Biostimulants Application Alleviates Water Stress Effects on Yield and Chemical Composition of Greenhouse Green Bean (Phaseolus vulgaris L.)
Abstract
1. Introduction
2. Materials and Methods
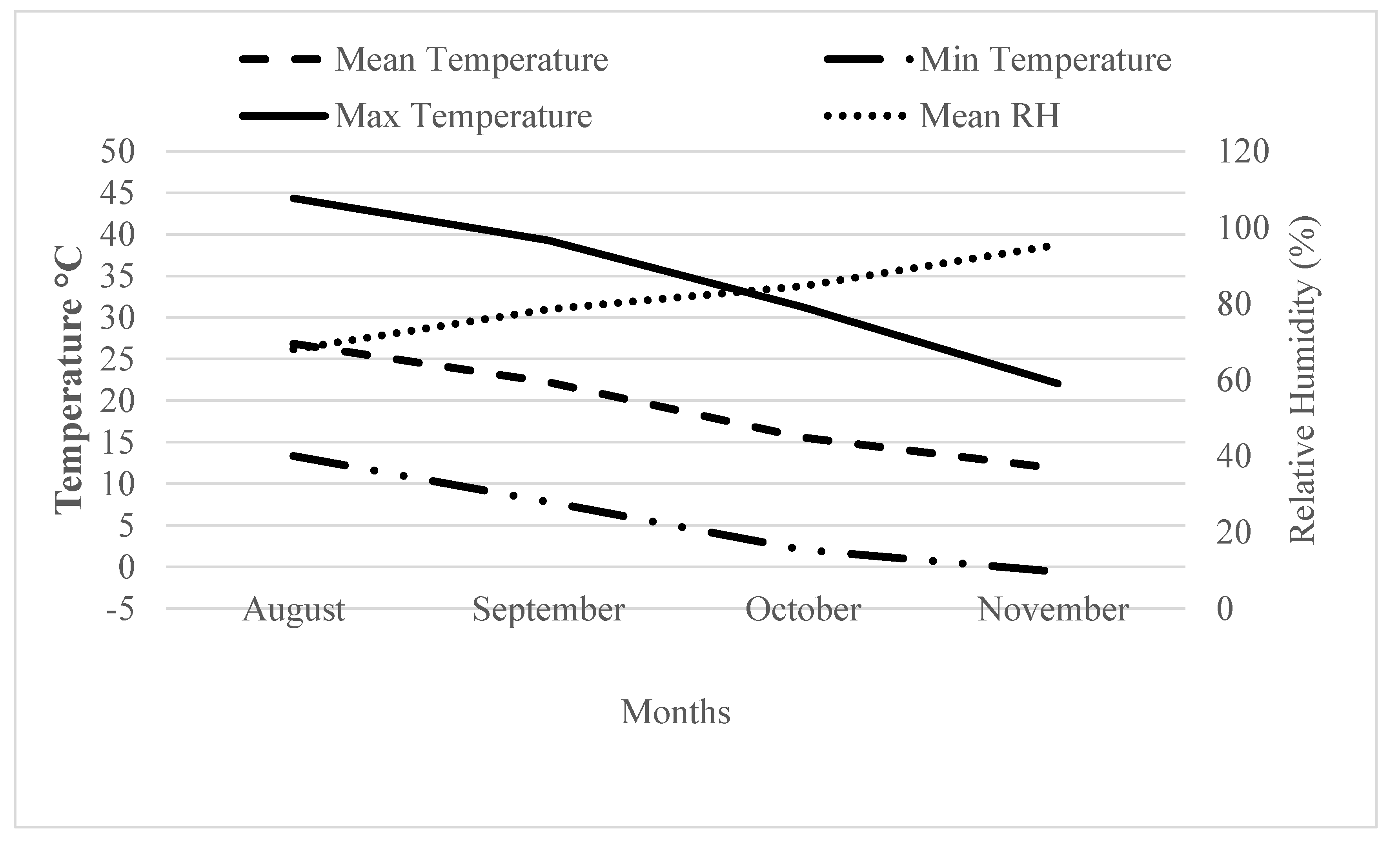
2.1. Plant Material and Growing Conditions
2.2. Chemical Analyses
2.2.1. Nutritional Value
2.2.2. Minerals Composition
2.2.3. Tocopherols
2.2.4. Organic Acids
2.2.5. Free Sugars
2.2.6. Fatty Acids
2.3. Statistical Analysis
2.3.1. Experimental Layout and Statistical Treatment
2.3.2. Linear Discriminant Analysis (LDA)
3. Results and Discussion
3.1. Yield and Growth Parameters
3.2. Nutritional Value
3.3. Mineral Composition
3.4. Tocopherols
3.5. Organic Acids
3.6. Free Sugars
3.7. Fatty Acids
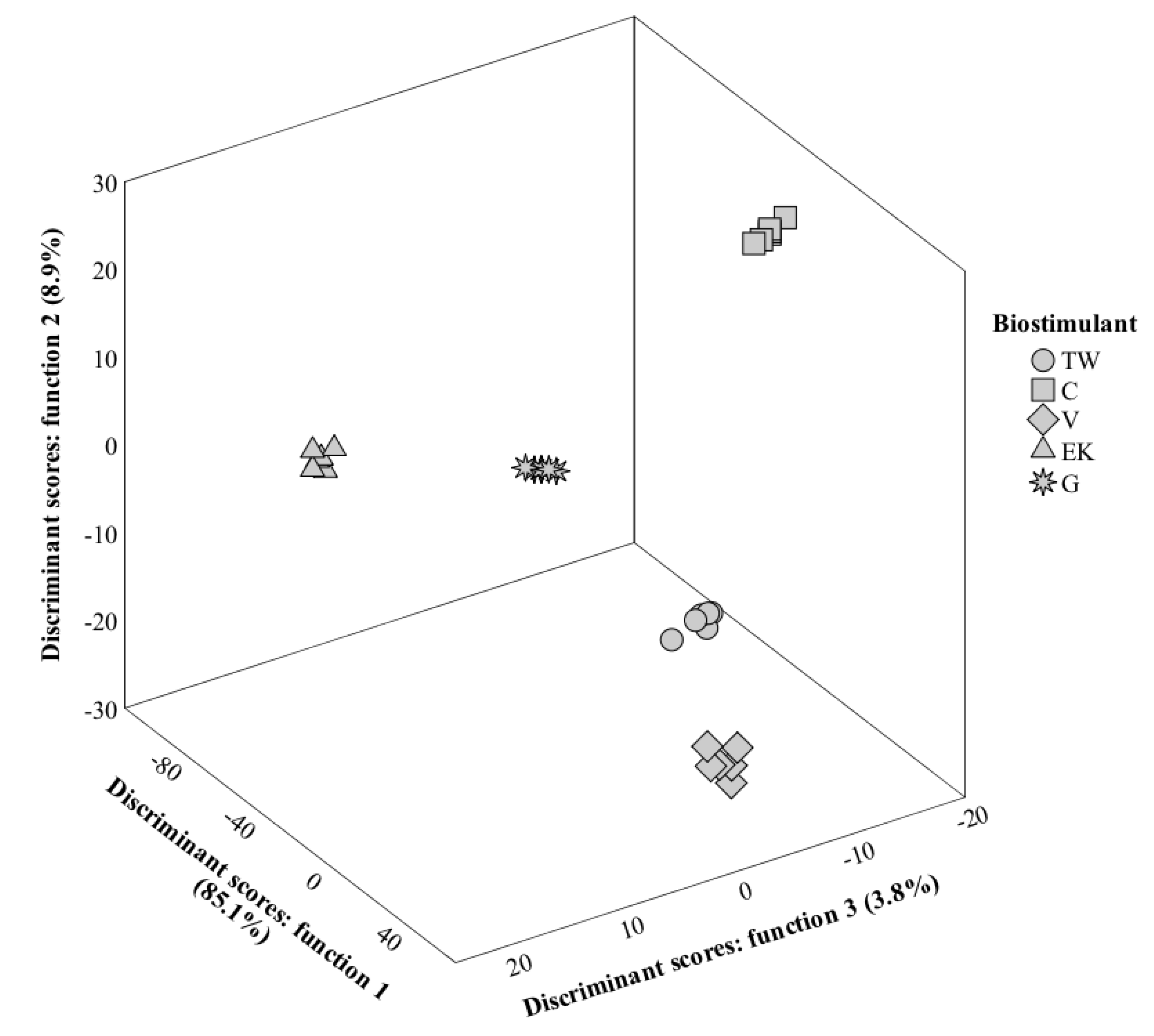
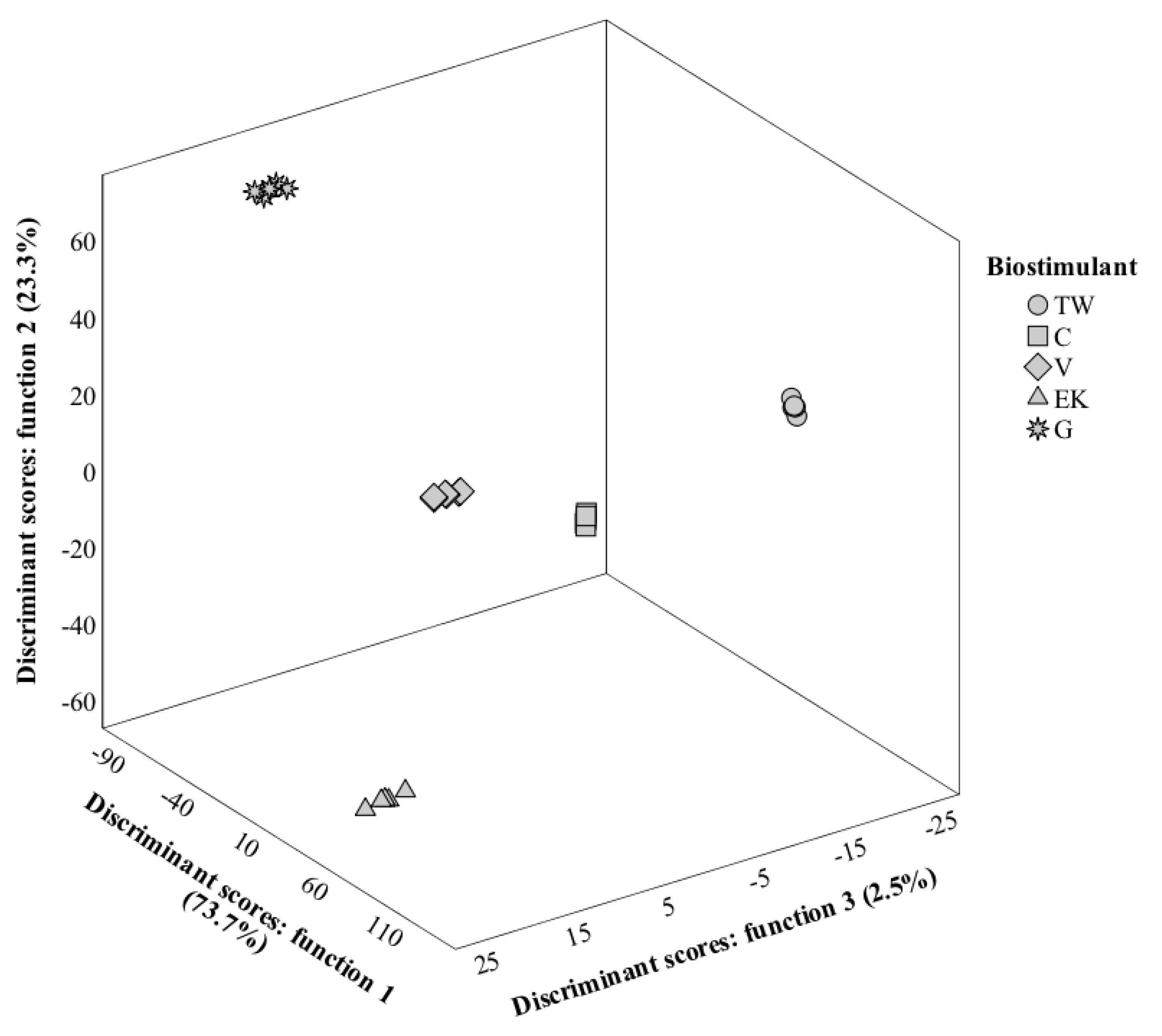
3.8. LDA Analysis
3.8.1. First Harvest
3.8.2. Second Harvest
3.8.3. Seeds
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Huang, Z.A.; Jiang, D.A.; Yang, Y.; Sun, J.W.; Jin, S.H. Effects of nitrogen deficiency on gas exchange, chlorophyll fluorescence, and antioxidant enzymes in leaves of rice plants. Photosynthetica 2004, 42, 357–364. [Google Scholar] [CrossRef]
- Zhao, D.; Reddy, K.R.; Kakani, V.G.; Reddy, V.R. Nitrogen deficiency effects on plant growth, leaf photosynthesis, and hyperspectral reflectance properties of sorghum. Eur. J. Agron. 2005, 22, 391–403. [Google Scholar] [CrossRef]
- Liebman, M.; Davis, A.S. Integration of soil, crop and weed management in low-external-input farming systems. Weed Res. 2000, 40, 27–47. [Google Scholar] [CrossRef]
- Postel, S.L. Entering an era of water scarcity: The challenges ahead. Ecol. Appl. 2000, 10, 941–948. [Google Scholar] [CrossRef]
- Bulgari, R.; Cocetta, G.; Trivellini, A.; Vernieri, P.; Ferrante, A. Biostimulants and crop responses: A review. Biol. Agric. Hortic. 2015, 31, 1–17. [Google Scholar] [CrossRef]
- Van Oosten, M.J.; Pepe, O.; De Pascale, S.; Silletti, S.; Maggio, A. The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem. Biol. Technol. Agric. 2017, 4, 1–12. [Google Scholar] [CrossRef]
- Ziosi, V.; Zandoli, R.; Di Nardo, A.; Biondi, S.; Antognoni, F.; Calandriello, F. Biological activity of different botanical extracts as evaluated by means of an array of in vitro and in vivo bioassays. Acta Hortic. 2012, 1009, 61–66. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.H.; Chaoxing, H. Does inoculation with Glomus mosseae improve salt tolerance in pepper plants? J. Plant Growth Regul. 2014, 33, 644–653. [Google Scholar] [CrossRef]
- Abu Seif, Y.I.; El-Miniawy, S.E.D.M.; Abu El-Azm, N.A.I.; Hegazi, A.Z. Response of snap bean growth and seed yield to seed size, plant density and foliar application with algae extract. Ann. Agric. Sci. 2016, 61, 187–199. [Google Scholar] [CrossRef]
- Ahmad, P.; Hashem, A.; Abd-Allah, E.F.; Alqarawi, A.A.; John, R.; Egamberdieva, D.; Gucel, S. Role of Trichoderma harzianum in mitigating NaCl stress in Indian mustard (Brassica juncea L) through antioxidative defense system. Front. Plant Sci. 2015, 6, 1–15. [Google Scholar] [CrossRef]
- Halpern, M.; Bar-Tal, A.; Ofek, M.; Minz, D.; Muller, T.; Yermiyahu, U. The use of biostimulants for enhancing nutrient uptake. Adv. Agron. 2015, 130, 141–174. [Google Scholar]
- Koleška, I.; Hasanagić, D.; Todorović, V.; Murtić, S.; Klokić, I.; Paradiković, N.; Kukavica, B. Biostimulant prevents yield loss and reduces oxidative damage in tomato plants grown on reduced NPK nutrition. J. Plant. Interact. 2017, 12, 209–218. [Google Scholar] [CrossRef]
- Rouphael, Y.; Franken, P.; Schneider, C.; Schwarz, D.; Giovannetti, M.; Agnolucci, M.; De Pascale, S.; Bonini, P.; Colla, G. Arbuscular mycorrhizal fungi act as biostimulants in horticultural crops. Sci. Hortic. (Amsterdam). 2015, 196, 91–108. [Google Scholar] [CrossRef]
- Kunicki, E.; Grabowska, A.; Sękara, A.; Wojciechowska, R. The effect of cultivar type, time of cultivation, and biostimulant treatment on the yield of spinach (Spinacia oleracea L.). Folia Hortic. 2010, 22, 9–13. [Google Scholar] [CrossRef]
- Pereira, J.L.; Queiroz, R.M.L.; Charneau, S.O.; Felix, C.R.; Ricart, C.A.O.; Lopes Da Silva, F.; Steindorff, A.S.; Ulhoa, C.J.; Noronha, E.F. Analysis of Phaseolus vulgaris response to its association with Trichoderma harzianum (ALL-42) in the presence or absence of the phytopathogenic fungi Rhizoctonia solani and Fusarium solani. PLoS ONE 2014, 9, 1–23. [Google Scholar] [CrossRef]
- Rouphael, Y.; Giordano, M.; Cardarelli, M.; Cozzolino, E.; Mori, M.; Kyriacou, M.C.; Bonini, P.; Colla, G. Plant-and seaweed-based extracts increase yield but differentially modulate nutritional quality of greenhouse spinach through biostimulant action. Agronomy 2018, 8, 126. [Google Scholar] [CrossRef]
