Warming Reduces Net Carbon Gain and Productivity in Medicago sativa L. and Festuca arundinacea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Species and Experimental Design
2.2. Photosynthetic Capacity
2.3. Respiration
2.4. Leaf Level Water Use Efficiency (WUE)
2.5. Biomass and Shoot Nutrients
2.6. Statistics
3. Results
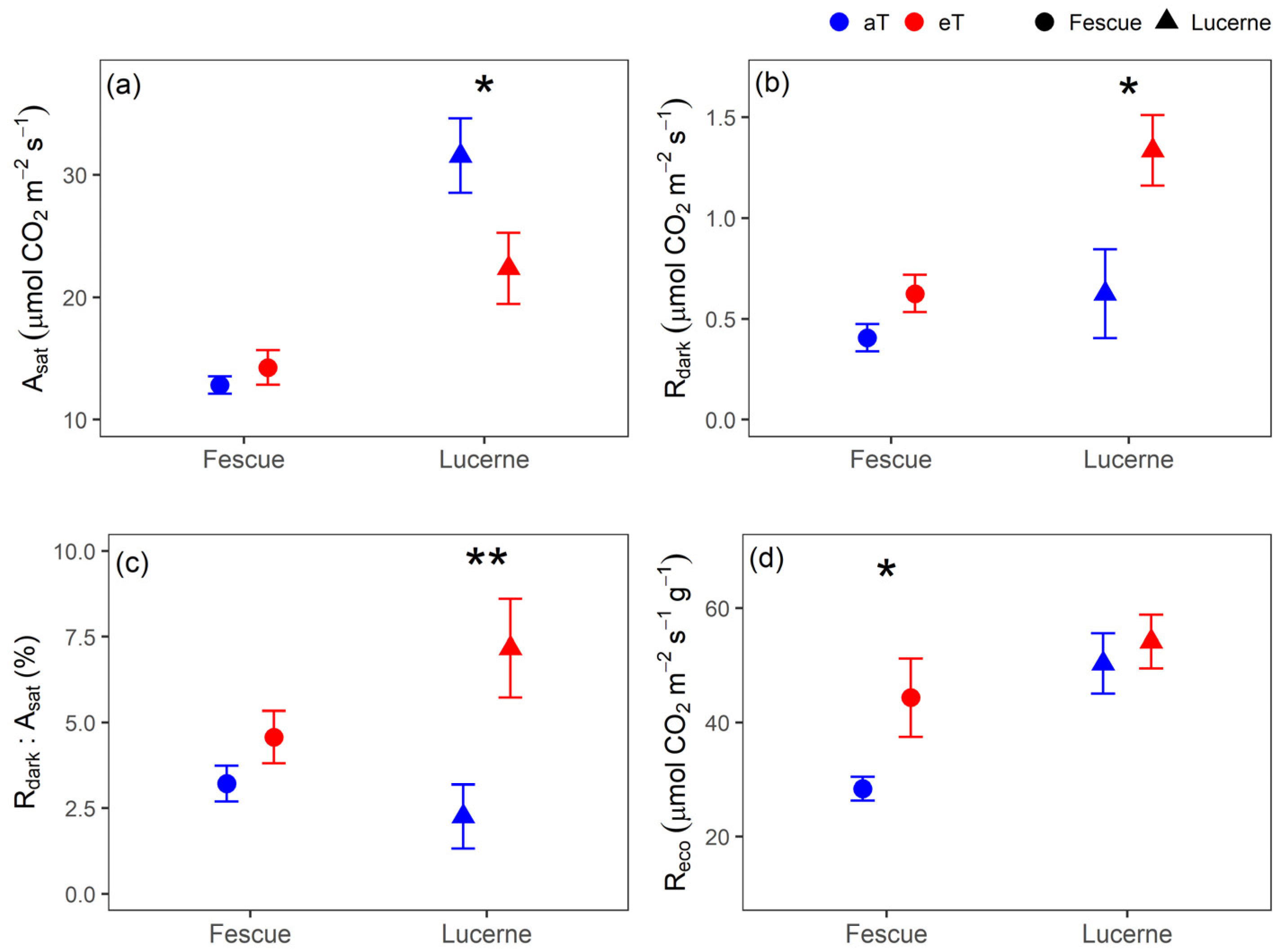
3.1. Carbon Balance
3.2. Productivity
3.3. Photosynthetic Capacity
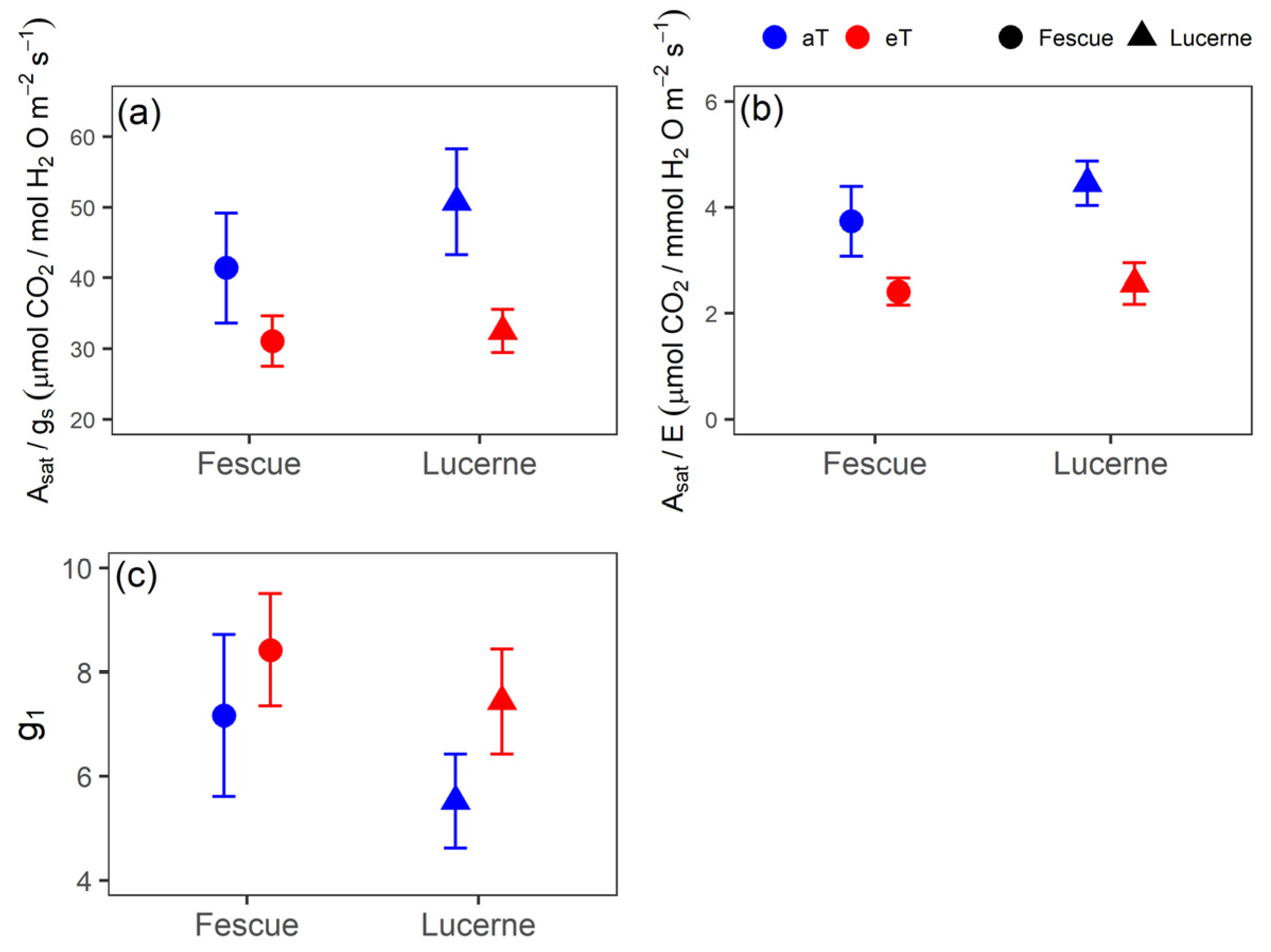
3.4. WUE
3.5. Shoot Stoichiometry
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bernstein, L.; Bosch, P.; Canziani, O.; Chen, Z.; Christ, R.; Davidson, O.; Hare, W.; Huq, S.; Karoly, D.; Kattsov, V.; et al. Climate Change 2007: Synthesis Report: An Assessment of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2008. [Google Scholar]
- Farquhar, G.D.; Von Caemmerer, S.; Berry, J.A. A Biochemical Model of Photosynthetic CO2 Assimilation. Planta 1980, 90, 78–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atkin, O.K.; Tjoelker, M.G. Thermal Acclimation and the Dynamic Response of Plant Respiration to Temperature. Trends Plant Sci. 2003, 8, 343–351. [Google Scholar] [CrossRef]
- Liang, J.; Xia, J.; Liu, L.; Wan, S. Global Patterns of the Responses of Leaf-Level Photosynthesis and Respiration in Terrestrial Plants to Experimental Warming. J. Plant Ecol. 2013, 6, 437–447. [Google Scholar] [CrossRef] [Green Version]
- Ryan, E.M.; Ogle, K.; Peltier, D.; Walker, A.P.; De Kauwe, M.G.; Medlyn, B.E.; Williams, D.G.; Parton, W.; Asao, S.; Guenet, B.; et al. Gross Primary Production Responses to Warming, Elevated CO2, and Irrigation: Quantifying the Drivers of Ecosystem Physiology in a Semiarid Grassland. Glob. Chang. Biol. 2017, 23, 3092–3106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, L.; Li, J.; Fan, Y.; Xu, S.; Zhang, Z. High Temperature Effects on Photosynthesis, PSII Functionality and Antioxidant Activity of Two Festuca arundinacea Cultivars with Different Heat Susceptibility. Bot. Stud. 2006, 47, 61–69. [Google Scholar]
- Cullen, B.R.; Johnson, I.R.; Eckard, R.J.; Lodge, G.M.; Walker, R.G.; Rawnsley, R.P.; McCaskill, M.R. Climate Change Effects on Pasture Systems in South-Eastern Australia. Crop Pasture Sci. 2009, 60, 933–942. [Google Scholar] [CrossRef]
