Practical Methods for Breaking Seed Dormancy in a Wild Ornamental Tulip Species Tulipa thianschanica Regel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Seed Collection
2.2. Water Imbibition and Seed Coat Removal Tests
2.3. Temperature Test
2.4. Plant Growth Regulators Test
2.5. Stratification Test
2.6. Seed Sowing Depth Test
2.7. Statistical Analysis
3. Results
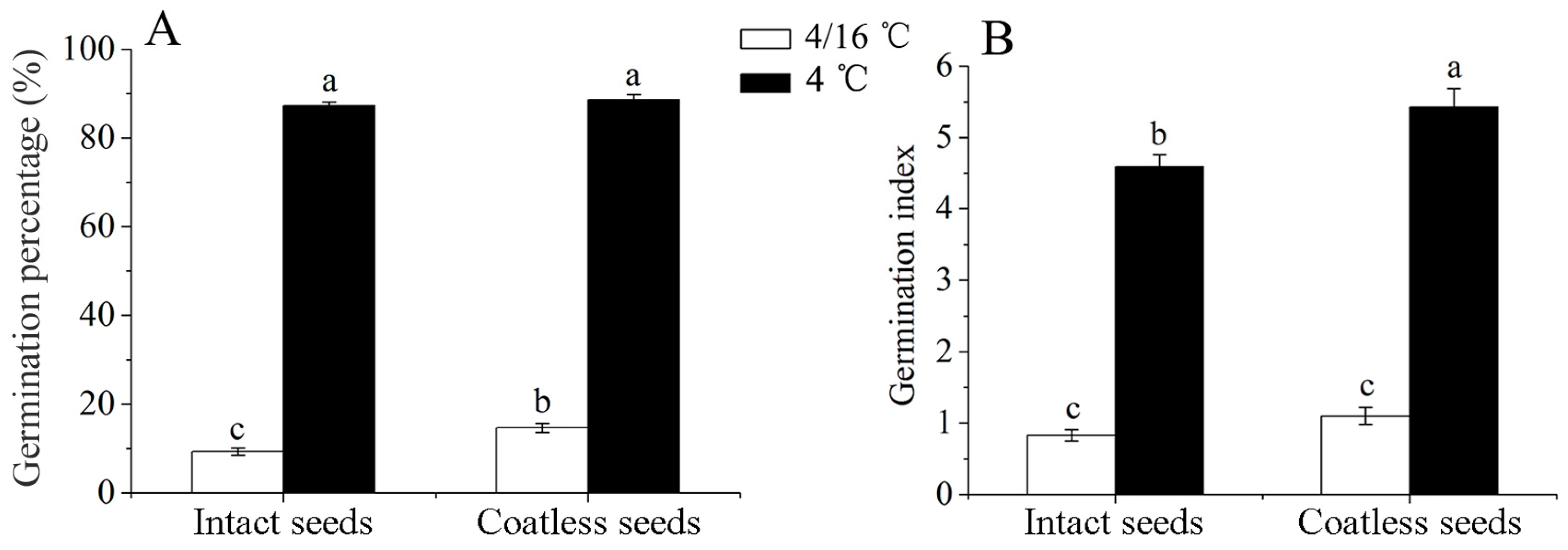
3.1. Water Absorption and Testa Influence on Seed Germination
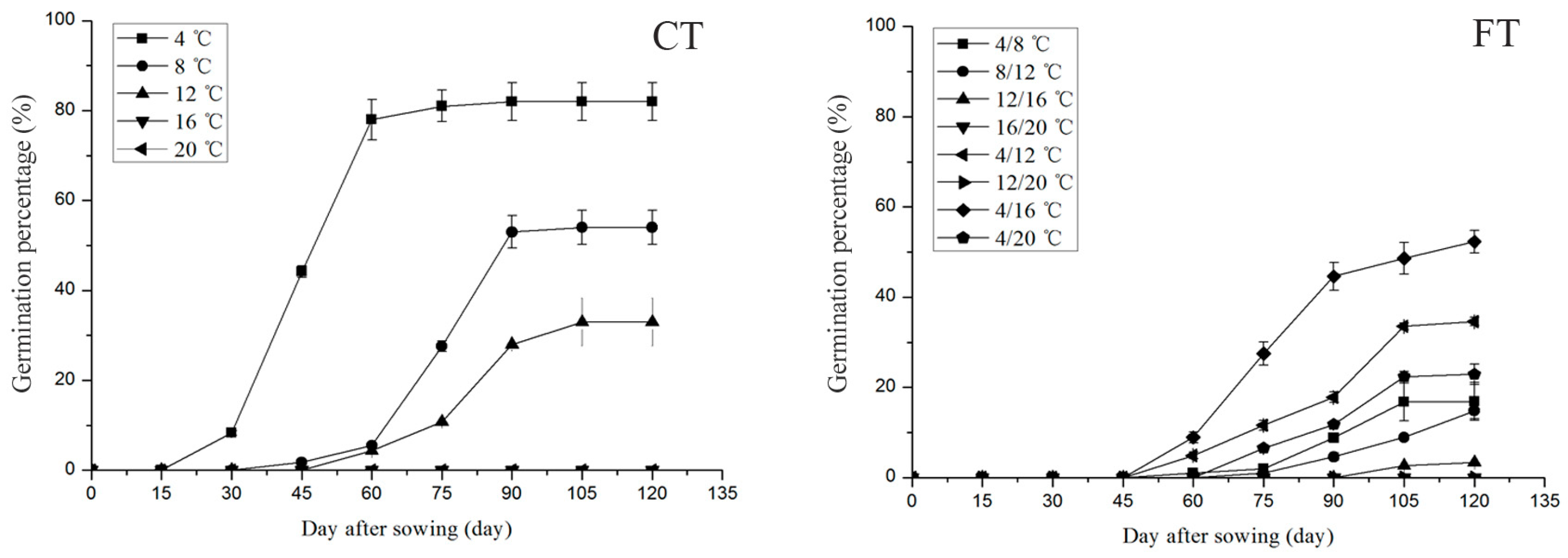
3.2. Effect of Temperature on Seed Germination
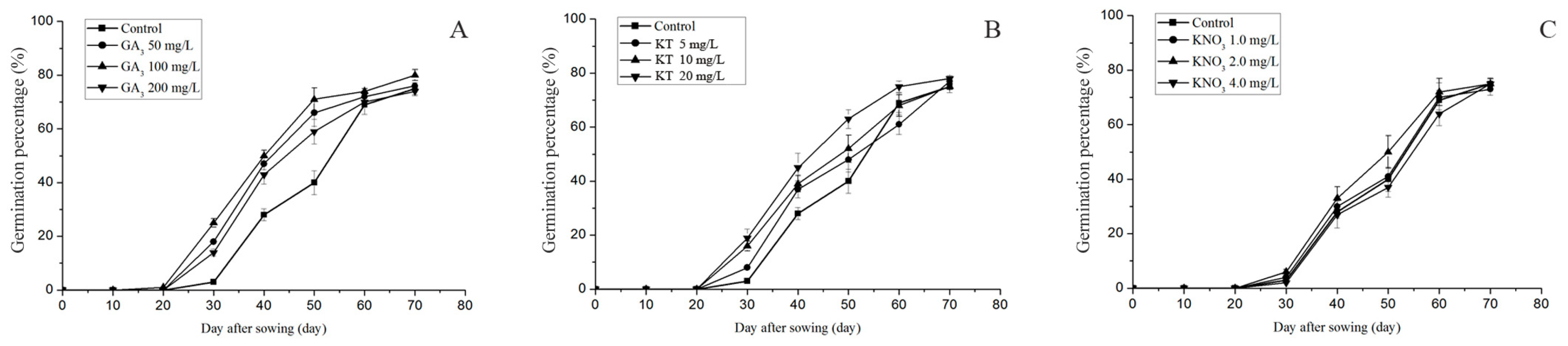
3.3. Effect of Exogenous Plant Growth Regulators on Seed Germination
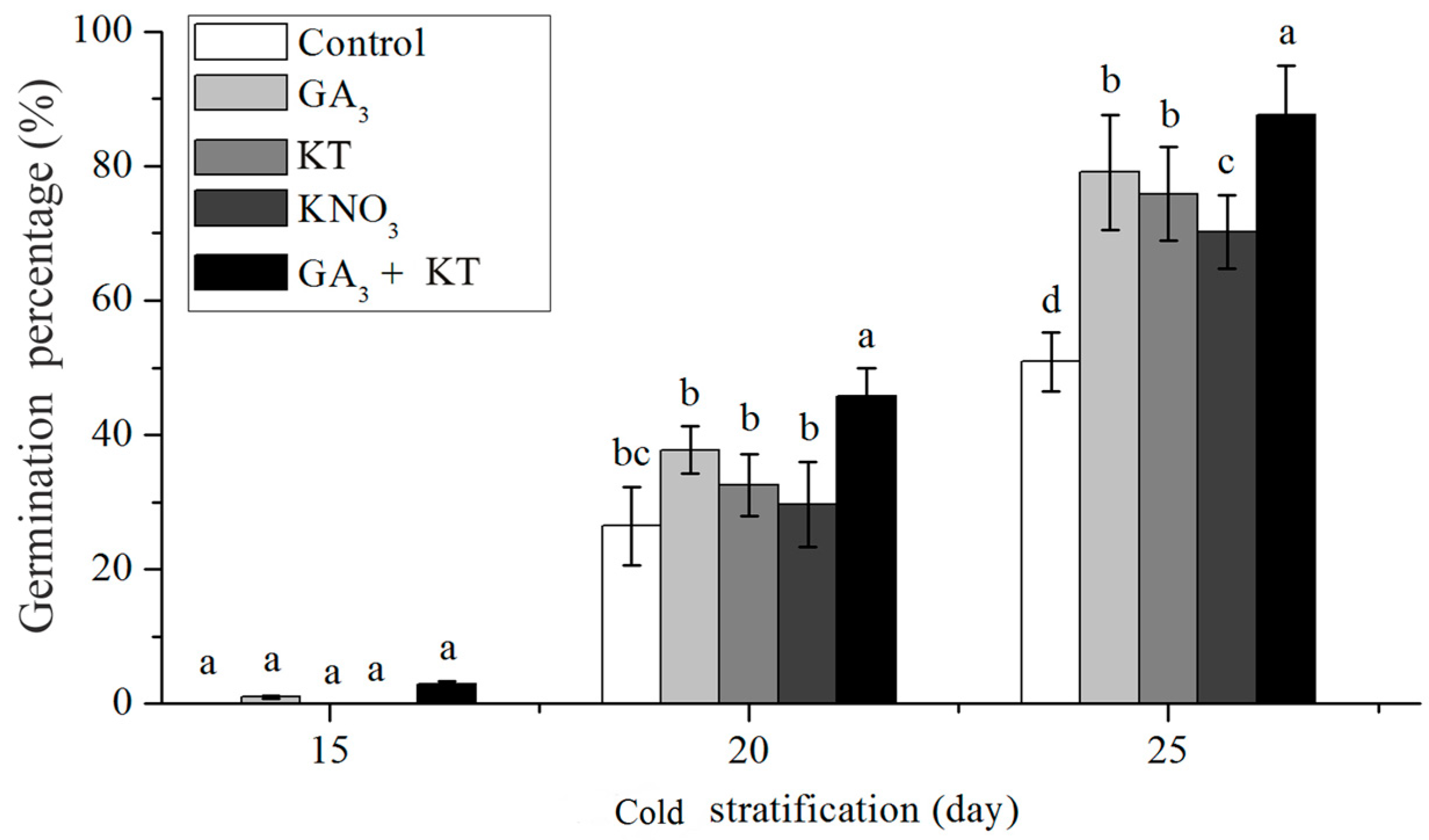
3.4. Effect of Cold Stratification on Seed Germination
3.5. Effect of Seed Sowing Depth on Seedling Emergence
4. Discussion
4.1. Temperature Regulation on Plant Seed Germination
4.2. Plant Growth Regulators Regulation on Plant Seed Germination
4.3. Cold Stratification Regulation on Plant Seed Germination
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Xing, G.M.; Qu, L.W.; Zhang, Y.Q.; Xue, L.; Su, J.W.; Lei, J.J. Collection and evaluation of wild tulip (Tulipa spp.) resources in China. Genet Resour. Crop Evol. 2017, 64, 641–652. [Google Scholar] [CrossRef]
- Qu, L.W.; Xue, L.; Xing, G.M.; Zhang, Y.Q.; Chen, J.J.; Zhang, W.; Lei, J.J. Karyotype analysis of eight wild Tulipa species native to China and the interspecific hybridization with tulip cultivars. Euphytica 2018, 214, 2–12. [Google Scholar] [CrossRef]
- Rhie, Y.H.; Lee, S.Y. Seed dormancy and germination of Epimedium koreanum Nakai. Sci. Hortic. 2020, 272, 109600. [Google Scholar] [CrossRef]
- Loddo, D.; Carlesi, S.; Adérito, T.P.C. Germination of Chloris barbata, Cynodon dactylon, and Cyperus rotundus from Angola at constant and alternate temperatures. Agronomy 2019, 9, 615. [Google Scholar] [CrossRef] [Green Version]