- Xu, C.; Leskovar, D.I. Effects of A. nodosum seaweed extracts on spinach growth, physiology and nutrition value under drought stress. Sci. Hortic. (Amsterdam) 2015, 183, 39–47. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Xylia, P.; Anastasiou, M.; Pantelides, I.; Tzortzakis, N. Effects of Ascophyllum nodosum seaweed extracts on lettuce growth, physiology and fresh-cut salad storage under potassium deficiency. J. Sci. Food Agric. 2018, 98, 5861–5872. [Google Scholar] [CrossRef]
- Galvão, Í.M.; dos Santos, O.F.; de Souza, M.L.C.; de Jesus Guimarães, J.; Kühn, I.E.; Broetto, F. Biostimulants action in common bean crop submitted to water deficit. Agric. Water Manag. 2019, 225, 105762. [Google Scholar] [CrossRef]
- De Pascale, S.; Rouphael, Y.; Colla, G. Plant biostimulants: Innovative tool for enhancing plant nutrition in organic farming. Eur. J. Hortic. Sci. 2018, 82, 277–285. [Google Scholar] [CrossRef]
- Karimzadeh Soureshjani, H.; Nezami, A.; Kafi, M.; Tadayon, M. Responses of two common bean (Phaseolus vulgaris L.) genotypes to deficit irrigation. Agric. Water Manag. 2019, 213, 270–279. [Google Scholar] [CrossRef]
- Hummel, M.; Hallahan, B.F.; Brychkova, G.; Ramirez-Villegas, J.; Guwela, V.; Chataika, B.; Curley, E.; McKeown, P.C.; Morrison, L.; Talsma, E.F.; et al. Reduction in nutritional quality and growing area suitability of common bean under climate change induced drought stress in Africa. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Arruda, I.M.; Moda-Cirino, V.; Koltun, A.; dos Santos, O.J.A.P.; Moreira, R.S.; Moreira, A.F.P.; Gonçalves, L.S.A. Physiological, biochemical and morphoagronomic characterization of drought-tolerant and drought-sensitive bean genotypes under water stress. Physiol. Mol. Biol. Plants 2018, 24, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Sarma, R.K.; Saikia, R. Alleviation of drought stress in mung bean by strain Pseudomonas aeruginosa GGRJ21. Plant. Soil 2014, 377, 111–126. [Google Scholar] [CrossRef]
- Sadak, M.S.; Abdelhamid, M.T.; Schmidhalter, U. Effect of foliar application of aminoacids on plant yield and some physiological parameters in bean plants irrigated with seawater. Acta Biol. Colomb. 2015, 20, 141–152. [Google Scholar]
- Kumar, M.; Mishra, S.; Dixit, V.; Kumar, M.; Agarwal, L.; Chauhan, P.S.; Nautiyal, C.S. Synergistic effect of Pseudomonas putida and Bacillus amyloliquefaciens ameliorates drought stress in chickpea (Cicer arietinum L.). Plant. Signal. Behav. 2016, 11, 1–9. [Google Scholar] [CrossRef]
- German, M.A.; Burdman, S.; Okon, Y.; Kigel, J. Effects of Azospirillum brasilense on root morphology of common bean (Phaseolus vulgaris L.) under different water regimes. Biol. Fertil. Soils 2000, 32, 259–264. [Google Scholar] [CrossRef]
- Klimek-Kopyra, A.; Kliszcz, A.; Ślizowska, A.; Kot, D. Application of biostimulants influences shoot and root characteristics of seedlings of winter pea (Pisum sativum L.). Acta Agrobot. 2019, 72, 1–8. [Google Scholar] [CrossRef]
- Dourado-Neto, D.; Dario, G.J.A.; Barbieri, A.P.P.; Martin, T.N. Biostimulant action on agronomic efficiency of corn and common beans. Biosci. J. 2014, 30, 371–379. [Google Scholar]
- Rady, M.M.; Desoky, E.S.M.; Elrys, A.S.; Boghdady, M.S. Can licorice root extract be used as an effective natural biostimulant for salt-stressed common bean plants? S. Afr. J. Bot. 2019, 121, 294–305. [Google Scholar] [CrossRef]
- Rady, M.M.; Mohamed, G.F. Modulation of salt stress effects on the growth, physio-chemical attributes and yields of Phaseolus vulgaris L. plants by the combined application of salicylic acid and Moringa oleifera leaf extract. Sci. Hortic. (Amsterdam) 2015, 193, 105–113. [Google Scholar] [CrossRef]
- Selby, C.; Carmichael, E.; Sharma, H.S.S. Bio-refining of perennial ryegrass (Lolium perenne): Evaluation of aqueous extracts for plant defence elicitor activity using French bean cell suspension cultures. Chem. Biol. Technol. Agric. 2016, 3, 1–7. [Google Scholar] [CrossRef]
- Elzaawely, A.A.; Ahmed, M.E.; Maswada, H.F.; Xuan, T.D. Enhancing growth, yield, biochemical, and hormonal contents of snap bean (Phaseolus vulgaris L.) sprayed with moringa leaf extract. Arch. Agron. Soil Sci. 2017, 63, 687–699. [Google Scholar] [CrossRef]