- Aspinwall, M.J.; Varhammar, A.; Blackman, C.J.; Tjoelker, M.G.; Ahrens, C.; Byrne, M.; Tissue, D.T.; Rymer, P.D. Adaptation and Acclimation Both Influence Photosynthetic and Respiratory Temperature Responses in Corymbia calophylla. Tree Physiol. 2017, 37, 1095–1112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berry, J.; Bjorkman, O. Photosynthetic Response and Adaptation to Temperature in Higher Plants. Annu. Rev. Plant Physiol. 1980, 31, 491–543. [Google Scholar] [CrossRef]
- Drake, J.E.; Aspinwall, M.J.; Pfautsch, S.; Rymer, P.D.; Reich, P.B.; Smith, R.A.; Crous, K.Y.; Tissue, D.T.; Ghannoum, O.; Tjoelker, M.G. The Capacity to Cope with Climate Warming Declines from Temperate to Tropical Latitudes in Two Widely Distributed Eucalyptus Species. Glob. Chang. Biol. 2015, 21, 459–472. [Google Scholar] [CrossRef]
- Ishikawa, K.; Onoda, Y.; Hikosaka, K. Intraspecific Variation in Temperature Dependence of Gas Exchange Characteristics among Plantago asiatica Ecotypes from Different Temperature Regimes. New Phytol. 2007, 172, 356–364. [Google Scholar] [CrossRef]
- Kumarathunge, D.P.; Medlyn, B.E.; Drake, J.E.; Tjoelker, M.G.; Aspinwall, M.J.; Battaglia, M.; Cano, F.J.; Kelsey, C.R.; Cavaleri, M.A.; Cernusak, L.A.; et al. Acclimation and Adaptation Components of the Temperature Dependence of Plant Photosynthesis at the Global Scale. New Phytol. 2018, 222, 768–784. [Google Scholar] [CrossRef] [Green Version]
- Way, D.A.; Yamori, W. Thermal Acclimation of Photosynthesis: On the Importance of Adjusting Our Definitions and Accounting for Thermal Acclimation of Respiration. Photosynth. Res. 2014, 119, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.H.; Morgan, J.A. Photosynthesis of Grass Species Differing in Carbon Dioxide Fixation Pathways’ VI. Differential effects of temperature and light intensity on photorespiration in C3, C4, and intermediate species. Plant Physiol. 1980, 66, 541–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, A.P.; Beckerman, A.P.; Gu, L.; Kattge, J.; Cernusak, L.A.; Domingues, T.F.; Scales, J.C.; Wohlfahrt, G.; Wullschleger, S.D.; Woodward, F.I. The Relationship of Leaf Photosynthetic Traits—Vcmax and Jmax—to Leaf Nitrogen, Leaf Phosphorus, and Specific Leaf Area: A Meta-Analysis and Modeling Study. Ecol. Evol. 2014, 4, 3218–3235. [Google Scholar] [CrossRef] [Green Version]
