Grazing Intensity Impacts on Herbage Mass, Sward Structure, Greenhouse Gas Emissions, and Animal Performance: Analysis of Brachiaria Pastureland
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site and Grazing Management
2.2. Experiments and Treatments
2.3. Response Variables
2.4. Statistical Analysis
3. Results
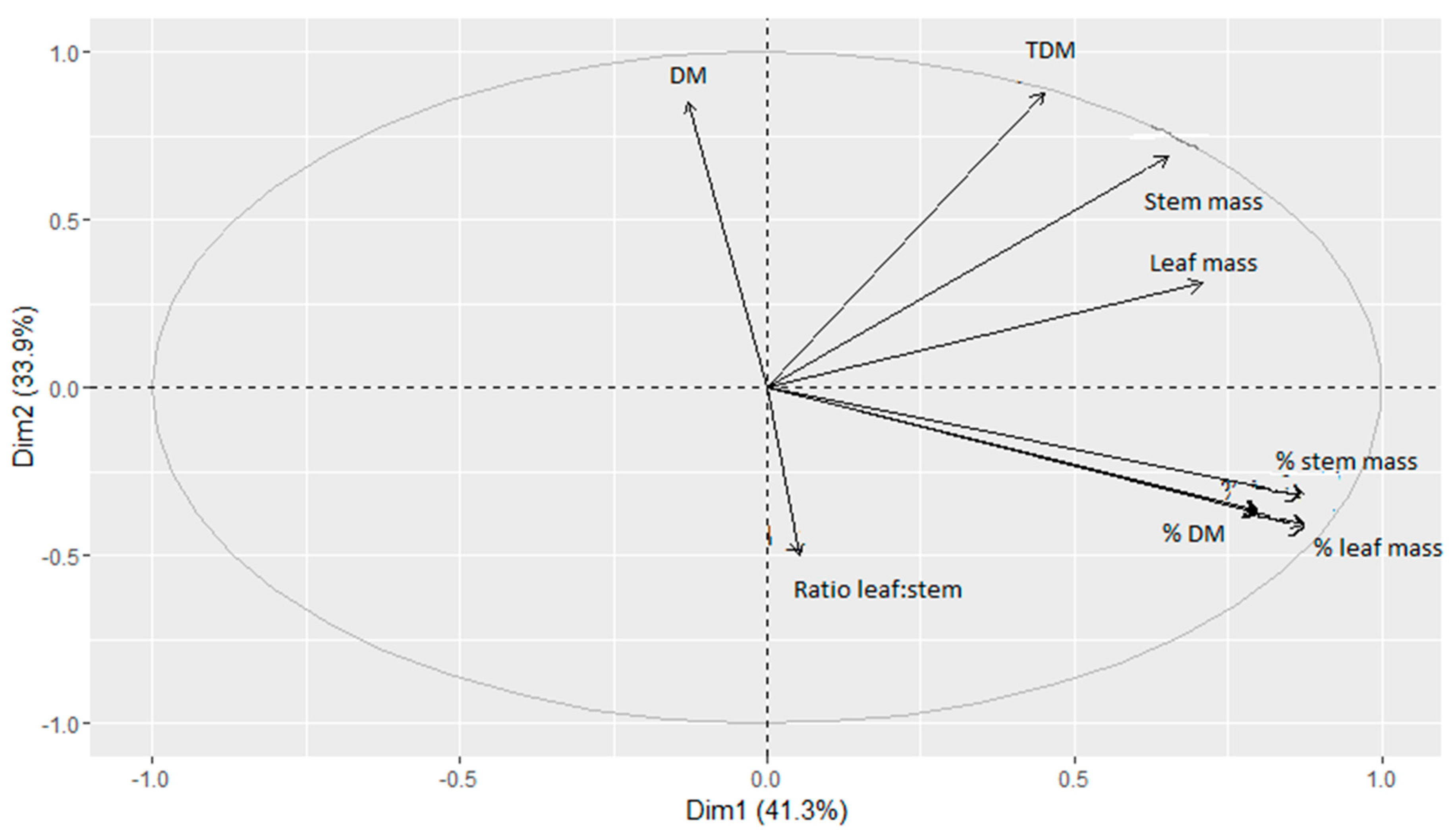
3.1. Forage Mass and Structure
3.2. Nutritive Value
3.3. Animal Performance
3.4. Enteric Methane
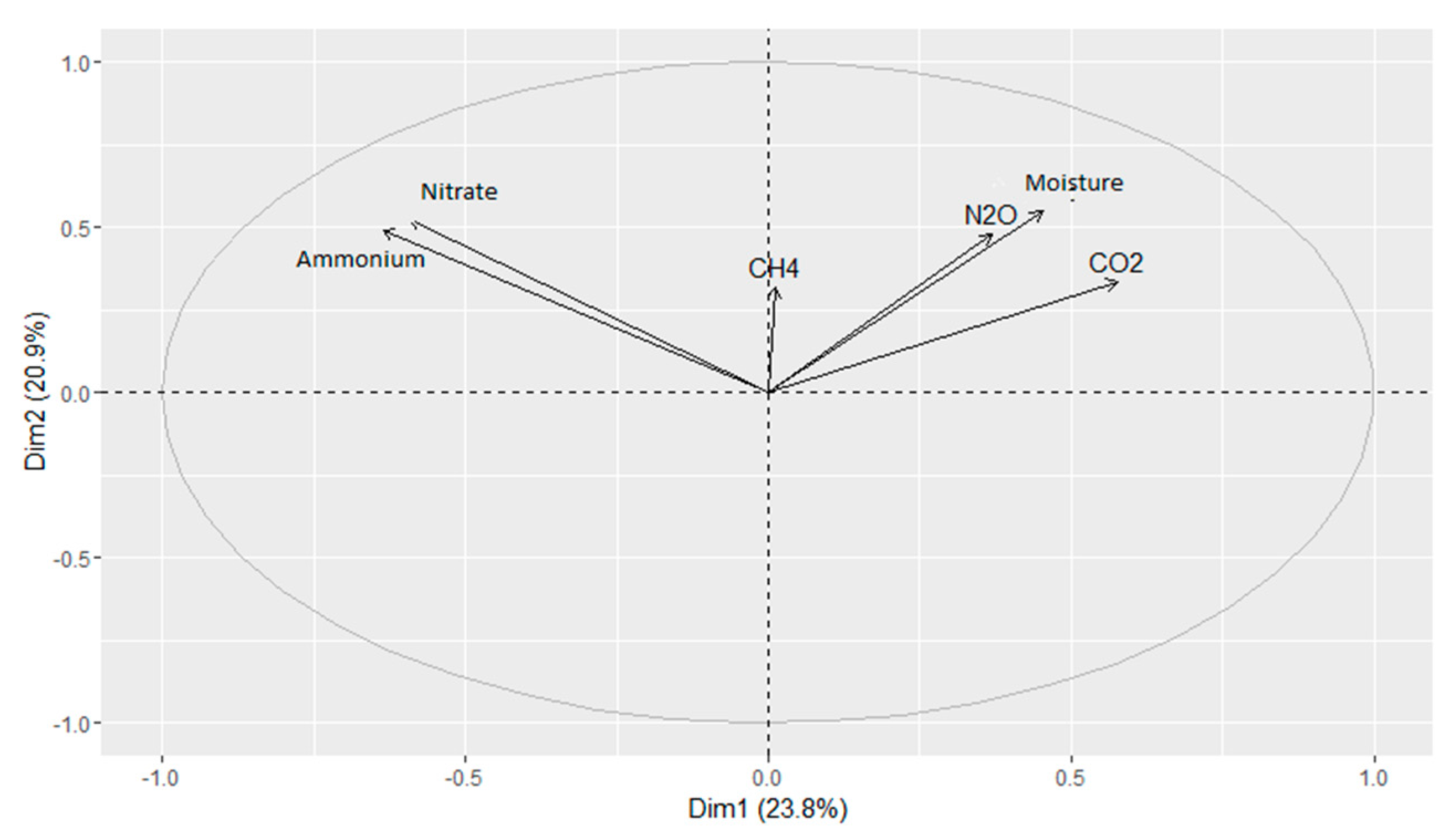
3.5. Greenhouse Gases
4. Discussion
4.1. Sward Structure
4.2. Nutritive Value
4.3. Animal Performance
4.4. Greenhouse Gases
4.5. Practical Implications for Pasture Management
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Phelps, L.N.; Kaplan, J.O. Land use for animal production in global change studies: Defining and characterizing a framework. Glob. Chang. Biol. 2017, 23, 4457–4471. [Google Scholar] [CrossRef]
- Cardoso, A.S.; Barbero, R.P.; Romanzini, E.P.; Teobaldo, R.W.; Ongaratto, F.; Fernandes, M.H.M.R.; Ruggieri, A.C.; Reis, R.A. Intensification: A key strategy to achieve great animal and environmental beef cattle production sustainability in Brachiaria grasslands. Sustainability 2020, 12, 6656. [Google Scholar] [CrossRef]
- Raposo, E.; Brito, L.F.; Janusckiewicz, E.R.; Oliveira, L.F.; Versuti, J.; Assumpção, F.M.; Cardoso, A.S.; Seniscalchi, D.; Delevatti, L.M.; Malheiros, E.B.; et al. Greenhouse gases emissions from tropical grasslands affected by nitrogen fertilizer management. Agron. J. 2020, 112. [Google Scholar] [CrossRef]
- Lima, D.M.; Abdalla Filho, A.L.; Lima, P.D.M.T.; Sakita, G.Z.; McManus, C.; Abdalla, A.L.; Louvandini, H. Morphological characteristics, nutritive quality, and methane production of tropical grasses in Brazil. Pesq. Agropec. Bras. 2018, 53, 323–331. [Google Scholar] [CrossRef]
- Euclides, V.P.B.; Valle, C.B.; Macedo, M.C.M.; Almeida, R.G.D.; Montagner, D.B.; Barbosa, R.A. Brazilian scientific progress in pasture research during the first decade of XXI century. Rev. Bras. Zootecn. 2010, 39, 151–168. [Google Scholar] [CrossRef]
- Rudel, T.K.; Paul, B.; White, D.; Rao, I.M.; Van Der Hoek, R.; Castro, A.; Peters, M. LivestockPlus: Forages, sustainable intensification, and food security in the tropics. Ambio 2015, 44, 685–693. [Google Scholar] [CrossRef]
- Euclides, V.P.B.; Euclides Filho, K.; Arruda, Z.D.; Figueiredo, G.D. Desempenho de novilhos em pastagens de Brachiaria decumbens submetidos a diferentes regimes alimentares. Rev. Bras. Zootec. 1998, 27, 246–254. [Google Scholar]
- Pedreira, B.C.; Pedreira, C.G.S.; da Silva, S.C. Estrutura do dossel e acúmulo de forragem de Brachiaria brizantha cultivar Xaraés em resposta a estratégias de pastejo. Pesq. Agropec. Bras. 2007, 42, 281–287. [Google Scholar] [CrossRef]
- Giacomini, A.A.; Silva, S.C.D.; Sarmento, D.O.D.L.; Zeferino, C.V.; Souza Júnior, S.J.; Trindade, J.K.D.; Nascimento Júnior, D.D. Growth of Marandu palisadegrass subjected to strategies of intermittent stocking. Sci. Agric. 2009, 66, 733–741. [Google Scholar] [CrossRef]
