Evaluation of Iraqi Rice Cultivars for Their Tolerance to Drought Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth
2.2. Evaluation of Rice Cultivars under PEG and Water-Deprived Conditions
2.3. Measurement of Chlorophyll Content (SPAD Value)
2.4. Quantitative Real-Time PCR (qRT-PCR) Analysis
2.5. Statistical Analysis
3. Results
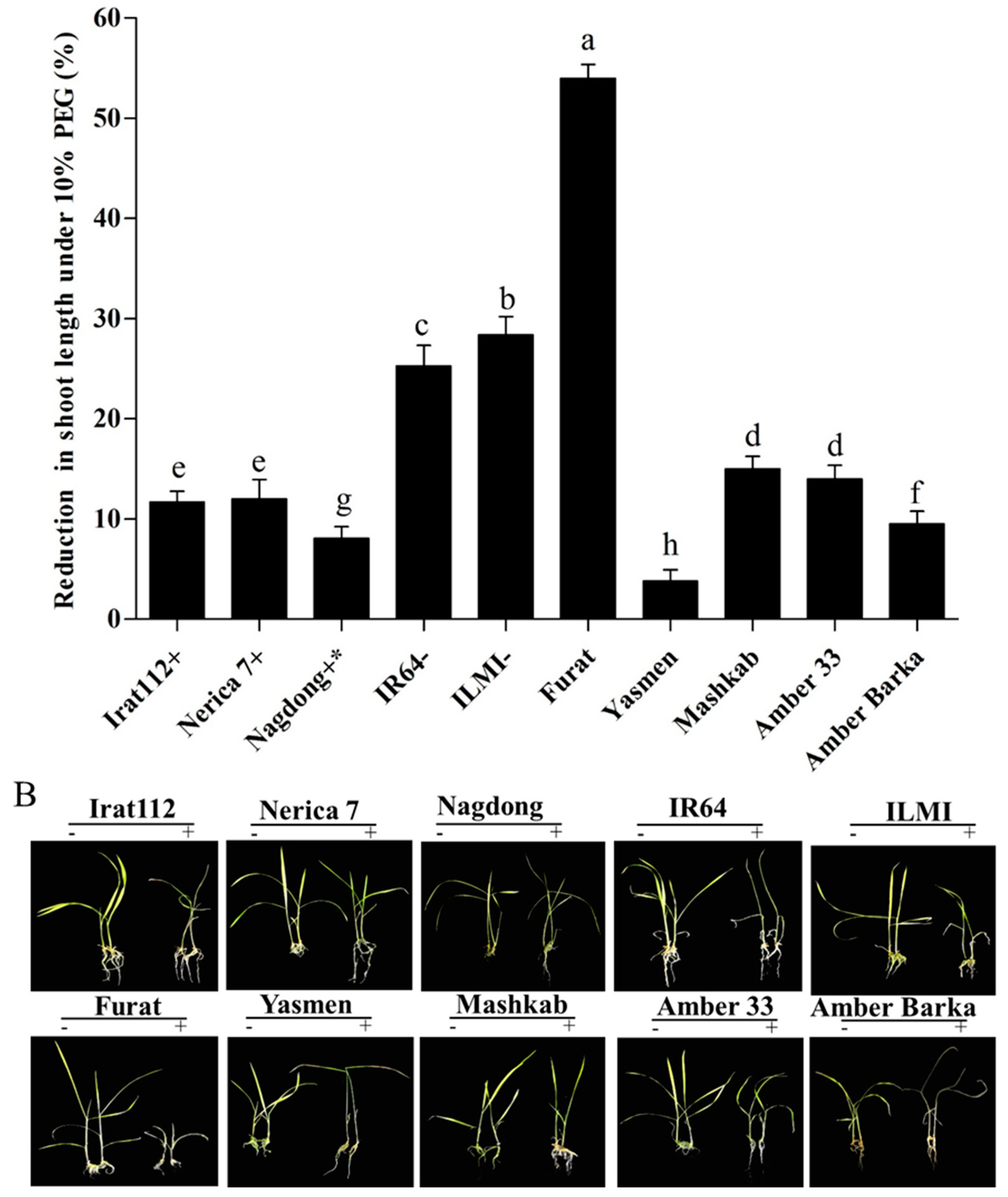
3.1. Evaluation of the Rice Cultivars after PEG-Induced Stress
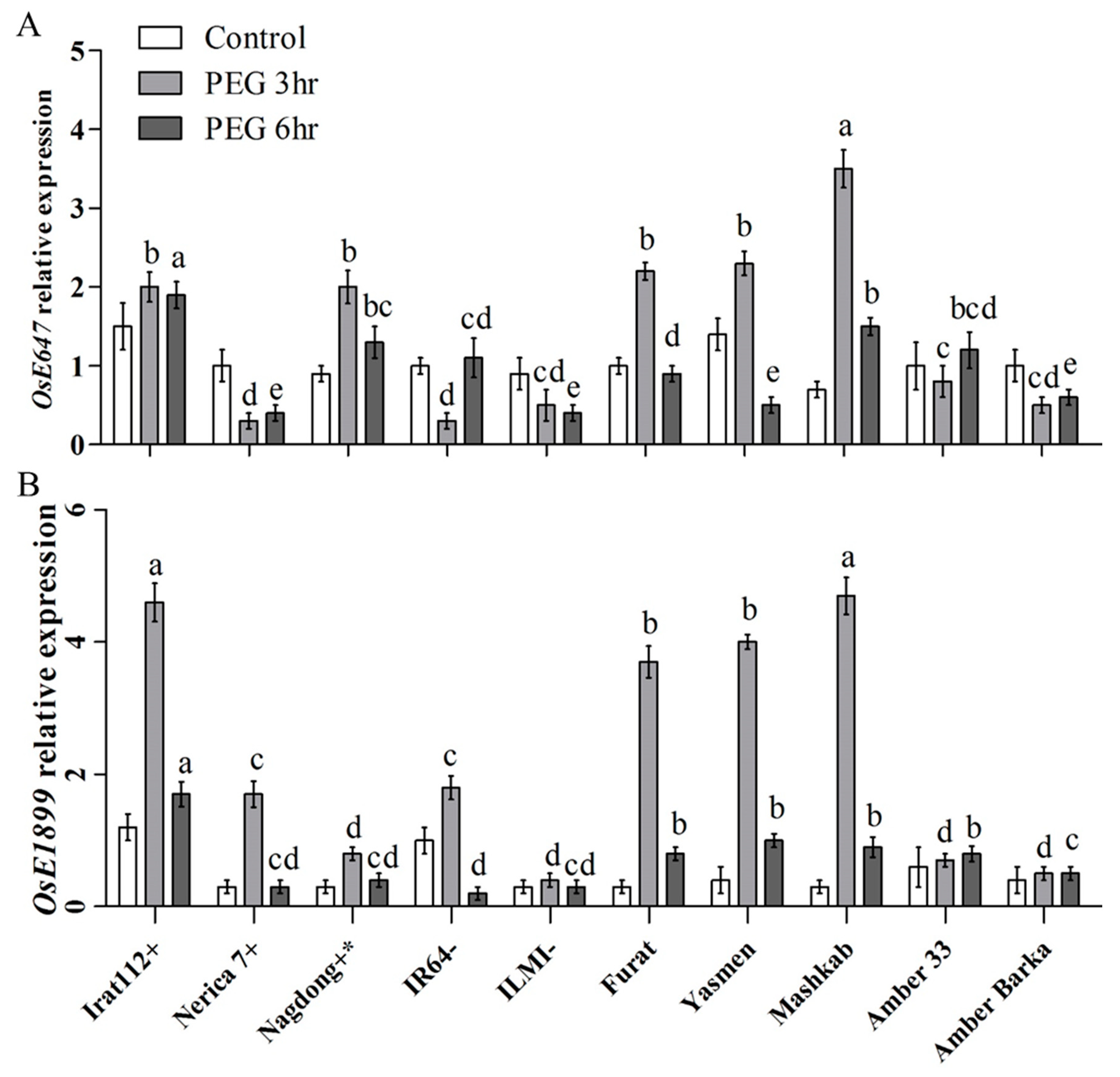
3.2. Molecular Characterization of the Rice Cultivars under PEG-Induced Drought Stress
3.3. Phenotypic Evaluation of Rice Cultivars under Water Deficit Conditions (Greenhouse)
3.3.1. Induction of Drought Stress under Greenhouse Conditions
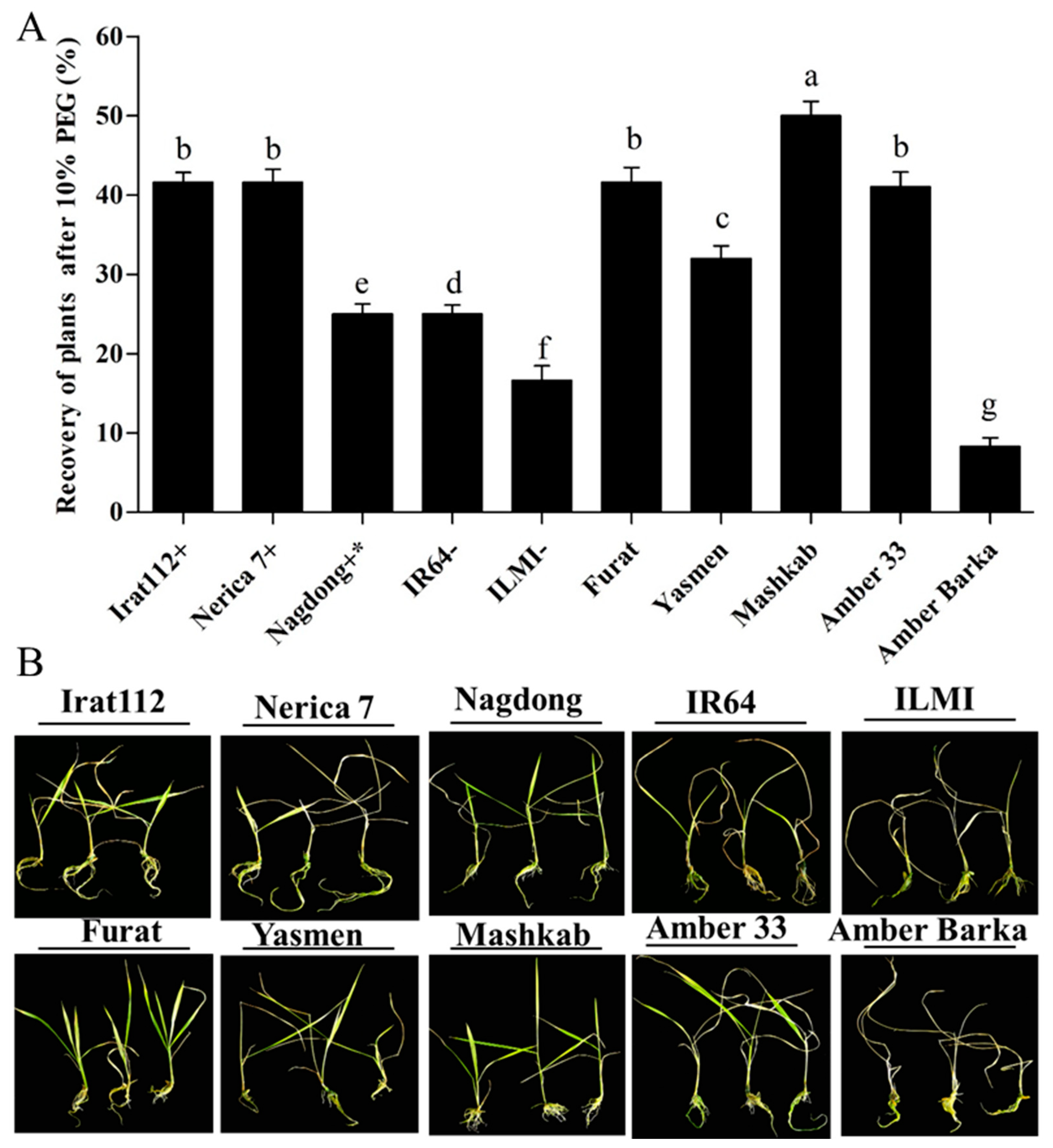
3.3.2. The Recovery Phase of Drought-Stressed Plants
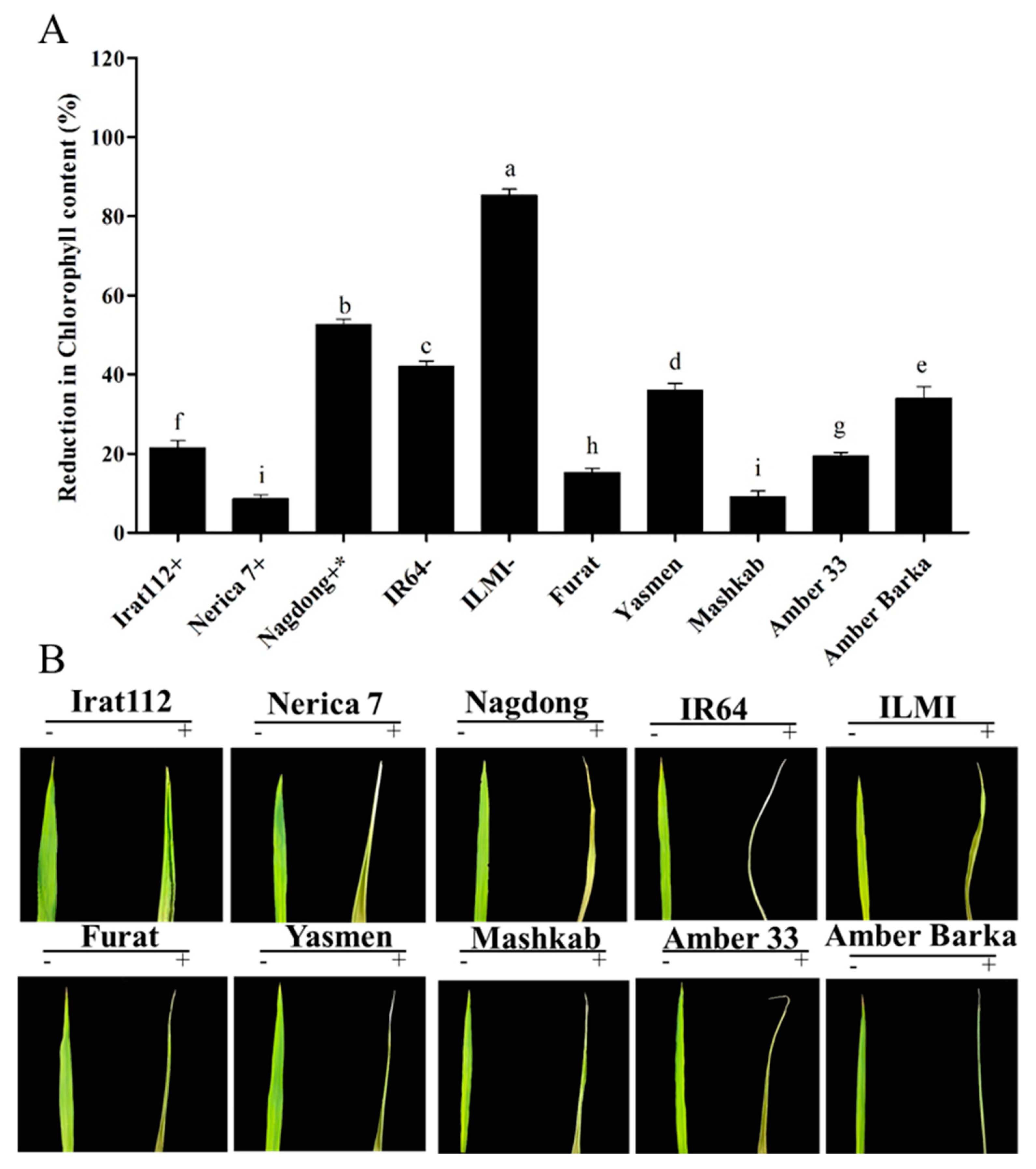
3.4. Chlorophyll Content of the Plants
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Awasthi, S.; Lal, J. Validation of SSR markers associated with drought tolerant QTLs in rice (Oryza sativa L.). Int. J. Sci. Footprints 2014, 2, 99–113. [Google Scholar]
- Khush, G.S. What it will take to Feed 5.0 Billion Rice consumers in 2030. Plant. Mol. Biol. 2005, 59, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Jaleel, C.A.; Manivannan, P.; Wahid, A.; Farooq, M.; Al-Juburi, H.J.; Somasundaram, R.; Panneerselvam, R. Drought stress in plants: A review on morphological characteristics and pigments composition. Int. J. Agric. Biol. 2009, 11, 100–105. [Google Scholar]
- Wilhite, D.A.; Pulwarty, R.S. Drought and Water Crises: Lessons Drawn, Some Lessons Learned, and the Road Ahead. In Drought and Water Crises: Integrating Science, Management, and Policy; CRC Press: Boca Raton, FL, USA, 2018; pp. 513–528. [Google Scholar]
- Al-Faraj, F.A.; Al-Dabbagh, B.N. Assessment of collective impact of upstream watershed development and basin-wide successive droughts on downstream flow regime: The Lesser Zab transboundary basin. J. Hydrol. 2015, 530, 419–430. [Google Scholar] [CrossRef]
- Al-Timimi, Y.K.; Al-Jiboori, M.H. Assessment of spatial and temporal drought in Iraq during the period 1980–2010. Int. J. Energ. Environ. 2013, 4, 291–302. [Google Scholar]
- Lindsey, K.; Jones, M. Plant. Biotechnology in Agriculture; Open University Press: Berkshire, UK, 1989. [Google Scholar]
- Buchanan, C.D.; Lim, S.; Salzman, R.A.; Kagiampakis, I.; Morishige, D.T.; Weers, B.D.; Klein, R.R.; Pratt, L.H.; Cordonnier-Pratt, M.-M.; Klein, P.E.; et al. Sorghum bicolor’s Transcriptome Response to Dehydration, High Salinity and ABA. Plant. Mol. Biol. 2005, 58, 699–720. [Google Scholar] [CrossRef]