- Elzaawely, A.A.; Ahmed, M.E.; Maswada, H.F.; Al-Araby, A.A.; Xuan, T.D. Growth traits, physiological parameters and hormonal status of snap bean (Phaseolus vulgaris L.) sprayed with garlic cloves extract. Arch. Agron. Soil Sci. 2018, 64, 1068–1082. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Taofiq, O.; Fernandes, Â.; Tzortzakis, N.; Ciric, A.; Sokovic, M.; Barros, L.; Ferreira, I.C. Bioactive properties of greenhouse-cultivated green beans (Phaseolus vulgaris L.) under biostimulants and water-stress effect. J. Sci. Food Agric. 2019, 99, 6049–6059. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.; Dias, M.I.; Petropoulos, S.A.; Plexida, S.; Chrysargyris, A.; Tzortzakis, N.; Calhelha, R.C.; Ivanov, M.; Stojković, D.; Soković, M.; et al. The Effects of Biostimulants, Biofertilizers and Water-Stress on Nutritional Value and Chemical Composition of Two Spinach Genotypes (Spinacia oleracea L.). Molecules 2019, 24, 4494. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis of AOAC International; Horwitz, W., Latimer, G., Eds.; AOAC International: Gaithersburg, MD, USA, 2016. [Google Scholar]
- Chrysargyris, A.; Nikolaidou, E.; Stamatakis, A.; Tzortzakis, N. Vegetative, physiological, nutritional and antioxidant behavior of spearmint (Mentha spicata L.) in response to different nitrogen supply in hydroponics. J. Appl. Res. Med. Aromat. Plants 2017, 6, 52–61. [Google Scholar] [CrossRef]
- Pereira, C.; Barros, L.; Carvalho, A.M.; Ferreira, I.C.F.R. Use of UFLC-PDA for the analysis of organic acids in thirty-five species of food and medicinal plants. Food Anal. Methods 2013, 6, 1337–1344. [Google Scholar] [CrossRef]
- Barros, L.; Pereira, E.; Calhelha, R.C.; Dueñas, M.; Carvalho, A.M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Bioactivity and chemical characterization in hydrophilic and lipophilic compounds of Chenopodium ambrosioides L. J. Funct. Foods 2013, 5, 1732–1740. [Google Scholar] [CrossRef]
- Barros, L.; Pereira, C.; Ferreira, I.C.F.R. Optimized analysis of organic acids in edible mushrooms from Portugal by Ultra-Fast Liquid Chromatography and Photodiode Array Detection. Food Anal. Methods 2013, 6, 309–316. [Google Scholar] [CrossRef]
- Aimo, S.; Gosetti, F.; D’Agostino, G.; Gamalero, E.; Gianotti, V.; Bottaro, M.; Gennaro, M.C.; Berta, G. Use of arbuscular mycorrhizal fungi and beneficial soil bacteria to improve yield and quality of saffron (Crocus sativus L.). Acta Hortic. 2010, 159–164. [Google Scholar] [CrossRef]
- Weber, N.; Schmitzer, V.; Jakopic, J.; Stampar, F. First fruit in season: Seaweed extract and silicon advance organic strawberry (Fragaria×ananassa Duch.) fruit formation and yield. Sci. Hortic. (Amsterdam) 2018, 242, 103–109. [Google Scholar] [CrossRef]
- Arthur, G.D.; Stirk, W.A.; Van Staden, J. Effect of a seaweed concentrate on the growth and yield of three varieties of Capsicum annuum. S. Afr. J. Bot. 2003, 69, 207–211. [Google Scholar] [CrossRef]
- Alam, M.Z.; Braun, G.; Norrie, J.; Hodges, D.M. Effect of Ascophyllum extract application on plant growth, fruit yield and soil microbial communities of strawberry. Can. J. Plant. Sci. 2013, 93, 23–36. [Google Scholar] [CrossRef]
- Alam, M.Z.; Braun, G.; Norrie, J.; Mark Hodges, D. Ascophyllum extract application can promote plant growth and root yield in carrot associated with increased root-zone soil microbial activity. Can. J. Plant. Sci. 2014, 94, 337–348. [Google Scholar] [CrossRef]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed extracts as biostimulants in horticulture. Sci. Hortic. (Amsterdam) 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Korir, H.; Mungai, N.W.; Thuita, M.; Hamba, Y.; Masso, C. Co-inoculation effect of rhizobia and plant growth promoting rhizobacteria on common bean growth in a low phosphorus soil. Front. Plant. Sci. 2017, 08, 1–10. [Google Scholar] [CrossRef]
- Farouk, S.; Abdul Qados, A.M.S. Enhancing seed quality and productivity as well as physio-anatomical responses of pea plants by folic acid and/or hydrogen peroxide application. Sci. Hortic. (Amsterdam) 2018, 240, 29–37. [Google Scholar] [CrossRef]
- Poorter, H.; Nagel, O. The role of biomass allocation in the growth response of plants to different levels of light, CO2, nutrients and water: A quantitative review. Aust. J. Plant. Physiol. 2000, 27, 595–607. [Google Scholar]
- Colla, G.; Nardi, S.; Cardarelli, M.; Ertani, A.; Lucini, L.; Canaguier, R.; Rouphael, Y. Protein hydrolysates as biostimulants in horticulture. Sci. Hortic. (Amsterdam) 2015, 196, 28–38. [Google Scholar] [CrossRef]
- Przygocka-Cyna, K.; Grzebisz, W. Effect of biofertilizers on nutrient uptake by vegetables grown in a short cropping sequence. J. Elem. 2018, 23, 807–823. [Google Scholar] [CrossRef]
- Elsheikh, E.A.E.; El Tinay, A.H.; Fadul, I.A. Effect of nutritional status of faba bean on proximate composition, anti-nutritional factors and in vitro protein digestibility (IVPD). Food Chem. 1999, 67, 379–383. [Google Scholar] [CrossRef]
- Kocira, A.; Świeca, M.; Kocira, S.; Złotek, U.; Jakubczyk, A. Enhancement of yield, nutritional and nutraceutical properties of two common bean cultivars following the application of seaweed extract (Ecklonia maxima). Saudi, J. Biol. Sci. 2018, 25, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Castellanos-Barriga, L.G.; Santacruz-Ruvalcaba, F.; Hernández-Carmona, G.; Ramírez-Briones, E.; Hernández-Herrera, R.M. Effect of seaweed liquid extracts from Ulva lactuca on seedling growth of mung bean (Vigna radiata). J. Appl. Phycol. 2017, 29, 2479–2488. [Google Scholar] [CrossRef]