- Delevatti, L.M.; Cardoso, A.S.; Barbero, R.P.; Leite, R.G.; Romanzini, E.P.; Ruggieri, A.; Reis, R.A. Effect of nitrogen application rate on yield, forage quality, and animal performance in a tropical pasture. Sci. Rep. 2019, 9, 7596. [Google Scholar] [CrossRef]
- Berça, A.S.; Cardoso, A.S.; Longhini, V.Z.; Tedeschi, L.O.; Boddey, R.M.; Berndt, A.; Reis, R.A.; Ruggieri, A.C. Methane production and nitrogen balance of dairy heifers grazing palisade grass cv. Marandu alone or with forage peanut. J. Anim. Sci. 2019, 97, 4625–4634. [Google Scholar] [CrossRef]
- Cardoso, S.A.; Brito, L.F.; Janusckiewicz, E.R.; Morgado, E.S.; Barbero, R.P.; Koscheck, J.F.W.; Reis, R.A.; Ruggieri, A.C. Impact of grazing intensity and seasons on greenhouse gas emissions in tropical grassland. Ecosystems 2017, 20, 845–859. [Google Scholar] [CrossRef]
- Cardoso, A.S.; Oliveira, S.C.; Janusckiewicz, E.R.; Brito, L.F.; Morgado, E.S.; Reis, R.A.; Ruggieri, A.C. Effect of season on ammonia, nitrous oxide, and methane emission factors for beef cattle excreta and urea fertilizer applied to tropical pasture. Soil Till. Res. 2019, 194, 104341. [Google Scholar] [CrossRef]
- Martin, C.; Morgavi, D.; Doreau, M. Methane mitigation in ruminants: From microbe to the farm scale. Animal 2010, 4, 351–365. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPCC). 2019 Refinement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories. 2019. Available online: https://www.ipcc-nggip.iges.or.jp/public/2019rf/pdf/4_Volume4/19R_V4_Ch11_Soils_N2O_CO2.pdf (accessed on 19 July 2020).
- IUSS. World Reference Base for Soil Resources, 1st ed.; World Soil Resources Reports; FAO: Rome, Italy, 2006. [Google Scholar]
- Van Raij, B.; Cantarella, H.; Quaggio, J.A.; Furlani, A.M.C. Recomendações de Adubação e Calagem Para o Estado de São Paulo, 2nd ed.; IAC. Boletim Técnico, 100; Instituto Agronômico, Campinas: Brasilia, Brazil, 1997.
- Mott, G.O.; Lucas, H.L. The design, conduct and interpretation of grazing trials on cultivated and improved pastures. In Proceedings of the 6th International Grassland Congress, Philadelphia, PA, USA, 17–23 August 1952; pp. 1380–1395. [Google Scholar]
- Barbero, R.P.; Malheiros, E.B.; Araújo, T.L.R.; Nave, R.L.G.; Mulliniks, J.T.; Berchielli, T.T.; Reis, R.A. Combining Marandu grass grazing height and supplementation level to optimize growth and productivity of yearling bulls. Anim. Feed Sci. Technol. 2015, 209, 110–118. [Google Scholar] [CrossRef]
- Casagrande, D.R.; Ruggieri, A.C.; Moretti, M.H.; Berchielli, T.T.; Vieira, B.R.; Roth, A.P.T.P.; Reis, R.A. Sward sward structure and performance of beef heifers under supplementation in Brachiaria brizantha cv. Marandu pastures maintained with three grazing intensities in a continuous stocking system. Rev. Bras. Zootecn. 2011, 40, 2074–2082. [Google Scholar] [CrossRef]
- Barbero, R.P.; Malheiros, E.B.; Nave, R.L.; Mulliniks, J.T.; Delevatti, L.M.; Koscheck, J.F.; Ruggieri, A.C.; Reis, R.A. Influence of post-weaning management system during the finishing phase on grasslands or feedlot on aiming to improvement of the beef cattle production. Agric. Syst. 2017, 153, 23–31. [Google Scholar] [CrossRef]
