Fertility Impact of Separate and Combined Treatments with Biochar, Sewage Sludge Compost and Bacterial Inocula on Acidic Sandy Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experiment Setup
2.3. Chemical and Biological Analysis
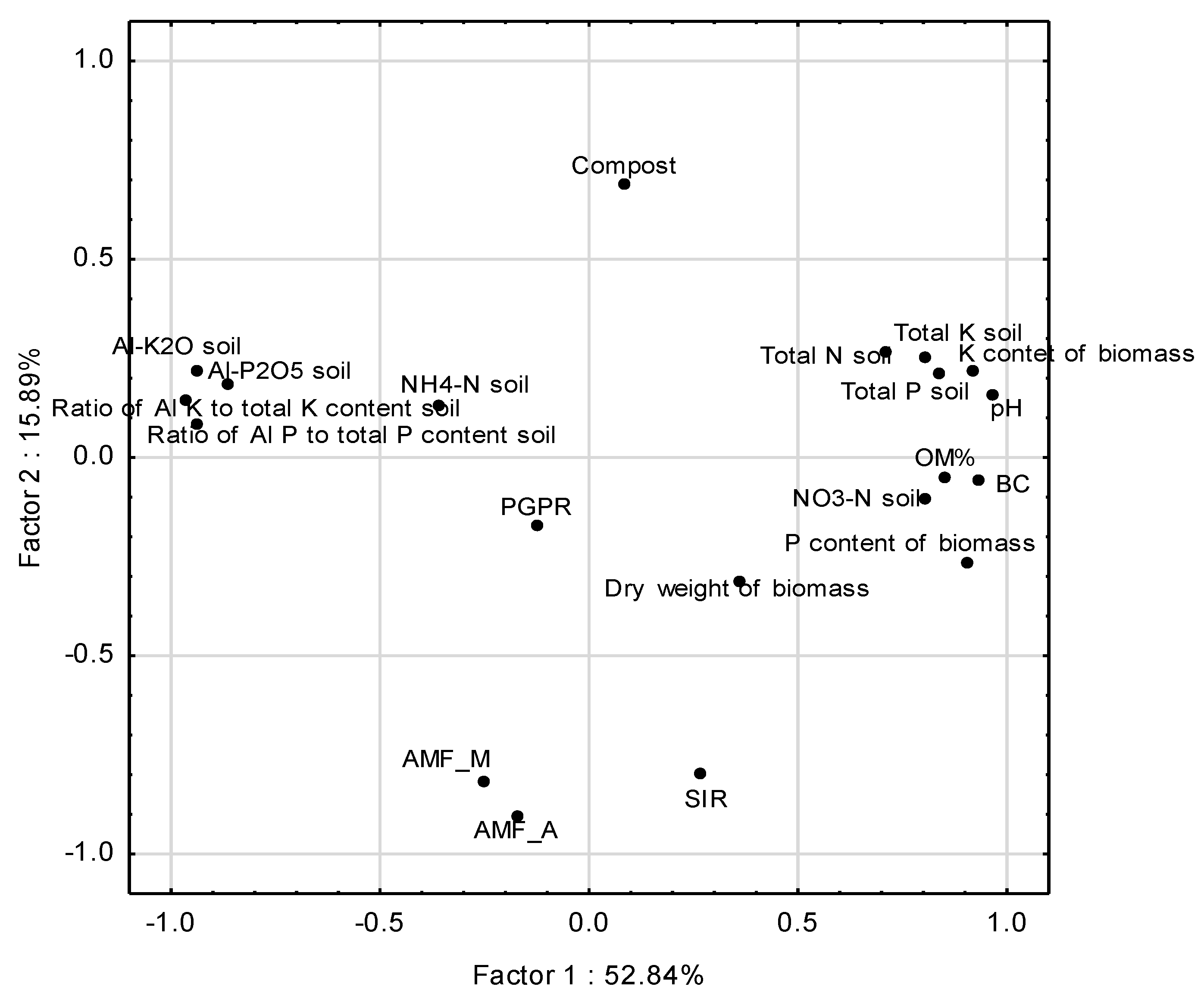
2.4. Statistical Analysis
3. Results
3.1. Soil pH and OM Content
3.2. N, P and K Contents in Soil and Plants
3.3. Maize Biomass, SIR and AMF Colonisation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Aranyos, J.T.; Tomócsik, A.; Makádi, M.; Mészáros, J.; Blaskó, L. Changes in physical properties of sandy soil after long-term compost treatment. Int. Agrophys. 2016, 30, 269–274. [Google Scholar] [CrossRef] [Green Version]
- Pare, T.; Gregorich, E.G. Soil textural effects on mineralization of nitrogen from crop residues and the added nitrogen interaction. Commun. Soil Sci. Plant Anal. 1999, 30, 145–157. [Google Scholar] [CrossRef]
- Tahir, S.; Marschner, P. Clay addition to sandy soil reduces nutrient leaching—Effect of clay concentration and ped size. Commun. Soil Sci. Plant Anal. 2017, 48, 1813–1821. [Google Scholar] [CrossRef]
- Hussain, F.; Hussain, I.; Khan, A.H.A.; Muhammad, Y.S.; Iqbal, M.; Soja, G.; Reichenauer, T.G.; Zeshan; Yousaf, S. Combined application of biochar, compost, and bacterial consortia with Italian ryegrass enhanced phytoremediation of petroleum hydrocarbon contaminated soil. Environ. Exp. Bot. 2018, 153, 80–88. [Google Scholar] [CrossRef]
- Sadet-Bourgeteau, S.; Hout, S.; Karimi, B.; Mathieu, O.; Mercier, V.; Montenach, D.; Morvan, T.; Sappin-Didier, V.; Watteau, V.; Nowak, V.; et al. Microbial communities from different soil types respond differently to organic waste input. Appl. Soil Ecol. 2019, 143, 70–79. [Google Scholar] [CrossRef]
- Verheijen, F.; Jeffery, S.; Bastos, A.C.; Van Der Velde, M.; Diafas, I. Biochar Application to Soils—A Critical Scientific Review of Effects on Soil Properties, Processes and Functions; EUR 24099 EN; Office for the Official Publications of the European Communities: Luxembourg, 2010; p. 149. ISBN 978-92-79-14293-2. [Google Scholar] [CrossRef]
- Thies, J.E.; Rillig, M.C. Characteristics of biochar: Biological properties, In Biochar for Environmental Management: Science and Technology; Lehmann, J., Joseph, S., Eds.; Earthscan Books Ltd.: London, UK; New York, NY, USA, 2009; pp. 85–105. ISBN 978-1-84407-658-1. [Google Scholar]
- Egamberdieva, D.; Hua, M.; Reckling, M.; Wirth, S.; Bellingrath-Kimura, S.D. Potential effects of biochar-based microbial inoculants in agriculture. Environ. Sustain. 2018, 1, 19–24. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Subbotina, I.; Chen, H.; Bogomolova, I.; Xu, X. Black carbon decomposition and incorporation into soil microbial biomass estimated by 14C labeling. Soil Biol. Biochem. 2009, 41, 210–219. [Google Scholar] [CrossRef]
