Biochar and Its Effects on Plant–Soil Macronutrient Cycling during a Three-Year Field Trial on Sandy Soil with Peppermint (Mentha piperita L.). Part I: Yield and Macro Element Content in Soil and Plant Biomass
Abstract
1. Introduction
2. Material and Methods
2.1. Field Trial
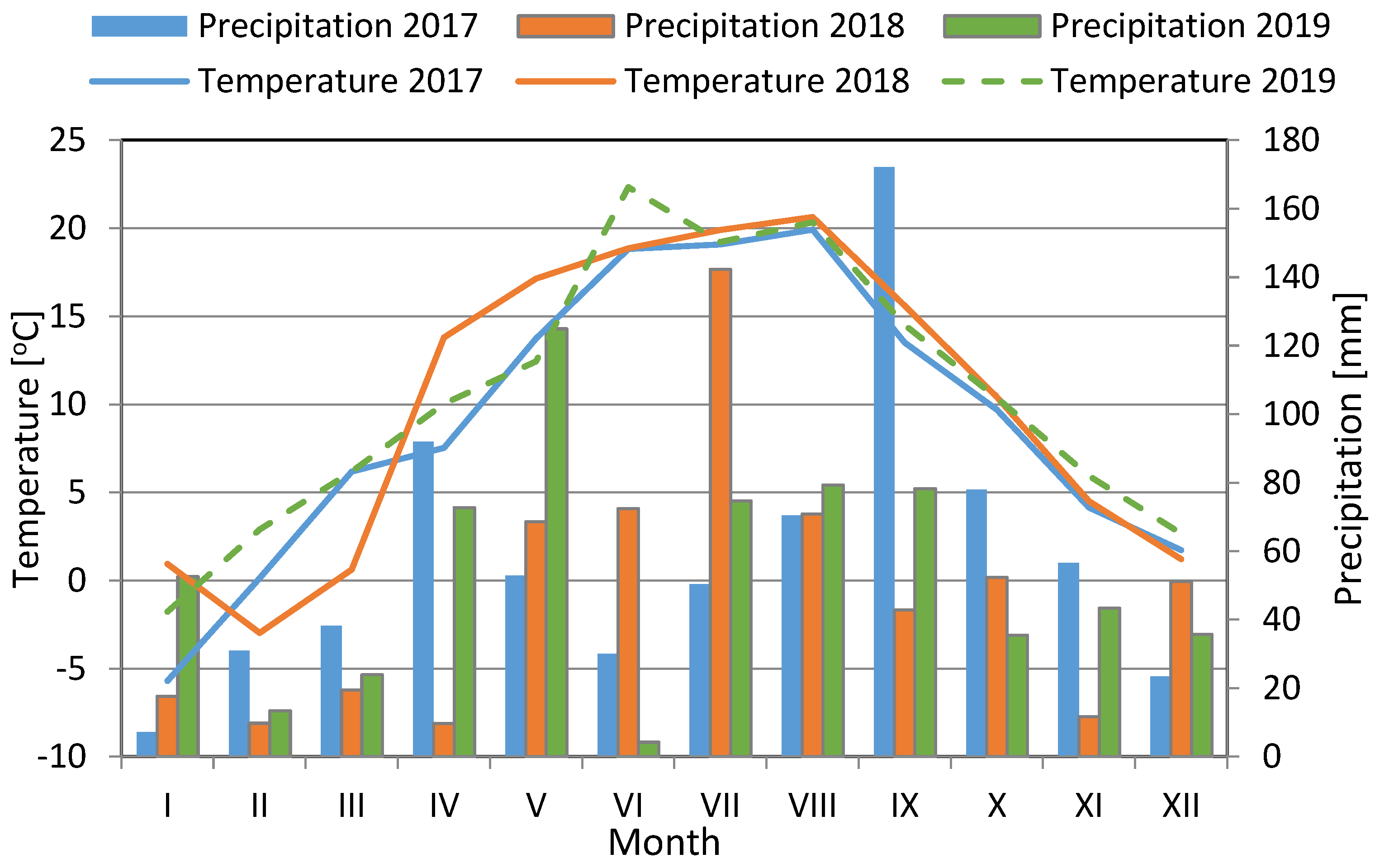
2.2. Climatic Condition
2.3. Plant Analysis
2.4. Soil Analysis
2.5. Statistical Analysis
3. Results and Discussion
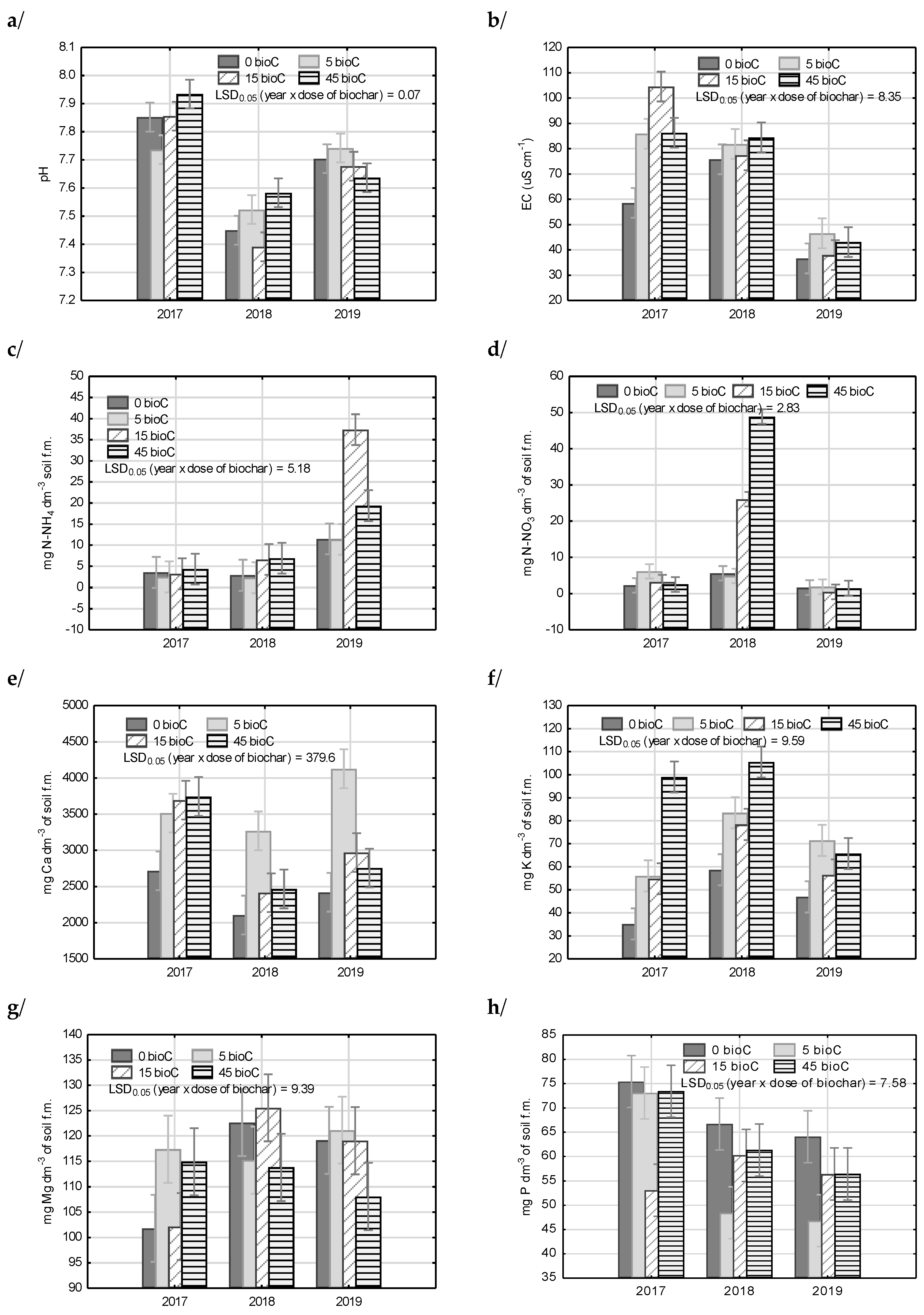
3.1. Chemical Properties of Soil
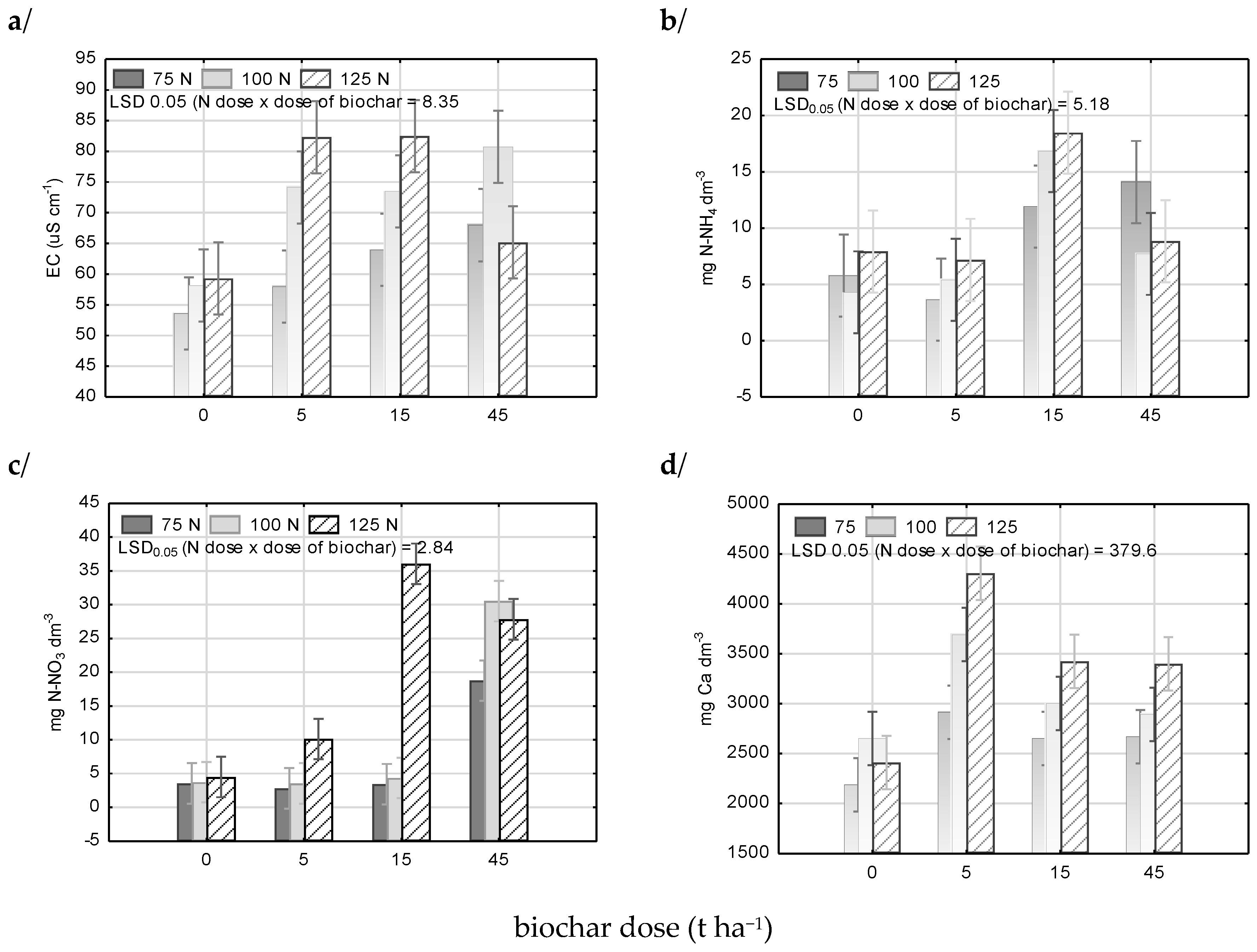
3.2. Soil Reaction (pH) and Salts Concentration in Soil Solution
3.3. Mineral Nitrogen Content in Soil
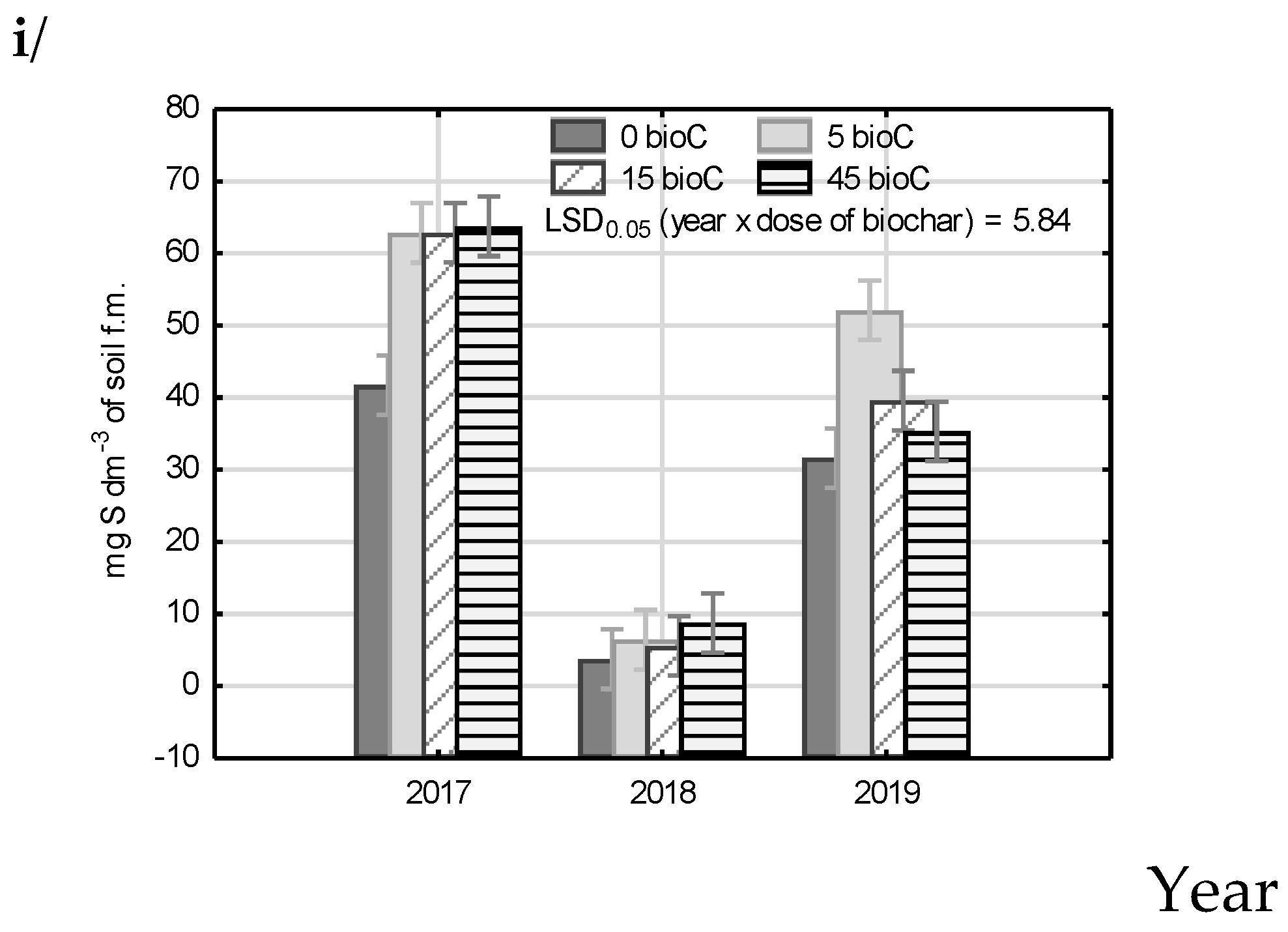
3.4. Available Macronutrients in Soil
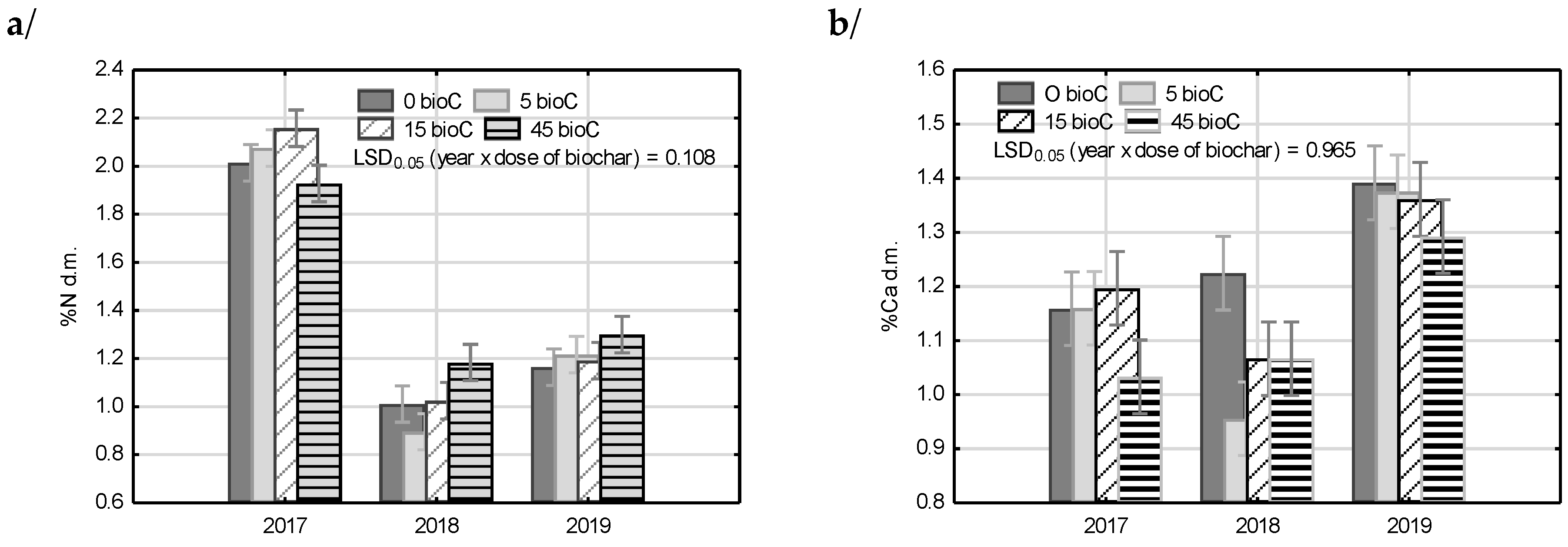
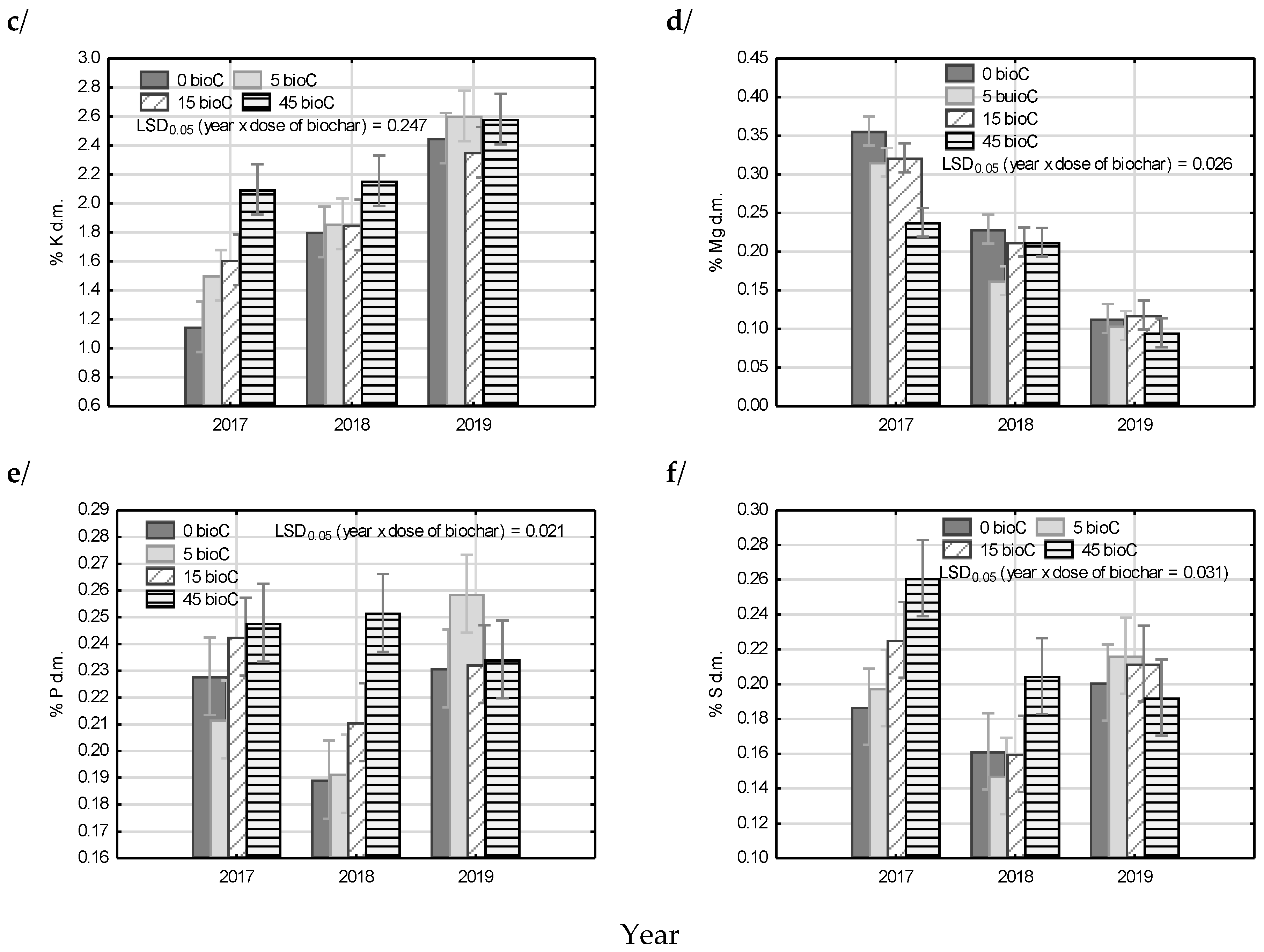
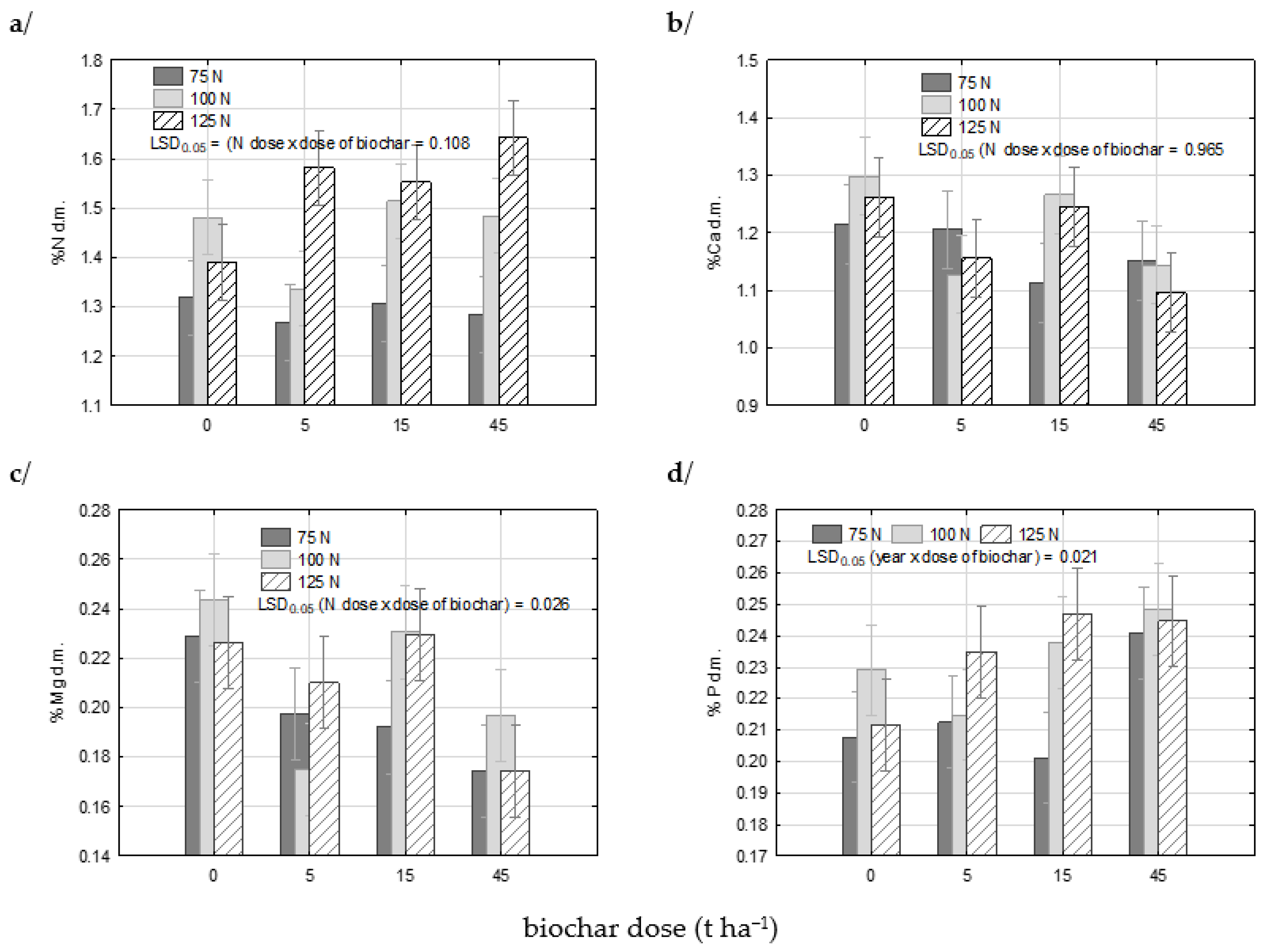
3.5. Plant Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lehmann, J.; da Silva, J.P.; Steiner, C.; Nehls, T.; Zech, W.; Glaser, B. Nutrient availability and leaching in an archaeological anthrosol and a ferralsol of the central amazon basin: Fertilizer. manure and charcoal amendments. Plant Soil 2003, 249, 343–357. [Google Scholar] [CrossRef]
- Sohi, S.P.; Krull, E.; Lopez-Capel, E.; Bol, R. A review of biochar and its use and function in soil. In Advances in Agronomy; Sparks, D.L., Ed.; Elsevier Academic Press Inc.: San Diego, CA, USA, 2010; Volume 105, pp. 47–82. [Google Scholar]
- Jones, D.L.; Rousk, J.; Edwards-Jones, G.; DeLuca, T.H.; Murphy, D.V. Biochar-mediated changes in soil quality and plant growth in a three year field trial. Soil Biol. Biochem. 2012, 45, 113–124. [Google Scholar] [CrossRef]
- Clough, T.J.; Condron, L.M.; Kammann, C.; Müller, C. Review of Biochar and Soil Nitrogen Dynamics. Agronomy 2013, 3, 275–293. [Google Scholar] [CrossRef]
- Riedel, T.; Hennessy, P.; Iden, S.C.; Koschinsky, A. Leaching of soil-derived major and trace elements in an arable topsoil after the addition of biochar. Eur. J. Soil Sci. 2015, 66, 823–834. [Google Scholar] [CrossRef]
- Bista, P.; Ghimire, R.; Machado, S.; Pritchett, L. Biochar effects on soil properties and wheat biomass vary with fertility management. Agronomy 2019, 9, 623. [Google Scholar] [CrossRef]
- Baigorri, R.; San Francisco, S.; Urrutia, O.; Garcia-Mina, J.M. Biochar-Ca and Biochar-Al/-Fe-Medited Phosphate Exchange Capacity are Main Drivers of the Different Biochar Effects on Plant in Acidic and Alkaline Soils. Agronomy 2020, 10, 968. [Google Scholar] [CrossRef]