- Medlyn, B.E.; Dreyer, E.; Ellsworth, D.; Forstreuter, M.; Harley, P.C.; Kirschbaum, M.U.F.; Le Roux, X.; Montpied, P.; Strassemeyer, J.; Walcroft, A.; et al. Temperature Response of Parameters of a Biochemically Based Model of Photosynthesis. II. A Review of Experimental Data. Plant Cell Environ. 2002, 25, 1167–1179. [Google Scholar] [CrossRef] [Green Version]
- Joseph, T.; Whitehead, D.; Turnbull, M.H. Soil Water Availability Influences the Temperature Response of Photosynthesis and Respiration in a Grass and a Woody Shrub. Funct. Plant Biol. 2014, 41, 468–481. [Google Scholar] [CrossRef]
- Hikosaka, K.; Ishikawa, K.; Borjigidai, A.; Muller, O.; Onoda, Y. Temperature Acclimation of Photosynthesis: Mechanisms Involved in the Changes in Temperature Dependence of Photosynthetic Rate. J. Exp. Bot. 2006, 57, 291–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wright, I.J.; Reich, P.B.; Atkin, O.K.; Lusk, C.H.; Tjoelker, M.G.; Westoby, M. Irradiance, Temperature and Rainfall Influence Leaf Dark Respiration in Woody Plants: Evidence from Comparisons across 20 Sites. New Phytol. 2006, 169, 309–319. [Google Scholar] [CrossRef]
- Brown, R.H.; Bouton, J.H.; Rigsby, L.; Rigler, M. Photosynthesis of Grass Species Differing in Carbon Dioxide Fixation Pathways. Plant Physiol. 1983, 71, 425–431. [Google Scholar] [CrossRef] [Green Version]
- Way, D.A.; Sage, R.F. Thermal Acclimation of Photosynthesis in Black Spruce [Picea mariana (Mill.) B.S.P.]. Plant Cell Environ. 2008, 31, 1250–1262. [Google Scholar] [CrossRef] [PubMed]
- Ow, L.F.; Whitehead, D.; Walcroft, A.S.; Turnbull, M.H. Thermal Acclimation of Respiration but Not Photosynthesis in Pinus radiata. Funct. Plant Biol. 2008, 35, 448–461. [Google Scholar] [CrossRef] [PubMed]
- Valencia, E.; Quero, J.L.; Maestre, F.T. Functional Leaf and Size Traits Determine the Photosynthetic Response of 10 Dryland Species to Warming. J. Plant Ecol. 2016, 9, 773–783. [Google Scholar] [CrossRef] [Green Version]
- Parmesan, C. Influences of Species, Latitudes and Methodologies on Estimates of Phenological Response to Global Warming. Glob. Chang. Biol. 2007, 13, 1860–1872. [Google Scholar] [CrossRef]