- Barre, P.; Turner, L.B.; Escobar-Gutiérrez, A.J. Leaf length variation in perennial forage grasses. Agriculture 2015, 5, 682–696. [Google Scholar] [CrossRef]
- Calvano, M.P.C.A.; Euclides, V.P.B.; Montagner, D.B.; Lempp, B.; Difante, G.S.; Flores, R.S.; Galbeiro, S. Perfilhamento e acúmulo de forragem do capim-marandu submetido a diferentes intensidades de pastejo. Ceres 2011, 58, 781–789. [Google Scholar] [CrossRef]
- Nunes, S.G.; Boock, A.; Penteado, M.D.O.; Gomes, D.T. Brachiaria Brizantha Cv. Marandu, 1st ed.; Embrapa Gado de Corte, Documentos (INFOTECA-E): Campo Grande, Brazil, 1984. [Google Scholar]
- Poppi, D.P.; McLennan, S.R.; Bediye, S.; Vega, A.; Zorrilla-Rios, J. Forage quality: Strategies for increasing nutritive value of forages. In International Grassland Congress; Buchanan-Smith, J.G., Bailey, L.D., McCaughey, P., Eds.; Winnipeg & Saskatoon: Winnipeg, MB, Canada, 1997. [Google Scholar]
- Magalhães, J.A.; Carneiro, M.S.S.; Andrade, A.C.; Pereira, E.S.; Rodrigues, B.H.N.; Costa, N.L.; Townsend, C.R. Bromatological composition the Marandu grass under effect of different irrigation and nitrogen fertilization. Semina Ciênc. Agrár. 2015, 36, 933–942. [Google Scholar]
- Araújo, R.A.D.; Rodrigues, R.C.; Costa, C.D.S.; Santos, F.N.S.; Costa, F.O.; Silva, I.R.D.; Rodrigues, M.M. Chemical composition and bromatologic degradability in situ of Marandu grass in silvopastoral systems formed by babassu and monoculture systems. Rev. Bras. Saúde Prod. Anim. 2016, 17, 401–412. [Google Scholar] [CrossRef][Green Version]
- McRoberts, K.C.; Parsons, D.; Ketterings, Q.M.; Hai, T.T.; Quan, N.H.; Ba, N.X.; Cherney, D.J.R. Urea and composted cattle manure affect forage yield and nutritive value in sandy soils of south-central Vietnam. Grass Forage Sci. 2017, 73, 132–145. [Google Scholar] [CrossRef]
- Da Silva, S.C.; Sbrissia, A.F.; Pereira, L.E.T. Ecophysiology of C4 forage grasses-understanding plant growth for optimizing their use and management. Agriculture 2015, 5, 598–625. [Google Scholar] [CrossRef]
- Hongyu, K.S.; Sandanielo’s, V.L.M.; Oliveira Junior, G.J. Análise de Componentes Principais: Resumo teórico, aplicação e interpretação. Eng. Sci. 2015, 1, 83–90. [Google Scholar] [CrossRef]
- Larsen, K.S.; Andresen, L.C.; Beier, C.; Jonasson, S.; Albert, K.R.; Ambus, P.E.R.; Ibrom, A. Reduced N cycling in response to elevated CO2, warming, and drought in a Danish heathland: Synthesizing results of the Climaite project after two years of treatments. Glob. Chang. Biol. 2011, 17, 1884–1899. [Google Scholar] [CrossRef]
- Xu, Z.Z.; Zhou, G.S. Combined effects of water stress and high temperature on photosynthesis, nitrogen metabolism and lipid peroxidation of a perennial grass. Leymus Chinensis Planta 2006, 224, 1080–1090. [Google Scholar] [CrossRef]
- Erice, G.; Irigoyen, J.J.; Pérez, P.; Martínez-Carrasco, R.; Sánchez-Díaz, M. Effect of elevated CO2, temperature and drought on photosynthesis of nodulated alfalfa during a cutting regrowth cycle. Physiol. Plantarum 2006, 126, 458–468. [Google Scholar] [CrossRef]
- Abd-Elgawad, M. Yield losses by phytonematodes: Challenges and opportunities with special reference to Egypt. Egypt. J. Agronematol. 2014, 13, 75–94. [Google Scholar] [CrossRef]