- Jeffery, S.; Verheijen, F.G.A.; van der Velde, M.; Bastos, A.C. A quantitative review of the effects of biochar application to soils on crop productivity using meta-analysis. Agr. Ecosyst. Environ. 2011, 144, 175–187. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, Y.; Liu, S.; Li, Z.; Tan, X.; Zeng, G.; Zhou, L.; Zheng, B. Biochar to improve soil fertility. A review. Agron. Sustain. Dev. 2016, 36, 36. [Google Scholar] [CrossRef]
- Zornoza, R.; Moreno-Barriga, F.; Acosta, J.A.; Muñoz, M.A.; Faz, A. Stability, nutrient availability and hydrophobicity of biochars derived from manure. crop residues. and municipal solid waste for their use as soil amendments. Chemosphere 2016, 144, 122–130. [Google Scholar] [CrossRef]
- Lima, J.R.S.; Silva, W.M.; Medeiros, E.V.; Duda, G.P.; Corrêa, M.M.; Filho, A.P.M.; Clermont-Dauphin, C.; Antonino, A.C.D.; Hammecker, C. Effect of biochar on physicochemical properties of a sandy soil and maize growth in a greenhouse experiment. Geoderma 2018, 319, 14–23. [Google Scholar] [CrossRef]
- Edeh, G.; Ondřej, M.; Bussb, W. A meta-analysis on biochar's effects on soil water properties—New insights and future research challenges. Sci. Total Environ. 2020, 714, 136857. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Lu, T.; Wang, Y.; Chen, Y.; Lei, T. Sewage sludge biochar: Nutrient composition and its effect on the leaching of soil nutrients. Geoderma 2016, 267, 17–23. [Google Scholar] [CrossRef]
- Mukherjee, A.; Zimmerman, A.R. Organic carbon and nutrient release from a range of laboratory-produced biochars and biochar-soil mixtures. Geoderma 2013, 193–194, 122–130. [Google Scholar] [CrossRef]
- Brassard, P.; Godbout, S.; Palacios, J.H.; Jeanne, T.; Hogue, R.; Dubé, P.; Limousy, L.; Raghavan, V. Effect of six engineered biochars on GHG emissions from two agricultural soils: A short-term incubation study. Geoderma 2018, 327, 73–84. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota—A review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Mukherjee, A.; Lal, R. The biochar dilemma. Soil Res. 2014, 52, 217–230. [Google Scholar] [CrossRef]
- Rajkovich, S.; Enders, A.; Hanley, K.; Hyland, C.; Zimmerman, A.R.; Lehmann, J. Corn growth and nitrogen nutrition after additions of biochars with varying properties to a temperate soil. Bio Fert. Soils 2012, 48, 271–284. [Google Scholar] [CrossRef]
