Adaptive Multi-Paddock Grazing Lowers Soil Greenhouse Gas Emission Potential by Altering Extracellular Enzyme Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites and Soil Collection
2.2. Soil Processing and Characterization
2.3. Soil Preparation, Incubation, Gas Sampling, and Analysis
2.4. Measurements of Microbial Activities and Soil Parameters
2.5. Data Preparation
2.6. Statistical Analyses
3. Results
3.1. Basic Soil Properties
3.2. Effects of Grazing, Soil Temperature, and Moisture on Cumulative GHG Fluxes
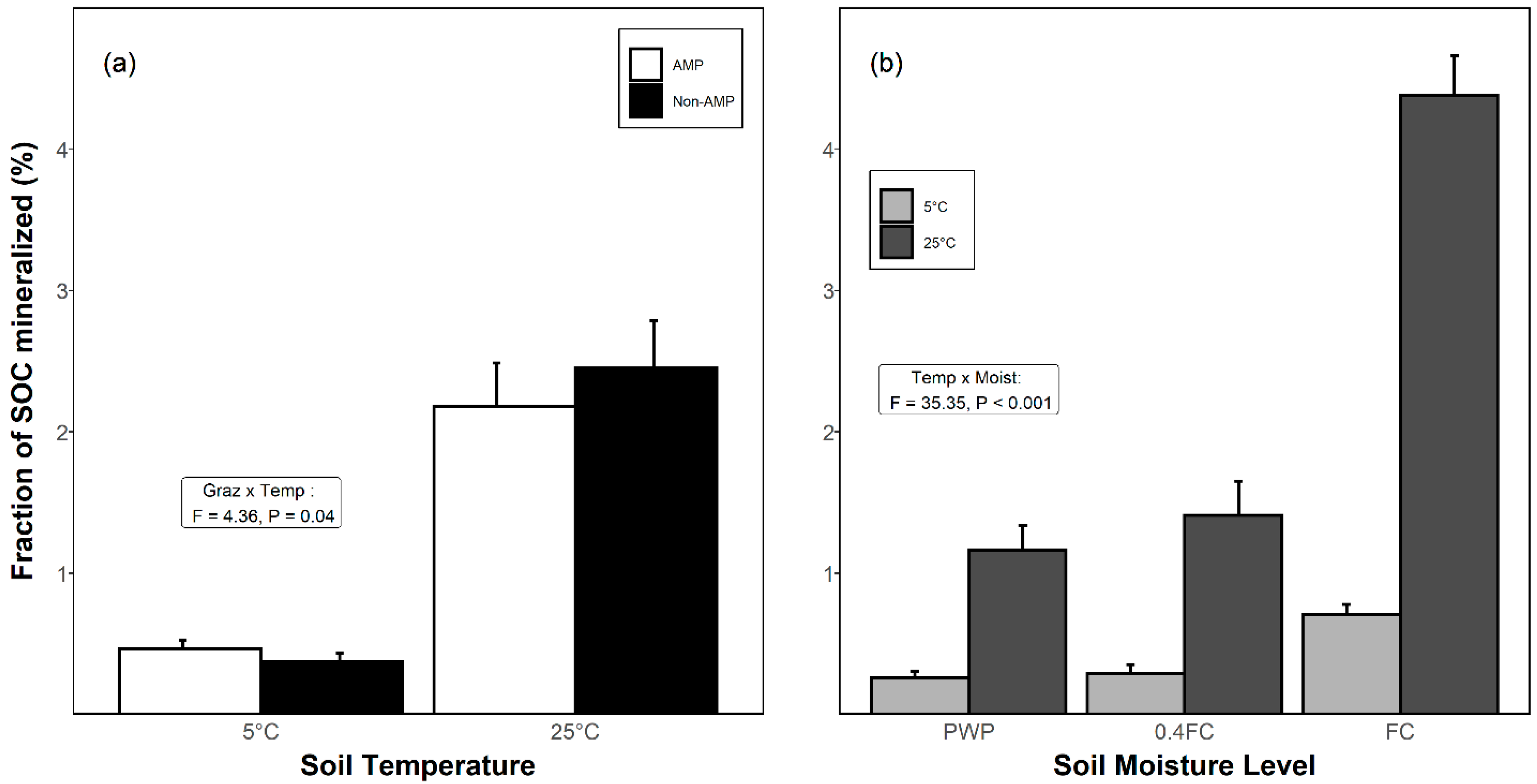
3.3. Proportion of SOC Mineralized as CO2
3.4. Factors Affecting GHG Fluxes
4. Discussion
4.1. Effects of Grazing Systems, Soil Temperature and Moisture on GHG Flux
4.2. SOC Mineralization and the Temperature Sensitivity of CO2 Emissions
4.3. Relative Effects of Grazing, Temperature and Moisture on EEA and GHG Fluxes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Ethical Statement
References
- WRI. A Guide to World Resources 2000–2001: People and Ecosystems; World Resources Institute: Washington, DC, USA, 2001. [Google Scholar]
- Leahy, P.; Kiely, G.; Scanlon, T.M. Managed Grasslands: A Greenhouse Gas Sink or Source? Geophys. Res. Lett. 2004, 31. [Google Scholar] [CrossRef]
- Jones, S.K.; Rees, R.M.; Skiba, U.M.; Ball, B.C. Greenhouse Gas Emissions from a Managed Grassland. Glob. Planet. Chang. 2005, 47, 201–211. [Google Scholar] [CrossRef]
- Asgedom, H.; Kebreab, E. Beneficial Management Practices and Mitigation of Greenhouse Gas Emissions in the Agriculture of the Canadian Prairie: A Review. Agron. Sustain. Dev. 2011, 31, 433–451. [Google Scholar] [CrossRef]
- Laca, E.A.; McEachern, M.-B.; Demment, M.W. Global Grazinglands and Greenhouse Gas Fluxes. Rangel. Ecol. Manag. 2010, 63, 1–3. [Google Scholar] [CrossRef]
- Follett, R.F.; Reed, D.A. Soil Carbon Sequestration in Grazing Lands: Societal Benefits and Policy Implications. Rangel. Ecol. Manag. 2010, 63, 4–15. [Google Scholar] [CrossRef]
- Bork, E.W.; Attaeian, B.; Cahill, J.F.; Chang, S.X. Soil Nitrogen and Greenhouse Gas Dynamics in a Temperate Grassland under Experimental Warming and Defoliation. Soil Sci. Soc. Am. J. 2019, 83, 780–790. [Google Scholar] [CrossRef]
- Tang, S.; Wang, K.; Xiang, Y.; Tian, D.; Wang, J.; Liu, Y.; Cao, B.; Guo, D.; Niu, S. Heavy Grazing Reduces Grassland Soil Greenhouse Gas Fluxes: A Global Meta-Analysis. Sci. Total Environ. 2019, 654, 1218–1224. [Google Scholar] [CrossRef]
- Liebig, M.A.; Gross, J.R.; Kronberg, S.L.; Phillips, R.L. Grazing Management Contributions to Net Global Warming Potential: A Long—Term Evaluation in the Northern Great Plains. J. Environ. Qual. 2010, 39, 799–809. [Google Scholar] [CrossRef]