- Holmgren, M.; Gómez-Aparicio, L.; Quero, J.L.; Valladares, F. Non-Linear Effects of Drought under Shade: Reconciling Physiological and Ecological Models in Plant Communities. Oecologia 2012, 169, 293–305. [Google Scholar] [CrossRef] [Green Version]
- Xiong, F.S.; Mueller, E.C.; Day, T.A. Photosynthetic and Respiratory Acclimation and Growth Response of Antarctic Vascular Plants to Contrasting Temperature Regimes. Am. J. Bot. 2000, 87, 700–710. [Google Scholar] [CrossRef]
- Dermody, O.; Weltzin, J.F.; Engel, E.C.; Allen, P.; Norby, R.J. How Do Elevated [CO2], Warming, and Reduced Precipitation Interact to Affect Soil Moisture and LAI in an Old Field Ecosystem? Plant Soil 2007, 301, 255–266. [Google Scholar] [CrossRef] [Green Version]
- Gunderson, C.A.; O’hara, K.H.; Campion, C.M.; Walker, A.V.; Edwards, N.T. Thermal Plasticity of Photosynthesis: The Role of Acclimation in Forest Responses to a Warming Climate. Glob. Chang. Biol. 2010, 16, 2272–2286. [Google Scholar] [CrossRef]
- AMTHOR, J.S. Respiration in a Future, Higher-CO2 World. Plant Cell Environ. 1991, 14, 13–20. [Google Scholar] [CrossRef]
- Salvucci, M.E.; Crafts-Brandner, S.J. Inhibition of Photosynthesis by Heat Stress: The Activation State of Rubisco as a Limiting Factor in Photosynthesis. Physiol. Plant. 2004, 120, 179–186. [Google Scholar] [CrossRef]
- Lewis, J.D.; Phillips, N.G.; Logan, B.A.; Hricko, C.R.; Tissue, D.T. Leaf Photosynthesis, Respiration and Stomatal Conductance in Six Eucalyptus Species Native to Mesic and Xeric Environments Growing in a Common Garden. Tree Physiol. 2011, 31, 997–1006. [Google Scholar] [CrossRef] [Green Version]
- Atkin, O.K.; Holly, C.; Ball, M.C. Acclimation of Snow Gum (Eucalyptus pauciflora) Leaf Respiration to Seasonal and Diurnal Variations in Temperature: The Importance of Changes in the Capacity and Temperature Sensitivity of Respiration. Plant Cell Environ. 2000, 23, 15–26. [Google Scholar] [CrossRef]
- Tjoelker, M.G.; Oleksyn, J.; Reich, P.B. Modelling Respiration of Vegetation: Evidence for a General Temperature-Dependent Q10. Glob. Chang. Biol. 2001, 7, 223–230. [Google Scholar] [CrossRef]