- Domingues, R.R.; Trugilho, P.F.; Silva, C.A. Properties of biochar derived from wood and high nutrient biomasses with the aim of agronomic and environmental benefits. PLoS ONE 2017, 12, 1–19. [Google Scholar] [CrossRef]
- Tsai, W.T.; Huang, P.C.; Lin, Y.Q. Characterization of Biochars Produced from Dairy Manure at High Pyrolysis Temperatures. Agronomy 2019, 9, 634. [Google Scholar] [CrossRef]
- Bruun, E.W.; Hauggaardd-Nielsen, H.; Ibradhim, N.; Egsgaard, H.; Ambus, P.; Jensen, A.P.; Dam-Johanse, K. Influence of fast pyrolysis temperature on biochar labile fraction and short-term carbon loss in a loamy soil. Biomass Bioenerg. 2011, 35, 1182–1189. [Google Scholar] [CrossRef]
- Gul, S.; Whalen, J.K. Biochemical cycling of nitrogen and phosphorus in biochar-amended soils. Soil Biol. Biochem. 2016, 103, 1–15. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, Z.; Deng, X.; Zhao, J.; Luo, Y.; Novak, J.; Herbert, S.; Xing, B. Characteristics and nutrient values of biochars produced from giant reed at different temperatures. Bioresour. Technol. 2013, 130, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Quosai, P.; Anstey, A.; Mohanty, A.K.; Misra, M. Characterization of biocarbon generated by high- and low-temperature pyrolysis of soy hulls and cofee chaf: For polymer composite applications. R. Soc. Open Sci. 2018, 5, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Bruun, E.; Cross, A.; Hammond, J.; Nelissen, V.; Rasse, D.P.; Hauggaard-Nielsen, H. Biochar carbon stability and effect on greenhouse gas emissions. In Biochar in European Soils and Agriculture: Science and Practice; Shackley, S., Ruysschaert, G., Zwart, K., Glaser, B., Eds.; Earthscan: London, UK, 2016; pp. 165–183. [Google Scholar]
- Libutti, A.; Mucci, M.; Francavilla, M.; Monteleone, M. Effect of biochar amendment on nitrate retention in a silty clay loam soil. Ital. J. Agro. 2016, 11, 273–276. [Google Scholar] [CrossRef]
- Libutti, A.; Cammerino, R.A.; Francavilla, M.; Monteleone, M. Soil Amendment with Biochar Affects Water Drainage and Nutrient Losses by Leaching: Experimental Evidence under Field-Grown Conditions. Agronomy 2019, 9, 758. [Google Scholar] [CrossRef]
- Spokas, K.A.; Novak, J.M.; Venterea, R.T. Biochar’s role as an alternative N-fertilizer: Ammonia capture. Plant Soil 2012, 350, 35–42. [Google Scholar] [CrossRef]
- Nguyen, T.T.N.; Xu, C.Y.; Tahmasbian, I.; Che, R.; Xu, Z.; Zhou, X.; Wallace, H.M.; Bai, S.H. Effects of biochar on soil available inorganic nitrogen: A review and metaanalysis. Geoderma 2017, 288, 79–96. [Google Scholar] [CrossRef]