- Braun, M.; Bai, Y.; McConkey, B.; Farrell, R.; Romo, J.T.; Pennock, D. Greenhouse Gas Flux in a Temperate Grassland as Affected by Landform and Disturbance. Landsc. Ecol. 2013, 28, 709–723. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, C.; Matthew, C.; Wood, B.; Hou, F. Key Sources and Seasonal Dynamics of Greenhouse Gas Fluxes from Yak Grazing Systems on the Qinghai-Tibetan Plateau. Sci. Rep. 2017, 7, 40857. [Google Scholar] [CrossRef]
- García-Marco, S.; Ravella, S.R.; Chadwick, D.; Vallejo, A.; Gregory, A.S.; Cárdenas, L.M. Ranking Factors Affecting Emissions of GHG from Incubated Agricultural Soils. Eur. J. Soil Sci. 2014, 65, 573–583. [Google Scholar] [CrossRef]
- Teague, R.; Grant, B.; Wang, H.-H. Assessing Optimal Configurations of Multi-Paddock Grazing Strategies in Tallgrass Prairie Using a Simulation Model. J. Environ. Manag. 2015, 150, 262–273. [Google Scholar] [CrossRef]
- Teague, R.; Provenza, F.; Kreuter, U.; Steffens, T.; Barnes, M. Multi-Paddock Grazing on Rangelands: Why the Perceptual Dichotomy between Research Results and Rancher Experience? J. Environ. Manag. 2013, 128, 699–717. [Google Scholar] [CrossRef]
- Teague, W.R.; Dowhower, S.L.; Baker, S.A.; Haile, N.; DeLaune, P.B.; Conover, D.M. Grazing Management Impacts on Vegetation, Soil Biota and Soil Chemical, Physical and Hydrological Properties in Tall Grass Prairie. Agric. Ecosyst. Environ. 2011, 141, 310–322. [Google Scholar] [CrossRef]
- Savory, A.; Butterfield, J. Holistic Management: A New Framework for Decision Making; Island Press: Washington, DC, USA, 1998. [Google Scholar]
- Wang, Z.; Yun, X.J.; Wei, Z.J.; Schellenberg, M.P.; Wang, Y.F.; Yang, X.; Hou, X.Y. Responses of Plant Community and Soil Properties to Inter-Annual Precipitation Variability and Grazing Durations in a Desert Steppe in Inner Mongolia. J. Integr. Agric. 2014, 13, 1171–1182. [Google Scholar] [CrossRef]
- Byrnes, R.C.; Eastburn, D.J.; Tate, K.W.; Roche, L.M. A Global Meta-Analysis of Grazing Impacts on Soil Health Indicators. J. Integr. Agric. 2018, 47, 758–765. [Google Scholar] [CrossRef]
- Hillenbrand, M.; Thompson, R.; Wang, F.; Apfelbaum, S.; Teague, R. Impacts of Holistic Planned Grazing with Bison Compared to Continuous Grazing with Cattle in South Dakota Shortgrass Prairie. Agric. Ecosyst. Environ. 2019, 279, 156–168. [Google Scholar] [CrossRef]
- Roesch, A.; Weisskopf, P.; Oberholzer, H.; Valsangiacomo, A.; Nemecek, T. An Approach for Describing the Effects of Grazing on Soil Quality in Life-Cycle Assessment. Sustainability 2019, 11, 4870. [Google Scholar] [CrossRef]
