Biochar Alters Soil Physical Characteristics, Arbuscular Mycorrhizal Fungi Colonization, and Glomalin Production
Abstract
1. Introduction
- (i)
- Biochar may change the levels and availability of nutrients (C, N, P, K) by changing the physicochemical parameters of the soil (e.g., cation exchange capacity, pH, water holding capacity), which affect both the host plant and the fungus.
- (ii)
- Biochar may change the rhizosphere microbiome that may promote plant growth (e.g., mycorrhizal helper bacteria, phosphate mobilizing bacteria).
- (iii)
- Biochar may alter the processes of plant-AMF signaling (transport and concentration of signal molecules) or the absorption of allelochemicals leading to changes in AM fungal root colonization.
- (iv)
- Biochar may serve as a microrefugia and shelter for hyphae consumers.
2. Materials and Methods
2.1. General Soil and Experimental Sites’ Data
2.2. Biochar Used in the Experiment
2.3. Experimental Setup
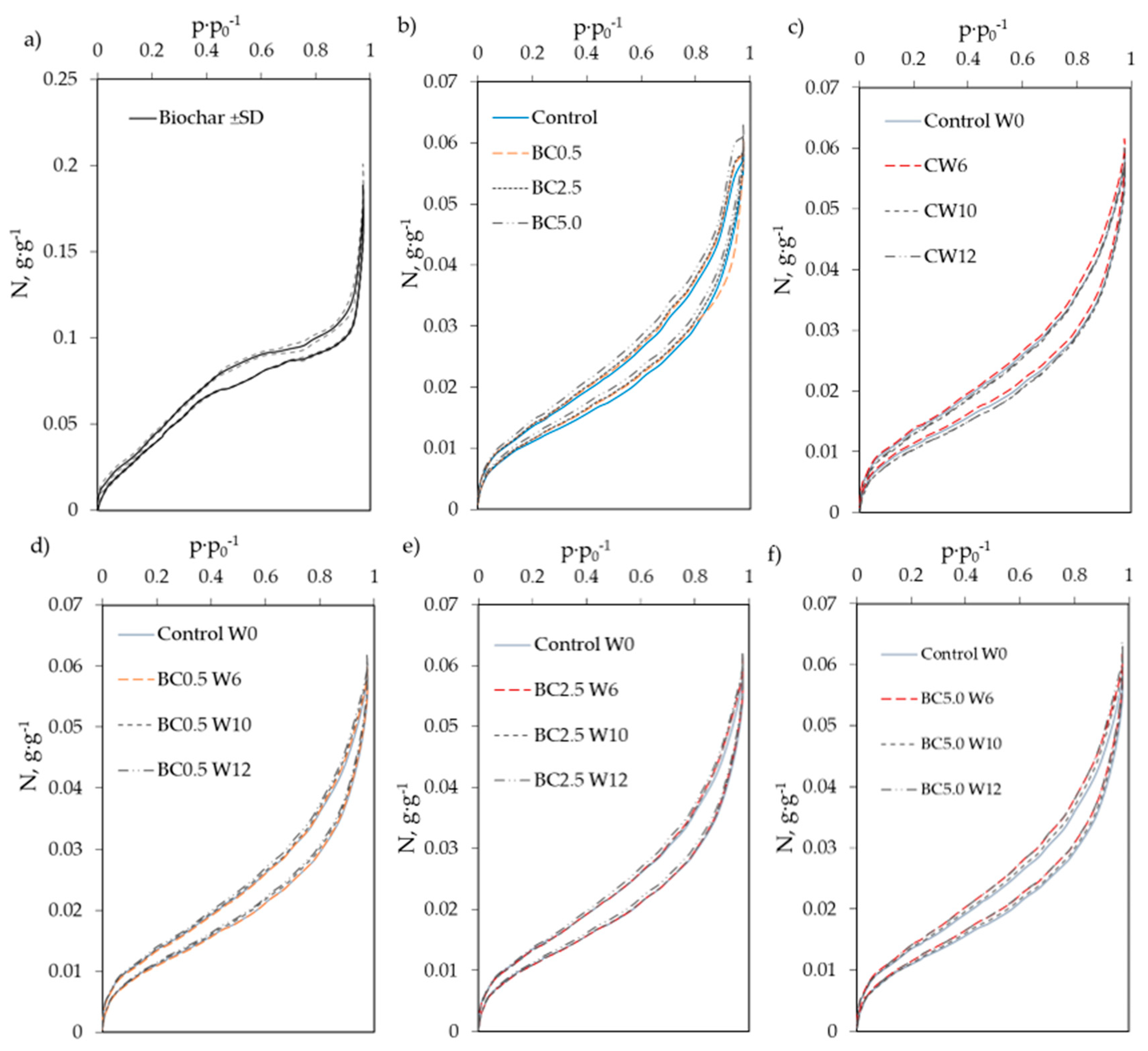
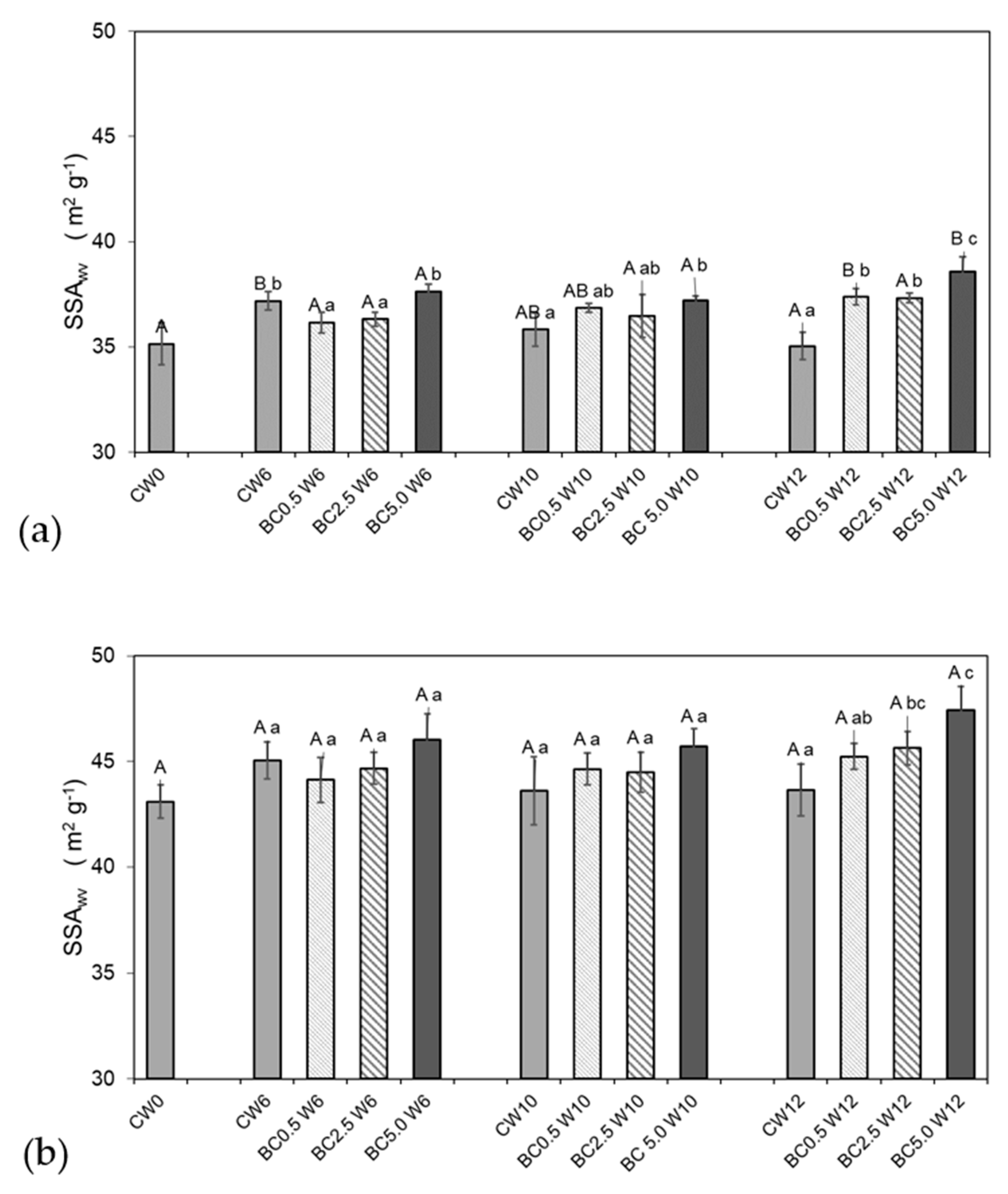
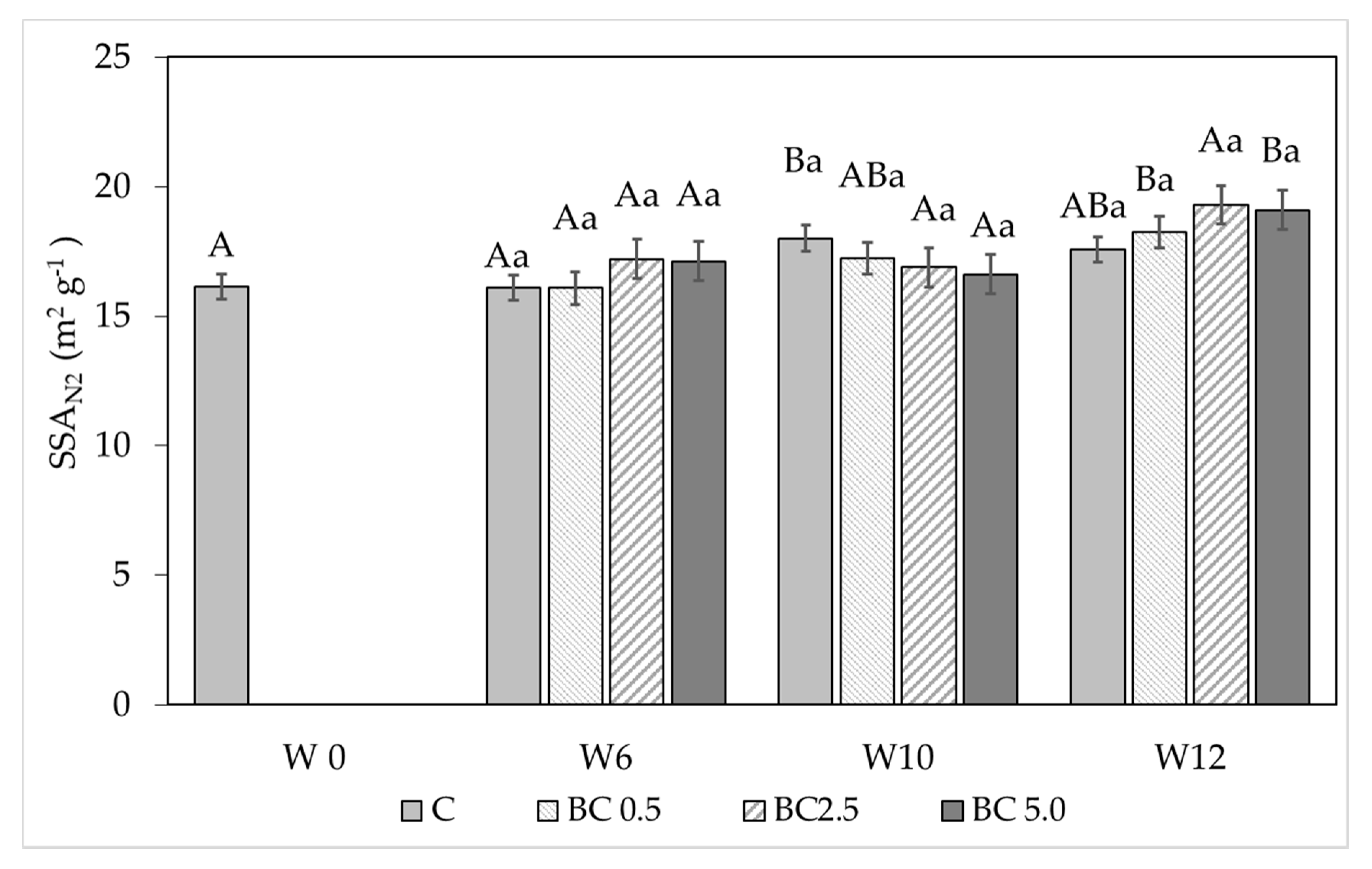
2.4. Soil Physical Measurements—Water Vapor Sorption and Soil Specific Surface Area
2.5. Arbuscular Mycorrhizal Fungal (AMF) and Glomalin Measurements
2.6. Statistical Analyses
3. Results
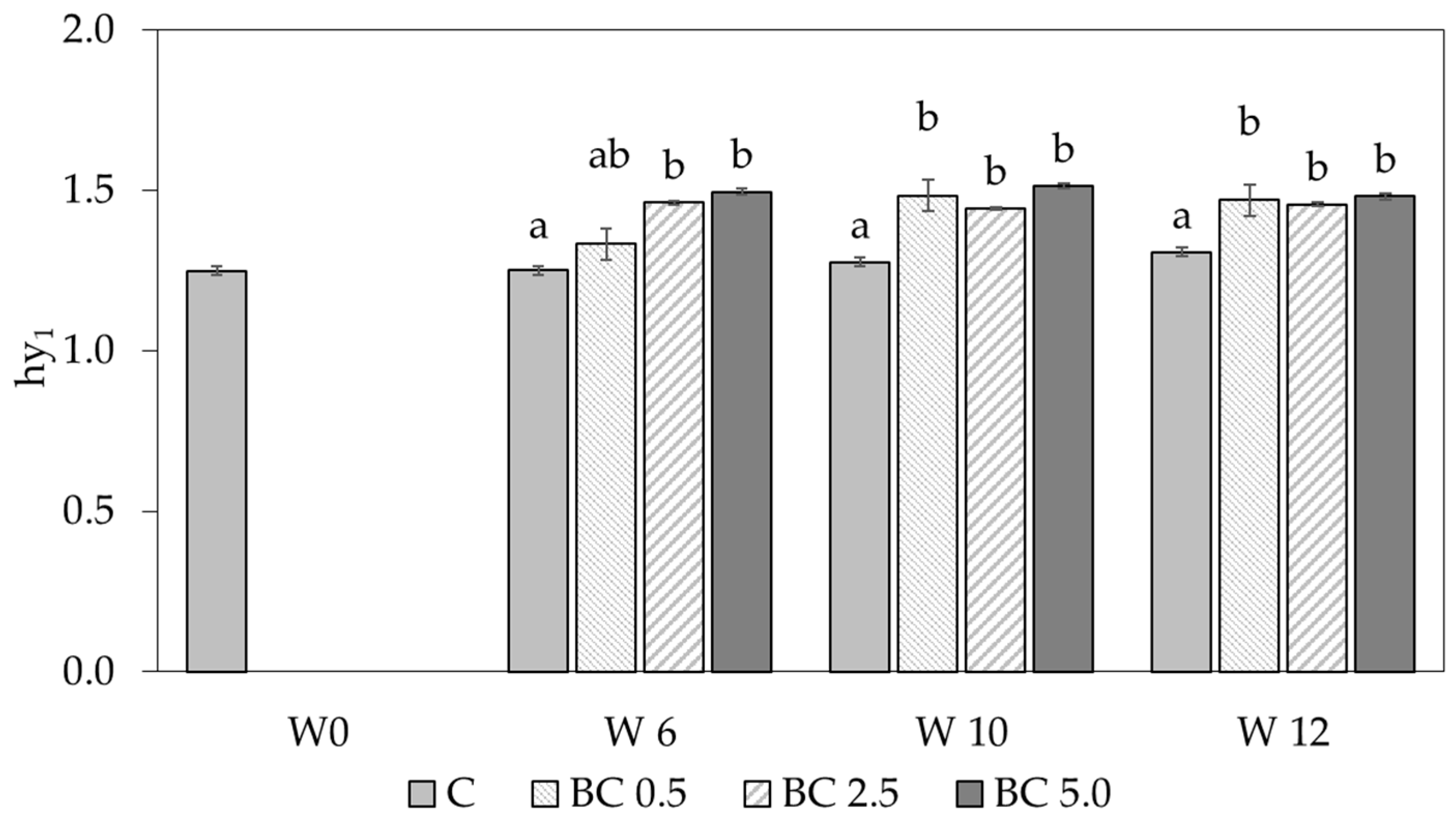
3.1. Hygroscopic Water Content and Water Vapor Sorption Changes
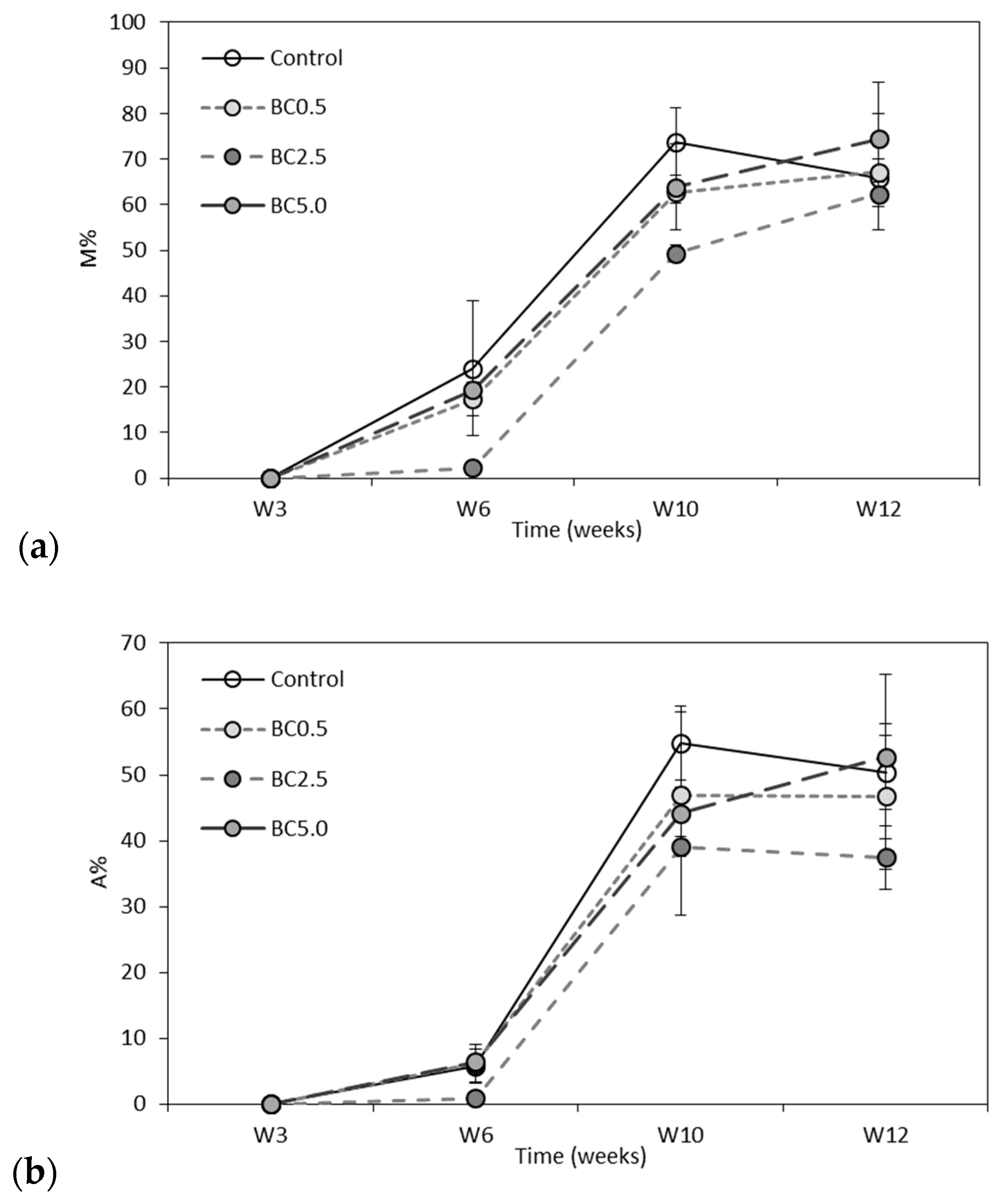
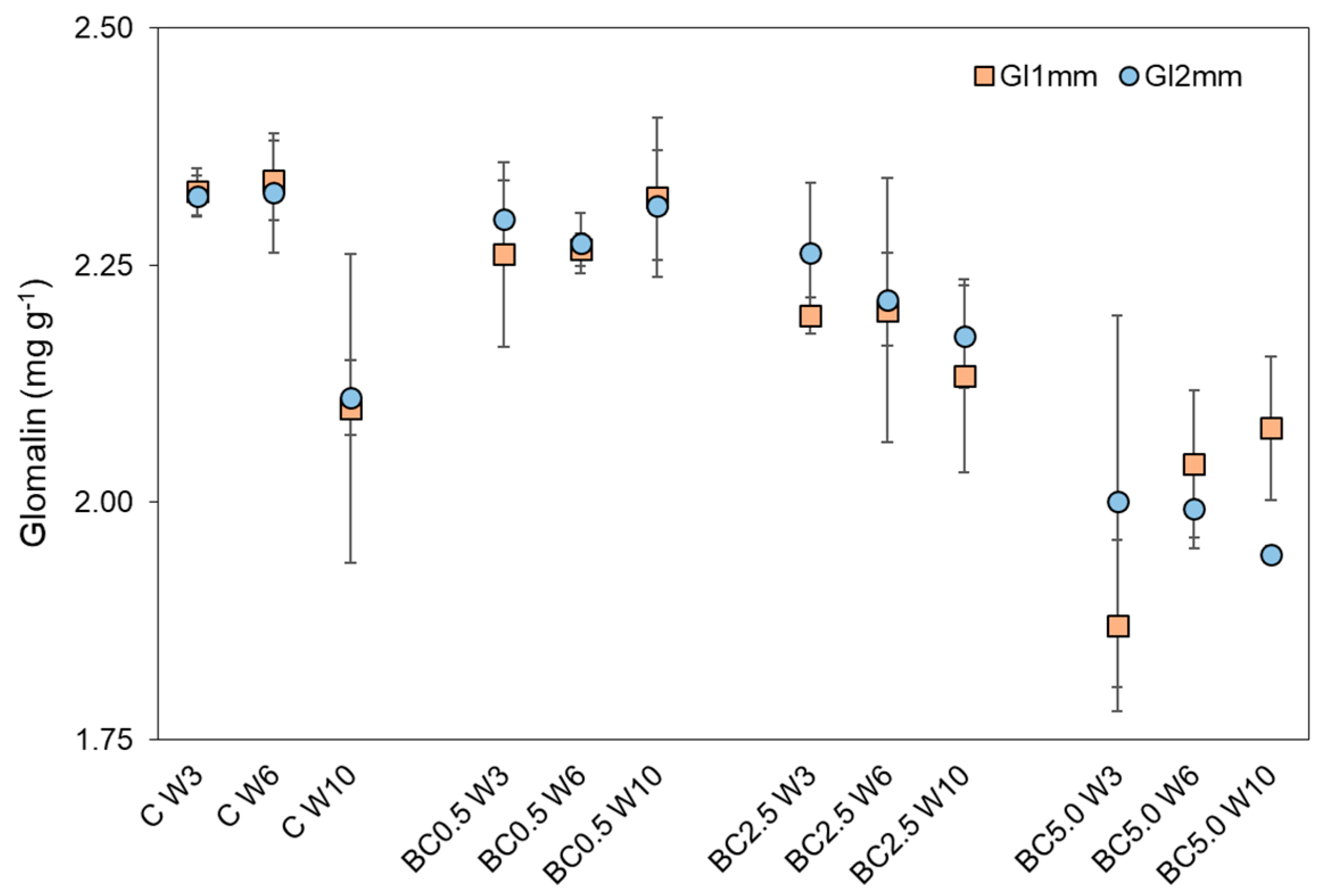
3.2. Arbuscular Mycorrhizal Fungal (AMF) Root Colonization and Glomalin Production
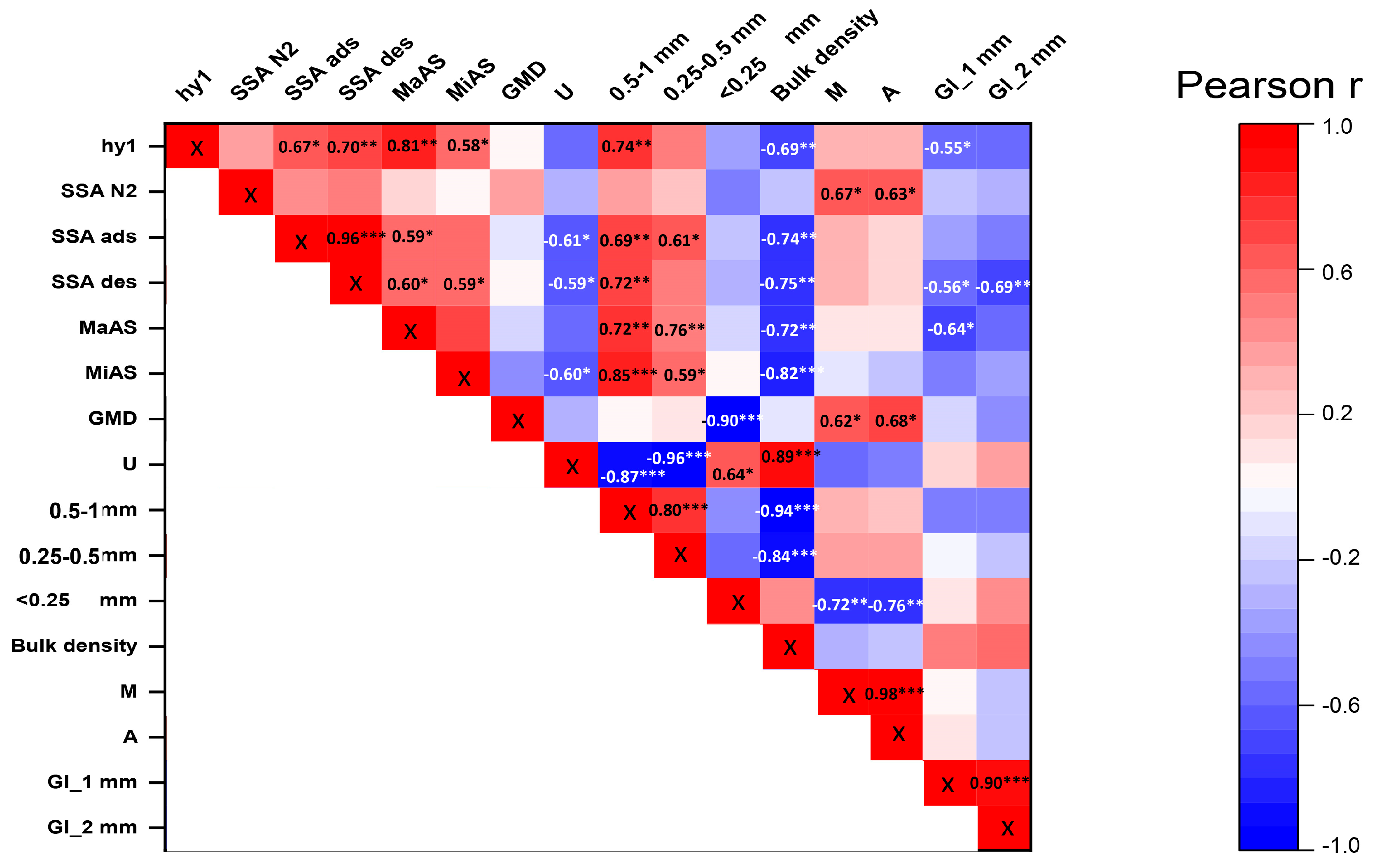
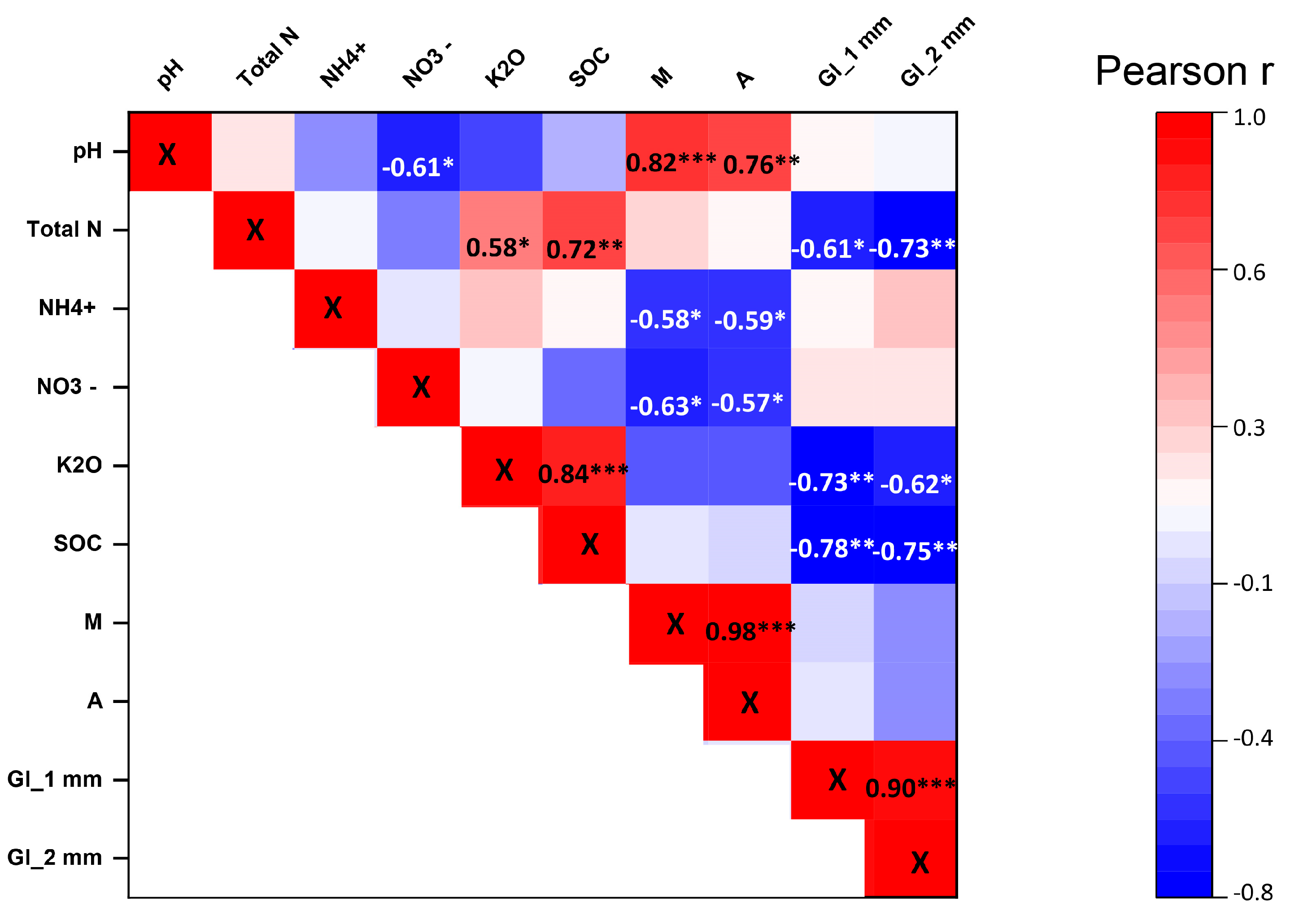
3.3. Relationships between the Soil Physical, Chemical, and Biological Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ye, S.; Zeng, G.; Wu, H.; Liang, J.; Zhang, C.; Dai, J.; Xiong, W.; Song, B.; Wu, S.; Yu, J. The effects of activated biochar addition on remediation efficiency of co-composting with contaminated wetland soil. Resour. Conserv. Recycl. 2019, 140, 278–285. [Google Scholar] [CrossRef]
- Makó, A.; Barna, G.; Horel, Á. Soil physical properties affected by biochar addition at different plant phaenological phases. Part II. Int. Agrophys. 2019, 1, 1–7. [Google Scholar] [CrossRef]
- Horák, J.; Šimanský, V.; Igaz, D. Biochar and Biochar with N Fertilizer Impact on Soil Physical Properties in a Silty Loam Haplic Luvisol. J. Ecol. Eng. 2019, 20, 31–38. [Google Scholar] [CrossRef]