- Steiner, C.; Glaser, B.; Teixeira, W.G.; Lehmann, J.; Blum, W.E.H.; Zech, W. Nitrogen retention and plant uptake on a highly weathered central Amazonian Ferralsol amended with compost and charcoal. J. Plant Nutr. Soil Sci. 2008, 171, 893–899. [Google Scholar] [CrossRef]
- Chan, K.Y.; Xu, Z. Biochar: Nutrient properties and their enhancement. In Biochar for Environmental Management: Science and Technology; Lehmann, J., Joseph, S., Eds.; Earthscan: London, UK, 2009; Volume 1, pp. 67–84. [Google Scholar]
- Yao, Y.; Gao, B.; Zhang, M.; Inyang, M.; Zimmerman, A.R. Effect of biochar amendment on sorption and leaching of nitrate, ammonium, and phosphate in a sandy soil. Chemosphere 2012, 89, 1467–1471. [Google Scholar] [CrossRef]
- Mukherjee, A.; Zimmerman, A.R.; Harris, W.G. Surface chemistry variations among a series of laboratory-produced biochars. Geoderma 2011, 163, 247–255. [Google Scholar] [CrossRef]
- Kabała, C.; Charzyński, P.; Chodorowski, J.; Drewnik, M.; Glina, B.; Greinert, A.; Hulisz, P.; Jankowski, M.; Jonczak, J.; Łabaz, B.; et al. Polish Soil Classification, 6th edition—Principles, classification, scheme and correlations. Soil Sci. Annual. 2019, 70, 71–97. [Google Scholar] [CrossRef]
- Krasowicz, S.; Oleszek, W.; Horabik, J.; Dębicki, R.; Jankowiak, J.; Stuczyński, T.; Jadczyszyn, J. Rational management of the soil environment in Poland. Pol. J. Agronomy. 2011, 7, 43–58. [Google Scholar]
- Petrovska, B.B. Historical review of medicinal plants’ usage. Pharmacognosy Rev. 2012, 6, 1–5. [Google Scholar] [CrossRef] [PubMed]
- McKay, D.L.; Blumberg, J.B. A review of the bioactivity and potential health benefits of peppermint tea (Mentha piperita L.). Phytother. Res. Int. J. Devot. Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2006, 20, 619–633. [Google Scholar] [CrossRef] [PubMed]
- Muciarelli, M.; Camusso, W.; Bertea, C.M.; Maffei, M.E. Effect of (+)-pulegone and other oil components of Mentha x Piperita on cucumber respiration. Phytochemistry 2001, 57, 91–98. [Google Scholar] [CrossRef]
- Sady, W. Field Vegetable Fertilization; Plantpress: Kraków, Poland, 2000. (In Polish) [Google Scholar]
- Ostrowska, A.; Gawliński, S.; Szczubiałka, Z. (Eds.) Soil and Plant Analysis Procedures; Institute Environment Protection: Warszawa, Poland, 1991. (In Polish) [Google Scholar]
- Poshtdar, A.; Mashhadie, A.R.A.; Moradi, F.; Siadat, S.A.; Bakhshandeh, A. Effect of source and rate of nitrogen fertilizer on yield and water and nitrogen use efficiency of peppermint (Mentha piperita L.). Iran. J. Crop Sci. 2016, 18, 13–31. [Google Scholar]