- Halde, C.; Hammermeister, A.M.; Mclean, L.N.; Webb, K.T.; Martin, R.C. Soil Compaction under Varying Rest Periods and Levels of Mechanical Disturbance in a Rotational Grazing System. Can. J. Soil Sci. 2011, 91, 957–964. [Google Scholar] [CrossRef]
- Ahmed, R.S.; Mario, E.B.; Carolyn, E.G. Grazing Intensity Effects on Litter Decomposition and Soil Nitrogen Mineralization. J. Range Manag. 1994, 47, 444. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Pan, H.; Hernández, M.; Guan, X.; Wang, W.; Zhang, Q.; Luo, Y.; Di, H.; Xu, J. Impact of Grazing on Shaping Abundance and Composition of Active Methanotrophs and Methane Oxidation Activity in a Grassland Soil. Biol. Fertil. Soils 2020, 56, 799–810. [Google Scholar] [CrossRef]
- Chuan, X.; Carlyle, C.N.; Bork, E.W.; Chang, S.X.; Hewins, D.B. Long-Term Grazing Accelerated Litter Decomposition in Northern Temperate Grasslands. Ecosystems 2018, 21, 1321–1334. [Google Scholar] [CrossRef]
- Briske, D.D.; Derner, J.D.; Brown, J.R.; Fuhlendorf, S.D.; Teague, W.R.; Gillen, R.L.; Ash, A.J.; Havstad, K.M.; Willms, W.D. Rotational Grazing on Rangelands: Reconciliation of Perception and Experimental Evidence. Rangel. Ecol. Manag. 2008, 61, 3. [Google Scholar] [CrossRef]
- Carter, J.; Jones, A.; Brien, M.; Ratner, J.; Wuerthner, G. Holistic Management: Misinformation on the Science of Grazed Ecosystems. Int. J. Biodivers. 2014, 2014, 1–10. [Google Scholar] [CrossRef]
- Semmartin, M.; Garibaldi, L.A.; Chaneton, E.J. Grazing History Effects on above- and Below-Ground Litter Decomposition and Nutrient Cycling in Two Co-Occurring Grasses. Plant Soil 2008, 303, 177–189. [Google Scholar] [CrossRef]
- Srinivasa Rao, C.; Grover, M.; Kundu, S.; Desai, S. Soil Enzymes. In Encyclopedia of Soil Science, 3rd ed.; Lal, R., Ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 2100–2107. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Hill, B.H.; Follstad Shah, J.J. Ecoenzymatic Stoichiometry of Microbial Organic Nutrient Acquisition in Soil and Sediment. Nature 2009, 462, 795–798. [Google Scholar] [CrossRef]
- Hewins, D.B.; Broadbent, T.; Carlyle, C.N.; Bork, E.W. Extracellular Enzyme Activity Response to Defoliation and Water Addition in Two Ecosites of the Mixed Grass Prairie. Agric. Ecosyst. Environ. 2016, 230, 79–86. [Google Scholar] [CrossRef]
- Bell, T.H.; Henry, H.A.L. Fine Scale Variability in Soil Extracellular Enzyme Activity Is Insensitive to Rain Events and Temperature in a Mesic System. Pedobiologia 2011, 54, 141–146. [Google Scholar] [CrossRef]