- Poroyko, V.; Hejlek, L.; Spollen, W.; Springer, G.; Nguyen, H.; Sharp, R.; Bohnert, H.J. The Maize Root Transcriptome by Serial Analysis of Gene Expression1[w]. Plant. Physiol. 2005, 138, 1700–1710. [Google Scholar] [CrossRef]
- Taheri, S. Effects of drought stress condition on the yield of spring wheat (Triticum aestivum) lines. Afr. J. Biotechnol. 2011, 10, 18339–18348. [Google Scholar] [CrossRef]
- Serraj, R.; McNally, K.L.; Slamet-Loedin, I.; Kohli, A.; Haefele, S.; Atlin, G.; Kumar, A. Drought Resistance Improvement in Rice: An Integrated Genetic and Resource Management Strategy. Plant. Prod. Sci. 2011, 14, 1–14. [Google Scholar] [CrossRef]
- Penna, S. Building stress tolerance through over-producing trehalose in transgenic plants. Trends Plant. Sci. 2003, 8, 355–357. [Google Scholar] [CrossRef]
- Reddy, A.R.; Chaitanya, K.V.; Vivekanandan, M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant. Physiol. 2004, 161, 1189–1202. [Google Scholar] [CrossRef]
- Abid, M.; Ali, S.; Qi, L.K.; Zahoor, R.; Tian, Z.; Jiang, D.; Snider, J.L.; Dai, T. Physiological and biochemical changes during drought and recovery periods at tillering and jointing stages in wheat (Triticum aestivum L.). Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef]
- Fukai, S.; Cooper, M. Development of drought-resistant cultivars using physiomorphological traits in rice. Field Crop. Res. 1995, 40, 67–86. [Google Scholar] [CrossRef]
- Xu, K.; Chen, S.; Li, T.; Ma, X.; Liang, X.; Ding, X.; Liu, H.; Luo, L.J. OsGRAS23, a rice GRAS transcription factor gene, is involved in drought stress response through regulating expression of stress-responsive genes. BMC Plant. Biol. 2015, 15, 141. [Google Scholar] [CrossRef]
- Qin, F.; Sakuma, Y.; Tran, L.-S.P.; Maruyama, K.; Kidokoro, S.; Fujita, Y.; Fujita, M.; Umezawa, T.; Sawano, Y.; Miyazono, K.-I.; et al. Arabidopsis DREB2A-Interacting Proteins Function as RING E3 Ligases and Negatively Regulate Plant Drought Stress–Responsive Gene Expression. Plant. Cell 2008, 20, 1693–1707. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, L.G.G.; Riaño-Pachón, D.M.; Schrago, C.G.; Dos Santos, R.V.; Mueller-Roeber, B.; Vincentz, M. The Role of bZIP Transcription Factors in Green Plant Evolution: Adaptive Features Emerging from Four Founder Genes. PLoS ONE 2008, 3, e2944. [Google Scholar] [CrossRef]