- Turnbull, M.H.; Whitehead, D.; Tissue, D.T.; Schuster, W.S.F.; Brown, K.J.; Griffin, K.L. Responses of Leaf Respiration to Temperature and Leaf Characteristics in Three Deciduous Tree Species Vary with Site Water Availability. Tree Physiol. 2001, 21, 571–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitehead, D.; Griffin, K.L.; Turnbull, M.H.; Tissue, D.T.; Engel, V.C.; Brown, K.J.; Schuster, W.S.F.; Walcroft, A.S. Response of Total Night-Time Respiration to Differences in Total Daily Photosynthesis for Leaves in a Quercus rubra L. Canopy: Implications for Modelling Canopy CO2 Exchange. Glob. Chang. Biol. 2004, 10, 925–938. [Google Scholar] [CrossRef]
- Katja, H.; Irina, B.; Hiie, I.; Olav, K.; Tiit, P.; Bahtijor, R.; Mari, T.; Ülo, N. Temperature Responses of Dark Respiration in Relation to Leaf Sugar Concentration. Physiol. Plant. 2012, 144, 320–334. [Google Scholar] [CrossRef]
- Rodríguez-Calcerrada, J.; Limousin, J.M.; Martin-Stpaul, N.K.; Jaeger, C.; Rambal, S. Gas Exchange and Leaf Aging in an Evergreen Oak: Causes and Consequences for Leaf Carbon Balance and Canopy Respiration. Tree Physiol. 2012, 32, 464–477. [Google Scholar] [CrossRef]
- Xu, C.Y.; Griffin, K.L. Seasonal Variation in the Temperature Response of Leaf Respiration in Quercus rubra: Foliage Respiration and Leaf Properties. Funct. Ecol. 2006, 20, 778–789. [Google Scholar] [CrossRef]
- Turnbull, M.H.; Tissue, D.T.; Griffin, K.L.; Richardson, S.J.; Peltzer, D.A.; Whitehead, D. Respiration Characteristics in Temperate Rainforest Tree Species Differ along a Long-Term Soil-Development Chronosequence. Oecologia 2005, 143, 271–279. [Google Scholar] [CrossRef]
- Slot, M.; Zaragoza-Castells, J.; Atkin, O.K. Transient Shade and Drought Have Divergent Impacts on the Temperature Sensitivity of Dark Respiration in Leaves of Geum urbanum. Funct. Plant Biol. 2008, 35, 1135–1146. [Google Scholar] [CrossRef]
- Griffin, K.L.; Turnbull, M.; Murthy, R. Canopy Position Affects the Temperature Response of Leaf Respiration in Populus deltoides. New Phytol. 2002, 154, 609–619. [Google Scholar] [CrossRef]
- Turnbull, M.H.; Whitehead, D.; Tissue, D.T.; Schuster, W.S.F.; Brown, K.J.; Griffin, K.L. Scaling Foliar Respiration in Two Contrasting Forest Canopies. Funct. Ecol. 2003, 17, 101–114. [Google Scholar] [CrossRef]
- Atkin, O.K.; Bruhn, D.; Hurry, V.M.; Tjoelker, M.G. Evans Review No. 2—The Hot and the Cold: Unravelling the Variable Response of Plant Respiration to Temperature. Funct. Plant Biol. 2005, 32, 87–105. [Google Scholar] [CrossRef] [Green Version]