- Butar, R.R.B.; Sitepu, F.E.; Hasanah, Y. Growth Response of Peppermint (Mentha piperita L.) Stem Cuttings on Several Composition of Planting Media. Indones. J. Agric. Res. 2018, 1, 187–192. [Google Scholar] [CrossRef][Green Version]
- Olszewski, M.W.; Eisenman, S.W. Influence of biochar amendment on herb growth in a green roof substrate. Hortic. Environ. Biotechnol. 2017, 58, 406–413. [Google Scholar] [CrossRef]
- Hasanah, Y.; Sitepu, F.E.; Butar, R.B. Morphological characters and N uptake of Mint (Mentha piperita) with the growing media composition treatment. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2019; p. 012147. [Google Scholar]
- Zheljazkov, V.D.; Cantrell, C.L.; Astatkie, T.; Ebelhar, M.W. Peppermint productivity and oil composition as a function of nitrogen. growth stage. and harvest time. Agronom. J. 2010, 102, 124–128. [Google Scholar] [CrossRef]
- Ram, D.; Ram, M.; Singh, R. Optimization of water and nitrogen application to menthol mint (Mentha arvensis L.) through sugarcane trash mulch in a sandy loam soil of semi-arid subtropical climate. Bioresour. Technol. 2006, 97, 886–893. [Google Scholar] [CrossRef]
- Mansoori, I. The effect of plant density and harvesting time on growth and essential oil of peppermint (Mentha piperita L.). J. Med. Bioeng. 2014, 3. [Google Scholar] [CrossRef][Green Version]
- Verma, R.K.; Verma, R.S.; Rahman, L.U.; Kalra, A.; Patra, D.D. Integrated Nutrient Management on Biomass. Oil Yields and Essential Oil Composition of Peppermint (Mentha piperita L.) and Residual Fertility in a Hilly Soil. J. Essential Oil Bearing Plants 2016, 19, 582–591. [Google Scholar] [CrossRef]
- Marks, A.N.; Mattan, S.; Alcañiz, J.M.; Pérez-Herrero, E.; Domene, X. Gasifier biochar effects on nutrient availability. organic matter mineralization. and soil fauna activity in a multi-year Mediterranean trial. Agric. Ecosyst. Environ. 2016, 215, 30–39. [Google Scholar] [CrossRef]
- Gundale, M.J.; Deluca, T.H. Charcoal Effects on Soil Solution Chemistry and Growth of Koeleria macrantha in the Ponderosa Pine/Douglas-fir Ecosystem. Biol. Fert. Soils 2007, 43, 303–311. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. Biochar for environmental management: An introduction. In Biochar for Environmental Management. Science and Technology; Lehmann, J., Joseph, S., Eds.; Earthscan: London, UK, 2009; pp. 1–12. [Google Scholar]