- Sokołowska, Z.; Szewczuk-Karpisz, K.; Turski, M.; Tomczyk, A.; Cybulak, M.; Skic, K. Effect of Wood Waste and Sunflower Husk Biochar on Tensile Strength and Porosity of Dystric Cambisol Artificial Aggregates. Agronomy 2020, 10, 244. [Google Scholar] [CrossRef]
- Toková, L.; Igaz, D.; Horák, J.; Aydin, E. Effect of Biochar Application and Re-Application on Soil Bulk Density, Porosity, Saturated Hydraulic Conductivity, Water Content and Soil Water Availability in a Silty Loam Haplic Luvisol. Agronomy 2020, 10, 1005. [Google Scholar] [CrossRef]
- Horel, A.; Gelybó, G.; Potyó, I.; Pokovai, K.; Bakacsi, Z. Soil Nutrient Dynamics and Nitrogen Fixation Rate Changes over Plant Growth in Temperate Soil. Agronomy 2019, 9, 179. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, M.; Wang, J.; Liu, X.; Guo, W.; Zheng, J.; Bian, R.; Wang, G.; Zhang, X.; Cheng, K.; et al. The responses of soil organic carbon mineralization and microbial communities to fresh and aged biochar soil amendments. GCB Bioenergy 2019, 11, 1408–1420. [Google Scholar] [CrossRef]
- Pokovai, K.; Tóth, E.; Horel, Á. Growth and Photosynthetic Response of Capsicum annuum L. in Biochar Amended Soil. Appl. Sci. 2020, 10, 4111. [Google Scholar] [CrossRef]
- Farkas, É.; Feigl, V.; Gruiz, K.; Vaszita, E.; Fekete-Kertész, I.; Tolner, M.; Kerekes, I.; Pusztai, É.; Kari, A.; Uzinger, N.; et al. Long-term effects of grain husk and paper fibre sludge biochar on acidic and calcareous sandy soils—A scale-up field experiment applying a complex monitoring toolkit. Sci. Total. Environ. 2020, 731. [Google Scholar] [CrossRef]
- Sokołowska, Z.; Jamroz, J.; Bańka, P. Apparent surface area of selected meal extrudates. Int. Agrophys. 2008, 22, 75–80. [Google Scholar]
- Greenland, D.J.; Hayes, M.H.B. The Chemistry of Soil Processes; J. Wiley: Hoboken, NJ, USA, 1981. [Google Scholar]
- Chenu, C.; Stotzky, G. Interactions between Microorganisms and Soil Particles. An Overview. In Interactions Between Soil Particles and Microorganisms; Wiley-VCH-Verlag: Weinheim, Germany, 2002. [Google Scholar]
- Arthur, E.; Tuller, M.; Moldrup, P.; De Jonge, L.W. Effects of biochar and manure amendments on water vapor sorption in a sandy loam soil. Geoderma 2015, 243–244, 175–182. [Google Scholar] [CrossRef]