- Way, D.A.; Oren, R. Differential Responses to Changes in Growth Temperature between Trees from Different Functional Groups and Biomes: A Review and Synthesis of Data. Tree Physiol. 2010, 30, 669–688. [Google Scholar] [CrossRef] [Green Version]
- Wythers, K.R.; Reich, P.B.; Tjoelker, M.G.; Bolstad, P.B. Foliar Respiration Acclimation to Temperature and Temperature Variable Q10 Alter Ecosytem Carbon Balance. Glob. Chang. Biol. 2005, 11, 435–449. [Google Scholar] [CrossRef]
- Yu, J.; Chen, L.; Xu, M.; Huang, B. Effects of Elevated CO2 on Physiological Responses of Tall Fescue to Elevated Temperature, Drought Stress, and the Combined Stresses. Crop Sci. 2012, 52, 1848–1858. [Google Scholar] [CrossRef]
- Vong, N.Q.; Murata, Y. Studies on the Physiological Characteristics of C3 and C4 Crop Species: I. The Effects of Air Temperature on the Apparent Photosynthesis, Dark Respiration, and Nutrient Absorption of Some Crops. Jpn. J. Crop Sci. 1977, 46, 45–52. [Google Scholar] [CrossRef] [Green Version]
- Bolton, J.K.; Harold Brown, R. Photosynthesis of Grass Species Differing in Carbon Dioxide Fixation Pathways. V. Response of Panicum maximum, Panicum milioides, and Tall Fescue (Festuca arundinacea) To Nitrogen Nutrition. Plant Physiol. 1980, 66, 97–100. [Google Scholar] [CrossRef] [Green Version]
- Huang, B.; Fu, J. Photosynthesis, Respiration, and Carbon Allocation of Two Cool-Season Perennial Grasses in Response to Surface Soil Drying. Plant Soil 2000, 227, 17–26. [Google Scholar] [CrossRef]
- Bélanger, G.; Gastal, F.; Warembourg, F.R. Carbon Balance of Tall Fescue (Festuca arundinacea Schreb.): Effects of Nitrogen Fertilization and the Growing Season. Ann. Bot. 1994, 74, 653–659. [Google Scholar] [CrossRef]
- Skinner, R.H. Nitrogen Fertilization Effects on Pasture Photosynthesis, Respiration, and Ecosystem Carbon Content. Agric. Ecosyst. Environ. 2013, 172, 35–41. [Google Scholar] [CrossRef]
- Ludlow, M.M. Studies on the Productivity of Tropical Pasture Plants. V.* Effect of Shading on Growth, Photosynthesis and Respiration in Two Grasses and Two Legumes. Aust. J. Agric. Res. 1974, 25, 425–433. [Google Scholar] [CrossRef]