- Savage, K.; Phillips, R.; Davidson, E. High Temporal Frequency Measurements of Greenhouse Gas Emissions from Soils. Biogeosciences 2014, 11, 2709–2720. [Google Scholar] [CrossRef]
- Curtin, D.; Beare, M.H.; Hernandez-Ramirez, G. Temperature and Moisture Effects on Microbial Biomass and Soil Organic Matter Mineralization. Soil Sci. Soc. Am. J. 2012, 76, 2055–2067. [Google Scholar] [CrossRef]
- Lang, M.; Cai, Z.; Chang, S.X. Effects of Land Use Type and Incubation Temperature on Greenhouse Gas Emissions from Chinese and Canadian Soils. J. Soils Sediments 2011, 11, 15–24. [Google Scholar] [CrossRef]
- Canadian Society of Soil Science. Soils of Canada. Available online: https://soilsofcanada.ca/orders/chernozemic-soils.php (accessed on 5 July 2020).
- Mbogga, M.; Wang, T.; Hansen, C.; Hamann, A. A Comprehensive Set of Interpolated Climate Data for Alberta; Alberta Sustainable Resource Development, Government of Alberta: Edmonton, AB, Canada, 2010; p. 7. [Google Scholar]
- ClimateAB. Historical and Projected Climate Data for Alberta. Available online: https://sites.ualberta.ca/~ahamann/data/climateab.html (accessed on 15 September 2020).
- Wang, T.; Hamann, A.; Spittlehouse, D.L.; Aitken, S.N. Development of Scale-Free Climate Data for Western Canada for Use in Resource Management. Int. J. Climatol. 2006, 26, 383–397. [Google Scholar] [CrossRef]
- Hendershot, W.; Lalande, H.; Duquette, M. Soil Reaction and Exchangeable Acidity. In Soil Sampling and Methods of Analysis; Carter, M.R., Gregorich, E.G., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 173–178. [Google Scholar] [CrossRef]
- Hao, X.; Ball, B.; Culley, J.; Carter, M.; Parkin, G. Soil Density and Porosity. In Soil Sampling and Methods of Analysis; Carter, M.R., Gregorich, E.G., Eds.; CRC Press: Boca Raton, FL, USA, 2008; pp. 743–760. [Google Scholar] [CrossRef]
- Kroetsch, D.; Wang, C. Particle Size Distribution. In Soil Sampling and Methods of Analysis, 2nd ed.; Carter, M.R., Gregorich, E.G., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 161–178. [Google Scholar]
- Reynolds, W.D.; Topp, G.C. Soil Water Desorption and Imbibition: Tension and Pressure Techniques. In Soil Sampling and Methods of Analysis, 2nd ed.; Carter, M.R., Gregorich, E.G., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 981–1005. [Google Scholar]
- Buchan, G.D.; Tonkin, P.J. Estimation of Field Capacity and Wilting Point of Some New Zealand Soils from Their Saturation Percentages. N. Z. J. Crop Hortic. Sci. 1990, 18, 241–246. [Google Scholar] [CrossRef]
- Dane, J.H.; Hopmans, J.W. Water Retention and Storage. In Methods of Soil Analysis: Part 4 Physical Methods; SSSA Book Series; Dane, J.H., Topp, C.G., Eds.; SSSA: Madison, WI, USA, 2002; pp. 671–795. [Google Scholar] [CrossRef]