- Hardie, M.; Clothier, B.; Bound, S.; Oliver, G.; Close, D. Does biochar influence soil physical properties and soil water availability? Plant. Soil 2014, 376, 347–361. [Google Scholar] [CrossRef]
- Horel, A.; Tóth, E.; Gelybó, G.; Dencső, M.; Farkas, C. Biochar Amendment Affects Soil Water and CO2 Regime during Capsicum Annuum Plant Growth. Agronomy 2019, 9, 58. [Google Scholar] [CrossRef]
- Gray, M.; Johnson, M.G.; Dragila, M.I.; Kleber, M. Water uptake in biochars: The roles of porosity and hydrophobicity. Biomass Bioenergy 2014, 61, 196–205. [Google Scholar] [CrossRef]
- Skic, K.; Boguta, P.; Sokołowska, Z. Analysis of the sorption properties of different soils using water vapour adsorption and potentiometric titration methods. Int. Agrophys. 2016, 30, 369–374. [Google Scholar] [CrossRef]
- Cybulak, M.; Sokołowska, Z.; Boguta, P. Hygroscopic moisture content of podzolic soil with biochar. Acta Agrophys. 2016, 23, 533–543. [Google Scholar]
- Antal, M.J.; Grønli, M. The Art, Science, and Technology of Charcoal Production. Ind. Eng. Chem. Res. 2003, 42, 1619–1640. [Google Scholar] [CrossRef]
- Lu, N.; Zhang, C. Separating External and Internal Surface Areas of Soil Particles. J. Geotech. Geoenviron. Eng. 2020, 146. [Google Scholar] [CrossRef]
- Galarneau, A.; Mehlhorn, D.; Guenneau, F.; Coasne, B.; Villemot, F.; Minoux, D.; Aquino, C.C.; Dath, J.-P. Specific Surface Area Determination for Microporous/Mesoporous Materials: The Case of Mesoporous FAU-Y Zeolites. Langmuir 2018, 34, 14134–14142. [Google Scholar] [CrossRef]
- De Jong, E. Comparison of three methods of measuring surface area of soils. Can. J. Soil Sci. 1999, 79, 345–351. [Google Scholar] [CrossRef]
- Paul, B.; Vanlauwe, B.; Ayuke, F.O.; Gassner, A.; Hoogmoed, M.; Hurisso, T.; Koala, S.; Lelei, D.K.; Ndabamenye, T.; Six, J.; et al. Medium-term impact of tillage and residue management on soil aggregate stability, soil carbon and crop productivity. Agric. Ecosyst. Environ. 2013, 164, 14–22. [Google Scholar] [CrossRef]
- Amézketa, E. Soil Aggregate Stability: A Review. J. Sustain. Agric. 1999, 14, 83–151. [Google Scholar] [CrossRef]