- Kumarathunge, D.P.; Drake, J.E.; Tjoelker, M.G.; López, R.; Pfautsch, S.; Vårhammar, A.; Medlyn, B.E. The Temperature Optima for Tree Seedling Photosynthesis and Growth Depend on Water Inputs. Glob. Chang. Biol. 2020, 26, 2544–2560. [Google Scholar] [CrossRef] [PubMed]
- Tarin, T.; Nolan, R.H.; Medlyn, B.E.; Cleverly, J.; Eamus, D. Water-Use Efficiency in a Semi-Arid Woodland with High Rainfall Variability. Glob. Chang. Biol. 2020, 26, 496–508. [Google Scholar] [CrossRef] [PubMed]
- Yoo, C.Y.; Pence, H.E.; Hasegawa, P.M.; Mickelbart, M.V. Regulation of Transpiration to Improve Crop Water Use. Crit. Rev. Plant Sci. 2009, 28, 410–431. [Google Scholar] [CrossRef]
- Will, R.E.; Wilson, S.M.; Zou, C.B.; Hennessey, T.C. Increased Vapor Pressure Deficit Due to Higher Temperature Leads to Greater Transpiration and Faster Mortality during Drought for Tree Seedlings Common to the Forest-Grassland Ecotone. New Phytol. 2013, 200, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Duursma, R.A.; Huang, G.; Smith, R.A.; Choat, B.; O’Grady, A.P.; Tissue, D.T. Elevated [CO2] Does Not Ameliorate the Negative Effects of Elevated Temperature on Drought-Induced Mortality in Eucalyptus radiata Seedlings. Plant Cell Environ. 2014, 37, 1598–1613. [Google Scholar] [CrossRef]
- Von Caemmerer, S.; Farquhar, G.D. Some Relationships between the Biochemistry of Photosynthesis and the Gas Exchange of Leaves. Planta 1981, 153, 376–387. [Google Scholar] [CrossRef]
- Fischer, R.; Turner, N.C. Plant productivity in the arid and semiarid zones. Ann. Rev. Plant Physiol. 1978, 29, 277–317. [Google Scholar] [CrossRef]
- Monteith, J.L.; Greenwood, D.J. How Do Crops Manipulate Water Supply and Demand? Philos. Trans. R. Soc. Lond. Ser. A Math. Phys. Sci. 1986, 316, 245–259. [Google Scholar] [CrossRef]
- Medlyn, B.E.; Duursma, R.A.; Eamus, D.; Ellsworth, D.S.; Prentice, I.C.; Barton, C.V.M.; Crous, K.Y.; De Angelis, P.; Freeman, M.; Wingate, L. Reconciling the Optimal and Empirical Approaches to Modelling Stomatal Conductance. Glob. Chang. Biol. 2011, 17, 2134–2144. [Google Scholar] [CrossRef] [Green Version]
- Bouton, J.H. An Overview of the Role of Lucerne (Medicago sativa L.) in Pastoral Agriculture. Crop Pasture Sci. 2012, 63, 734–738. [Google Scholar] [CrossRef]