- Lata, C.; Muthamilarasan, M.; Prasad, M. Drought Stress Responses and Signal Transduction in Plants. In Elucidation of Abiotic Stress Signaling in Plants; Springer Science and Business Media LLC: Berlin, Germany, 2015; pp. 195–225. [Google Scholar]
- Yamaguchi-Shinozaki, K.; Shinozaki, K. Transcriptional Regulatory Networks In Cellular Responses And Tolerance To Dehydration And Cold Stresses. Annu. Rev. Plant. Biol. 2006, 57, 781–803. [Google Scholar] [CrossRef]
- Lafitte, R.; Blum, A.; Atlin, G. Using secondary traits to help identify drought-tolerant genotypes. In Breeding Rice for Drought-Prone Environments; IRRI: Laguna, Philippines, 2003; pp. 37–48. [Google Scholar]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant Drought Stress: Effects, Mechanisms and Management. In Sustainable Agriculture; Springer: Dordrecht, The Netherlands, 2009; Volume 29, pp. 153–188. [Google Scholar] [CrossRef]
- Ni, J.; Colowit, P.M.; Mackill, D.J. Evaluation of genetic diversity in rice subspecies using microsatellite markers. Crop. Sci. 2002, 42, 601–607. [Google Scholar]
- Okoshi, M.; Hu, J.; Ishikawa, R.; Fujimura, T. Polymorphic Analysis of Landraces of Japanese Rice using Microsatellite Markers. Breed. Res. 2004, 6, 125–133. [Google Scholar] [CrossRef][Green Version]
- Collins, N.C.; Tardieu, F.; Tuberosa, R. Quantitative Trait Loci and Crop Performance under Abiotic Stress: Where Do We Stand?: Table I. Plant. Physiol. 2008, 147, 469–486. [Google Scholar] [CrossRef]
- Elmajid, A.; Omer, A.B. Characterization Of Drought Tolerance Traits In Selected Rice (Oriza Sativa L.) Genotypes Grown In Sudan Using Simple Sequence Repeat (Ssr) Markers. Master’s Thesis, Jomo Kenyatta University of Agriculture and Technology, Juja, Kenya, 2018. [Google Scholar]
- Singh, N.; Dang, T.T.M.; Vergara, G.V.; Pandey, D.M.; Sanchez, D.; Neeraja, C.N.; Septiningsih, E.M.; Mendioro, M.; Tecson-Mendoza, E.M.; Ismail, A.M.; et al. Molecular marker survey and expression analyses of the rice submergence-tolerance gene SUB1A. Theor. Appl. Genet. 2010, 121, 1441–1453. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, S.; Satoh, K.; Nagata, T.; Kawagashira, N.; Doi, K.; Kishimoto, N.; Yazaki, J.; Ishikawa, M.; Yamada, H.; Ooka, H.; et al. Collection, Mapping, and Annotation of Over 28,000 cDNA Clones from japonica Rice. Science 2003, 301, 376–379. [Google Scholar] [CrossRef] [PubMed]
- Jayashree, B.; Punna, R.; Prasad, P.; Bantte, K.; Hash, C.T.; Chandra, S.; A Hoisington, D.; Varshney, R.K. A database of simple sequence repeats from cereal and legume expressed sequence tags mined in silico: Survey and evaluation. Silico Biol. 2006, 6, 607–620. [Google Scholar]