- Costa, O.Y.A.; Raaijmakers, J.M.; Kuramae, E.E. Microbial Extracellular Polymeric Substances: Ecological Function and Impact on Soil Aggregation. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C.; Mummey, D.L. Mycorrhizas and soil structure. New Phytol. 2006, 171, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Totsche, K.U.; Amelung, W.; Gerzabek, M.H.; Guggenberger, G.; Klumpp, E.; Knief, C.; Lehndorff, E.; Mikutta, R.; Peth, S.; Prechtel, A.; et al. Microaggregates in soils. J. Plant. Nutr. Soil Sci. 2018, 181, 104–136. [Google Scholar] [CrossRef]
- Pal, A.; Pandey, S. Role of Glomalin in Improving Soil Fertility: A Review. Int. J. Plant. Soil Sci. 2014, 3, 1112–1129. [Google Scholar] [CrossRef] [PubMed]
- Magurno, F.; Malicka, M.; Posta, K.; Wozniak, G.; Lumini, E.; Piotrowska-Seget, Z. Glomalin gene as molecular marker for functional diversity of arbuscular mycorrhizal fungi in soil. Biol. Fertil. Soils 2019, 55, 411–417. [Google Scholar] [CrossRef]
- Rillig, M.C. Arbuscular mycorrhizae, glomalin, and soil aggregation. Can. J. Soil Sci. 2004, 84, 355–363. [Google Scholar] [CrossRef]
- Rosier, C.L.; Piotrowski, J.S.; Hoye, A.T.; Rillig, M.C. Intraradical protein and glomalin as a tool for quantifying arbuscular mycorrhizal root colonization. Pedobiologia 2008, 52, 41–50. [Google Scholar] [CrossRef]
- Wilson, G.W.T.; Rice, C.W.; Rillig, M.C.; Springer, A.; Hartnett, D.C. Soil aggregation and carbon sequestration are tightly correlated with the abundance of arbuscular mycorrhizal fungi: Results from long-term field experiments. Ecol. Lett. 2009, 12, 452–461. [Google Scholar] [CrossRef]
- Koide, R.T. Chapter 25—Biochar—Arbuscular Mycorrhiza Interaction in Temperate Soils. In Mycorrhizal Mediation of Soil; Johnson, N.C., Gehring, C., Jansa, J., Eds.; Elsevier: Berlin, Germany, 2017; pp. 461–477. [Google Scholar] [CrossRef]
- Warnock, D.D.; Lehmann, J.; Kuyper, T.W.; Rillig, M.C. Mycorrhizal responses to biochar in soil—Concepts and mechanisms. Plant. Soil 2007, 300, 9–20. [Google Scholar] [CrossRef]
- Dövényi, Z. Magyarország Kistájainak Katasztere; MTA Földrajztudományi Kutatóintézet: Budapest, Hungary, 2010; p. 876. (In Hungarian) [Google Scholar]
- Uzinger, N.; Takács, T.; Szili-Kovács, T.; Radimszky, L.; Füzy, A.; Draskovits, E.; Szűcs-Vásárhelyi, N.; Molnár, M.; Farkas, É.; Kutasi, J.; et al. Fertility Impact of Separate and Combined Treatments with Biochar, Sewage Sludge Compost and Bacterial Inocula on Acidic Sandy Soil. Agronomy 2020, 10, 1612. [Google Scholar] [CrossRef]