- McMinn, D. Chromatography: Gas Detectors: General (Flame Ionization Detectors and Thermal Conductivity Detectors). In Encyclopedia of Separation Science; Wilson, I.D., Ed.; Academic Press: Oxford, UK, 2000; pp. 443–447. [Google Scholar] [CrossRef]
- Lovelock, J.E. The Electron Capture Detector: Theory and Practice. J. Chromatogr. A 1974, 99, 3–12. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Saiya-Cork, K.; Long, T.; Osgood, M.P.; Neher, D.A.; Zak, D.R.; Norby, R.J. Soil Microbial Activity in a Liquidambar Plantation Unresponsive to CO2-Driven Increases in Primary Production. Appl. Soil Ecol. 2003, 24, 263–271. [Google Scholar] [CrossRef]
- Kaliaskar, D. Cultivation and Grazing Impacts on Extracellular Enzyme Activity in Alberta Grasslands; University of Alberta: Edmonton, AB, Canada, 2020. [Google Scholar]
- Brookes, P.C.; Landman, A.; Pruden, G.; Jenkinson, D.S. Chloroform Fumigation and the Release of Soil Nitrogen: A Rapid Direct Extraction Method to Measure Microbial Biomass Nitrogen in Soil. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An Extraction Method for Measuring Soil Microbial Biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Miranda, K.M.; Espey, M.G.; Wink, D.A. A Rapid, Simple Spectrophotometric Method for Simultaneous Detection of Nitrate and Nitrite. Nitric Oxide 2001, 5, 62–71. [Google Scholar] [CrossRef]
- Keeney, D.R.; Nelson, D.W. Nitrogen-Inorganic Forms. In Methods of Soil Analysis Part 2, Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy Inc. and Soil Science Society of America Inc.: Madison, WI, USA, 1982; pp. 643–698. [Google Scholar]
- Ding, F.; Huang, Y.; Sun, W.; Jiang, G.; Chen, Y. Decomposition of Organic Carbon in Fine Soil Particles Is Likely More Sensitive to Warming Than in Coarse Particles: An Incubation Study with Temperate Grassland and Forest Soils in Northern China. PLoS ONE 2014, 9, e95348. [Google Scholar] [CrossRef]
- Shrestha, B.M.; Sitaula, B.K.; Singh, B.R.; Bajracharya, R.M. Fluxes of CO2 and CH4 in Soil Profiles of a Mountainous Watershed of Nepal as Influenced by Land Use, Temperature, Moisture and Substrate Addition. Nutr. Cycl. Agroecosyst. 2004, 68, 155–164. [Google Scholar] [CrossRef]
- Myhre, G.; Shindell, D.; Bréon, F.-M.; Collins, W.; Fuglestvedt, J.; Huang, J.; Koch, D.; Lamarque, J.-F.; Lee, D.; Mendoza, B.; et al. Anthropogenic and Natural Radiative Forcing. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- RStudio Team. Rstudio: Integrated Development for R. Available online: http://www.rstudio.com/ (accessed on 5 July 2020).