- Mancuso, S.; Azzarello, E.; Mugnai, S.; Briand, X. Marine bioactive substances (IPA extract) improve foliar ion uptake and water stress tolerance in potted Vitis vinifera plants. Adv. Hortic. Sci. 2006, 20, 156–161. [Google Scholar]
- Gosling, P.; Mead, A.; Proctor, M.; Hammond, J.P.; Bending, G.D. Contrasting arbuscular mycorrhizal communities colonizing different host plants show a similar response to a soil phosphorus concentration gradient. New Phytol. 2013, 198, 546–556. [Google Scholar] [CrossRef]
- Colla, G.; Cardarelli, M.; Bonini, P.; Rouphael, Y. Foliar applications of protein hydrolysate, plant and seaweed extracts increase yield but differentially modulate fruit quality of greenhouse tomato. HortScience 2017, 52, 1214–1220. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Rior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Kellős, T.; Tímár, I.; Szilágyi, V.; Szalai, G.; Galiba, G.; Kocsy, G. Stress hormones and abiotic stresses have different effects on antioxidants in maize lines with different sensitivity. Plant. Biol. 2008, 10, 563–572. [Google Scholar] [CrossRef]
- Kan, L.; Nie, S.; Hu, J.; Wang, S.; Bai, Z.; Wang, J.; Zhou, Y.; Jiang, J.; Zeng, Q.; Song, K. Comparative study on the chemical composition, anthocyanins, tocopherols and carotenoids of selected legumes. Food Chem. 2018, 260, 317–326. [Google Scholar] [CrossRef]
- Elkelish, A.A.; Alnusaire, T.S.; Soliman, M.H.; Gowayed, S.; Senousy, H.H.; Fahad, S. Calcium availability regulates antioxidant system, physio-biochemical activities and alleviates salinity stress mediated oxidative damage in soybean seedlings. J. Appl. Bot. Food Qual. 2019, 92, 258–266. [Google Scholar]
- El-Bassiouny, H.M.S.; El-Monem, A.A.A.; Abdallah, M.M.-S.; Soliman, K.M. Role of arbuscular mycorrhiza, α-tocopherol and nicotinamide on the nitrogen containing compounds and adaptation of sunflower plant to Water stress. Biosci. Res. 2018, 15, 2068–2088. [Google Scholar]
- Laxa, M.; Liebthal, M.; Telman, W.; Chibani, K.; Dietz, K.J. The role of the plant antioxidant system in drought tolerance. Antioxidants 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Casadesús, A.; Polo, J.; Munné-Bosch, S. Hormonal effects of an enzymatically hydrolyzed animal protein-based biostimulant (pepton) in water-stressed tomato plants. Front. Plant. Sci. 2019, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zushi, K.; Matsuzoe, N.; Kitano, M. Developmental and tissue-specific changes in oxidative parameters and antioxidant systems in tomato fruits grown under salt stress. Sci. Hortic. (Amsterdam) 2009, 122, 362–368. [Google Scholar] [CrossRef]
- Palmieri, F.; Estoppey, A.; House, G.L.; Lohberger, A.; Bindschedler, S.; Chain, P.S.G.; Junier, P. Chapter Two - Oxalic acid, a molecule at the crossroads of bacterial-fungal interactions. Adv. Appl. Microbiol. 2019, 106, 49–77. [Google Scholar]
- Weber, H.; Borisjuk, L.; Wobus, U. Sugar import and metabolism during seed development. Trends Plant. Sci. 1997, 2, 169–174. [Google Scholar] [CrossRef]
- Rathinasabapathi, B. Metabolic engineering for stress tolerance: Installing osmoprotectant synthesis pathways. Ann. Bot. 2000, 86, 709–716. [Google Scholar] [CrossRef]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Ahmed, N.; Zhang, L. Role of Arbuscular Mycorrhizal Fungi in Plant Growth Regulation: Implications in Abiotic Stress Tolerance. Front. Plant. Sci. 2019, 10, 1–15. [Google Scholar] [CrossRef]
- Rosa, M.; Prado, C.; Podazza, G.; Interdonato, R.; González, J.A.; Hilal, M.; Prado, F.E. Soluble sugars-metabolism, sensing and abiotic stress. A complex network in the life of plants. Plant. Signal. Behav. 2009, 4, 388–393. [Google Scholar] [CrossRef]
- Petropoulos, S.; Ntatsi, G.; Levizou, E.; Barros, L.; Ferreira, I. Nutritional profile and chemical composition of Cichorium spinosum ecotypes. LWT - Food Sci. Technol. 2016, 73, 95–101. [Google Scholar] [CrossRef]
- López-Bucio, J.; Pelagio-Flores, R.; Herrera-Estrella, A. Trichoderma as biostimulant: Exploiting the multilevel properties of a plant beneficial fungus. Sci. Hortic. (Amsterdam) 2015, 196, 109–123. [Google Scholar] [CrossRef]

| Product | Composition | Application Method | Dose |
|---|---|---|---|
| Nomoren (G) | 20% of arbuscular mycorrhizal fungi (AMF) (Glomus spp.) | Irrigation water | 5 L/ha |
| Twin-Antistress (TW) | Natural microorganisms based on Bacillus subtilis, and yeast and Ascophyllum nodosum extracts, as well as N (organic): 1%, organic carbon: 10%, and organic matter (<50 kDa): 30% | Irrigation water | 5 L/ha |