- Lim, T.; Spokas, K.A.; Feyereisen, G.W.; Novak, J. Predicting the impact of biochar additions on soil hydraulic properties. Chemosphere 2016, 142, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Alburquerque, J.A.; Calero, J.M.; Barrón, V.; Torrent, J.; Del Campillo, M.C.; Gallardo, A.; Villar, R. Effects of biochars produced from different feedstocks on soil properties and sunflower growth. J. Plant. Nutr. Soil Sci. 2014, 177, 16–25. [Google Scholar] [CrossRef]
- Schon, M.; Compton, M.P.; Bell, E.; Burns, I. Nitrogen Concentrations Affect Pepper Yield and Leachate Nitrate-nitrogen from Rockwool Culture. HortScience 1994, 29, 1139–1142. [Google Scholar] [CrossRef]
- Meier, U. Growth Stages of Mono- and Dicotyledonous Plants. BBCH-Monograph, 2nd ed.; Blackwell Science: Berlin, Germany, 2001. [Google Scholar]
- Di Gléria, J.; Klimes-Szmik, A.; Dvoracsek, M. Bodenphysik und Bodenkolloidik; Akadémiai Kiadó: Budapest, Hungary, 1962; p. 795. [Google Scholar]
- ALFA-WERO. Polish Standard PN-Z-19010–1 Soil Quality. In Determination of Specific Surface Area of Soils by Water Sorption (BET); ALFA-WERO: Warsaw, Poland, 1997. (In Polish) [Google Scholar]
- Mooney, R.W.; Keenan, A.G.; Wood, L.A. Adsorption of Water Vapor by Montmorillonite. I. Heat of Desorption and Application of BET Theory1. J. Am. Chem. Soc. 1952, 74, 1367–1371. [Google Scholar] [CrossRef]
- Phillips, J.; Hayman, D. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Trouvelot, A.; Kought, J.L.; Gianinazzi-Pearson, V. Mesure du taux de mycorhization VA d’un systeme radiculaire. Recherche de methodes d’estimation ayant une signification fonctionnelle. In Physiological and Genetical Aspects of Mycorrhizae; Gianinazzi, S., Gianinazzi-Pearson, V., Eds.; INRA: Paris, France, 1986; pp. 217–221. ISBN 285340-774-8. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-Dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Wright, S.; Upadhyaya, A. A survey of soils for aggregate stability and glomalin, a glycoprotein produced by hyphae of arbuscular mycorrhizal fungi. Plant. Soil 1998, 198, 97–107. [Google Scholar] [CrossRef]
- Brunauer, S.; Deming, L.S.; Deming, W.E.; Teller, E. On a Theory of the van der Waals Adsorption of Gases. J. Am. Chem. Soc. 1940, 62, 1723–1732. [Google Scholar] [CrossRef]
- Skic, K.; Sokołowska, Z.; Boguta, P.; Skic, A. The effect of application of digestate and agro-food industry sludges on Dystric Cambisol porosity. PLoS ONE 2020, 15, e0238469. [Google Scholar] [CrossRef] [PubMed]