- Kill-Silveira, R.; Jangarelli, M. How the calving order of cows affects the performance of Nellore calves. Acta Sci. Anim. Sci. 2018, 40, e34519. [Google Scholar] [CrossRef][Green Version]
- Underwood, K.R.; Tong, J.F.; Price, P.L.; Roberts, A.J.; Grings, E.E.; Hess, B.W.; Means, W.J.; Du, M. Nutrition during mid to late gestation affects growth, adipose tissue deposition, and tenderness in cross-bred beef steers. Meat Sci. 2010, 86, 588–593. [Google Scholar] [CrossRef]
- Greenwood, P.L.; Cafe, L.M.; Hearnshaw, H.; Hennessy, D.W. Consequences of nutrition and growth retardation early in life for growth and composition of cattle and eating quality of beef. RAAN Aus. 2015, 15, 183–195. [Google Scholar]
- Waldo, D.R.; Jorgensen, N.A. Forages for high animal production: Nutritional factors and effects of conservation. J. Dairy Sci. 1981, 64, 1207–1229. [Google Scholar] [CrossRef]
- Poppi, D.P.; Hughes, T.P.; L’Huillier, P.J. Intake of Pasture by Grazing Ruminants. Livestock Feeding on Pasture; Hamilton Publishing: Hamilton, New Zealand, 1987; pp. 55–64. [Google Scholar]
- McMeniman, J.P.; Defoor, P.J.; Galyean, M.L. Evaluation of the National Research Council (1996) dry matter intake prediction equations and relationships between intake and performance by feedlot cattle. J. Anim. Sci. 2009, 87, 1138–1146. [Google Scholar] [CrossRef]
- Tóth, E.; Deák, B.; Valkó, O.; Kelemen, A.; Miglécz, T.; Tóthmérész, B.; Török, P. Livestock type is more crucial than grazing intensity: Traditional cattle and sheep grazing in short-grass steppes. Land Degrad. Dev. 2018, 29, 231–239. [Google Scholar] [CrossRef]
- Nave, R.L.G.; Sulc, R.M.; Barker, D.J. Relationships of forage nutritive value to cool-season grass canopy characteristics. Crop. Sci. 2013, 53, 341–348. [Google Scholar] [CrossRef]
- Vieira, B.R.; Azenha, M.V.; Casagrande, D.R.; Costa, D.F.A.; Ruggieri, A.C.; Berchielli, T.T.; Reis, R.A. Ingestive behavior of supplemented Nellore heifers grazing palisade grass pastures managed with different sward heights. Anim. Sci. J. 2017, 88, 696–704. [Google Scholar] [CrossRef]
- Carvalho, P.C.F.; Moraes, A. Comportamento ingestivo de ruminantes: Bases para o manejo sustentável do pasto. In Simpósio sobre Manejo Sustentável em Pastagem; Cecato, U., Barbosa, M.A.A.F., Galbeiro, S., Paris, W., Grecco, F.C.A.R., Soares Filho, C.V., Teixeira, S., Eds.; EdUEM: Maringá, Brazil, 2005; pp. 1–20. [Google Scholar]
- Cardoso, A.S.; Berndt, A.; Leytem, A.; Alves, B.J.; Carvalho, I.D.N.D.; Soares, L.H.D.B.; Urquiaga, S.; Boddey, R.M. Impact of the intensification of beef production in Brazil on greenhouse gas emissions and land use. Agric. Syst. 2016, 143, 86–96. [Google Scholar] [CrossRef]
- Sauvant, D.; Giger-Reverdin, S. Modélisation des interactions digestives et de la production de méthane. Inra Prod. Anim. 2009, 22, 375–384. [Google Scholar] [CrossRef]
- José Neto, A.; Messana, J.D.; Granja-Salcedo, Y.T.; Castagnino, P.S.; Fiorentini, G.; Reis, R.A.; Berchielli, T.T. Effect of starch level in supplement with or without oil source on diet and apparent digestibility, rumen fermentation and microbial population of Nellore steers grazing tropical grass. Livest. Sci. 2017, 202, 171–179. [Google Scholar] [CrossRef]