- Ichitani, K.; Namigoshi, K.; Sato, M.; Taura, S.; Aoki, M.; Matsumoto, Y.; Saitou, T.; Marubashi, W.; Kuboyama, T. Fine mapping and allelic dosage effect of Hwc1, a complementary hybrid weakness gene in rice. Theor. Appl. Genet. 2007, 114, 1407–1415. [Google Scholar] [CrossRef] [PubMed]
- Gholizadeh, A. Heterologous Expression of Stress-Responsive DUF538 Domain Containing Protein and its Morpho-Biochemical Consequences. Protein J. 2011, 30, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Zheng, X.; Chen, L.; Gao, H.; Yang, H.; Long, P.; Rong, J.; Lu, B.; Li, J.; Luo, L.J. Genetic Differentiation Revealed by Selective Loci of Drought-Responding EST-SSRs between Upland and Lowland Rice in China. PLoS ONE 2014, 9, e106352. [Google Scholar] [CrossRef]
- Hirayama, T.; Shinozaki, K. Research on plant abiotic stress responses in the post-genome era: Past, present and future. Plant J. 2010, 61, 1041–1052. [Google Scholar] [CrossRef]
- Singh, K. Transcription factors in plant defense and stress responses. Curr. Opin. Plant. Biol. 2002, 5, 430–436. [Google Scholar] [CrossRef]
- Shinozaki, K. Molecular responses to dehydration and low temperature: Differences and cross-talk between two stress signaling pathways. Curr. Opin. Plant. Biol. 2000, 3, 217–223. [Google Scholar] [CrossRef]
- Joo, J.; Lee, Y.H.; Song, S.I. Overexpression of the rice basic leucine zipper transcription factor OsbZIP12 confers drought tolerance to rice and makes seedlings hypersensitive to ABA. Plant. Biotechnol. Rep. 2014, 8, 431–441. [Google Scholar] [CrossRef]
- Aboukhadrah, S.; Abd Allah, A.; Gharib, H.; Sakran, R.M. Effect of Soil Water Deficit on Yield and Its Components at The Different Growth Stages in Rice (Oryza sativa). Egyptian J. Agro. 2015, 37, 79–92. [Google Scholar]
- Kim, T.; Hur, Y.-J.; Han, S.-I.; Cho, J.-H.; Kim, K.-M.; Lee, J.-H.; Song, Y.-C.; Kwon, Y.-U.; Shin, D. Drought-tolerant QTL qVDT11 leads to stable tiller formation under drought stress conditions in rice. Plant. Sci. 2017, 256, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Casartelli, A.; Riewe, D.; Hubberten, H.M.; Altmann, T.; Hoefgen, R.; Heuer, S. Exploring traditional aus-type rice for metabolites conferring drought tolerance. Rice 2018, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Wade, L.J.; McLaren, C.G.; Quintana, L.; Harnpichitvitaya, D.; Rajatasereekul, S.; Sarawgi, A.K.; Kumar, A.; Ahmed, H.U.; Sarwoto; Singh, A.K.; et al. Genotype by environment interactions across diverse rainfed lowland rice environments. Field Crop. Res. 1999, 64, 35–50. [Google Scholar] [CrossRef]