- Horel, Á.; Barna, G.; Makó, A. Soil physical properties affected by biochar addition at different plant phaenological phases. Part I. Int. Agrophys. 2019, 33, 255–262. [Google Scholar] [CrossRef]
- Boguta, P.; Sokołowska, Z.; Skic, K.; Tomczyk, A. Chemically engineered biochar—Effect of concentration and type of modifier on sorption and structural properties of biochar from wood waste. Fuel 2019, 256. [Google Scholar] [CrossRef]
- Bonelli, P.R.; Buonomo, E.L.; Cukierman, A.L. Pyrolysis of Sugarcane Bagasse and Co-pyrolysis with an Argentinean Subbituminous Coal. Energy Sourc. Part A Recover. Util. Environ. Eff. 2007, 29, 731–740. [Google Scholar] [CrossRef]
- Katyal, S.; Thambimuthu, K.; Valix, M. Carbonisation of bagasse in a fixed bed reactor: Influence of process variables on char yield and characteristics. Renew. Energy 2003, 28, 713–725. [Google Scholar] [CrossRef]
- Cybulak, M.; Sokołowska, Z.; Boguta, P. Impact of Biochar on Physicochemical Properties of Haplic Luvisol Soil under Different Land Use: A Plot Experiment. Agronomy 2019, 9, 531. [Google Scholar] [CrossRef]
- Baiamonte, G.; Crescimanno, G.; Parrino, F.; De Pasquale, C. Effect of biochar on the physical and structural properties of a sandy soil. Catena 2019, 175, 294–303. [Google Scholar] [CrossRef]
- Cao, T.; Chen, W.; Yang, T.; He, T.; Liu, Z.; Meng, J. Surface Characterization of Aged Biochar Incubated in Different Types of Soil. Bioresources 2017, 12, 6366–6377. [Google Scholar] [CrossRef]
- Agegnehu, G.; Srivastava, A.; Bird, M.I. The role of biochar and biochar-compost in improving soil quality and crop performance: A review. Appl. Soil Ecol. 2017, 119, 156–170. [Google Scholar] [CrossRef]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Ahmed, N.; Zhang, L. Role of Arbuscular Mycorrhizal Fungi in Plant Growth Regulation: Implications in Abiotic Stress Tolerance. Front. Plant. Sci. 2019, 10. [Google Scholar] [CrossRef]
- Garmendia, I.; Goicoechea, N.; Aguirreolea, J. Effectiveness of three Glomus species in protecting pepper (Capsicum annuum L.) against verticillium wilt. Biol. Control. 2004, 31, 296–305. [Google Scholar] [CrossRef]