- Tang, A.J.; Tian, M.H.; Long, C.L. Seed dormancy and germination of three herbaceous perennial desert ephemerals from the Junggar Basin, China. Seed Sci. Res. 2009, 19, 183–189. [Google Scholar] [CrossRef]
- Rouhi, H.R.; Karimi, F.A.; Shahbodaghlo, A.R.; Sheikhalian, M.; Rahmatabadi, R.; Samadi, M.; Karimi, F. Effects of sulfuric acid, stratification, phytohormone and potassium nitrate on dormancy breaking and germination of water lily tulip (Tulipa kaufmanniana Regel.). Int. J. Agric. Sci. 2012, 2, 136–142. [Google Scholar]
- Huarte, H.R.; Benech-Arnold, R.L. Hormonal nature of seed responses to fluctuating temperatures in Cynara cardunculus (L.). Seed Sci. Res. 2010, 20, 39–45. [Google Scholar] [CrossRef]
- El-Keblawy, A.; Gairola, S. Dormancy regulating chemicals alleviate innate seed dormancy and promote germination of desert annuals. J. Plant Growth Regul. 2017, 36, 300–312. [Google Scholar] [CrossRef]
- Yang, L.; Müller, K.; El-kassaby, Y.A.; Kermode, A.R. Changes in hormone flux and signaling in white spruce (Picea glauca) seeds during the transition from dormancy to germination in response to temperature cues. BMC Plant Biol. 2015, 15, 292. [Google Scholar]
- Lee, K.P.; Piskurewicz, U.; Turecková, V.; Strnad, M.; Lopez-Molina, L. A seed coat bedding assay shows that RGL2- dependent release of abscisic acid by the endosperm controls embryo growth in Arabidopsis dormant seeds. Proc. Natl. Acad. Sci. USA 2010, 107, 19108–19113. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Liu, T.; Zhou, L.L. Research on the biological and germinating characters of wild Tulipa. J. Shihezi Univ. 2001, 5, 197–200. [Google Scholar]
- Wróbel, S.; Kęsy, J.; Treder, K. Effect of growth regulators and ethanol on termination of dormancy in Potato tubers. Am. J. Potato Res. 2017, 94, 544–555. [Google Scholar] [CrossRef] [Green Version]
- Ozel, C.A. Effective seed dormancy breaking and regeneration of Centaurea Tchiatcheffii fisch ET. Mey. Fresen. Environ. Bull. 2017, 3, 2197–2204. [Google Scholar]