- Zimmerman, A.R.; Gao, B.; Ahn, M.-Y. Positive and negative carbon mineralization priming effects among a variety of biochar-amended soils. Soil Biol. Biochem. 2011, 43, 1169–1179. [Google Scholar] [CrossRef]
- Van Zwieten, L.; Kimber, S.; Morris, S.; Chan, K.Y.; Downie, A.; Rust, J.; Joseph, S.D.; Cowie, A. Effects of biochar from slow pyrolysis of papermill waste on agronomic performance and soil fertility. Plant Soil 2010, 327, 235–246. [Google Scholar] [CrossRef]
- El-Naggar, A.; Lee, S.S.; Rinklebe, J.; Farooq, M.; Song, H.; Sarmah, A.K.; Zimmerman, A.R.; Ahmad, M.; Shaheen, S.M.; Ok, Y.S. Biochar application to low fertility soils: A review of current status, and future prospects. Geoderma 2019, 337, 536–554. [Google Scholar] [CrossRef]
- Bista, P.; Ghimire, R.; Machado, S.; Pritchett, L. Biochar effects on soil properties and wheat biomass vary with fertility management. Agronomy 2019, 9, 623. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhang, L.; Yang, H.; Yan, G.; Xu, Z.; Chen, C.; Zhang, D. Biochar nutrient availability rather than its water holding capacity governs the growth of both C3 and C4 plants. J. Soils Sedim. 2016, 16, 801–810. [Google Scholar] [CrossRef] [Green Version]
- Cortellini, L.; Toderi, G.; Baldoni, G.; Nassisi, A. Effects on the content of organic matter, nitrogen, phosphorus and heavy metals in soil and plants after application of compost and sewage sludge. In The Science of Composting; de Bertoldi, M., Sequi, P., Lemmes, B., Papi, T., Eds.; Springer: Dordrecht, The Netherlands, 1996; pp. 457–468. [Google Scholar] [CrossRef]
- Wu, H.; Zeng, G.; Liang, J.; Chen, J.; Xu, J.; Dai, J.; Li, V.; Chen, M.; Xu, P.; Zhou, Y.; et al. Responses of bacterial community and functional marker genes of nitrogen cycling to biochar, compost and combined amendments in soil. Appl. Microbiol. Biotechnol. 2016, 100, 8583–8591. [Google Scholar] [CrossRef] [PubMed]
- Brodowski, S.; John, B.; Flessa, H.; Amelung, W. Aggregate-occluded black carbon in soil. Eur. J. Soil Sci. 2006, 57, 539–546. [Google Scholar] [CrossRef]
- Yadav, A. Microbial inoculants for sustainable agriculture. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 800–804. [Google Scholar] [CrossRef]
- Rékási, M.; Szili-Kovács, T.; Takács, T.; Bernhardt, B.; Puspán, I.; Kovács, R.; Kutasi, J.; Draskovits, E.; Molnár, S.; Molnár, M.; et al. Improving the fertility of sandy soils in the temperate region by combined biochar and microbial inoculant treatments. Arch. Agron. Soil Sci. 2018, 65, 44–57. [Google Scholar] [CrossRef]
- Moland, S.; Robicheau, B.M.; Browne, R.; Newell, R.; Walker, A.K. Determining the effects of biochar and an arbuscular mycorrhizal inoculant on the growth of fowl mannagrass (Glyceria striata) (Poaceae). FACETS 2018, 3, 441–454. [Google Scholar] [CrossRef]
- Nadeem, S.M.; Imran, M.; Naveed, M.; Khan, M.Y.; Ahmad, M.; Zahir, Z.A.; Crowley, D.E. Synergistic use of biochar, compost and plant growth-promoting rhizobacteria for enhancing cucumber growth under water deficit conditions. J. Sci. Food Agric. 2017, 97, 5139–5145. [Google Scholar] [CrossRef]