- Hernádi, I.; Sasvári, Z.; Albrechtová, J.; Vosátka, M.; Posta, K. Arbuscular Mycorrhizal Inoculant Increases Yield of Spice Pepper and Affects the Indigenous Fungal Community in the Field. HortScience 2012, 47, 603–606. [Google Scholar] [CrossRef]
- Latef, A.A.H.A.; Chaoxing, H. Does Inoculation with Glomus mosseae Improve Salt Tolerance in Pepper Plants? J. Plant. Growth Regul. 2014, 33, 644–653. [Google Scholar] [CrossRef]
- Regvar, M.; Vogel-Mikuš, K.; Ševerkar, T. Effect of AMF inoculum from field isolates on the yield of green pepper, parsley, carrot, and tomato. Folia Geobot. Phytotaxon. 2003, 38, 223–234. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Editorial: Biostimulants in Agriculture. Front. Plant. Sci. 2020, 11. [Google Scholar] [CrossRef]
- Selvakumar, G.; Yi, P.H.; Lee, S.E.; Shagol, C.C.; Han, S.G.; Sa, T.; Chung, B.N. Effects of Long-Term Subcultured Arbuscular Mycorrhizal Fungi on Red Pepper Plant Growth and Soil Glomalin Content. Mycobiology 2018, 46, 122–128. [Google Scholar] [CrossRef]
- Sensoy, S.; Demir, S.; Türkmen, Ö.; Erdinc, C.; Savur, O.B. Responses of some different pepper (Capsicum annuum L.) genotypes to inoculation with two different arbuscular mycorrhizal fungi. Sci. Hortic. 2007, 113, 92–95. [Google Scholar] [CrossRef]
- Wu, Q.-S.; Cao, M.-Q.; Zou, Y.-N.; Chengjun, J. Direct and indirect effects of glomalin, mycorrhizal hyphae and roots on aggregate stability in rhizosphere of trifoliate orange. Sci. Rep. 2015, 4. [Google Scholar] [CrossRef]
- Rillig, M.C.; Steinberg, P.D. Glomalin production by an arbuscular mycorrhizal fungus: A mechanism of habitat modification? Soil Biol. Biochem. 2002, 34, 1371–1374. [Google Scholar] [CrossRef]
- Lee, J.-E.; Eom, A.-H. Effect of Organic Farming on Spore Diversity of Arbuscular Mycorrhizal Fungi and Glomalin in Soil. Mycobiology 2009, 37, 272–276. [Google Scholar] [CrossRef]
- Steinberg, P.D.; Rillig, M.C. Differential decomposition of arbuscular mycorrhizal fungal hyphae and glomalin. Soil Biol. Biochem. 2003, 35, 191–194. [Google Scholar] [CrossRef]
- Amendola, C.; Montagnoli, A.; Terzaghi, M.; Trupiano, D.; Oliva, F.; Baronti, S.; Miglietta, F.; Chiatante, D.; Scippa, G. Short-term effects of biochar on grapevine fine root dynamics and arbuscular mycorrhizae production. Agric. Ecosyst. Environ. 2017, 239, 236–245. [Google Scholar] [CrossRef]