- Ubaidillah, M.; Faperta, M.; Kim, K.-M. Identification of phytohormone changes and its related genes under abiotic stresses in transgenic rice. BIOCELL 2019, 43, 215–224. [Google Scholar] [CrossRef]
- Kaneda, C. Breeding and Dissemination Efforts of “NERICA”. Jpn. J. Trop. Agric. 2007, 51, 145–151. [Google Scholar]
- Futakuchi, K.; Fofana, M.; Sie, M. Varietal Differences in Lodging Resistance of African Rice (Oryza glaberrima Steud.). Asian J. Plant. Sci. 2008, 7, 569–573. [Google Scholar] [CrossRef]
- Badro, H.; Furtado, A.; Henry, R.J. Relationships between Iraqi Rice Varieties at the Nuclear and Plastid Genome Levels. Plants 2019, 8, 481. [Google Scholar] [CrossRef]
- Huang, J.; Wang, M.-M.; Jiang, Y.; Bao, Y.; Huang, X.; Sun, H.; Xu, D.; Lan, H.-X.; Zhang, H. Expression analysis of rice A20/AN1-type zinc finger genes and characterization of ZFP177 that contributes to temperature stress tolerance. Gene 2008, 420, 135–144. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circular. Calif. Agric. Experim. Stn. 1950, 347, 32. [Google Scholar]
- Grimault, A.; Gendrot, G.; Chamot, S.; Widiez, T.; Rabillé, H.; Gérentes, M.-F.; Creff, A.; Thévenin, J.; Dubreucq, B.; Ingram, G.; et al. ZmZHOUPI, an endosperm-specific basic helix-loop-helix transcription factor involved in maize seed development. Plant J. 2015, 84, 574–586. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Liang, G.; Yu, D. Activated Expression of WRKY57 Confers Drought Tolerance in Arabidopsis. Mol. Plant. 2012, 5, 1375–1388. [Google Scholar] [CrossRef] [PubMed]
- Ses, I. Standard Evaluation System; International Rice Research Institute: Manila, Philippines, 2002; pp. 11–30. [Google Scholar]
- De Datta, S.; Malabuyoc, J.; Aragon, E. A field screening technique for evaluating rice germplasm for drought tolerance during the vegetative stage. Field Crop. Res. 1988, 19, 123–134. [Google Scholar] [CrossRef]
- Yang, P.-M.; Huang, Q.-C.; Qin, G.-Y.; Zhao, S.-P.; Zhou, J.G. Different drought-stress responses in photosynthesis and reactive oxygen metabolism between autotetraploid and diploid rice. Photosynthetica 2014, 52, 193–202. [Google Scholar] [CrossRef]
- Khan, M.A.; Asaf, S.; Khan, A.L.; Adhikari, A.; Jan, R.; Ali, S.; Imran, M.; Kim, K.-M.; Lee, I.-J. Halotolerant Rhizobacterial Strains Mitigate the Adverse Effects of NaCl Stress in Soybean Seedlings. BioMed Res. Int. 2019, 2019, 1–15. [Google Scholar] [CrossRef]