- Ohsowski, B.M.; Dunfield, K.; Klironomos, J.N.; Hart, M.M. Plant response to biochar, compost, and mycorrhizal fungal amendments in post-mine sandpits. Restor. Ecol. 2018, 26, 63–72. [Google Scholar] [CrossRef]
- Molnár, M.; Vaszita, E.; Farkas, É.; Ujaczki, É.; Fekete-Kertész, I.; Tolner, M.; Klebercz, O.; Kirchkeszner, C.; Gruiz, K.; Uzinger, N.; et al. Acidic sandy soil improvement with biochar—A microcosm study. Sci. Total Environ. 2016, 563–564, 855–865. [Google Scholar] [CrossRef]
- Horel, A.; Barna, G.; Mako, A. Soil physical properties affected by biochar addition at different plant phaenological phases. Part I. Int. Agrophys. 2019, 33, 255–262. [Google Scholar] [CrossRef]
- Soil Bacteriae for Inoculating Stress Soils. WO2015/118516. Available online: https://patentscope.wipo.int/ (accessed on 26 February 2017).
- Soil Bacteria Containing Fertilizer and Method for Its Preparation. WO 2012/093374. Available online: https://patentscope.wipo.int/ (accessed on 26 February 2017).
- Box, G.E.P.; Wilson, K.B. On the experimental attainment of optimum condition. J. R. Stat. Soc. Ser. B. 1951, 13, 1–38. [Google Scholar] [CrossRef]
- Major, J. Guidelines on Practical Aspects of Biochar Application to Field Soil in Various Soil Management Systems; International Biochar Initiative: Washington, DC, USA, 2010; Available online: www.biochar-international.org (accessed on 26 February 2017).
- Kang, S.; Shi, W.; Zhang, J. An improved water-use efficiency for maize grown under regulated deficit irrigation. Field Crops Res. 2000, 67, 207–214. [Google Scholar] [CrossRef]
- Jalota, S.K.; Singh, S.; Chahal, G.B.S.; Ray, S.S.; Panigraghy, S.; Bhupinder-Singh; Singh, K.B. Soil texture, climate and management effects on plant growth, grain yield and water use by rainfed maize–wheat cropping system: Field and simulation study. Agric. Water Manag. 2010, 97, 83–90. [Google Scholar] [CrossRef]
- ISO 10390:2005 Soil Quality—Determination of pH; ISO: Geneva, Switzerland, 2005.
- Environmental Protection. Testing of Soils. Determination of Organic Matter; MSZ 21470-52:1983; Hungarian Standard Association: Budapest, Hungary, 1984. [Google Scholar]
- ISO 10694:1995. Soil Quality—Determination of Organic and Total Carbon after Dry Combustion; ISO: Geneva, Switzerland, 1995. [Google Scholar]
- Evaluation of Some Chemical Properties of the Soil. Laboratory Tests. (pH Value, Phenolphtalein Alkalinity Expressed in Soda, Total Water Soluble Salt Content, Hydrolytic (y1 Value) and Exchangeable Acidity (y2 Value); MSZ-08-0206/2, 1978; Hungarian Standard Association: Budapest, Hungary, 1978. (In Hungarian) [Google Scholar]
- Determination of the Soluble Nutrient Element Content of the Soil; MSZ 20135, 1999; Hungarian Standard Association: Budapest, Hungary, 1999.