- Šimanský, V. Effects of biochar and biochar with nitrogen on soil organic matter and soil structure in haplic Luvisol. Acta Fytotech. Zootech. 2016, 19, 129–138. [Google Scholar] [CrossRef]
- LeCroy, C.; Masiello, C.A.; Rudgers, J.A.; Hockaday, W.C.; Silberg, J.J. Nitrogen, biochar, and mycorrhizae: Alteration of the symbiosis and oxidation of the char surface. Soil Biol. Biochem. 2013, 58, 248–254. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, X.; Zhong, S.; Yin, G.; Gao, Y.; He, X. Recalcitrant carbon components in glomalin-related soil protein facilitate soil organic carbon preservation in tropical forests. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef]
| Chemical and Physical Parameters at t = 0 | Soil | SD | Biochar | SD |
|---|---|---|---|---|
| 2–0.052 mm (%) | 25.84 | ±0.64 | 84.52 | ±0.08 |
| 0.052–0.0066 mm (%) | 50.03 | ±0.76 | 13.90 | ±0.70 |
| <0.0066 mm (%) | 24.13 | ±0.15 | 1.57 | ±4.23 |
| pH(H2O) | 7.97 | ±0.04 | 10.33 | ±0 |
| Total N (%) | 0.14 | ±0.02 | 1.0 | ±0.1 |
| NH4+ (mg kg−1) | 5.84 | ±1.01 | 1.9 | ±0.1 |
| CaCO3 (%) | 10.41 | ±0.34 | 5.75 a | − |
| P2O5 (mg kg−1) | 977.9 | ±158.1 | 5031.1 | ±32.6 |
| K2O (mg kg−1) | 443.1 | ±96.2 | 13,570.3 | ±59.0 |
| OC (%) | 0.93 | ±0.07 | 27.89 | ±1.73 |
| EC 2.5 (mS cm−1) * | 0.2 | ±0.01 | 3.03 | − |
| VWCinitial (%) | 26.51 | ±3.7 | − | − |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barna, G.; Makó, A.; Takács, T.; Skic, K.; Füzy, A.; Horel, Á. Biochar Alters Soil Physical Characteristics, Arbuscular Mycorrhizal Fungi Colonization, and Glomalin Production. Agronomy 2020, 10, 1933. https://doi.org/10.3390/agronomy10121933
Barna G, Makó A, Takács T, Skic K, Füzy A, Horel Á. Biochar Alters Soil Physical Characteristics, Arbuscular Mycorrhizal Fungi Colonization, and Glomalin Production. Agronomy. 2020; 10(12):1933. https://doi.org/10.3390/agronomy10121933
Chicago/Turabian StyleBarna, Gyöngyi, András Makó, Tünde Takács, Kamil Skic, Anna Füzy, and Ágota Horel. 2020. "Biochar Alters Soil Physical Characteristics, Arbuscular Mycorrhizal Fungi Colonization, and Glomalin Production" Agronomy 10, no. 12: 1933. https://doi.org/10.3390/agronomy10121933
APA StyleBarna, G., Makó, A., Takács, T., Skic, K., Füzy, A., & Horel, Á. (2020). Biochar Alters Soil Physical Characteristics, Arbuscular Mycorrhizal Fungi Colonization, and Glomalin Production. Agronomy, 10(12), 1933. https://doi.org/10.3390/agronomy10121933