- Lin, Y.; Munroe, P.; Joseph, S.; Henderson, R.; Ziolkowski, A. Water extractable organic carbon in untreated and chemical treated biochars. Chemosphere 2012, 87, 151–157. [Google Scholar] [CrossRef]
- Rietra, R.P.J.J.; Heinen, M.; Dimkpa, C.O.; Bindraban, P.S. Effects of Nutrient Antagonism and Synergism on Yield and Fertilizer Use Efficiency. Commun. Soil Sci. Plant Anal. 2017, 48, 1895–1920. [Google Scholar] [CrossRef]
- Kunhikrishnan, A.; Thangarajan, R.; Bolan, N.S.; Xu, Y.; Mandal, S.; Gleeson, D.B.; Seshadri, B.; Zaman, M.; Barton, L.; Tang, C.; et al. Functional Relationships of Soil Acidification. Liming. and Greenhouse Gas Flux. Adv. Agron. 2016, 139, 1–71. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, B.; Gao, Q.; Gao, Y.; Liu, S. Adsorption of phosphorus by different biochars. Spectrosc. Lett. 2017, 50, 73–80. [Google Scholar] [CrossRef]
- Arrobas, M.; Ferreira, Q.; Afonso, S.; Rodrigues, A. Sufficiency ranges and crop nutrient removals for peppermint (Mentha x piperita L.) established from field and pot fertilizer experiments. Commun. Soil Sci. Plant Anal. 2018, 49, 1719–1730. [Google Scholar] [CrossRef]
- Martinsen, V.; Shitumbanuma, V.; Alling, V.; Breedveld, G.; Rutherford, D.; Sparrevik, M.; Hale, S.; Obia, A.; Mulder JCornelissen, G. Biochar effect on maize yield and soil characteristics in five conservation farming sites in Zambia. Agronomy 2013, 3, 256–274. [Google Scholar] [CrossRef]
- Oram, N.J.; van de Voorde, T.F.J.; Ouwehand, G.J.; Bezemer, T.M.; Mommer, L.; Jeffery, S.; Van Groenigen, J.W. Soil amendment with biochar increases the competitive ability of legumes via increased potassium availability. Agric. Ecosyst. Environ. 2014, 191, 92–98. [Google Scholar] [CrossRef]
- Marschner, P. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar] [CrossRef]
- Senbayram, M.; Gransee, A.; Wahle, V.; Thiel, H. Role of magnesium fertilisers in agriculture: Plant–soil continuum. Crop Pasture Sci. 2015, 66, 1219–1229. [Google Scholar] [CrossRef]
- Noyce, G.L.; Jones, T.; Fulthorpe, R.; Basiliko, N. Phosphorus uptake and availability and short-term seedling growth in three Ontario soils amended with ash and biochar. Can. J. Soil Sci. 2017, 97, 678–691. [Google Scholar] [CrossRef]

| Field | pH | EC | % C | P | N-NH4 | N-NO3 | K | Mg | Ca | S |
|---|---|---|---|---|---|---|---|---|---|---|
| Before cultivation | 7.03 | 0.05 | 1.03 | 107 | 3.50 | 2.82 | 45.7 | 189 | 2199 | 23.5 |
| Standard numbers * | - | - | 20–50 | - | - | 80–120 | 60–120 | 800–1000 | - |