- ISO. ISO 11261:1995 Soil Quality—Determination of Total Nitrogen—Modified Kjeldahl Method; ISO: Geneva, Switzerland, 1995. [Google Scholar]
- Environmental Testing of Soils. Determination of Total and Soluble Toxic Element, Heavy Metal and Chromium (VI) Content; MSZ 21470-50:2006; Hungarian Standard Association: Budapest, Hungary, 2006. [Google Scholar]
- Anderson, J.P.E.; Domsch, K.H. A physiological method for the quantitative measurement of microbial biomass in soils. Soil Biol. Biochem. 1978, 10, 215–221. [Google Scholar] [CrossRef]
- Szili-Kovács, T.; Zsuposné Oláh, Á.; Kátai, J.; Villányi, I.; Takács, T. Correlations between biological and chemical soil properties in soils from long-term experiments. Agrokém. Talajt. 2011, 60, 241–254. [Google Scholar] [CrossRef]
- Trouvelot, A.; Kough, J.L.; Gianinazzi-Pearson, V. Mesure du taux de mycorhization VA d’un système radiculaire. Recherches et methods d’estimation ayant une signification fonctionnelle. In Physiological and Genetical Aspects of Mycorrhizae; Gianinazzi-Pearson, V., Gianinazzi, S., Eds.; INRA: Paris, France, 1986; pp. 217–221. [Google Scholar]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Masto, R.E.; Kumar, S.; Rout, T.K.; Sarkar, P.; George, J.; Ram, L.C. Biochar from water hyacinth (Eichornia crassipes) and its impact on soil biological activity. CATENA 2013, 111, 64–71. [Google Scholar] [CrossRef]
- Denmead, O.T.; Shaw, R.H. The effects of soil moisture stress at different stages of growth on the development and yield of corn. Agron. J. 1960, 52, 272–274. [Google Scholar] [CrossRef]
- Jones, D.L.; Rousk, J.; Edwards-Jones, G.; DeLuca, T.H.; Murphy, D.V. Biochar-mediated changes in soil quality and plant growth in a three year field trial. Soil Biol. Biochem. 2012, 45, 113–124. [Google Scholar] [CrossRef]
- Whitman, T.; Singh, B.P.; Zimmerman, A.R. Priming effects in biochar-amended soils: Implications of biochar-soil organic matter interactions for carbon storage. In Biochar for Environmental Management: Science, Technology and Implementation; Lehmann, J., Joseph, S., Eds.; Routledge: New York, NY, USA, 2015; pp. 455–488. ISBN 978-0-415-70415-1. [Google Scholar]
- Cross, A.; Sohi, S.P. The priming potential of biochar products in relation to labile carbon contents and soil organic matter status. Soil Biol. Biochem. 2011, 43, 2127–2134. [Google Scholar] [CrossRef]
- Jien, S.H.; Wang, C.S. Effects of biochar on soil properties and erosion potential in a highly weathered soil. CATENA 2013, 110, 225–233. [Google Scholar] [CrossRef] [Green Version]
- Tan, Z.; Lin, C.S.K.; Ji, X.; Rainey, T.J. Returning biochar to fields: A review. Appl. Soil Ecol. 2017, 116, 1–11. [Google Scholar] [CrossRef]
- Von der Weid, I.; Alviano, D.S.; Santos, A.L.S.; Soares, R.M.A.; Alviano, C.S.; Seldin, L. Antimicrobial activity of Paenibacillus peoriae strain NRRL BD-62 against a broad spectrum of phytopathogenic bacteria and fungi. J. Appl. Microbiol. 2003, 95, 1143–1151. [Google Scholar] [CrossRef]