- Grace, J.B. Structural Equation Modeling and Natural Systems; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Lefcheck, J.S. Piecewisesem: Piecewise Structural Equation modelling in R for Ecology, Evolution, and Systematics. Methods Ecol. Evol. 2016, 7, 573–579. [Google Scholar] [CrossRef]
- Hamdi, S.; Moyano, F.; Sall, S.; Bernoux, M.; Chevallier, T. Synthesis Analysis of the Temperature Sensitivity of Soil Respiration from Laboratory Studies in Relation to Incubation Methods and Soil Conditions. Soil Biol. Biochem. 2013, 58, 115–126. [Google Scholar] [CrossRef]
- Ghimire, R.; Bista, P.; Machado, S. Long-Term Management Effects and Temperature Sensitivity of Soil Organic Carbon in Grassland and Agricultural Soils. Sci. Rep. 2019, 9, 12151. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Chang, S.X.; Ma, B.; Bork, E.W. Watering Increased DOC Concentration but Decreased N2O Emission from a Mixed Grassland Soil under Different Defoliation Regimes. Biol. Fertil. Soils 2016, 52, 987–996. [Google Scholar] [CrossRef]
- Fang, C.; Moncrieff, J.B. The Dependence of Soil CO2 Efflux on Temperature. Soil Biol. Biochem. 2001, 33, 155–165. [Google Scholar] [CrossRef]
- Wu, X.; Yao, Z.; Brüggemann, N.; Shen, Z.Y.; Wolf, B.; Dannenmann, M.; Zheng, X.; Butterbach-Bahl, K. Effects of Soil Moisture and Temperature on CO2 and CH4 Soil–Atmosphere Exchange of Various Land Use/Cover Types in a Semi-Arid Grassland in Inner Mongolia, China. Soil Biol. Biochem. 2010, 42, 773–787. [Google Scholar] [CrossRef]
- Zhou, W.; Hui, D.; Shen, W. Effects of Soil Moisture on the Temperature Sensitivity of Soil Heterotrophic Respiration: A Laboratory Incubation Study. PLoS ONE 2014, 9, e92531. [Google Scholar] [CrossRef]
- Smith, K.A.; Ball, T.; Conen, F.; Dobbie, K.E.; Massheder, J.; Rey, A. Exchange of Greenhouse Gases between Soil and Atmosphere: Interactions of Soil Physical Factors and Biological Processes. Eur. J. Soil Sci. 2003, 54, 779–791. [Google Scholar] [CrossRef]
- Stanley, P.L.; Rowntree, J.E.; Beede, D.K.; DeLonge, M.S.; Hamm, M.W. Impacts of Soil Carbon Sequestration on Life Cycle Greenhouse Gas Emissions in Midwestern USA Beef Finishing Systems. Agric. Syst. 2018, 162, 249–258. [Google Scholar] [CrossRef]
- Dowhower, S.L.; Teague, W.R.; Casey, K.D.; Daniel, R. Soil Greenhouse Gas Emissions as Impacted by Soil Moisture and Temperature under Continuous and Holistic Planned Grazing in Native Tallgrass Prairie. Agric. Ecosyst. Environ. 2020, 287, 106647. [Google Scholar] [CrossRef]
- Schuman, G.; Ingram, L.; Stahl, P.; Derner, J.; Vance, G.; Morgan, J. Influence of Management on Soil Organic Carbon Dynamics in Northern Mixed-Grass Rangeland. Soil Carbon Sequestration Greenh. Effect 2009, 57, 169–180. [Google Scholar]
- Ussiri, D.; Lal, R. Mitigation Options for Livestock and Pasture Lands. In Soil Emission of Nitrous Oxide and Its Mitigation; Springer: Berlin/Heidelberg, Germany, 2013; pp. 277–313. [Google Scholar]
- Oertel, C.; Matschullat, J.; Zurba, K.; Zimmermann, F.; Erasmi, S. Greenhouse Gas Emissions from Soils—A Review. Geochemistry 2016, 76, 327–352. [Google Scholar] [CrossRef]
- Zhuang, Q.; Chen, M.; Xu, K.; Tang, J.; Saikawa, E.; Lu, Y.; Melillo, J.M.; Prinn, R.G.; McGuire, A.D. Response of Global Soil Consumption of Atmospheric Methane to Changes in Atmospheric Climate and Nitrogen Deposition. Glob. Biogeochem. Cycles 2013, 27, 650–663. [Google Scholar] [CrossRef]