| Factor | Yield of Fresh Matter (t ha−1) | Dry Matter Yield (t ha−1) | |
|---|---|---|---|
| Year of research | 2017 | 12.3 a* | 3.32 a |
| 2018 | 22.5 c | 6.24 c | |
| 2019 | 16.0 b | 4.38 b | |
| A dose of biochar (Mg∙ha−1) | 0 | 16.7 a | 4.68 a |
| 5 | 16.5 a | 4.46 a | |
| 15 | 17.2 a | 4.63 a | |
| 45 | 17.4 a | 4.82 a | |
| Nitrogen level (kg∙ha−1) | 75 | 14.8 a | 4.10 a |
| 100 | 17.6 ab | 4.78 ab | |
| 125 | 18.5 b | 5.07 b | |
| Year | N Level (kg ha−1) | A Dose of Biochar (t ha−1) | Average | |||
|---|---|---|---|---|---|---|
| 0 | 5 | 15 | 45 | |||
| 2017 | 75 | 13.7 | 11.8 | 11.8 | 9.9 | 11.8 a |
| 100 | 14.5 | 13.3 | 11.0 | 11.2 | 12.5 a | |
| 125 | 14.9 | 12.3 | 12.4 | 10.9 | 12.6 a | |
| Average | 14.3 b* | 12.5 a | 11.8 a | 10.7 a | - | |
| 2018 | 75 | 17.5 | 18.5 | 20.6 | 21.3 | 19.5 a |
| 100 | 20.4 | 21.7 | 27.0 | 24.8 | 23.5 b | |
| 125 | 23.8 | 22.8 | 26.5 | 25.3 | 24.6 b | |
| Average | 20.6 a | 21.0 a | 23.8 b | 24.7 b | - | |
| 2019 | 75 | 12.2 | 12.5 | 13.5 | 14.0 | 13.1 a |
| 100 | 15.0 | 17.5 | 17.0 | 17.5 | 16.8 ab | |
| 125 | 18.5 | 18.0 | 15.2 | 21.7 | 18.3 b | |
| Average | 15.2 a | 16.0 a | 15.2 a | 17.8 a | - | |
| Year | N Level (kg ha−1) | Biochar Dose (t ha−1) | Average | |||
|---|---|---|---|---|---|---|
| 0 | 5 | 15 | 45 | |||
| 2017 | 75 | 4.14 | 3.30 | 3.46 | 2.46 | 3.33 a |
| 100 | 4.63 | 3.06 | 2.77 | 2.99 | 3.37 a | |
| 125 | 3.73 | 3.50 | 3.12 | 2.68 | 3.26 a | |
| Average | 4.17 c* | 3.29 b | 3.11 ab | 2.71 a | - | |
| 2018 | 75 | 4.96 | 5.36 | 5.67 | 5.59 | 5.39 a |
| 100 | 5.74 | 5.70 | 7.47 | 6.31 | 6.30 b | |
| 125 | 6.55 | 5.94 | 7.05 | 8.63 | 7.04 c | |
| Average | 5.75 a | 5.67 a | 6.73 b | 6.84 b | - | |
| 2019 | 75 | 3.34 | 3.31 | 3.68 | 3.93 | 3.93 a |
| 100 | 4.17 | 4.92 | 4.62 | 4.94 | 4.94 b | |
| 125 | 4.83 | 5.00 | 3.92 | 5.84 | 5.84 b | |
| Average | 4.11 a | 4.41 a | 4.08 a | 4.91 a | - | |
| Factor | pH | EC | N-NH4 | N-NO3 | Ca | K | Mg | P | S-SO4 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Year | 2017 | 7.84 c* | 84 b | 3.4 a | 3.5 b | 3418 c | 61 a | 109 a | 69 b | 58 c |
| 2018 | 7.49 a | 80 b | 4.7 a | 21.3 c | 2564 a | 82 b | 119 b | 59 a | 6 a | |
| 2019 | 7.69 b | 41 a | 19.9 b | 1.4 a | 3068 b | 60 a | 117 b | 56 a | 40 b | |
| Biochar dose | 0 | 7.67 ab | 57 a | 6.0 a | 3.2 a | 2414 a | 47 a | 115 a | 69 b | 26 a |
| 5 | 7.67 ab | 71 b | 5.4 a | 4.3 a | 3637 c | 70 b | 118 a | 56 a | 40 c | |
| 15 | 7.64 a | 73 b | 15.7 c | 9.9 b | 3026 b | 63 b | 116 a | 57 a | 36 b | |
