicum Peppers grown in Brazil. Int. J. Food Prop. 2016, 19, 1292–1302. [Google Scholar] [CrossRef]
- Giuffrida, D.; Dugo, P.; Torre, G.; Bignardi, C.; Cavazza, A.; Corradini, C.; Dugo, G. Characterization of 12 Capsicum varieties by evaluation of their carotenoid profile and pungency determination. Food Chem. 2013, 140, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Jeeatid, N.; Techawongstien, S.; Suriharn, B.; Chanthai, S.; Bosland, P.W.; Techawongstien, S. Influence of water stresses on capsaicinoid production in hot pepper (Capsicum chinense Jacq.) cultivars with different pungency levels. Food Chem. 2018, 245, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Barbero, G.F.; Ruiz, A.G.; Liazid, A.; Palma, M.; Vera, J.C.; Barroso, C.G. Evolution of total and individual capsaicinoids in peppers during ripening of the Cayenne pepper plant (Capsicum annuum L.). Food Chem. 2014, 153, 200–206. [Google Scholar] [CrossRef]
- Contreras-Padilla, M.; Yahia, E.M. Changes in capsaicinoids during development, maturation, and senescence of Chile Peppers and relation with peroxidase activity. J. Agric. Food Chem. 1998, 46, 2075–2079. [Google Scholar] [CrossRef]
- Barbero, G.F.; de Aguiar, A.C.; Carrera, C.; Olachea, Á.; Ferreiro-González, M.; Martínez, J.; Palma, M.; Barroso, C.G. Evolution of capsaicinoids in Peter Pepper (Capsicum annuum var. annuum) during fruit ripening. Chem. Biodiver. 2016, 13, 1068–1075. [Google Scholar] [CrossRef]
- Fayos, O.; de Aguiar, A.C.; Jiménez-Cantizano, A.; Ferreiro-González, M.; Garcés-Claver, A.; Martínez, J.; Mallor, C.; Ruiz-Rodríguez, A.; Palma, M.; Barroso, C.G.; et al. Ontogenetic variation of individual and total capsaicinoids in Malagueta peppers (Capsicum frutescens) during fruit maturation. Molecules 2017, 22, 736. [Google Scholar] [CrossRef]
- Boersch, A.; Callingham, B.A.; Lembeck, F.; Sharman, D.F. Enzymic oxidation of capsaicin. Biochem. Pharmacol. 1991, 41, 1863–1869. [Google Scholar] [CrossRef]
- Bernal, M.A.; Calderón, A.A.; Pedreño, M.A.; Muñoz, R.; Barceló, A.R.; de Cáceres, F.M. Capsaicin oxidation by peroxidase from Capsicum annuum (Var. annuum) fruits. J. Agric. Food Chem. 1993, 41, 1041–1044. [Google Scholar] [CrossRef]
- Lema, A.; Martinez, T.; Garcés, A.; Fayos, O.; Pomar, F.; González, S.; Silvar, C.; Mallor, C.; Barbero, G.F. Study of the oxidation of capsicinoids by basic peroxidases from pepper. In Proceedings of the XIV Spanish-Portuguese Congress of Plant Physiology, SEFV, Toledo, Spain, 14–17 June 2015; p. 188, ISBN 978-84-606-8883-9. [Google Scholar]
- Moirangthem, S.S.; Gogoi, S.; Thongbam, P.D.; Ramya, K.T.; Fiyaz, R.A.; Pandey, D.S. Effect of sowing time and crop geometry on the Casaicinoid content in Bhoot Jolokia (Capsicum chinense Jacq.). J. Sci. Technol. 2014, 51, 1974–1981. [Google Scholar] [CrossRef][Green Version]
- Meghvansi, M.K.; Siddiqui, S.; Khan, M.H.; Gupta, V.K.; Vairale, M.G.; Gogoi, H.K.; Singh, L. Naga chilli: A potential source of capsaicinoids with broad-spectrum ethnopharmacological applications. J. Ethnopharmacol. 2010, 132, 1–14. [Google Scholar] [CrossRef]
- Purkayastha, J.; Alam, S.I.; Gogoi, H.K.; Singh, L.; Veer, V. Molecular characterization of ‘Bhut Jolokia’ the hottest chilli. J. Biosci. 2012, 37, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.L.B.; Arroio Sergio, C.S.; Santos, P.; Barbero, G.F.; Rezende, C.A.; Martínez, J. Effect of ultrasound on the supercritical CO2 extraction of bioactive compounds from dedo de moça pepper (Capsicum baccatum. Var. pendulum). Ultrason. Sonochem. 2016, 31, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, J.P.; Barbero, G.F.; Avellán, O.F.; Garcés-Claver, A.; Godoy, H.T.; Palma, M.; Barroso, C.G. Use of multivariate statistical techniques to optimize the separation of 17 capsinoids by ultra-performance liquid chromatography using different columns. Talanta 2015, 134, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, J.P.; Barbero, G.F.; Godoy, H.T.; Palma, M.; Barroso, C.G. Multivariate optimization by statistical methods of ultra-high-performance liquid chromatography conditions for the separation of 17 capsaicinoids. Anal. Methods 2016, 8, 1659–1666. [Google Scholar] [CrossRef]