- Lee, J.W.; Jo, I.H.; Kim, J.U.; Hong, C.E.; Kim, Y.C.; Kim, D.H.; Park, Y.D. Improvement of seed dehiscence and germination in ginseng by stratification, gibberellin, and/or kinetin treatments. Hortic. Environ. Biotechnol. 2018, 59, 293–301. [Google Scholar] [CrossRef]
- Yang, L.W.; Liu, S.Y.; Lin, R.C. The role of light in regulating seed dormancy and germination. J. Integr. Plant Biol. 2020, 62, 1310–1326. [Google Scholar] [CrossRef] [PubMed]
- Renzi, J.P.; Brus, J.; Pirintsos, S.; Erdős, L.; Duchoslav, M.; Smýkal, P. Release of Medicago truncatula Gaertn. and Pisum sativum subsp. elatius (M. Bieb.) Asch. et Graebn. Seed dormancy tested in soil conditions. Agronomy 2020, 10, 1–16. [Google Scholar]
- Nie, X.X.; Kang, X.S.; Lu, T.; Li, W.; Li, F.; Xiong, Q. Study on the seed germination characteristics of Tulipa Iliensis. Seed 2016, 35, 70–73. [Google Scholar]
- Li, Y.; Wu, Y.; Elmongy, M.; Lyu, X.S.; Ren, Z.M.; Sun, M.Y.; Xia, Y.P. Aseptic germination and bulblet formation of wild tulip (Tulipa sinkiangensi). J. Zhejiang Univ. 2017, 43, 307–316. [Google Scholar]
- Zhang, A.Q.; Jing, H.; Yin, C.M.; Li, G.X. Seed dormancy characteristics and germination condition of Tulipa gesnersiana. Pratacultural Sci. 2010, 27, 48–53. [Google Scholar]
- Qu, L.W.; Su, J.W.; Li, S.L.; Zhang, Y.Q.; Lu, J.J. The sowing technology of hybridization seeds of tulip. Mod. Landsc. Archit. 2014, 11, 42–44. [Google Scholar]
- Hu, X.W.; Huang, X.H.; Wang, Y.R. Hormonal and temperature regulation of seed dormancy and germination in Leymus chinensis. Plant Growth Regul. 2012, 67, 199–207. [Google Scholar] [CrossRef]
- Huang, W.C.; Maytona, H.S.; Amirkhani, M.; Wang, D.C.; Taylor, A.G. Seed dormancy, germination and fungal infestation of eastern gamagrass seed. Ind. Crop Prod. 2017, 99, 109–116. [Google Scholar] [CrossRef]
- Nijenstein, H.; Don, R.; Nydam, J. Comparison of oven moisture test at 130 °C vs. 103 °C. Seed Sci. Technol. 2002, 30, 102–106. [Google Scholar]