- Khan, M.; Imran, Q.M.; Shahid, M.; Mun, B.-G.; Lee, I.-J.; Khan, M.A.; Hussain, A.; Lee, I.-J.; Yun, B.-W. Nitric oxide- induced AtAO3 differentially regulates plant defense and drought tolerance in Arabidopsis thaliana. BMC Plant. Biol. 2019, 19, 602–619. [Google Scholar] [CrossRef]
- Revalska, M.; Vassileva, V.; Zechirov, G.; Iantcheva, A. Is the auxin influx carrierLAX3essential for plant growth and development in the model plantsMedicago truncatula, Lotus japonicusandArabidopsis thaliana? Biotechnol. Biotechnol. Equip. 2015, 29, 786–797. [Google Scholar] [CrossRef]
- Al-Ansari, N.; Ali, A.; Knutsson, S. Present Conditions and Future Challenges of Water Resources Problems in Iraq. J. Water Resour. Prot. 2014, 6, 1066–1098. [Google Scholar] [CrossRef]
- da Cruz, R.P.; Milach, S.C.K. Tolerância ao frio no estádio de germinação em arroz: Métodos de avaliação e caracterização de genótipos. Sci. Agric. 2004, 61, 1–8. [Google Scholar]
- Krishnasamy, V.; Seshu, D.V. Seed Germination Rate and Associated Characters in Rice. Crop. Sci. 1989, 29, 904–908. [Google Scholar] [CrossRef]
- Singh, B.N.; Mackill, D.J. Genetics of leaf rolling under drought stress. In Rice Genetics Collection; World Scientific Pub Co Pte Ltd.: Singapore, 2008; pp. 159–166. [Google Scholar]
- Madabula, F.P.; Dos Santos, R.S.; Machado, N.; Pegoraro, C.; Kruger, M.M.; Da Maia, L.C.; De Sousa, R.O.; De Oliveira, A.C. Rice genotypes for drought tolerance: Morphological and transcriptional evaluation of auxin-related genes. Bragantia 2016, 75, 428–434. [Google Scholar] [CrossRef]
- Okçu, G.; Kaya, M.D.; Atak, M. Effects of salt and drought stresses on germination and seedling growth of pea (Pisum sativum L.). Turkish J. Agric. Forestry 2005, 29, 237–242. [Google Scholar]
- Abegunewardene, N.; Schmidt, K.-H.; Vosseler, M.; Dreher, M.; Keller, T.; Hoffmann, N.; Veit, K.; Petersen, S.E.; Lehr, H.; Schreiber, W.G.; et al. Local Transient Myocardial Liposomal Gene Transfer of Inducible Nitric Oxide Synthase Does Not Aggravate Myocardial Function and Fibrosis and Leads to Moderate Neovascularization in Chronic Myocardial Ischemia in Pigs. Microcirculation 2010, 17, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Manschadi, A.M.; Hammer, G.L.; Christopher, J.T.; Devoil, P. Genotypic variation in seedling root architectural traits and implications for drought adaptation in wheat (Triticum aestivum L.). Plant. Soil 2007, 303, 115–129. [Google Scholar] [CrossRef]