| 45 | 7.72 b | 71 b | 10.2 b | 17.6 c | 2988 b | 90 c | 112 a | 64 b | 36 b | |
| N dose | 75 | 7.68 a | 61 a | 8.9 a | 5.2 a | 2605 a | 64 a | 115 a | 64 b | 30 a |
| 100 | 7.66 a | 72 b | 8.6 a | 7.5 b | 3059 a | 67 ab | 117 a | 64 b | 36 b | |
| 125 | 7.68 a | 72 b | 10.6 a | 13.6 c | 3385 a | 71 b | 112 a | 56 a | 38 b | |
| Factor | N | Ca | K | Mg | P | S | |
|---|---|---|---|---|---|---|---|
| Year | 2017 | 2.04 c* | 1.14 b | 1.59 a | 0.31 c | 0.23 b | 0.22 b |
| 2018 | 1.03 a | 1.08 a | 1.92 b | 0.20 b | 0.21 a | 0.17 a | |
| 2019 | 1.22 b | 1.36 c | 2.50 c | 0.11 a | 0.24 b | 0.21 b | |
| Biochar dose | 0 | 1.39 a | 1.26 c | 1.80 a | 0.23 c | 0.21 a | 0.18 a |
| 5 | 1.40 ab | 1.16 ab | 1.99 b | 0.19 a | 0.22 ab | 0.19 a | |
| 15 | 1.46 bc | 1.21 bc | 1.94 ab | 0.22 b | 0.23 b | 0.20 a | |
| 45 | 1.47 c | 1.13 a | 2.28 c | 0.18 a | 0.24 c | 0.22 b | |
| N dose | 75 | 1.29 a | 1.17 a | 1.99 a | 0.20 a | 0.22 a | 0.20 a |
| 100 | 1.45 b | 1.21 a | 2.01 a | 0.21 a | 0.23 b | 0.20 a | |
| 125 | 1.54 c | 1.19 a | 2.00 a | 0.21 a | 0.23 b | 0.19 a | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadowska, U.; Domagała-Świątkiewicz, I.; Żabiński, A. Biochar and Its Effects on Plant–Soil Macronutrient Cycling during a Three-Year Field Trial on Sandy Soil with Peppermint (Mentha piperita L.). Part I: Yield and Macro Element Content in Soil and Plant Biomass. Agronomy 2020, 10, 1950. https://doi.org/10.3390/agronomy10121950
Sadowska U, Domagała-Świątkiewicz I, Żabiński A. Biochar and Its Effects on Plant–Soil Macronutrient Cycling during a Three-Year Field Trial on Sandy Soil with Peppermint (Mentha piperita L.). Part I: Yield and Macro Element Content in Soil and Plant Biomass. Agronomy. 2020; 10(12):1950. https://doi.org/10.3390/agronomy10121950
Chicago/Turabian StyleSadowska, Urszula, Iwona Domagała-Świątkiewicz, and Andrzej Żabiński. 2020. "Biochar and Its Effects on Plant–Soil Macronutrient Cycling during a Three-Year Field Trial on Sandy Soil with Peppermint (Mentha piperita L.). Part I: Yield and Macro Element Content in Soil and Plant Biomass" Agronomy 10, no. 12: 1950. https://doi.org/10.3390/agronomy10121950
APA StyleSadowska, U., Domagała-Świątkiewicz, I., & Żabiński, A. (2020). Biochar and Its Effects on Plant–Soil Macronutrient Cycling during a Three-Year Field Trial on Sandy Soil with Peppermint (Mentha piperita L.). Part I: Yield and Macro Element Content in Soil and Plant Biomass. Agronomy, 10(12), 1950. https://doi.org/10.3390/agronomy10121950