- Wnuk, E.; Walkiewicz, A.; Bieganowski, A. Methanogenesis and Aerobic Methanotrophy in Arable Soils Contaminated with Cadmium. CATENA 2020, 189, 104480. [Google Scholar] [CrossRef]
- Serrano-Silva, N.; Sarria-GuzmÁN, Y.; Dendooven, L.; Luna-Guido, M. Methanogenesis and Methanotrophy in Soil: A Review. Pedosphere 2014, 24, 291–307. [Google Scholar] [CrossRef]
- Horz, H.-P.; Rich, V.; Avrahami, S.; Bohannan, B.J.M. Methane-Oxidizing Bacteria in a California Upland Grassland Soil: Diversity and Response to Simulated Global Change. Appl. Environ. Microbiol. 2005, 71, 2642–2652. [Google Scholar] [CrossRef]
- Wang, D.; He, N.; Wang, Q.; LÜ, Y.; Wang, Q.; Xu, Z.; Zhu, J. Effects of Temperature and Moisture on Soil Organic Matter Decomposition Along Elevation Gradients on the Changbai Mountains, Northeast China. Pedosphere 2016, 26, 399–407. [Google Scholar] [CrossRef]
- Vaieretti, M.V.; Iamamoto, S.; Pérez Harguindeguy, N.; Cingolani, A.M. Livestock Grazing Affects Microclimate Conditions for Decomposition Process through Changes in Vegetation Structure in Mountain Grasslands. Acta Oecol. 2018, 91, 101–107. [Google Scholar] [CrossRef]
- Kirschbaum, M.U.F. The Temperature Dependence of Soil Organic Matter Decomposition, and the Effect of Global Warming on Soil Organic C Storage. Soil Biol. Biochem. 1995, 27, 753–760. [Google Scholar] [CrossRef]
- Naeth, M.A.; Bailey, A.W.; Pluth, D.J.; Chanasyk, D.; Hardin, R.T. Grazing Impacts on Litter and Soil Organic Matter in Mixed Prairie and Fescue Grassland Ecosystems of Alberta. J. Range Manag. 1991, 44, 7–12. [Google Scholar] [CrossRef]
- Piñeiro, G.; Paruelo, J.M.; Oesterheld, M.; Jobbágy, E.G. Pathways of Grazing Effects on Soil Organic Carbon and Nitrogen. Rangel. Ecol. Manag. 2010, 63, 109–119. [Google Scholar] [CrossRef]
- Parton, W.; Morgan, J.; Kelly, R.; Ojima, D.; Follett, R. Modeling Soil C Responses to Environmental Change in Grassland Systems. In The Potential of US Grazing Lands to Sequester Carbon and Mitigate the Greenhouse Effect; Lewis Publication: New York, NY, USA, 2000; pp. 371–398. [Google Scholar]
- Hartmann, A.A.; Buchmann, N.; Niklaus, P.A. A Study of Soil Methane Sink Regulation in Two Grasslands Exposed to Drought and N Fertilization. Plant Soil 2011, 342, 265–275. [Google Scholar] [CrossRef]
- Jacinthe, P.A.; Lal, R. Methane Oxidation Potential of Reclaimed Grassland Soils as Affected by Management. Soil Sci. 2006, 171. [Google Scholar] [CrossRef]
- Cenini, V.L.; Fornara, D.A.; McMullan, G.; Ternan, N.; Carolan, R.; Crawley, M.J.; Clément, J.-C.; Lavorel, S. Linkages between Extracellular Enzyme Activities and the Carbon and Nitrogen Content of Grassland Soils. Soil Biol. Biochem. 2016, 96, 198–206. [Google Scholar] [CrossRef]
- Xiao, W.; Chen, X.; Jing, X.; Zhu, B. A Meta-Analysis of Soil Extracellular Enzyme Activities in Response to Global Change. Soil Biol. Biochem. 2018, 123, 21–32. [Google Scholar] [CrossRef]
- Allison, S.D.; Vitousek, P.M. Responses of Extracellular Enzymes to Simple and Complex Nutrient Inputs. Soil Biol. Biochem. 2005, 37, 937–944. [Google Scholar] [CrossRef]
- Bork, E.W.; Dobert, T.F.; Grenke, J.S.J.; Carlyle, C.N.; Cahill, J.C.; Boyce, M.S. Comparative Pasture Management Regimes on Cattle Ranches with and without Adaptive Multi-Paddock (Amp) Grazing. Rangel. Ecol. Manag. submitted.