- Mazzilli, S.R.; Kemanian, A.R.; Ernst, O.R.; Jackson, R.B.; Piñeiro, G. Greater humification of belowground than aboveground biomass carbon into particulate soil organic matter in no-till corn and soybean crops. Soil Biol. Biochem. 2015, 85, 22–30. [Google Scholar] [CrossRef]
- Shepherd, M.A. Factors affecting nitrate leaching from sewage sludges applied to a sandy soil in arable agriculture. Agric. Ecosyst. Environ. 1996, 58, 171–185. [Google Scholar] [CrossRef]
- Lehmann, J.; Pereira da Silva, J., Jr.; Steiner, C.; Nehls, T.; Zech, W.; Glaser, B. Nutrient availability and leaching in an archaeological Anthrosol and Ferralsol of the Central Amazon basin: Fertilizer, manure and charcoal amendments. Plant Soil 2003, 249, 343–357. [Google Scholar] [CrossRef]
- Shepherd, J.G.; Buss, W.; Sohi, S.P.; Heal, K.V. Bioavailability of phosphorus, other nutrients and potentially toxic elements from marginal biomass-derived biochar assessed in barley (Hordeum vulgare) growth experiments. Sci. Total Environ. 2017, 584, 448–457. [Google Scholar] [CrossRef] [Green Version]
- Quilliam, R.S.; Glanville, H.C.; Wade, S.C.; Jones, D.L. Life in the ‘charosphere’—Does biochar in agricultural soil provide a significant habitat for microorganisms? Soil Biol. Biochem. 2013, 65, 287–293. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Marschner, P. Soil respiration, microbial biomass and nutrient availability in soil after repeated addition of low and high C/N plant residues. Biol. Fertil. Soils 2016, 52, 165–176. [Google Scholar] [CrossRef]
- Brimecombe, M.J.; De Leij, F.A.; Lynch, J.M. The effect of root exudates on rhizosphere microbial populations. In The Rhizosphere: Biochemistry and Organic Substances at the Soil-Plant Interface; Pinton, R., Varanini, Z., Nannipieri, P., Eds.; Marcel-Dekker, Inc.: New York, NY, USA, 2001; pp. 95–140. ISBN 0-8247-0427-4. [Google Scholar]
- Ziadi, N.; Whalen, J.K.; Messiga, A.J.; Morel, C. Assessment and modeling of soil available phosphorus in sustainable cropping systems. Adv. Agron. 2013, 122, 85–126. [Google Scholar] [CrossRef]
- Satyaprakash, M.; Nikitha, T.; Reddi, E.U.B.; Sadhana, B.; Vani, S.S. Phosphorous and phosphate solubilising bacteria and their role in plant nutrition. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 2133–2144. [Google Scholar]
- Parniske, M. Arbuscular mycorrhiza: The mother of plant root endosymbioses. Nat. Rev. Microbiol. 2008, 6, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Priyadharsini, P.; Muthukumar, T. Interactions between arbuscular mycorrhizal fungi and potassium-solubilizing microorganisms on agricultural productivity. In Potassium Solubilizing Microorganisms for Sustainable Agriculture; Meena, V.S., Maurya, B.R., Verma, J.P., Meena, R.S., Eds.; Springer: New Delhi, India, 2016; pp. 111–125. ISBN 978-81-322-2774-8. [Google Scholar] [CrossRef]