| Veramin Ca (V) | Amino acid complex of vegetable origin with Aloe vera extract, and CaO: 15.6% | Foliar spraying | 500 g/100 L H2O |
| EKOprop (EK) | Mixture of arbuscular mycorrhizal fungi (Glomus spp: 1%), rhizospere symbiotic bacteria (Bacillus spp., Streptomyces spp., Pseudomonas spp.; 1.6 × 109 CFU/g in total), and saprophytic fungi (Trichoderma spp.: 5 × 105 CFU/g) | Irrigation water | 1 kg/ha |
| 1st Harvest | 2nd Harvest | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | Number of Pods/Plant | Mean Pod Weight (g) | Yield (kg/ha) | Number of Pods/Plant | Mean Pod Weight (g) | Yield (kg/ha) | Number of Pods/Plant | Mean Pod Weight (g) | Yield (kg/ha) | 100 Seeds Weight (g) | Seeds per Pot |
| CW+ ¥ | 24 ± 1c | 9 ± 1b | 4296 ± 105d | 17 ± 4c | 9 ± 1b | 3138 ± 43c | 41 ± 4d | 9 ± 1c | 7434 ± 120d | 101 ± 2f | 5.4 ± 0.5a |
| VW+ | 26 ± 2b | 9.7 ± 0.6a | 4933 ± 154b | 18 ± 1b | 9 ± 1b | 3318 ± 89b | 44 ± 4b | 10 ± 1b | 8311 ± 233b | 112 ± 3d | 4.8 ± 0.9b |
| EKW+ | 27 ± 1a | 9.7 ± 0.8a | 5284 ± 120a | 18.6 ± 0.8a | 10 ± 1a | 3701 ± 88a | 46 ± 3a | 10 ± 2a | 8985 ± 196a | 108 ± 2e | 5.0 ± 0.8b |
| GW+ | 26 ± 2b | 9 ± 1b | 4621 ± 85.0c | 16.4 ± 0.8c | 10 ± 2a | 3318 ± 81b | 42 ± 2c | 9 ± 1c | 7939 ± 160c | 116 ± 2b | 5.5 ± 0.9a |
| TWW+ | 24 ± 2c | 8 ± 1d | 3799 ± 109e | 17.5 ± 0.9c | 10 ± 2a | 3357 ± 96b | 42 ± 3c | 9 ± 1c | 7156 ± 187e | 116 ± 2b | 5.5 ± 0.9a |
| CW- | 14 ± 3f | 8.8 ± 0.9c | 2456 ± 75h | 13 ± 3f | 9 ± 2b | 2435 ± 77e | 27 ± 2h | 9 ± 1c | 4891 ± 137h | 114 ± 3bc | 5.0 ± 0.9b |
| VW- | 14 ± 2f | 8 ± 1d | 2213 ± 90i | 12.0 ± 0.6g | 7.3 ± 0.8d | 1749 ± 59f | 26 ± 2i | 7.8 ± 0.8f | 3962 ± 147j | 115 ± 3b | 4.4 ± 0.5c |
| EKW- | 16.7 ± 0.8e | 8 ± 1d | 2515 ± 50h | 14.1 ± 0.9e | 8 ± 2c | 2147 ± 62c | 31 ± 2g | 7.6 ± 0.7g | 4662 ± 80i | 122 ± 2a | 5.0 ± 0.6b |
| GW- | 20.2 ± 0.9d | 8 ± 1d | 3172 ± 79f | 15 ± 1d | 8 ± 2c | 2519 ± 81de | 36 ± 2e | 8 ± 1e | 5691 ± 139f | 113 ± 3cd | 5.1 ± 0.5b |
| TWW- | 16.7 ± 0.8e | 8.2 ± 0.8d | 2748 ± 64g | 15.4 ± 0.8d | 8 ± 2c | 2603 ± 71d | 32 ± 2f | 8.3 ± 0.9d | 5351 ± 127g | 114 ± 2bc | 5.0 ± 0.7b |
| 1st Harvest of Pods | ||||||
|---|---|---|---|---|---|---|
| Treatment | Moisture (%) | Fat | Proteins | Ash | Carbohydrates | Energy |
| CW+ ¥ | 96.4 ± 0.7a | 48.2 ± 0.8e | 165 ± 6h | 59.6 ± 0.4h | 728 ± 3b | 4630 ± 20f |
| VW+ | 94.2 ± 0.4c | 45.6 ± 0.6g | 201 ± 1a | 79 ± 1a | 674 ± 2i | 4821 ± 3a |
| EKW+ | 95.0 ± 0.6b | 48 ± 2e | 197 ± 4b | 73.7 ± 0.2b | 681 ± 1h | 4790 ± 20b |
| GW+ | 92.5 ± 0.7de | 53.7 ± 0.2b | 174.5 ± 0.1e | 71.6 ± 0.5c | 700 ± 1f | 4658 ± 1e |
| TWW+ | 93 ± 1d | 50.1 ± 0.5c | 193 ± 3c | 70 ± 3d | 688 ± 1g | 4760 ± 10c |
| CW- | 93 ± 2d | 49.4 ± 0.8cd | 150 ± 02i | 66 ± 2f | 735 ± 2a | 4553 ± 8h |
| VW- | 88 ± 1g | 48.7 ± 0.8de | 181.5 ± 0.5d | 67 ± 1e | 703 ± 2e | 4713 ± 1d |
| EKW- | 93 ± 1d | 45.5 ± 0.3g | 169.5 ± 0.4g | 61 ± 1g | 724 ± 1c | 4665 ± 1e |
| GW- | 89.4 ± 0.8f | 47 ± 3f | 171.6 ± 0.3f | 66 ± 2f | 715 ± 1d | 4671 ± 9e |
| TWW- | 92 ± 1e | 67 ± 1a | 173.2 ± 0.1e | 69.5 ± 0.1d | 690 ± 1g | 4599 ± 3g |
| 2nd Harvest of pods | ||||||
| CW+ | 91 ± 1b | 27.1 ± 0.2e | 204.6 ± 0.3b | 49.6 ± 0.7cd | 719 ± 1f | 4915 ± 2a |
| VW+ | 92 ± 2a | 32 ± 2d | 174 ± 1e | 56.9 ± 0.8b | 737 ± 1e | 4739 ± 2f |
| EKW+ | 91.1 ± 0.5b | 37.1 ± 0.2b | 195.9 ± 0.3c | 51 ± 1c | 716 ± 1f | 4831 ± 2d |
| GW+ | 90.7 ± 0.4c | 34 ± 2c | 207.1 ± 0.1a | 68.4 ± 0.5a | 690 ± 1g | 4898 ± 5b |
| TWW+ | 90 ± 2d | 27.3 ± 0.2e | 175 ± 1e | 49 ± 2d | 748 ± 1d | 4768 ± 2e |
| CW- | 91.1 ± 0.5b | 43 ± 2a | 150.2 ± 0.1h | 49 ± 1d | 758 ± 1c | 4580 ± 6g |
| VW- | 87.6 ± 0.8f | 32 ± 2d | 174.3 ± 0.2e | 52 ± 2c | 741 ± 3e | 4742 ± 4f |
| EKW- | 89 ± 1e | 21.4 ± 0.7f | 189.2 ± 0.4d | 45 ± 1e | 745 ± 1d | 4861 ± 1c |
| GW- | 90 ± 2d | 18 ± 1h | 164.0 ± 0.4g | 49 ± 2d | 769 ± 1a | 4747 ± 5f |
| TWW- | 90 ± 1d | 19.8 ± 0.8g | 171 ± 1f | 45 ± 2e | 764 ± 1b | 4776 ± 2e |
| Seeds | ||||||
| CW+ | 69.8 ± 0.3a | 40.0 ± 0.1c | 305 ± 6e | 51.4 ± 0.5cd | 604 ± 4a | 5360 ± 20g |
| VW+ | 66.7 ± 0.7d | 37 ± 1d | 310.2 ± 0.2d | 53 ± 2b | 600 ± 2a | 5403 ± 5f |
| EKW+ | 67.7 ± 0.9b | 33.2 ± 0.1g | 320 ± 2b | 53 ± 2b | 593 ± 3c | 5468 ± 6ab |
| GW+ | 67 ± 2c | 52.7 ± 0.1a | 337 ± 1a | 56 ± 1a | 554 ± 1e | 5474 ± 3a |
| TWW+ | 66.3 ± 0.7e | 45 ± 3b | 317.6 ± 0.1c | 49.7 ± 0.1f | 588 ± 2d | 5410 ± 7e |
| CW- | 62 ± 1g | 40.2 ± 0.1c | 322.7 ± 0.1b | 51 ± 1de | 587 ± 1d | 5453 ± 1c |
| VW- | 65.8 ± 0.9f | 35 ± 2e | 320.2 ± 0.1b | 51.7 ± 0.3c | 593 ± 2c | 5461 ± 6b |
| EKW- | 67.3 ± 0.6c | 34 ± 1f | 317 ± 1c | 48.1 ± 0.8g | 601 ± 1a | 5448 ± 6c |
| GW- | 69.8 ± 0.5a | 34 ± 1f | 317.1 ± 0.4c | 50.9 ± 0.4e | 598 ± 1b | 5450 ± 2c |
| TWW- | 66.2 ± 0.4e | 35.1 ± 0.6e | 316.5 ± 0.4c | 50 ± 1f | 598 ± 1b | 5442 ± 3d |