| Response Variable | Fixed Effect | Degree of Freedom¥ | F-Value | p-Value |
|---|---|---|---|---|
| Carbon dioxide (CO2) | Grazing (G) | 1, 10 | 0.41 | 0.534 |
| Temperature (T) | 1, 100 | 555.63 | <0.001 | |
| Moisture (M) | 2, 100 | 120.61 | <0.001 | |
| G × T | 1, 100 | 10.06 | 0.020 | |
| G × M | 2, 100 | 0.64 | 0.528 | |
| T × M | 2, 100 | 1.13 | 0.326 | |
| G × M × T | 2, 100 | 0.10 | 0.905 | |
| Nitrous oxide (N2O) | G | 1, 10 | 0.33 | 0.578 |
| T | 1, 100 | 47.69 | <0.001 | |
| M | 2, 100 | 45.21 | <0.001 | |
| G × T | 1, 100 | 3.06 | 0.084 | |
| G × M | 2, 100 | 0.42 | 0.660 | |
| T × M | 2, 100 | 0.37 | 0.689 | |
| G × M × T | 2, 100 | 0.44 | 0.645 | |
| Methane (CH4) | G | 1, 10 | 6.81 | 0.026 |
| T | 1, 100 | 44.88 | <0.001 | |
| M | 2, 100 | 24.65 | <0.001 | |
| G × T | 1, 100 | 0.26 | 0.609 | |
| G × M | 2, 100 | 0.68 | 0.510 | |
| T × M | 2, 100 | 0.08 | 0.927 | |
| G × M × T | 2, 100 | 0.16 | 0.852 | |
| Net GHG § | G | 1, 10 | 0.39 | 0.547 |
| T | 1, 100 | 581.50 | <0.001 | |
| M | 2, 100 | 122.82 | <0.001 | |
| G × T | 1, 100 | 10.55 | 0.002 | |
| G × M | 2, 100 | 0.64 | 0.528 | |
| T × M | 2, 100 | 1.04 | 0.358 | |
| G × M × T | 2, 100 | 0.11 | 0.898 | |
| Q10 (CO2) ‡ | G | 1, 10 | 12.19 | 0.006 |
| M | 2, 40 | 7.27 | 0.002 | |
| G × M | 2, 40 | 0.68 | 0.514 | |
| Cmin/SOC † | G | 1, 10 | 0.95 | 0.354 |
| T | 1, 100 | 436.89 | <0.001 | |
| M | 2, 100 | 110.22 | <0.001 | |
| G × T | 1, 100 | 4.36 | 0.039 | |
| G × M | 2, 100 | 1.69 | 0.190 | |
| T × M | 2, 100 | 35.35 | <0.001 | |
| G × M × T | 2, 100 | 0.35 | 0.703 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shrestha, B.M.; Bork, E.W.; Chang, S.X.; Carlyle, C.N.; Ma, Z.; Döbert, T.F.; Kaliaskar, D.; Boyce, M.S. Adaptive Multi-Paddock Grazing Lowers Soil Greenhouse Gas Emission Potential by Altering Extracellular Enzyme Activity. Agronomy 2020, 10, 1781. https://doi.org/10.3390/agronomy10111781
Shrestha BM, Bork EW, Chang SX, Carlyle CN, Ma Z, Döbert TF, Kaliaskar D, Boyce MS. Adaptive Multi-Paddock Grazing Lowers Soil Greenhouse Gas Emission Potential by Altering Extracellular Enzyme Activity. Agronomy. 2020; 10(11):1781. https://doi.org/10.3390/agronomy10111781
Chicago/Turabian StyleShrestha, Bharat M., Edward W. Bork, Scott X. Chang, Cameron N. Carlyle, Zilong Ma, Timm F. Döbert, Dauren Kaliaskar, and Mark S. Boyce. 2020. "Adaptive Multi-Paddock Grazing Lowers Soil Greenhouse Gas Emission Potential by Altering Extracellular Enzyme Activity" Agronomy 10, no. 11: 1781. https://doi.org/10.3390/agronomy10111781
APA StyleShrestha, B. M., Bork, E. W., Chang, S. X., Carlyle, C. N., Ma, Z., Döbert, T. F., Kaliaskar, D., & Boyce, M. S. (2020). Adaptive Multi-Paddock Grazing Lowers Soil Greenhouse Gas Emission Potential by Altering Extracellular Enzyme Activity. Agronomy, 10(11), 1781. https://doi.org/10.3390/agronomy10111781