- Meister, N.C.; Cardoso, A.S.; Alari, F.O.; Lemos, N.L.S.; Frighetto, R.T.S.; Malheiros, E.B.; Reis, R.A.; Ruggieri, A.C. Effect of pasture management on enteric methane emissions from goats. Trop. Anim. Health Prod. 2021. just accepted. [Google Scholar]
- Jones, F.M.; Phillips, F.A.; Naylor, T.; Mercer, N.B. Methane emissions from grazing Angus beef cows selected for divergent residual feed intake. Anim. Feed Sci. Technol. 2011, 166, 302–307. [Google Scholar] [CrossRef]
- Barrière, Y.; Laperche, A.; Barrot, L.; Aurel, G.; Briand, M.; Jouanin, L. QTL analysis of lignification and cell wall digestibility in the Bay-0 × Shahdara RIL progeny of Arabidopsis thaliana as a model system for forage plants. Plant. Sci. 2005, 168, 1235–1245. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change. Guidelines for National Greenhouse Gas Inventories. Greenhouse Gas Inventory Reference Manual 4. 2006. Available online: http://www.ipcc971nggip.iges.or.jp/public/2006gl/vol4.html (accessed on 19 July 2020).
- Carbone, M.S.; Still, C.J.; Ambrose, A.R.; Dawson, T.E.; Williams, A.P.; Boot, C.M.; Schaeffer, S.M.; Schimel, J.P. Seasonal and episodic moisture controls on plant and microbial contributions to soil respiration. Oecologia 2011, 167, 265–278. [Google Scholar] [CrossRef]
- Cardoso, A.S.; Quintana, B.G.; Janusckiewicz, E.; Brito, L.F.; Morgado, E.D.S.; Reis, R.A.; Ruggieri, A.C. How do methane rates vary with soil moisture and compaction, N compound and rate, and dung addition in a tropical soil? Int. J. Biometeorol. 2019, 63, 1533–1540. [Google Scholar] [CrossRef]
- Davidson, E.A.; Janssens, I.A.; Luo, Y. On the variability of respiration in terrestrial ecosystems: Moving beyond Q10. Glob. Chang. Biol. 2006, 12, 154–164. [Google Scholar] [CrossRef]
- Pezzopane, J.R.M.; Santos, P.M.; Evangelista, S.R.M.; Bosi, C.; Cavalcante, A.C.R.; Bettiol, G.M.; Pellegrino, G.Q. Panicum maximum cv. Tanzânia: Climate trends and regional pasture production in Brazil. Grass Forage Sci. 2017, 72, 104–117. [Google Scholar] [CrossRef]
- Jantalia, C.P.; Santos, H.P.; Urquiaga, S.; Boddey, R.M.; Alves, B.J. Fluxes of nitrous oxide from soil under different crop rotations and tillage systems in the South of Brazil. Nutr. Cycling Agroecosyst. 2008, 82, 161–173. [Google Scholar] [CrossRef]
- Mazzetto, A.M.; Barneze, A.S.; Feigl, B.J.; Van-Groenigen, J.W.; Oenema, O.; Cerri, C.C. Temperature and moisture affect methane and nitrous oxide emission from bovine manure patches in tropical conditions. Soil Biol. Biochem. 2014, 76, 242–248. [Google Scholar] [CrossRef]
- Bayer, C.; Gomes, J.; Zanatta, J.A.; Vieira, F.C.B.; Piccolo, M.C.; Dieckow, J.; Six, J. Soil nitrous oxide emissions as affected by long-term tillage, cropping systems and nitrogen fertilization in Southern Brazil. Soil Till. Res. 2015, 146, 213–222. [Google Scholar] [CrossRef]
- Ryden, J.C.; Lund, L.J. Nature and extent of directly measured denitrification losses from some irrigated vegetable crop production units 1. Soil Sci. Soc. Am. J. 1980, 44, 505–511. [Google Scholar] [CrossRef]