| Pods | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment | N (g/kg) | K (g/kg) | P (g/kg) | Na (g/kg) | Ca (g/kg) | Mg (mg/kg) | Cu (mg/kg) | Zn (mg/kg) | Mn (mg/kg) |
| CW+ ¥ | 26.9 ± 0.3a | 17.7 ± 0.3c | 6.3 ± 0.4b | 0.05 ± 0.03c | 6.6 ± 1.6e | 104 ± 5h | 138 ± 17c | 24 ± 1d | 4.1 ± 0.4b |
| VW+ | 24.6 ± 0.2b | 18.7 ± 0.4b | 4.2 ± 0.4e | 0.07 ± 0.06c | 4.3 ± 0.9h | 235 ± 17c | 78 ± 18e | 29 ± 2c | 4.5 ± 0.7a |
| EKW+ | 24.6 ± 0.3b | 19.1 ± 0.3b | 7.3 ± 0.2a | 0.16 ± 0.02a | 5.4 ± 0.7g | 131 ± 21g | 205 ± 18a | 38 ± 4a | 3.8 ± 0.4c |
| GW+ | 23.0 ± 0.5cd | 20.0 ± 0.4a | 6.2 ± 0.4b | 0.07 ± 0.03c | 6.1 ± 1f | 219 ± 46d | 170 ± 15b | 21 ± 1e | 4.5 ± 0.1a |
| TWW+ | 23.2 ± 0.8c | 19.0 ± 0.5b | 4.2 ± 0.4e | 0.17 ± 0.03a | 10.7 ± 3.2c | 314 ± 6b | 162 ± 19b | 33 ± 3b | 4.0 ± 0.5bc |
| CW- | 22.4 ± 0.3e | 16.7 ± 0.3d | 5.2 ± 0.7d | 0.03 ± 0.01d | 4 ± 1.3h | 341 ± 16a | 98 ± 18d | 29 ± 2c | 3.8 ± 0.2c |
| VW- | 26.5 ± 0.5a | 15.1 ± 0.2f | 6.0 ± 0.2c | 0.14 ± 0.02b | 11.5 ± 3.5b | 171 ± 12e | 46 ± 16f | 28 ± 2c | 4.2 ± 0.8ab |
| EKW- | 23.2 ± 0.2c | 17.9 ± 0.6c | 5.5 ± 0.1d | 0.16 ± 0.08a | 8.5 ± 2.6d | 147 ± 8f | 200 ± 27a | 28 ± 3c | 3.8 ± 0.5c |
| GW- | 21.5 ± 0.1f | 17.1 ± 0.8d | 5.3 ± 0.7d | 0.07 ± 0.01c | 10.2 ± 2.3c | 311 ± 14b | 169 ± 21b | 21.8 ± 0.3e | 4.4 ± 0.3a |
| TWW- | 22.8 ± 0.5d | 16.1 ± 0.3e | 5.9 ± 0.2c | 0.06 ± 0.01c | 13.2 ± 4.7a | 311 ± 35b | 145 ± 12c | 20 ± 3e | 4.1 ± 0.2b |
| Seeds | |||||||||
| CW+ | 42.9 ± 0.3b | 1.6 ± 0.2a | 13.3 ± 1.4b | 0.18 ± 0.08a | 11.1 ± 2.3e | 7 ± 1f | 158 ± 45c | 13 ± 2g | 5 ± 1a |
| VW+ | 41.3 ± 0.4d | 1.6 ± 0.3a | 9.4 ± 1.8g | 0.16 ± 0.05a | 6.8 ± 1.7f | 11 ± 3cd | 99 ± 38e | 21 ± 1b | 0.5 ± 0.2g |
| EKW+ | 41.9 ± 0.8c | 1.6 ± 0.2a | 12.7 ± 2.3c | 0.05 ± 0.01b | 10.9 ± 1.4e | 6 ± 3fg | 193 ± 11b | 13 ± 2g | 4.4 ± 0.9cd |
| GW+ | 41.9 ± 0.1c | 1.7 ± 0.3a | 14.0 ± 6.6a | 0.05 ± 0.01b | 12.6 ± 2.5c | 5 ± 4g | 165 ± 15c | 18 ± 3cd | 3.5 ± 0.4e |
| TWW+ | 41.9 ± 0.1c | 1.6 ± 0.3a | 8.0 ± 0.9h | 0.03 ± 0.01b | 15.2 ± 1.5a | 13 ± 1b | 199 ± 39a | 17 ± 3de | 4.6 ± 0.4bc |
| CW- | 39.5 ± 0.3e | 1.6 ± 0.4a | 10.0 ± 2.5f | 0.15 ± 0.04a | 10.9 ± 2.4e | 10.0 ± 0.6de | 120 ± 50d | 13 ± 1g | 5.1 ± 0.3a |
| VW- | 42.8 ± 0.5b | 1.6 ± 0.1a | 7.2 ± 0.5i | 0.15 ± 0.06a | 3.7 ± 1.3g | 15 ± 3a | 90 ± 8f | 22.6 ± 0.3a | 2 ± 1f |
| EKW- | 42.2 ± 0.8c | 1.6 ± 0.9a | 11.9 ± 2.6d | 0.03 ± 0.01b | 11.7 ± 1.7d | 2.9 ± 0.3h | 190 ± 49b | 16 ± 2ef | 4.2 ± 0.3d |
| GW- | 44.7 ± 0.2a | 1.6 ± 0.6a | 11.0 ± 1.2e | 0.04 ± 0.01b | 14.1 ± 1.3b | 9.2 ± 0.6e | 168 ± 30c | 19.2 ± 0.9c | 4.2 ± 0.4d |
| TWW- | 44.7 ± 0.2a | 1.6 ± 0.1a | 7.5 ± 1.2hi | 0.04 ± 0.01b | 12.3 ± 3.7c | 12 ± 2bc | 160 ± 43c | 15.1 ± 0.2f | 4.8 ± 0.5b |
| Treatment | α-Tocopherol | γ-Tocopherol | Total Tocopherols | |
|---|---|---|---|---|
| 1st Harvest of Pods | ||||
| CW+ ¥ | 770 ± 20f | 2970 ± 20i | 3740 ± 10i | |
| VW+ | 880 ± 40c | 4880 ± 30f | 5760 ± 10f | |
| EKW+ | 930 ± 30b | 5230 ± 50d | 6160 ± 20d | |
| GW+ | 306 ± 3g | 4020 ± 60g | 4330 ± 50g | |
| TWW+ | 760 ± 20f | 6060 ± 90b | 6820 ± 70b | |
| CW- | 798 ± 3e | 5416 ± 1c | 6214 ± 3c | |
| VW- | 810 ± 30d | 4990 ± 50e | 5800 ± 90e | |
| EKW- | 278 ± 3i | 3910 ± 30h | 4190 ± 20h | |
| GW- | 290 ± 20h | 2940 ± 60j | 3240 ± 80j | |
| TWW- | 1010 ± 10a | 6410 ± 40a | 7420 ± 30a | |
| 2nd Harvest of pods | ||||
| CW+ | 288 ± 6g | 1560 ± 60i | 1840 ± 60i | |
| VW+ | 236 ± 6h | 1670 ± 10h | 1900 ± 10h | |
| EKW+ | 266 ± 3g | 1530 ± 30j | 1800 ± 40j | |
| GW+ | 508 ± 6e | 3280 ± 20b | 3790 ± 30d | |
| TWW+ | 160 ± 10i | 1940 ± 40g | 2100 ± 30g | |
| CW- | 1810 ± 20a | 2820 ± 30d | 4630 ± 50b | |
| VW- | 1750 ± 20b | 3500 ± 20a | 5250 ± 10a | |
| EKW- | 720 ± 20d | 2440 ± 20e | 3160 ± 10e | |
| GW- | 326 ± 8f | 2000 ± 40f | 2320 ± 50f | |
| TWW- | 1210 ± 10c | 3090 ± 20c | 4300 ± 40c | |
| Seeds | ||||
| α-Tocopherol | γ-Tocopherol | δ-Tocopherol | Total Tocopherols | |
| CW+ | 0.96 ± 0.01a | 39.1 ± 0.1b | 2.48 ± 0.03a | 42.5 ± 0.1a |
| VW+ | 0.52 ± 0.01c | 39.8 ± 0.1a | 2.28 ± 0.01b | 42.7 ± 0.1a |
| EKW+ | 0.50 ± 0.01c | 38.5 ± 0.1c | 1.95 ± 0.02f | 40.9 ± 0.1c |
| GW+ | 0.52 ± 0.01c | 36.7 ± 0.1d | 2.21 ± 0.03c | 39.5 ± 0.1d |
| TWW+ | 0.53 ± 0.02c | 38.4 ± 0.1c | 2.01 ± 0.03e | 41.0 ± 0.1c |
| CW- | 0.46 ± 0.01d | 36.1 ± 0.1e | 2.16 ± 0.05d | 38.7 ± 0.1e |
| VW- | 0.57 ± 0.01b | 35.4 ± 0.1f | 1.91 ± 0.02f | 38.0 ± 0.1f |
| EKW- | 0.96 ± 0.05a | 32.0 ± 0.1h | 1.48 ± 0.02g | 34.5 ± 0.1h |
| GW- | 0.59 ± 0.02b | 39.0 ± 0.1b | 1.91 ± 0.02f | 41.5 ± 0.1b |
| TWW- | 0.45 ± 0.02d | 34.7 ± 0.1g | 2.14 ± 0.08d | 37.3 ± 0.1g |
| Oxalic Acid (g/kg dw) | Malic Acid (g/kg dw) | Ascorbic Acid (g/kg dw) | Total Organic Acids (g/kg dw) | Fructose (g/kg dw) | Glucose (g/kg dw) | Sucrose (g/kg dw) | Total Sugars (g/kg dw) | |