- Adesemoye, A.O.; Kloepper, J.W. Plant–microbes interactions in enhanced fertilizer-use efficiency. Appl. Microbiol. Biotechnol. 2009, 85, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammer, E.C.; Balogh-Brunstad, Z.; Jakobsen, I.; Olsson, P.A.; Stipp, S.L.; Rillig, M.C. A mycorrhizal fungus grows on biochar and captures phosphorus from its surfaces. Soil Biol. Biochem. 2014, 77, 252–260. [Google Scholar] [CrossRef]

| Parameter | Soil | BC | Compost |
|---|---|---|---|
| pH(H2O) | 4.9 | 10.4 | 7.08 |
| OM% | 0.64 | 22.5 * | 8.12 |
| CaCO3% | 0 | 5.75 | 6.81 |
| Total N% | 0.044 | 0.959 | 1.16 |
| Total P (mg/kg) | 260 | 6742 | 10,259 |
| Total K (mg/kg) | 1193 | 15,380 | 8243 |
| Plant-available K (AL-K2O, mg/kg) | 36.1 | 12,595 | 4778 |
| Plant-available P (AL-P2O5, mg/kg) | 68.9 | 5227 | 8196 |
| Total Ca (mg/kg) | 309 | 34,270 | 54,207 |
| Total Mg (mg/kg) | 1096 | 3539 | 9161 |
| Total Zn (mg/kg) | 41.6 | 53.3 | 449 |
| Treatment | Treatment Level Code | Amounts of Each Material Applied | ||
|---|---|---|---|---|
| BC (%w/w) | PGPR (CFU/pot) | Compost (%w/w) | ||
| SEPARATE APPLICATION (completely randomised block design) | ||||
| Control treatment (n = 3) | - | 0 | 0 | 0 |
| Compost treatments (n = 3) | C1 | 0 | 0 | 0.16 |
| C2 | 0 | 0 | 0.33 | |
| C3 | 0 | 0 | 0.5 | |
| BC treatments (n = 3) | BC1 | 0.5 | 0 | 0 |
| BC2 | 1 | 0 | 0 | |
| BC3 | 1.5 | 0 | 0 | |
| PGPR treatments (n = 3) | PGPR1 | 0 | 3.7 × 106 | 0 |
| PGPR2 | 0 | 7.5 × 106 | 0 | |
| PGPR3 | 0 | 1.2 × 107 | 0 | |
| COMBINED APPLICATION (Box and Wilson design) | ||||
| Alternating treatments (n = 3) | 1 | 1.5 | 1.2 × 107 | 0.25 |
| 2 | 0.5 | 1.2 × 107 | 0.25 | |
| 3 | 1.5 | 3.7 × 106 | 0.25 | |
| 4 | 0.5 | 3.7 × 106 | 0.25 | |
| 5 | 1.5 | 1.2 × 107 | 0.08 | |
| 6 | 0.5 | 1.2 × 107 | 0.08 | |
| 7 | 1.5 | 3.7 × 106 | 0.08 | |
| 8 | 0.5 | 3.7 × 106 | 0.08 | |
| Extreme treatments (n = 3) | 9 | 2 | 7.5 × 106 | 0.16 |
| 10 | 0 | 7.5 × 106 | 0.16 | |
| 11 | 1 | 2.4 × 107 | 0.16 | |
| 12 | 1 | 0 | 0.16 | |
| 13 | 1 | 7.5 × 106 | 0.33 | |
| 14 | 1 | 7.5 × 106 | 0 | |
| Central treatment (n = 10) | 15 | 1 | 7.5 × 106 | 0.16 |
| Properties | Levels | LSD5% | p | |||
|---|---|---|---|---|---|---|
| Control | BC1 | BC2 | BC3 | |||
| Soil | ||||||
| pH (H2O) | 4.93 ± 0.1 | 5.29 ± 0.02 | 5.71 ± 0.07 | 5.94 ± 0.05 | 0.18 | *** |
| OM% | 0.642 ± 0.06 | 0.67 ± 0.06 | 0.70 ± 0.06 | 0.77 ± 0.05 | 0.10 | * |
| Total K mg/kg | 1193 ± 14 | 1261 ± 217 | 1386 ± 85 | 1521 ± 171 | 148 | *** |
| AL-K2O mg/kg | 36.1 ± 4.0 | 62.8 ± 1.1 | 93.0 ± 4.3 | 145.3 ± 8.9 | 10.2 | *** |
| Total P mg/kg | 260 ± 2 | 279 ± 14 | 301 ± 19 | 302 ± 12 | 36 | * |
| AL-P2O5 mg/kg | 68.9 ± 1.3 | 85.9 ± 7.8 | 101.2 ± 7.5 | 111.3 ± 8.6 | 13.8 | *** |
| NO3-N mg/kg | 1.93 ± 0.20 | 2.27 ± 0.00 | 3.29 ± 0.17 | 3.75 ± 0.24 | 0.50 | *** |
| SIR (µg CO2-C/g soil/hour) | 0.72 ± 0.07 | 0.94 ± 0.12 | 1.07 ± 0.06 | 1.12 ± 0.10 | 0.25 | ** |
| Maize | ||||||
| AMF–M% | 52.1 ± 2.3 | 59.3 ± 2.0 | 58.4 ± 8.2 | 76.1 ± 6.8 | 9.5 | ** |
| AMF–A% | 38.6 ± 1.5 | 49.0 ± 5.0 | 48.2 ± 5.0 | 64.1 ± 5.4 | 9.9 | ** |
| Maize P mg/kg | 1596 ± 233 | 2196 ± 98 | 3096 ± 144 | 3865 ± 358 | 625 | *** |
| Maize K mg/kg | 10,804 ± 1184 | 24,270 ± 1050 | 32,728 ± 305 | 37,034 ± 1387 | 2913 | *** |