- Geng, J.; Cheng, S.; Fang, H.; Yu, G.; Li, X.; Si, G.; Yu, G. Soil nitrate accumulation explains the nonlinear responses of soil CO2 and CH4 fluxes to nitrogen addition in a temperate needle-broadleaved mixed forest. Ecol. Indic. 2017, 79, 28–36. [Google Scholar] [CrossRef]
| Period | Days with Rain | Rainfall | Temperature (°C) | ||
|---|---|---|---|---|---|
| (d) | (mm) | Max. | Min. | Mean | |
| December 2005/May 2006 | 82 | 1089 | 31.3 | 17.2 | 23.2 |
| December 2006/May 2007 | 95 | 1009 | 317 | 18.7 | 24.2 |
| December 2007/May 2008 | 86 | 1145 | 30.3 | 14.2 | 22.8 |
| December 2008/May 2009 | 87 | 1105 | 32.1 | 17.2 | 23.4 |
| October 2010/May 2011 | 92 | 1455 | 31.1 | 17.7 | 23.1 |
| January 2012/May 2012 | 47 | 454 | 31.8 | 18.2 | 25.0 |
| December 2012/May 2013 | 75 | 1047 | 29.8 | 18.4 | 23.1 |
| October 2013/April 2014 | 78 | 832 | 32.2 | 17.1 | 24.1 |
| Year | Fertilization and Lime Application | Top-Dressed Fertilization | Fertilization Schedule |
|---|---|---|---|
| 2006 | 200 kg ha−1 N (urea and 160 kg ha−1 K2O (Potassium chloride) | split over two applications | |
| 2007 | 50, 25, and 50 kg ha−1 of NPK | 50 kg ha−1 N (urea) | one application |
| 2008 | 50, 25, and 50 kg ha−1 of NPK | 250 kg ha−1 N (urea) | split over three applications |
| 2009 | 12, 42, and 24 kg ha−1 of NPK | 250 kg ha−1 N (urea) | split over two applications |
| 2011 | 8, 28, and 16 kg ha−1 of NPK | 180 kg ha−1 N (urea) | split over four applications |
| 2012 | 25, 25, and 25 kg ha−1 of NPK | 90 kg ha−1 N (urea) | split over two applications |
| 2013 | 7, 20, and 14 kg ha−1 of NPK | 160 kg ha–1 N (urea) | split over three applications |
| 2014 | 1000 kg ha−1 of dolomitic lime and 4 and 62 kg ha−1 of N and P | 180 and 40 kg ha−1 of formulated N and K | split over four applications |
| Sward Height (cm) | p-Value | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Year | 15 | 25 | 35 | Sward | Year | S*Y |
| Herbage mass (t DM ha−1) | 2008 | 7.22 | 8.86 | 10.03 | p < 0.001 | p < 0.001 | p = 0.298 |
| 2009 | 3.46 | 5.08 | 7.45 | ||||
| 2011 | 4.95 | 9.24 | 10.22 | ||||
| 2012 | 4.37 | 7.59 | 9.61 | ||||
| 2013 | 5.99 | 7.74 | 11.02 | ||||
| 2014 | 5.36 | 8.37 | 10.83 | ||||
| Mean | 5.23 | 7.81 | 9.86 | ||||
| Leaf mass (t DM ha−1) | 2008 | 1.64 | 1.79 | 2.26 | p < 0.001 | p < 0.001 | p = 0.435 |
| 2009 | 1.45 | 2.39 | 2.39 | ||||
| 2011 | 1.43 | 2.47 | 2.92 | ||||
| 2012 | 1.33 | 2.15 | 2.82 | ||||
| 2013 | 2.59 | 3.16 | 4.24 | ||||
| 2014 | 2.10 | 2.80 | 3.43 | ||||
| Mean | 1.76 | 2.46 | 3.01 | ||||
| Stem mass (t DM ha−1) | 2008 | 1.84 | 2.71 | 3.59 | p < 0.001 | p < 0.001 | p = 0.467 |
| 2009 | 1.10 | 1.70 | 2.80 | ||||
| 2011 | 1.44 | 3.46 | 3.65 | ||||
| 2012 | 1.24 | 2.30 | 2.95 | ||||
| 2013 | 2.37 | 3.33 | 5.26 | ||||
| 2014 | 1.49 | 2.41 | 3.60 | ||||
| Mean | 1.58 | 2.65 | 3.64 | ||||
| Dead material mass (t DM ha−1) | 2008 | 3.74 | 4.36 | 4.18 | p < 0.001 | p < 0.001 | p = 0.657 |
| 2009 | 1.24 | 1.75 | 2.26 | ||||
| 2011 | 2.08 | 3.31 | 3.65 | ||||
| 2012 | 1.80 | 3.14 | 3.85 | ||||
| 2013 | 1.03 | 1.24 | 1.52 | ||||
| 2014 | 1.76 | 3.17 | 3.84 | ||||
| Mean | 1.94 | 2.83 | 3.22 | ||||