- Franco, J.; Bañón, S.; Vicente, M.J.; Miralles, J.; Martínez-Sánchez, J.J. Review Article:Root development in horticultural plants grown under abiotic stress conditions – a review. J. Hortic. Sci. Biotechnol. 2011, 86, 543–556. [Google Scholar] [CrossRef]
- Gowda, V.R.; Henry, A.; Yamauchi, A.; Shashidhar, H.E.; Serraj, R. Root biology and genetic improvement for drought avoidance in rice. Field Crop. Res. 2011, 122, 1–13. [Google Scholar] [CrossRef]
- Rabello, A.R.; Guimarães, C.M.; Rangel, P.H.N.; Da Silva, F.R.; Seixas, D.; Souza, E.M.; Brasileiro, A.C.M.; Spehar, C.R.; Ferreira, M.E.; Mehta, A. Identification of drought-responsive genes in roots of upland rice (Oryza sativa L). BMC Genom. 2008, 9, 485. [Google Scholar] [CrossRef]
- Hadiarto, T.; Tran, L.-S.P. Progress studies of drought-responsive genes in rice. Plant. Cell Rep. 2011, 30, 297–310. [Google Scholar] [CrossRef]
- Dubouzet, J.G.; Sakuma, Y.; Ito, Y.; Kasuga, M.; Dubouzet, E.G.; Miura, S.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. OsDREB genes in rice, Oryza sativa L., encode transcription activators that function in drought-, high-salt- and cold-responsive gene expression. Plant J. 2003, 33, 751–763. [Google Scholar] [CrossRef]
- Jakoby, M.; Weisshaar, B.; Dröge-Laser, W.; Vicente-Carbajosa, J.; Tiedemann, J.; Kroj, T.; Parcy, F. bZIP transcription factors in Arabidopsis. Trends Plant. Sci. 2002, 7, 106–111. [Google Scholar] [CrossRef]
- Kraus, T.E.; McKersie, B.D.; Fletcher, R.A. Paclobutrazol-induced Tolerance of Wheat Leaves to Paraquat May Involve Increased Antioxidant Enzyme Activity. J. Plant. Physiol. 1995, 145, 570–576. [Google Scholar] [CrossRef]
- Sedeek, S.E.M.; El-Namaky, R.A.; Hammoud, S.A.A.; El-Habet, H.B. Genetical Studies On Root System And Yield And Its Components Traits Under Water Limit Condition In Rice (Oryza sativa L.). J. Agric. Chem. Biotechnol. 2012, 3, 447–460. [Google Scholar] [CrossRef]
- Hasan, M.; Bhuiyan, M.; Begum, S.; Raffi, S.; Razia, S. Morphological and molecular characterization of some NERICA mutant lines under drought condition. Progress. Agric. 2015, 26, 15–21. [Google Scholar] [CrossRef][Green Version]





| Cultivars | Drought Related Characteristics | Countries | Subspecies | References |
|---|---|---|---|---|
| IR64 | Drought susceptible | Philippines | O. sativa ssp. indica | [39] |
| Irat112 | Drought Tolerant | Cote dIvoire | O. sativa ssp. indica | [37] |
| Furat | Unknown | Iraq | O. sativa ssp. indica | [44] |