- Khodorova, N.; Boitel-Conti, M. The role of temperature in the growth and flowering of geophytes. Plant 2013, 2, 699–711. [Google Scholar] [CrossRef] [Green Version]
- Nabieva, A.Y.; Gerasimovich, L.V. The application of different reproduction techniques for rare species waterlily tulip (Tulipa kaufmanniana Regel.) propagation under ex situ conditions. Ornam. Hortic. 2019, 25, 450–460. [Google Scholar]
- Yuan, Y.J.; Mu, G.J. Features of spring climate change in Tianshan mountainous area for the recent 40 years and comparison with that in plain area of Xinjiang. Agric. Land Geogr. 2004, 27, 35–40. [Google Scholar]
- Shu, K.; Liu, X.D.; Xie, Q.; He, Z.H. Two faces of one seed: Hormonal regulation of dormancy and germination. Mol. Plant 2016, 9, 34–45. [Google Scholar] [CrossRef] [Green Version]
- Sohindji, F.S.; Sogbohossou, D.E.O.; Zohoungbogbo, H.P.F.; Houdegbe, C.A.; Achigan-Dako, E.G. Understanding molecular mechanisms of seed dormancy for improved germination in traditional leafy vegetables: An overview. Agronomy 2020, 10, 57. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Tian, Y.; Lu, X. Breeding of the dormant thermosensitive genic male-sterile lines of early rice to overcome pre-harvest sprouting of the hybrid seeds. Agronomy 2018, 8, 191. [Google Scholar] [CrossRef] [Green Version]
- Debeaujon, I.; Koornneef, M. Gibberellin requirement for Arabidopsis seed germination is determined both by testa characteristics and embryonic abscisic acid. Plant Physiol. 2000, 122, 415–424. [Google Scholar] [CrossRef] [Green Version]
- White, C.N.; Rivin, C.J. Gibberellins and seed development in maize. II. Gibberellin synthesis inhibition enhances abscisic acid signaling in cultured embryos. Plant Physiol. 2000, 122, 1089–1097. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Hu, P.W.; Huang, M.K.; Tang, Y.; Li, Y.; Li, L.; Hou, X.L. The NF-YC-RGL2 module integrates GA and ABA signalling to regulate seed germination in Arabidopsis. Nat. Commun. 2016, 7, 12768. [Google Scholar] [CrossRef] [Green Version]