| % leaf | 2008 | 47.0 | 40.1 | 38.8 | p = 0.006 | p < 0.001 | p = 0.499 |
| 2009 | 49.8 | 41.5 | 44.5 | ||||
| 2011 | 30.4 | 28.3 | 29.5 | ||||
| 2012 | 30.4 | 30.3 | 30.7 | ||||
| 2013 | 43.4 | 40.9 | 38.5 | ||||
| 2014 | 39.0 | 34.1 | 32.4 | ||||
| Mean | 40.0 | 35.9 | 35.7 | ||||
| % stem | 2008 | 53.0 | 60.0 | 61.0 | p < 0.016 | p < 0.001 | p = 0.626 |
| 2009 | 48.8 | 47.4 | 43.5 | ||||
| 2011 | 58.5 | 58.5 | 55.5 | ||||
| 2012 | 28.3 | 30.3 | 30.7 | ||||
| 2013 | 39.5 | 43.1 | 47.8 | ||||
| 2014 | 27.7 | 28.5 | 32.5 | ||||
| Mean | 42.6 | 44.6 | 45.2 | ||||
| % dead material | 2008 | 50.8 | 48.7 | 41.1 | p < 0.29 | p = 0.081 | p = 0.822 |
| 2009 | 38.9 | 35.1 | 30.1 | ||||
| 2011 | 41.5 | 34.2 | 35.4 | ||||
| 2012 | 41.3 | 41.4 | 39.8 | ||||
| 2013 | 17.2 | 16.0 | 13.8 | ||||
| 2014 | 33.3 | 37.4 | 35.1 | ||||
| Mean | 37.2 | 35.5 | 32.6 | ||||
| Sward Height (cm) | |||||||
|---|---|---|---|---|---|---|---|
| Variable | Year | 15 | 25 | 35 | Sward | Year | S*Y |
| Organic matter (g kg−1 DM) | 2008 | 90.0 | 90.6 | 90.8 | p = 0.0014 | p < 0.001 | p = 0.839 |
| 2009 | 90.1 | 90.8 | 90.7 | ||||
| 2011 | 91.4 | 90.7 | 90.9 | ||||
| 2012 | 91.5 | 91.6 | 91.7 | ||||
| 2013 | 91.0 | 92.5 | 92.4 | ||||
| 2014 | 91.3 | 91.3 | 91.4 | ||||
| Mean | 90.9 | 91.3 | 91.3 | ||||
| Crude protein (g kg−1 DM) | 2008 | 15.8 | 15.6 | 14.6 | p = 0.06 | p < 0.001 | p = 0.093 |
| 2009 | 14.7 | 14.5 | 13.6 | ||||
| 2011 | 16.3 | 15.3 | 14.7 | ||||
| 2012 | 13.5 | 13.4 | 12.6 | ||||
| 2013 | 15.1 | 13.9 | 13.7 | ||||
| 2014 | 12.7 | 11.2 | 14.7 | ||||
| Mean | 14.7 | 14.0 | 14.0 | ||||
| Neutral detergent fiber (g kg−1 DM) | 2008 | 57.2 | 60.4 | 59.9 | p = 0.0043 | p < 0.001 | p = 0.753 |
| 2009 | 60.6 | 61.1 | 63.0 | ||||
| 2011 | 54.7 | 54.6 | 55.6 | ||||
| 2012 | 57.6 | 57.5 | 58.9 | ||||
| 2013 | 57.7 | 60.0 | 59.9 | ||||
| 2014 | 59.1 | 61.2 | 60.0 | ||||
| Mean | 57.8 | 59.1 | 59.6 | ||||
| Acid detergent fiber (g kg−1 DM) | 2008 | 33.7 | 34.1 | 39.9 | p = 0.033 | p = 0.099 | p = 0.006 |
| 2009 | 28.7 | 28.8 | 30.1 | ||||
| 2011 | 28.5 | 28.4 | 35.6 | ||||
| 2012 | 26.0 | 26.0 | 26.2 | ||||
| 2013 | 28.9 | 29.3 | 29.7 | ||||
| 2014 | 34.4 | 34.0 | 34.1 | ||||
| Mean | 30.4 | 30.4 | 32.0 | ||||
| Lignin (g kg−1 DM) | 2008 | 4.6 | 3.9 | 5.7 | p = 0.78 | p < 0.01 | p = 0.31 |
| 2009 | 4.6 | 4.7 | 5.0 | ||||
| 2011 | 2.8 | 3.5 | 3.6 | ||||
| 2012 | 3.3 | 3.3 | 3.2 | ||||
| 2013 | - | - | - | ||||
| 2014 | 3.2 | 3.4 | 3.7 | ||||
| Mean | 3.7 | 3.8 | 4.2 | ||||
| Digestibility of dry matter (% DM) | 2008 | 75.8 | 78.7 | 77.0 | p = 0.055 | p < 0.001 | p = 0.805 |
| 2009 | 57.3 | 54.7 | 60.2 | ||||
| 2011 | 59.5 | 56.0 | 68.6 | ||||
| 2012 | 63.8 | 64.0 | 69.0 | ||||
| 2013 | 72.8 | 70.4 | 68.5 | ||||
| 2014 | 71.3 | 70.5 | 66.6 | ||||
| Mean | 65.4 | 64.6 | 68.2 | ||||
| Treatment (cm) | |||||||
|---|---|---|---|---|---|---|---|
| Variable | Year | 15 | 25 | 35 | Sward | Year | S*Y |
| Average daily gain (kg day−1) | 2008 | 0.32 | 0.63 | 0.76 | p = 0.027 | p < 0.001 | p = 0.644 |
| 2009 | 0.61 | 0.73 | 0.78 | ||||
| 2011 | 0.69 | 0.87 | 0.95 | ||||
| 2012 | 0.40 | 0.60 | 0.80 | ||||
| 2013 | 1.08 | 1.15 | 1.20 | ||||
| 2014 | 0.91 | 0.89 | 0.91 | ||||