| Properties | Levels | LSD5% | p | |||
|---|---|---|---|---|---|---|
| Control | C1 | C2 | C3 | |||
| Soil | ||||||
| pH (H2O) | 4.93 ± 0.1 | 5.08 ± 0.03 | 5.32 ± 0.01 | 5.51 ± 0.03 | 0.14 | *** |
| Total K mg/kg | 1193 ± 14 | 1255 ± 136 | 1144 ± 73 | 1353 ± 108 | 139 | ** |
| Total P mg/kg | 260 ± 2 | 273 ± 5 | 286 ± 4 | 294 ± 6 | 12 | *** |
| AL-P2O5 mg/kg | 68.9 ± 1.3 | 78.8 ± 2.1 | 101.3 ± 4.7 | 120.7 ± 7.0 | 10.5 | *** |
| Total N %w/w | 0.044 ± 0.002 | 0.039 ± 0.002 | 0.040 ± 0.002 | 0.041 ± 0.000 | 0.003 | ** |
| NO3-N mg/kg | 1.93 ± 0.20 | 0.73 ± 0.21 | 0.87 ± 0.36 | 0.90 ± 0.37 | 0.71 | ** |
| SIR (µg CO2-C/g soil/hour) | 0.72 ± 0.07 | 1.06 ± 0.11 | 1.12 ± 0.12 | 1.40 ± 0.10 | 0.28 | *** |
| Maize | ||||||
| AMF–A% | 38.6 ± 1.5 | 24.5 ± 4.4 | 17.5 ± 1.4 | 9.3 ± 1.8 | 5.7 | *** |
| Maize dry biomass (g/pot) | 2.68 ± 0.29 | 4.53 ± 0.21 | 5.89 ± 0.48 | 7.30 ± 0.25 | 0.75 | *** |
| Maize N %w/w | 0.478 ± 0.013 | 0.379 ± 0.011 | 0.389 ± 0.034 | 0.421 ± 0.015 | 0.052 | ** |
| Properties | Levels | LSD5% | p | |||
|---|---|---|---|---|---|---|
| Control | PGPR1 | PGPR2 | PGPR3 | |||
| Soil | ||||||
| AL-K2O mg/kg | 36.1 ± 4.0 | 27.5 ± 1.8 | 30.7 ± 0.9 | 24.4 ± 1.1 | 6.3 | ** |
| Maize | ||||||
| AMF–M% | 52.1 ± 2.3 | 64.8 ± 0.5 | 65.7 ± 6.3 | 71.6 ± 4.4 | 10.4 | * |
| Maize P mg/kg | 1596 ± 233 | 1861 ± 66 | 2020 ± 76 | 2033 ± 249 | 362 | * |
| Maize K mg/kg | 10,804 ± 1184 | 12,580 ± 102 | 14,007 ± 373 | 13,414 ± 523 | 1302 | *** |
| Treatment | Soil | Maize | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| pH (H2O) | OM (%) | AL-K2O (mg/kg) | Total P (mg/kg) | AL-P2O5 (mg/kg) | NO3-N (mg/kg) | Ratio of Plant- Available K to Total K (%) | Ratio of Plant- Available P to Total P (%) | K (mg/kg) | P (mg/kg) | |
| BC | + *** | + *** | + *** | + *** | + *** | + *** | + *** | + *** | + *** | + *** |
| PGPR | ||||||||||
| Compost | + * | + ** | + *** | |||||||
| BC × PGPR | + * | |||||||||
| BC × Compost | + ** | |||||||||
| Compost × PGPR | ||||||||||
| R2 | 92.56 | 62.86 | 93.59 | 70.46 | 83.79 | 63.98 | 89.47 | 75.71 | 81.77 | 74.11 |
| Treatment | Soil | Maize | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| pH (H2O) | OM (%) | AL-K2O (mg/kg) | Total P (mg/kg) | AL-P2O5 (mg/kg) | NO3-N (mg/kg) | Ratio of Available K to Total K Content (%) | Ratio of Available P to Total P Content (%) | K (mg/kg) | P (mg/kg) | |
| 1 | 6.4 ± 0.1 | 0.98 ± 0.04 | 196.7 ± 6.9 | 342 ± 9 | 184 ± 14.2 | 4.75 ± 0.49 | 11.14 ± 0.67 | 23.7 ± 1.58 | 61,566 ± 4210 | 3300 ± 500 |
| 2 | 5.7 ± 0.0 | 0.82 ± 0.05 | 93.2 ± 4.0 | 307 ± 9 | 122.0 ± 2.2 | 2.36 ± 0.21 | 6.09 ± 0.14 | 17.5 ± 0.40 | 38,474 ± 360 | 1627 ± 135 |
| 3 | 6.3 ± 0.0 | 1.00 ± 0.03 | 184.7 ± 4.5 | 356 ± 11 | 188.0 ± 5.0 | 3.79 ± 0.25 | 10.69 ± 0.29 | 23.2 ± 1.37 | 51,718 ± 307 | 2804 ± 63 |
| 4 | 5.7 ± 0.0 | 0.89 ± 0.03 | 81.7 ± 7.6 | 308 ± 9 | 129.7 ± 7.7 | 2.23 ± 0.39 | 5.93 ± 0.67 | 18.5 ± 1.09 | 39,365 ± 4768 | 2046 ± 290 |
| 5 | 6.2 ± 0.0 | 1.01 ± 0.04 | 177.3 ± 8.1 | 352 ± 4 | 166 ± 13.3 | 4.41 ± 0.36 | 11.13 ± 0.38 | 20.8 ± 1.47 | 47,364 ± 1062 | 3352 ± 144 |