- Carruggio, F.; Onofri, A.; Impelluso, C.; Gianpietro, G.G.D.; Scopece, G.; Cristaudo, A. Seed dormancy breaking and germination in Bituminaria basaltica and B. bituminosa (Fabaceae). Plants 2020, 9, 1110. [Google Scholar] [CrossRef] [PubMed]
- Aghilian, B.; Mohammad, K.H.; Sepideh, A. Evaluation of seed dormancy in forty medicinal plant species. Intl. J. Agric. Crop Sci. 2014, 7, 760–768. [Google Scholar]
- Jiao, F.; Liu, Q.; Sun, G.F.; Lin, Q.W.; Li, X.D.; Zhang, J.Z. Study on seed germination characteristics of two wild tulips in Xinjiang. North. Hortic. 2015, 2, 55–60. [Google Scholar]
- Ye, X.X.; Zhang, M.; Dong, S.; Ma, Y.Q. Conditioning duration and agents involved in broomrape seeds responding to germination stimulants. Plant Growth Regul. 2017, 81, 221–230. [Google Scholar] [CrossRef]
- Kelly, A.; Lacroix, C. Effects of seed age and dormancy breaking treatments on the viability and germination of the Gulf of Saint Lawrence aster (Symphyotrichum laurentianum). Botany 2019, 97, 699–705. [Google Scholar] [CrossRef]
- Gashi, B.; Osmani, M.; Aliu, S. Breaking seed dormancy of Tulipa scardica Bornm. and Tulipa kosovarica Kit Tan, Shuka & Krasniqi by pre-chilling, plant growth regulators and some chemical treatments. Acta Agric. Slov. 2019, 113, 203–210. [Google Scholar]
- Ikeya, S.; Aoyanagi, T.; Ishizuka, I.; Takeuchi, A.; Kozaki, A. Nitrate promotes germination under inhibition by NaCl or high concentration of glucose. Plants 2020, 9, 707. [Google Scholar] [CrossRef]
- Iralu, V.; Krishna, U. Seed dormancy, germination and seedling characteristics of Elaeocarpus prunifolius Wall. ex Müll. Berol.: A threatened tree species of north-eastern India. N. Z. J. For. Sci. 2018, 48, 16. [Google Scholar] [CrossRef]
- Yang, L.E.; Peng, D.L.; Li, Z.M.; Huang, L.; Yang, J.; Sun, H. Cold stratification, temperature, light, GA3, and KNO3 effects on seed germination of Primula beesiana from Yunnan, China. Plant Divers. 2020, 42, 168–173. [Google Scholar] [CrossRef]