|---|---|---|---|---|---|---|---|---|
| Treatment | 1st Harvest of pods | |||||||
| CW+ ¥ | 14.0 ± 0.1h | 18.4 ± 0.2d | tr | 32.5 ± 0.1h | 151 ± 4f | 84.5 ± 0.1g | 5.8 ± 0.4b | 241 ± 4h |
| VW+ | 26.3 ± 0.1a | 23.1 ± 0.1a | tr | 49.4 ± 0.1a | 168 ± 5e | 90 ± 2f | nd | 257 ± 7f |
| EKW+ | 21.8 ± 0.2c | 20.4 ± 0.5c | 0.10 ± 0.01b | 42.4 ± 0.4b | 194 ± 3b | 107 ± 3c | nd | 301 ± 6c |
| GW+ | 19.3 ± 0.1e | 21.7 ± 0.4b | tr | 41.0 ± 0.3d | 173 ± 0d | 85 ± 3g | 4.7 ± 0.3d | 263 ± 4e |
| TWW+ | 23.2 ± 0.1b | 18.1 ± 0.2de | 0.5 ± 0.1a | 41.8 ± 0.3c | 173 ± 1d | 101 ± 2d | 3.5 ± 0.1f | 277 ± 2d |
| CW- | 18.5 ± 0.1f | 16.5 ± 0.2f | 0.50 ± 0.03a | 35.5 ± 0.1f | 194 ± 2b | 135 ± 5a | 4.3 ± 0.1e | 333 ± 7a |
| VW- | 17.0 ± 0.1g | 20.6 ± 0.4c | 0.10 ± 0.01b | 37.7 ± 0.4e | 153 ± 8f | 84 ± 6g | 7.6 ± 0.1a | 250 ± 10g |
| EKW- | 16.4 ± 0.1g | 17.9 ± 0.3e | tr | 34.3 ± 0.3g | 171 ± 4d | 106 ± 3c | 3.1 ± 0.3g | 280 ± 8d |
| GW- | 20.9 ± 0.1d | 16.7 ± 0.1f | tr | 37.7 ± 0.2e | 184 ± 2c | 94 ± 6e | 3.1 ± 0.3g | 281 ± 8d |
| TWW- | 21.2 ± 0.1cd | 20.4 ± 0.3c | 0.50 ± 0.01a | 42.1 ± 0.4bc | 198 ± 1a | 110 ± 3b | 5.2 ± 0.4c | 314 ± 3b |
| 2nd Harvest of pods | ||||||||
| CW+¥ | 10.9 ± 0.1d | 15.0 ± 0.4h | tr | 25.9 ± 0.4h | 170 ± 2e | 82 ± 4g | 3.5 ± 0.4e | 256 ± 6h |
| VW+ | 10.7 ± 0.1e | 17.8 ± 0.1d | tr | 28.5 ± 0.1f | 180 ± 2d | 91 ± 2f | 2.4 ± 0.2f | 273 ± 4f |
| EKW+ | 10.5 ± 0.1f | 17.4 ± 0.1e | tr | 28.0 ± 0.1g | 181 ± 4d | 101 ± 3d | 4.3 ± 0.2d | 287 ± 6d |
| GW+ | 22.7 ± 0.2a | 24.1 ± 0.3b | tr | 46.9 ± 0.1a | 195 ± 1b | 105 ± 1c | 1.76 ± 0.01g | 301 ± 1c |
| TWW+ | 8.9 ± 0.2h | 16.4 ± 0.3g | tr | 25.3 ± 0.4j | 170 ± 1e | 106 ± 3c | 3.5 ± 0.7e | 280 ± 3e |
| CW- | 10.9 ± 0.2d | 20.6 ± 0.3c | tr | 31.5 ± 0.1d | 180 ± 5d | 96 ± 2e | 14 ± 2b | 290 ± 9d |
| VW- | 9.9 ± 0.2g | 24.6 ± 0.2a | tr | 34.5 ± 0.4c | 162 ± 6f | 90 ± 3f | 13.1 ± 0.5c | 265 ± 9g |
| EKW- | 8.3 ± 0.1i | 17.1 ± 0.2f | tr | 25.5 ± 0.3i | 191 ± 3c | 110 ± 10b | 13.3 ± 0.9c | 318 ± 14b |
| GW- | 16.3 ± 0.1b | 20.4 ± 0.2c | tr | 36.8 ± 0.2b | 232 ± 7a | 140 ± 7a | 4.2 ± 0.4d | 380 ± 10a |
| TWW- | 13.2 ± 0.1c | 17.3 ± 0.3e | tr | 30.6 ± 0.4e | 163 ± 4f | 77 ± 3h | 15.3 ± 0.8a | 256 ± 8h |
| Seeds | ||||||||
| Oxalic acid (mg/kg dw) | Malic acid (mg/kg dw) | Ascorbic acid (mg/kg dw) | Fumaric acid (mg/kg dw) | Total organic acids (mg/kg dw) | Sucrose (g/kg dw) | Total sugars (g/kg dw) | ||
| CW+¥ | 224 ± 3g | 1170 ± 20c | 657 ± 6b | tr | 2050 ± 30d | 19.5 ± 0.3c | 19.5 ± 0.3c | |
| VW+ | 293 ± 1f | 1383 ± 7b | 612 ± 3c | tr | 2290 ± 10b | 19.3 ± 0.9c | 19.3 ± 0.9c | |
| EKW+ | 185 ± 8h | 970 ± 30d | 524 ± 2e | tr | 1680 ± 40f | 22.3 ± 0.5b | 22.3 ± 0.5b | |
| GW+ | 600 ± 10b | 910 ± 60e | 715 ± 4a | tr | 2220 ± 60c | 16.6 ± 0.5f | 16.6 ± 0.5f | |
| TWW+ | 185 ± 3h | 316 ± 3h | 488 ± 9g | tr | 990 ± 20i | 18.9 ± 0.4d | 18.9 ± 0.4d | |
| CW- | 530 ± 10d | tr | 530 ± 10e | tr | 1040 ± 40h | 19.2 ± 0.6c | 19.2 ± 0.6c | |
| VW- | 752 ± 1a | 1440 ± 40a | 580 ± 10d | tr | 2780 ± 30a | 15.5 ± 0.7g | 15.5 ± 0.7g | |
| EKW- | 579 ± 8c | 690 ± 30f | 515 ± 7f | tr | 1780 ± 40e | 17.1 ± 0.5e | 17.1 ± 0.5e | |
| GW- | 530 ± 10d | tr | 396 ± 3i | tr | 964 ± 1j | 22.9 ± 0.7a | 22.9 ± 0.7a | |
| TWW- | 440 ± 10e | 490 ± 20g | 449 ± 9h | tr | 1380 ± 20g | 15 ± 1h | 15 ± 1h | |
| CW+ ¥ | VW+ | EKW+ | GW+ | TWW+ | CW- | VW- | EKW- | GW- | TWW- | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1st Harvest of pods | ||||||||||
| C16:0 | 21.72 ± 0.04d | 20.87 ± 0.07f | 19.35 ± 0.05i | 24.1 ± 0.1b | 18.4 ± 0.1j | 23.55 ± 0.06c | 19.9 ± 0.1h | 20.26 ± 0.05g | 25.6 ± 0.3a | 21.18 ± 0.09e |
| C18:0 | 5.30 ± 0.01c | 4.87 ± 0.01d | 3.95 ± 0.01g | 9.81 ± 0.02a | 3.65 ± 0.01h | 5.35 ± 0.03c | 4.23 ± 0.02f | 4.27 ± 0.01f | 5.59 ± 0.03b | 4.48 ± 0.01e |
| C18:1n9c | 1.75 ± 0.02d | 1.34 ± 0.01g | 1.51 ± 0.01f | 2.08 ± 0.01b | 1.60 ± 0.01e | 1.79 ± 0.01d | 1.72 ± 0.01d | 2.18 ± 0.01a | 1.93 ± 0.01c | 1.53 ± 0.01f |
| C18:2n6c | 27.60 ± 0.03f | 28.69 ± 0.04d | 28.65 ± 0.01d | 26.98 ± 0.01g | 29.94 ± 0.03b | 28.52 ± 0.05d | 28.60 ± 0.01d | 32.02 ± 0.01a | 27.74 ± 0.08e | 29.33 ± 0.04c |
| C18:3n3 | 38.50 ± 0.01f | 39.53 ± 0.04d | 42.21 ± 0.04b | 32.62 ± 0.01j | 42.76 ± 0.06a | 35.51 ± 0.02h | 41.36 ± 0.06c | 36.83 ± 0.05g | 33.1 ± 0.1i | 38.7 ± 0.1e |
| C22:0 | 1.27 ± 0.03b | 0.96 ± 0.09d | 0.82 ± 0.01f | 1.06 ± 0.06c | 0.77 ± 0.01g | 1.47 ± 0.05a | 0.86 ± 0.03e | 0.88 ± 0.05e | 1.46 ± 0.05a | 0.99 ± 0.02d |
| C24:0 | 1.09 ± 0.02f | 1.29 ± 0.02c | 1.15 ± 0.01e | 1.03 ± 0.02g | 1.04 ± 0.01g | 1.32 ± 0.04c | 1.24 ± 0.02d | 1.12 ± 0.02ef | 1.56 ± 0.02a | 1.39 ± 0.01b |
| SFA | 31.06 ± 0.03d | 29.33 ± 0.02e | 26.80 ± 0.07h | 37.64 ± 0.03a | 25.02 ± 0.07h | 33.35 ± 0.08c | 27.62 ± 0.06g | 27.87 ± 0.06f | 35.9 ± 0.2b | 29.42 ± 0.06e |