| Mean | 0.67 | 0.81 | 0.90 | ||||
| Gain per area (kg ha−1) | 2008 | 506 | 278 | 196 | p < 0.001 | p = 0.544 | p = 0.739 |
| 2009 | 744 | 672 | 576 | ||||
| 2011 | 778 | 715 | 602 | ||||
| 2012 | 492 | 528 | 468 | ||||
| 2013 | 778 | 578 | 470 | ||||
| 2014 | 597 | 410 | 322 | ||||
| Mean | 649 | 530 | 439 | ||||
| Stocking rate (AU ha−1) 1 Animal unit (AU) equals 450 kg of body weight | 2008 | 5.49 | 3.92 | 4.63 | p < 0.001 | p < 0.001 | p = 0.476 |
| 2009 | 5.01 | 4.96 | 5.86 | ||||
| 2011 | 5.33 | 4.32 | 3.20 | ||||
| 2012 | 6.64 | 4.51 | 3.42 | ||||
| 2013 | 8.20 | 5.81 | 4.42 | ||||
| 2014 | 7.01 | 4.93 | 3.80 | ||||
| Mean | 6.13 | 4.73 | 4.09 | ||||
| Sward Height (cm) | |||||||
|---|---|---|---|---|---|---|---|
| Variable | Year | 15 | 25 | 35 | Sward | Year | S*Y |
| Daily CH4 emissions (g animal−1 day−1) | 2012 | 107 | 179 | 184 | p = 0.002 | p < 0.0001 | p = 0.193 |
| 2013 | 129 | 132 | 123 | ||||
| 2014 | 106 | 119 | 155 | ||||
| Mean | 114 | 141 | 156 | ||||
| Emissions per gain (g kg−1 BW gain) | 2012 | 117 | 191 | 174 | p = 0.012 | p = 0.0003 | p = 0.459 |
| 2013 | 119 | 115 | 101 | ||||
| 2014 | 115 | 136 | 177 | ||||
| Mean | 114 | 149 | 154 | ||||
| Sward Height (cm) 2 | p-Value | ||||
|---|---|---|---|---|---|
| 15 | 25 | 35 | Effect | ||
| N2O | 0.73 (0.69) 2 | −1.11 (1.10) | −2.64 (1.05) | p < 0.01 | Linear |
| CH4 | 0.21 (0.06) | 0.11 (0.05) | 0.85 (0.50) | p < 0.01 | ns |
| CO2 | 44.2 (3.1) | 59.4 (3.9) | 80.0 (6) | p < 0.01 | Linear |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruggieri, A.C.; Cardoso, A.d.S.; Ongaratto, F.; Casagrande, D.R.; Barbero, R.P.; Brito, L.d.F.; Azenha, M.V.; Oliveira, A.A.; Koscheck, J.F.W.; Reis, R.A. Grazing Intensity Impacts on Herbage Mass, Sward Structure, Greenhouse Gas Emissions, and Animal Performance: Analysis of Brachiaria Pastureland. Agronomy 2020, 10, 1750. https://doi.org/10.3390/agronomy10111750
Ruggieri AC, Cardoso AdS, Ongaratto F, Casagrande DR, Barbero RP, Brito LdF, Azenha MV, Oliveira AA, Koscheck JFW, Reis RA. Grazing Intensity Impacts on Herbage Mass, Sward Structure, Greenhouse Gas Emissions, and Animal Performance: Analysis of Brachiaria Pastureland. Agronomy. 2020; 10(11):1750. https://doi.org/10.3390/agronomy10111750
Chicago/Turabian StyleRuggieri, Ana Cláudia, Abmael da Silva Cardoso, Fernando Ongaratto, Daniel Rume Casagrande, Rondineli Pavezzi Barbero, Liziane de Figueiredo Brito, Mariane Vieira Azenha, André Alves Oliveira, Jefferson Fabiano Werner Koscheck, and Ricardo Andrade Reis. 2020. "Grazing Intensity Impacts on Herbage Mass, Sward Structure, Greenhouse Gas Emissions, and Animal Performance: Analysis of Brachiaria Pastureland" Agronomy 10, no. 11: 1750. https://doi.org/10.3390/agronomy10111750
APA StyleRuggieri, A. C., Cardoso, A. d. S., Ongaratto, F., Casagrande, D. R., Barbero, R. P., Brito, L. d. F., Azenha, M. V., Oliveira, A. A., Koscheck, J. F. W., & Reis, R. A. (2020). Grazing Intensity Impacts on Herbage Mass, Sward Structure, Greenhouse Gas Emissions, and Animal Performance: Analysis of Brachiaria Pastureland. Agronomy, 10(11), 1750. https://doi.org/10.3390/agronomy10111750