- Wang, Z.K.; Ma, R.; Zhao, M.S.; Wang, F.F.; Zhang, N.; Si, H.J. NO and ABA interaction regulates tuber dormancy and sprouting in potato. Front. Plant Sci. 2020, 11, 311. [Google Scholar] [CrossRef]
- Vidal, E.A.; Alvarez, J.M.; Araus, V.; Riveras, E.; Brooks, M.D.; Krouk, G.; Ruffel, S.; Lejay, L.; Crawford, N.M.; Coruzzi, G.M.; et al. Nitrate in 2020: Thirty years from transport to signaling networks. Plant Cell 2020, 32, 2094–2119. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.P.; Cai, W.M. Functional characterization of the BnNCED3 gene in Brassica Napus. Plant Sci. 2017, 256, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Jan, K.; Paweł, S. Response of Amaranthus retroflexus L. seeds to gibberellic acid, ethylene and abscisic acid depending on duration of stratification and burial. Plant Growth Regul. 2013, 70, 15–26. [Google Scholar]
- Rouhi, H.R.; Afshari, R.T.; Shakarami, K. Seed treatments to overcome dormancy of waterlily tulip (Tulipa kaufmanniana Regel.). Aust. J. Crop Sci. 2010, 4, 718–721. [Google Scholar]
- Wang, C.X.; OuYang, T.; Jiang, Y.C. Aseptic germination and bulblet formation of interspecific hybrids between wild species and cultivar of tulip. Plant Physiol. Commun. 2009, 45, 1098–1100. [Google Scholar]
| Temperatures (°C) | Initial Germination Duration (Day) | T50 (Day) | Final Germination Duration (Day) |
|---|---|---|---|
| CTx | |||
| 4 | 33.8 ± 1.08 d | 46.1 ± 1.35 d | 57.3 ± 1.66 c |
| 8 | 61.3 ± 2.20 bc | 89.2 ± 2.11 b | 107.3 ± 2.45 b |
| 12 | 63.5 ± 2.35 b | 92.2 ± 2.01 b | 111.0 ± 1.88 b |
| 16 | --z | -- | -- |
| 20 | -- | -- | -- |
| FTy | |||
| 4/8 | 69.3 ± 3.32 a | 92.3 ± 2.89 b | 118.3 ± 4.05 ab |
| 8/12 | 72.0± 1.88 a | 97.2 ± 1.28 a | 128.6 ± 2.19 a |
| 12/16 | 73.3 ± 4.41 a | 95.9 ± 0.88 a | 126.0 ± 5.34 a |
| 16/20 | -- | -- | -- |
| 4/12 | 64.0± 6.24 b | 97.4 ± 2.33 a | 121.6 ± 3.71 a |
| 12/20 | -- | -- | -- |