| MUFA | 2.75 ± 0.06c | 2.30 ± 0.05f | 2.18 ± 0.02g | 2.62 ± 0.01d | 2.17 ± 0.01g | 2.43 ± 0.01e | 2.26 ± 0.02f | 3.12 ± 0.01a | 2.99 ± 0.02b | 2.25 ± 0.01f |
| PUFA | 66.19 ± 0.03f | 68.37 ± 0.07e | 71.02 ± 0.05b | 59.74 ± 0.02i | 72.81 ± 0.08a | 64.22 ± 0.09g | 70.12 ± 0.07c | 69.01 ± 0.07d | 61.1 ± 0.2h | 68.33 ± 0.06e |
| 2nd Harvest of pods | ||||||||||
| C16:0 | 18.8 ± 0.2g | 26.81 ± 0.08b | 22.4 ± 0.1d | 21.4 ± 0.1e | 28.90 ± 0.06a | 23.6 ± 0.1c | 17.09 ± 0.06i | 23.6 ± 0.1c | 19.1 ± 0.2f | 17.7 ± 0.1h |
| C18:0 | 4.38 ± 0.03f | 9.68 ± 0.02a | 4.81 ± 0.01d | 4.00 ± 0.01g | 6.78 ± 0.01b | 5.35 ± 0.02c | 3.69 ± 0.01h | 5.30 ± 0.03c | 4.68 ± 0.03e | 4.38 ± 0.01f |
| C18:1n9c | 1.48 ± 0.01h | 1.71 ± 0.01f | 1.75 ± 0.01f | 1.46 ± 0.01h | 2.29 ± 0.01b | 1.68 ± 0.01g | 1.95 ± 0.01e | 2.13 ± 0.01d | 2.21 ± 0.02c | 2.82 ± 0.01a |
| C18:2n6c | 28.46 ± 0.02d | 24.80 ± 0.01g | 28.46 ± 0.04d | 28.89 ± 0.05c | 26.04 ± 0.02f | 28.39 ± 0.01d | 32.46 ± 0.02b | 28.03 ± 0.04e | 34.33 ± 0.09a | 28.41 ± 0.01d |
| C18:3n3 | 43.3 ± 0.1a | 31.42 ± 0.01h | 35.0 ± 0.1g | 40.02 ± 0.07d | 29.67 ± 0.01i | 36.12 ± 0.03e | 40.43 ± 0.07c | 35.08 ± 0.03g | 35.63 ± 0.07f | 42.78 ± 0.02b |
| C22:0 | 0.64 ± 0.02h | 1.1 ± 0.1c | 1.7 ± 0.1a | 1.04 ± 0.04d | 0.927 ± 0.005f | 0.95 ± 0.02e | 1.18 ± 0.06b | 1.68 ± 0.06a | 0.46 ± 0.01i | 0.720 ± 0.008g |
| C24:0 | 0.901 ± 0.008f | 1.32 ± 0.01a | 1.03 ± 0.01e | 1.17 ± 0.02d | 1.27 ± 0.05b | 1.22 ± 0.03c | 0.91 ± 0.02f | 1.20 ± 0.04cd | 0.930 ± 0.006f | 0.84 ± 0.01g |
| SFA | 26.1 ± 0.1h | 40.95 ± 0.03a | 31.75 ± 0.02e | 29.1 ± 0.1f | 40.23 ± 0.09b | 32.94 ± 0.05d | 24.3 ± 0.1j | 33.6 ± 0.1c | 26.8 ± 0.2g | 25.50 ± 0.07i |
| MUFA | 1.93 ± 0.01h | 2.63 ± 0.04e | 4.6 ± 0.2a | 1.78 ± 0.01i | 3.90 ± 0.07b | 2.37 ± 0.03g | 2.52 ± 0.01f | 2.99 ± 0.02d | 3.08 ± 0.02c | 3.06 ± 0.02c |
| PUFA | 71.9 ± 0.1b | 56.42 ± 0.01i | 63.6 ± 0.1g | 69.1 ± 0.1e | 55.87 ± 0.02j | 64.68 ± 0.02f | 73.1 ± 0.1a | 63.43 ± 0.09h | 70.2 ± 0.2d | 71.44 ± 0.05c |
| Seeds | ||||||||||
| C16:0 | 11.60 ± 0.04b | 11.86 ± 0.03a | 11.16 ± 0.06c | 10.46 ± 0.04i | 10.73 ± 0.05g | 10.93 ± 0.04d | 10.58 ± 0.01h | 10.83 ± 0.05f | 10.89 ± 0.01e | 10.38 ± 0.02j |
| C18:0 | 2.61 ± 0.01b | 2.69 ± 0.01a | 2.19 ± 0.01h | 2.25 ± 0.01g | 2.10 ± 0.01i | 2.54 ± 0.01c | 2.38 ± 0.01e | 2.43 ± 0.01d | 2.33 ± 0.01f | 2.27 ± 0.01g |
| C18:1n9c | 2.35 ± 0.01b | 1.80 ± 0.01d | 1.95 ± 0.02c | 1.54 ± 0.01h | 1.58 ± 0.02g | 2.56 ± 0.01a | 1.70 ± 0.01e | 1.63 ± 0.01f | 1.46 ± 0.01i | 1.79 ± 0.01d |
| C18:2n6c | 29.54 ± 0.02a | 25.28 ± 0.01e | 27.36 ± 0.03b | 26.00 ± 0.01c | 24.89 ± 0.03h | 24.97 ± 0.01f | 25.84 ± 0.01d | 24.31 ± 0.03j | 24.52 ± 0.01i | 24.93 ± 0.02g |
| C18:3n3 | 51.48 ± 0.03i | 55.96 ± 0.02g | 55.54 ± 0.02h | 57.75 ± 0.05d | 58.41 ± 0.05c | 56.88 ± 0.01f | 57.46 ± 0.04e | 58.72 ± 0.03b | 59.08 ± 0.02a | 58.77 ± 0.03b |
| SFA | 16.15 ± 0.06b | 16.66 ± 0.01a | 14.63 ± 0.05f | 14.32 ± 0.03h | 14.45 ± 0.01g | 15.30 ± 0.02c | 14.66 ± 0.03f | 15.07 ± 0.07d | 14.78 ± 0.01e | 14.15 ± 0.02i |
| MUFA | 2.72 ± 0.01a | 2.02 ± 0.02e | 2.39 ± 0.03b | 1.84 ± 0.01f | 2.17 ± 0.06c | 2.77 ± 0.01a | 1.97 ± 0.02e | 1.82 ± 0.01f | 1.59 ± 0.01g | 2.08 ± 0.02d |
| PUFA | 81.13 ± 0.05i | 81.32 ± 0.03h | 82.98 ± 0.02f | 83.84 ± 0.05a | 83.39 ± 0.07d | 81.93 ± 0.01g | 83.37 ± 0.05d | 83.11 ± 0.06e | 83.63 ± 0.01c | 83.77 ± 0.04b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petropoulos, S.A.; Fernandes, Â.; Plexida, S.; Chrysargyris, A.; Tzortzakis, N.; Barreira, J.C.M.; Barros, L.; Ferreira, I.C.F.R. Biostimulants Application Alleviates Water Stress Effects on Yield and Chemical Composition of Greenhouse Green Bean (Phaseolus vulgaris L.). Agronomy 2020, 10, 181. https://doi.org/10.3390/agronomy10020181
Petropoulos SA, Fernandes Â, Plexida S, Chrysargyris A, Tzortzakis N, Barreira JCM, Barros L, Ferreira ICFR. Biostimulants Application Alleviates Water Stress Effects on Yield and Chemical Composition of Greenhouse Green Bean (Phaseolus vulgaris L.). Agronomy. 2020; 10(2):181. https://doi.org/10.3390/agronomy10020181
Chicago/Turabian StylePetropoulos, Spyridon A., Ângela Fernandes, Sofia Plexida, Antonios Chrysargyris, Nikos Tzortzakis, João C. M. Barreira, Lillian Barros, and Isabel C. F. R. Ferreira. 2020. "Biostimulants Application Alleviates Water Stress Effects on Yield and Chemical Composition of Greenhouse Green Bean (Phaseolus vulgaris L.)" Agronomy 10, no. 2: 181. https://doi.org/10.3390/agronomy10020181
APA StylePetropoulos, S. A., Fernandes, Â., Plexida, S., Chrysargyris, A., Tzortzakis, N., Barreira, J. C. M., Barros, L., & Ferreira, I. C. F. R. (2020). Biostimulants Application Alleviates Water Stress Effects on Yield and Chemical Composition of Greenhouse Green Bean (Phaseolus vulgaris L.). Agronomy, 10(2), 181. https://doi.org/10.3390/agronomy10020181