| Yasmin | Unknown | Iraq | O. sativa ssp. indica | [44] |
| Mashkab | Unknown | Iraq | O. sativa ssp. japonica | [44] |
| Amber 33 | Unknown | Iraq | O. sativa ssp. japonica | [44] |
| Amber Barka | Unknown | Iraq | O. sativa ssp. indica | [44] |
| Nerica 7 | Drought Tolerant | Cote dIvoire | Interspecific (O. glaberrima X O. sativa) | [42] |
| ILMI | Drought susceptible | Korea | O. sativa ssp. japonica | [41] |
| Nagdong | Moderate Tolerant | Korea | O. sativa ssp. japonica | [38] |
| Scale | Description (Leaf Drying) | Description (Drought Scale) 2 | Rate |
|---|---|---|---|
| 1 | No symptoms of stress effects. | No symptoms | Highly resistant |
| 2 | Slight tip drying. | Slight tip drying | Resistant |
| 3 | Tip drying extended up to ¼ length of all leaves. | Tip drying extended up to ¼ | Moderately Resistant |
| 4 | Tip drying extended to ¼ length in at most 50% of all leaves. | Tip drying extended up to ¼ | |
| 5 | Tip drying extended to ¼ length or more in 50% of all leave. | One -fourth to ½ of all leaves dried | Moderately susceptible |
| 6 | 50% of all leaves fully dried. | One -fourth to ½ of all leaves dried | |
| 7 | More than 50% but less than 70% of all leaves fully dried. | More than ⅔ of all leaves fully dried | Susceptible |
| 8 | 70% of all leaves fully dried. | More than ⅔ of all leaves fully dried | |
| 9 | More than 70% of all leaves fully dried. | All plants apparently dead | Highly susceptible |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Azzawi, T.N.I.; Khan, M.; Hussain, A.; Shahid, M.; Imran, Q.M.; Mun, B.-G.; Lee, S.-U.; Yun, B.-W. Evaluation of Iraqi Rice Cultivars for Their Tolerance to Drought Stress. Agronomy 2020, 10, 1782. https://doi.org/10.3390/agronomy10111782
Al Azzawi TNI, Khan M, Hussain A, Shahid M, Imran QM, Mun B-G, Lee S-U, Yun B-W. Evaluation of Iraqi Rice Cultivars for Their Tolerance to Drought Stress. Agronomy. 2020; 10(11):1782. https://doi.org/10.3390/agronomy10111782
Chicago/Turabian StyleAl Azzawi, Tiba Nazar Ibrahim, Murtaza Khan, Adil Hussain, Muhammad Shahid, Qari Muhammad Imran, Bong-Gyu Mun, Sang-Uk Lee, and Byung-Wook Yun. 2020. "Evaluation of Iraqi Rice Cultivars for Their Tolerance to Drought Stress" Agronomy 10, no. 11: 1782. https://doi.org/10.3390/agronomy10111782
APA StyleAl Azzawi, T. N. I., Khan, M., Hussain, A., Shahid, M., Imran, Q. M., Mun, B.-G., Lee, S.-U., & Yun, B.-W. (2020). Evaluation of Iraqi Rice Cultivars for Their Tolerance to Drought Stress. Agronomy, 10(11), 1782. https://doi.org/10.3390/agronomy10111782