| 4/16 | 59.2 ± 4.81 c | 78.3 ± 4.72 c | 102.3 ± 3.76 b |
| 4/20 | 72.6 ± 2.60 a | 91.5 ± 2.64 b | 126.6 ± 3.46 a |
| Treatments (mg/L) | Initial Germination Duration (Day) | T50 (Day) | Final Germination Duration (Day) | |
|---|---|---|---|---|
| Control | 0 | 35.7 ± 1.45 a | 57.4 ± 1.94 a | 65.8 ± 3.76 a |
| GA3 | 50 | 28.6 ± 1.24 cd | 42.7 ± 1.20 c | 53.3 ± 1.33 c |
| 100 | 26.9 ± 1.85 d | 41.3 ± 0.82 c | 52.6 ± 0.39 c | |
| 200 | 32.6 ± 1.53 b | 44.6 ± 1.20 bc | 57.3 ± 1.21 bc | |
| KT | 5 | 30.0 ± 1.52 c | 47.0 ± 1.63 b | 63.3 ± 0.89 ab |
| 10 | 28.5 ± 1.26 cd | 47.3 ± 1.45 b | 62.0 ± 0.58 ab | |
| 20 | 28.2 ± 1.18 cd | 46.6 ± 1.20 b | 60.7 ± 0.85 b | |
| KNO3 | 1.0 | 32.6 ± 1.45 b | 56.0 ± 1.15 a | 64.0 ± 2.08 ab |
| 2.0 | 31.0 ± 1.53 bc | 55.2 ± 2.59 a | 63.2 ± 3.21 ab | |
| 4.0 | 35.6 ± 2.72 a | 56.5 ± 2.34 a | 63.6 ± 8.37 ab | |
| PGRs (mg/L) | Initial Germination Duration (Day) | T50 (Day) | Final Germination Duration (Day) | |
|---|---|---|---|---|
| GA3 | KT | |||
| 0 | 0 | 36.2 ± 0.32 a | 55.3 ± 0.66 a | 69.5 ± 2.01 a |
| 50 | 5 | 29.3 ± 1.86 c | 43.0 ± 1.34 c | 64.0 ± 2.65 b |
| 10 | 31.4 ± 2.16 b | 44.3 ± 2.10 c | 55.6 ± 1.44 c | |
| 20 | 27.5 ± 1.44 cd | 42.6 ± 1.02 cd | 53.1 ± 0.96 d | |
| 100 | 5 | 27.8 ± 1.96 cd | 46.5 ± 1.22 b | 54.2 ± 1.32 cd |
| 10 | 25.3 ± 2.53 d | 42.3 ± 2.10 d | 49.8 ± 0.53 e | |
| 20 | 25.5 ± 1.19 d | 43.1 ± 1.34 c | 54.4 ± 1.34 cd | |
| 200 | 5 | 29.4 ± 2.64 c | 46.6 ± 2.36 b | 55. 5 ± 3.28 c |
| 10 | 26.0 ± 0.49 d | 43.2 ± 1.74 c | 52.6 ± 1.37 d | |
| 20 | 32.3 ± 3.02 b | 44.3 ± 2.67 c | 56.7 ± 2.58 c | |
| Significance | ||||
| GA3 | *** | ** | ** | |
| KT | ** | * | ns | |
| GA3 × KT | *** | *** | *** | |
| Cold Stratification Duration (Day) | Treatments (mg/L) | Initial Germination Duration (Day) | T50 (Day) | Final Germination Duration (Day) |
|---|---|---|---|---|
| 15 | Control (0) | -- z | -- | -- |
| GA3 (100) | 74.0 ± 3.60 a | 93.0 ± 2.65 a | 133.7 ± 3.18 a | |
| KT (20) | -- | -- | -- | |