- Barbero, G.F.; Liazid, A.; Palma, M.; Barroso, C.G. Ultrasound-assisted extraction of capsaicinoids from peppers. Talanta 2008, 75, 1332–1337. [Google Scholar] [CrossRef]
- Vázquez-Espinosa, M.; González-de-Peredo, A.V.; Ferreiro-González, M.; Barroso, C.G.; Palma, M.; Barbero, G.F.; Espada-Bellido, E. Optimizing and comparing ultrasound- and microwave-assisted extraction methods applied to the extraction of antioxidant capsinoids in peppers. Agronomy 2019, 9, 633. [Google Scholar] [CrossRef]
- Iwai, K.; Suzuki, T.; Fujiwake, H. Formation and accumulation of pungent principle of hot pepper fruits, capsaicin and its analogues, in Capsicum annuun var. annuun cv. Karayatsubusa at different growth stages after flowering. Agric. Biol. Chem. 1979, 43, 2493–2498. [Google Scholar] [CrossRef]
- Menichini, F.; Tundis, R.; Bonesi, M.; Loizzo, M.R.; Conforti, F.; Statti, G.; de Cindio, B.; Houghton, P.J.; Menichini, F. The influence of fruit ripening on the phytochemical content and biological activity of Capsicum chinense Jacq. cv Habanero. Food Chem. 2009, 114, 553–560. [Google Scholar] [CrossRef]
- Pino, J.; González, M.; Ceballos, L.; Centurión-Yah, A.R.; Trujillo-Aguirre, J.; Latournerie-Moreno, L.; Sauri-Duch, E. Characterization of total capsaicinoids, colour and volatile compounds of Habanero chilli pepper (Capsicum chinense Jack.) cultivars grown in Yucatan. Food Chem. 2007, 104, 1682–1686. [Google Scholar] [CrossRef]
- Bosland, P.W.; Baral, J.B. ‘Bhut Jolokia’-The world’s hottest known chile pepper is a putative naturally occurring interspecific hybrid. HortScience 2007, 42, 222–224. [Google Scholar] [CrossRef]
- Díaz, J.; Pomar, F.; Bernal, Á.; Merino, F. Peroxidases and the metabolism of capsaicin in Capsicum annuum L. Phytochem. Rev. 2004, 3, 141–157. [Google Scholar] [CrossRef]
- Estrada, B.; Bernal, M.A.; Díaz, J.; Pomar, F.; Merino, F. Fruit development in Capsicum annuum: Changes in capsaicin, lignin, free phenolics, and peroxidase patterns. J. Agric. Food Chem. 2000, 48, 6234–6239. [Google Scholar] [CrossRef] [PubMed]
- Olguín-Rojas, J.A.; Fayos, O.; Vázquez-León, L.A.; Ferreiro-González, M.; Rodríguez-Jimenes, G.C.; Palma, M.; Garcés-Claver, A.; Barbero, G.F. Progression of the total and individual capsaicinoids content in the fruits of three different cultivars of capsicum chinense Jacq. Agronomy 2019, 9, 141. [Google Scholar] [CrossRef]
- Sganzerla, M.; Coutinho, J.P.; de Melo, A.M.T.; Godoy, H.T. Fast method for capsaicinoids analysis from Capsicum chinense fruits. Food Res. Int. 2014, 64, 718–725. [Google Scholar] [CrossRef]
- Lavorgna, M.; Orlo, E.; Nugnes, R.; Piscitelli, C.; Russo, C.; Isidori, M. Capsaicin in hot chili peppers: In vitro evaluation of its antiradical, antiproliferative and apoptotic activities. Plant Foods Hum. Nutr. 2019, 74, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Harvell, K.P.; Bosland, P.W. The environment produces a significant effect on pungency of chiles. HortScience 1997, 32, 1292. [Google Scholar] [CrossRef]
- Fayos, O.; Ochoa-Alejo, N.; de la Vega, O.M.; Savirón, M.; Orduna, J.; Mallor, C.; Barbero, G.F.; Garcés-Claver, A. Assessment of capsaicinoid and capsinoid accumulation patterns during fruit development in three chili pepper genotypes (Capsicum spp.) carrying pun1 and pAMT alleles related to pungency. J. Agric. Food Chem. 2019, 67, 12219–12227. [Google Scholar] [CrossRef] [PubMed]