| 6 | 5.4 ± 0.0 | 0.76 ± 0.00 | 74.9 ± 5.8 | 286 ± 7 | 102.0 ± 2.4 | 2.38 ± 0.54 | 5.94 ± 0.56 | 15.7 ± 0.44 | 36,828 ± 1842 | 2101 ± 274 |
| 7 | 6.2 ± 0.0 | 1.09 ± 0.02 | 153.7 ± 8.7 | 320 ± 8 | 147.3 ± 2.4 | 4.01 ± 0.41 | 9.75 ± 0.73 | 20.3 ± 0.73 | 46,200 ± 3020 | 3216 ± 334 |
| 8 | 5.4 ± 0.0 | 0.73 ± 0.04 | 67.5 ± 3.8 | 280 ± 6 | 101.5 ± 2.7 | 3.49 ± 0.25 | 4.61 ± 0.70 | 16.0 ± 0.76 | 38,245 ± 2988 | 2305 ± 88 |
| 9 | 6.3 ± 0.1 | 0.95 ± 0.03 | 199 ± 13.7 | 344 ± 13 | 169 ± 15.3 | 5.44 ± 0.12 | 13.56 ± 1.71 | 21.7 ± 1.15 | 48,114 ± 3068 | 3612 ± 425 |
| 10 | 5.1 ± 0.1 | 0.71 ± 0.04 | 49.7 ± 3.4 | 269 ± 8 | 93.2 ± 1.3 | 1.76 ± 0.38 | 3.64 ± 0.32 | 15.2 ± 0.57 | 22,846 ± 566 | 1422 ± 273 |
| 11 | 6.1 ± 0.2 | 0.88 ± 0.12 | 130 ± 10.0 | 311 ± 9 | 127 ± 13.5 | 5.18 ± 0.40 | 8.16 ± 0.51 | 18.1 ± 1.71 | 47,896 ± 2853 | 2740 ± 228 |
| 12 | 5.9 ± 0.1 | 0.87 ± 0.03 | 128.7 ± 9.7 | 304 ± 9 | 134.0 ± 5.1 | 6.03 ± 0.75 | 8.05 ± 0.88 | 19.4 ± 0.83 | 49,954 ± 1638 | 2645 ± 460 |
| 13 | 6.1 ± 0.1 | 0.88 ± 0.05 | 128.7 ± 5.7 | 313 ± 5 | 162 ± 16.9 | 4.95 ± 0.42 | 7.83 ± 0.29 | 22.8 ± 2.62 | 45,830 ± 2825 | 2275 ± 114 |
| 14 | 5.9 ± 0.1 | 0.92 ± 0.01 | 119 ± 10.2 | 293 ± 7 | 114.0 ± 4.2 | 4.45 ± 0.60 | 7.80 ± 0.64 | 17.1 ± 1.03 | 48,398 ± 1562 | 2721 ± 620 |
| 15 | 6.0 ± 0.1 | 0.87 ± 0.07 | 115.6 ± 8.5 | 312 ± 18 | 132.2 ± 6.7 | 3.80 ± 0.72 | 7.73 ± 0.90 | 18.6 ± 0.98 | 44,006 ± 3180 | 2724 ± 291 |
| LSD5% | 0.1 | 0.08 | 11.3 | 17 | 12.5 | 0.74 | 0.54 | 0.82 | 3933 | 449 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uzinger, N.; Takács, T.; Szili-Kovács, T.; Radimszky, L.; Füzy, A.; Draskovits, E.; Szűcs-Vásárhelyi, N.; Molnár, M.; Farkas, É.; Kutasi, J.; et al. Fertility Impact of Separate and Combined Treatments with Biochar, Sewage Sludge Compost and Bacterial Inocula on Acidic Sandy Soil. Agronomy 2020, 10, 1612. https://doi.org/10.3390/agronomy10101612
Uzinger N, Takács T, Szili-Kovács T, Radimszky L, Füzy A, Draskovits E, Szűcs-Vásárhelyi N, Molnár M, Farkas É, Kutasi J, et al. Fertility Impact of Separate and Combined Treatments with Biochar, Sewage Sludge Compost and Bacterial Inocula on Acidic Sandy Soil. Agronomy. 2020; 10(10):1612. https://doi.org/10.3390/agronomy10101612
Chicago/Turabian StyleUzinger, Nikolett, Tünde Takács, Tibor Szili-Kovács, László Radimszky, Anna Füzy, Eszter Draskovits, Nóra Szűcs-Vásárhelyi, Mónika Molnár, Éva Farkas, József Kutasi, and et al. 2020. "Fertility Impact of Separate and Combined Treatments with Biochar, Sewage Sludge Compost and Bacterial Inocula on Acidic Sandy Soil" Agronomy 10, no. 10: 1612. https://doi.org/10.3390/agronomy10101612
APA StyleUzinger, N., Takács, T., Szili-Kovács, T., Radimszky, L., Füzy, A., Draskovits, E., Szűcs-Vásárhelyi, N., Molnár, M., Farkas, É., Kutasi, J., & Rékási, M. (2020). Fertility Impact of Separate and Combined Treatments with Biochar, Sewage Sludge Compost and Bacterial Inocula on Acidic Sandy Soil. Agronomy, 10(10), 1612. https://doi.org/10.3390/agronomy10101612