| KNO3 (2.0) | -- | -- | -- | |
| GA3 (100) + KT (10) | 73.6 ± 4.07 a | 91.6 ± 5.02 a | 132.3 ± 5.24 a | |
| Significance | ns | ns | ns | |
| 20 | Control (0) | 59.7 ± 2.19 a | 76.7 ± 3.93 a | 97.6 ± 4.91 a |
| GA3 (100) | 53.8 ± 2.08 b | 70.2 ± 2.08 b | 85.7 ± 7.80 b | |
| KT (20) | 57.3 ± 2.73 a | 74.5 ± 3.76 a | 93.0 ± 5.20 a | |
| KNO3 (2.0) | 57.2 ± 2.19 a | 75.7 ± 2.81 a | 95.3 ± 1.35 a | |
| GA3 (100) + KT (10) | 51.4 ± 3.46 b | 64.9 ± 2.18 c | 80.3 ± 2.08 c | |
| Significance | * | ** | ** | |
| 25 | Control (0) | 43.5 ± 1.53 a | 61.3 ± 4.04 a | 77.5 ± 3.69 a |
| GA3 (100) | 31.3 ± 0.89 c | 48.3 ± 1.76 c | 56.5 ± 1.20 c | |
| KT (20) | 36.2 ± 3.06 b | 53.7 ± 3.84 b | 67.5 ± 2.31 b | |
| KNO3 (2.0) | 38.0 ± 1.15 b | 54.3 ± 2.03 b | 65.0 ± 2.32 b | |
| GA3 (100) + KT (10) | 27.5 ± 2.08 d | 43.6 ± 3.18 d | 50.7 ± 1.20 d | |
| Significance | *** | *** | *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Qu, L.-W.; Zhao, J.; Xue, L.; Dai, H.-P.; Xing, G.-M.; Lei, J.-J. Practical Methods for Breaking Seed Dormancy in a Wild Ornamental Tulip Species Tulipa thianschanica Regel. Agronomy 2020, 10, 1765. https://doi.org/10.3390/agronomy10111765
Zhang W, Qu L-W, Zhao J, Xue L, Dai H-P, Xing G-M, Lei J-J. Practical Methods for Breaking Seed Dormancy in a Wild Ornamental Tulip Species Tulipa thianschanica Regel. Agronomy. 2020; 10(11):1765. https://doi.org/10.3390/agronomy10111765
Chicago/Turabian StyleZhang, Wei, Lian-Wei Qu, Jun Zhao, Li Xue, Han-Ping Dai, Gui-Mei Xing, and Jia-Jun Lei. 2020. "Practical Methods for Breaking Seed Dormancy in a Wild Ornamental Tulip Species Tulipa thianschanica Regel" Agronomy 10, no. 11: 1765. https://doi.org/10.3390/agronomy10111765
APA StyleZhang, W., Qu, L.-W., Zhao, J., Xue, L., Dai, H.-P., Xing, G.-M., & Lei, J.-J. (2020). Practical Methods for Breaking Seed Dormancy in a Wild Ornamental Tulip Species Tulipa thianschanica Regel. Agronomy, 10(11), 1765. https://doi.org/10.3390/agronomy10111765