- Lema, A.; Martínez-Cortés, T.; Garcés, A.; Mallor, C.; Fayos, O.; Barbero, G.F.; Silvar, C.; Pomar, F. 5-5’ dicapsiate: Product of the oxidation of capsiate by cationic peroxidases from pepper (Capsicum annuum L.). In Proceedings of the XVIth EUCARPIA Capsicum and Eggplant Working Group Meeting in memoriam Dr. Alain Palloix, Kecskemet, Hungary, 12–14 September 2016; pp. 500–505, ISBN 978-615-5270-27-7. [Google Scholar] [CrossRef]
- Tanaka, Y.; Hosokawa, M.; Otsu, K.; Watanabe, T.; Yazawa, S. Assessment of capsiconinoid composition, nonpungent capsaicinoid analogues, in Capsicum cultivars. J. Agric. Food Chem. 2009, 57, 5407–5412. [Google Scholar] [CrossRef] [PubMed]
- Sutoh, K.; Kobata, K.; Watanabe, T. Stability of capsinoid in various solvents. J. Agric. Food Chem. 2001, 49, 4026–4030. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Yoneda, H.; Hosokawa, M.; Miwa, T.; Yazawa, S. Application of marker-assisted selection in breeding of a new fresh pepper cultivar (Capsicum annuum) containing capsinoids, low-pungent capsaicinoid analogs. Sci. Hortic. 2014, 165, 242–245. [Google Scholar] [CrossRef]
- Jang, S.; Han, K.; Jo, Y.D.; Jeong, H.-J.; Siddiqui, M.I.; Kang, B.-C. Substitution of a dysfunctional pAMT allele results in low-pungency but high levels of capsinoid in Capsicum chinense ‘Habanero’. Plant Breed. Biotechnol. 2015, 3, 119–128. [Google Scholar] [CrossRef]
- Jarret, R.L.; Bolton, J.; Perkins, L.B. 509-45-1, a Capsicum annuum pepper germplasm containing high concentrations of capsinoids. HortScience 2014, 49, 107–108. [Google Scholar] [CrossRef]

| Code | Fruit Sprouting | Dpa | Visual State |
|---|---|---|---|
| M-1 | 18/09 | 12 | Green color |
| M-2 | 11/09 | 19 | Green color |
| M-3 | 04/09 | 26 | Green color |
| M-4 | 28/08 | 33 | Green color |
| M-5 | 21/08 | 40 | Yellow color |
| M-6 | 14/08 | 47 | Orange color |
| M-7 | 07/08 | 54 | Red color |
| M-8 | 31/07 | 61 | Red color |
| M-9 | 24/07 | 68 | Red color/over-ripeness |
| Dpa | n-DHC | C | DHC | h-C | h-DHC |
|---|---|---|---|---|---|
| 12 | 1.03 | 0.48 | 0.31 | 0.29 | 0.12 |
| 19 | 27.59 | 24.04 | 25.83 | 46.03 | 16.88 |
| 26 | 62.01 | 48.44 | 51.38 | 71.82 | 59.82 |
| 33 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| 40 | 3.65 | 3.90 | 3.56 | 3.83 | 0.00 |
| 47 | 50.96 | 34.55 | 33.71 | 37.83 | 37.97 |
| 54 | 30.34 | 26.89 | 19.79 | 25.32 | 31.55 |
| 61 | 19.46 | 20.25 | 15.02 | 20.22 | 16.92 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vázquez-Espinosa, M.; Olguín-Rojas, J.A.; Fayos, O.; V. González-de-Peredo, A.; Espada-Bellido, E.; Ferreiro-González, M.; G. Barroso, C.; F. Barbero, G.; Garcés-Claver, A.; Palma, M. Influence of Fruit Ripening on the Total and Individual Capsaicinoids and Capsiate Content in Naga Jolokia Peppers (Capsicum chinense Jacq.). Agronomy 2020, 10, 252. https://doi.org/10.3390/agronomy10020252
Vázquez-Espinosa M, Olguín-Rojas JA, Fayos O, V. González-de-Peredo A, Espada-Bellido E, Ferreiro-González M, G. Barroso C, F. Barbero G, Garcés-Claver A, Palma M. Influence of Fruit Ripening on the Total and Individual Capsaicinoids and Capsiate Content in Naga Jolokia Peppers (Capsicum chinense Jacq.). Agronomy. 2020; 10(2):252. https://doi.org/10.3390/agronomy10020252
Chicago/Turabian StyleVázquez-Espinosa, Mercedes, José Arturo Olguín-Rojas, Oreto Fayos, Ana V. González-de-Peredo, Estrella Espada-Bellido, Marta Ferreiro-González, Carmelo G. Barroso, Gerardo F. Barbero, Ana Garcés-Claver, and Miguel Palma. 2020. "Influence of Fruit Ripening on the Total and Individual Capsaicinoids and Capsiate Content in Naga Jolokia Peppers (Capsicum chinense Jacq.)" Agronomy 10, no. 2: 252. https://doi.org/10.3390/agronomy10020252
APA StyleVázquez-Espinosa, M., Olguín-Rojas, J. A., Fayos, O., V. González-de-Peredo, A., Espada-Bellido, E., Ferreiro-González, M., G. Barroso, C., F. Barbero, G., Garcés-Claver, A., & Palma, M. (2020). Influence of Fruit Ripening on the Total and Individual Capsaicinoids and Capsiate Content in Naga Jolokia Peppers (Capsicum chinense Jacq.). Agronomy, 10(2), 252. https://doi.org/10.3390/agronomy10020252