- Yang, S.; Gao, M.; Xu, C.; Gao, J.; Deshpande, S.; Lin, S.; Roe, B.A.; Zhu, H. Alfalfa Benefits from Medicago truncatula: The RCT1 Gene from M. truncatula Confers Broad-Spectrum Resistance to Anthracnose in Alfalfa. Proc. Natl. Acad. Sci. USA 2008, 105, 12164–12169. [Google Scholar] [CrossRef] [Green Version]
- Wigley, K.; Ridgway, H.J.; Humphries, A.W.; Ballard, R.A.; Moot, D.J. Increased Lucerne Nodulation in Acid Soils with Sinorhizobium meliloti and Lucerne Tolerant to Low PH and High Aluminium. Crop Pasture Sci. 2018, 69, 1031–1040. [Google Scholar] [CrossRef]
- Kørup, K.; Lærke, P.E.; Baadsgaard, H.; Andersen, M.N.; Kristensen, K.; Münnich, C.; Didion, T.; Jensen, E.S.; Mårtensson, L.M.; Jørgensen, U. Biomass Production and Water Use Efficiency in Perennial Grasses during and after Drought Stress. GCB Bioenergy 2018, 10, 12–27. [Google Scholar] [CrossRef] [Green Version]
- Mcdonald, W.; Nikandrow, A.; Bishop, A.; Lattimore, M.; Gardner, P.; Williams, R.; Hyson, L. Lucerne for Pasture and Fodder; NSW Agriculture: Orange, NSW, Australia, 2003; Agfact P2. 2.25. [Google Scholar]
- Harris, C.; Clark, S.; Culvenor, R.; Li, G.; Gardner, M.; Hayes, R.; Nie, Z.; Norton, M.; Clark, B. New Tall Fescue Cultivars for Medium to Low Rainfall Environments in Southern Australia. In Proceedings of the 16th Australian Agronomy Conference on Capturing Opportunities and Overcoming Obstacles in Australian Agronomy, Armidale, NSW, Australia, 14–18 October 2012. [Google Scholar]
- Yang, F.; Li, J.; Gan, X.; Qian, Y.; Wu, X.; Yang, Q. Assessing Nutritional Status of Festuca arundinacea by Monitoring Photosynthetic Pigments from Hyperspectral Data. Comput. Electron. Agric. 2010, 70, 52–59. [Google Scholar] [CrossRef]
- Hill, M.J.; Pearson, C.J.; Kirby, A.C. Germination and Seedling Growth of Prairie Grass, Tall Fescue and Italian Ryegrass at Different Temperatures. Aust. J. Agric. Res. 1985, 36, 13–24. [Google Scholar] [CrossRef]
- Aspinwall, M.J.; Drake, J.E.; Campany, C.; Vårhammar, A.; Ghannoum, O.; Tissue, D.T.; Reich, P.B.; Tjoelker, M.G. Convergent Acclimation of Leaf Photosynthesis and Respiration to Prevailing Ambient Temperatures under Current and Warmer Climates in Eucalyptus tereticornis. New Phytol. 2016, 212, 354–367. [Google Scholar] [CrossRef]
- Ghannoum, O.; Phillips, N.G.; Sears, M.A.; Logan, B.A.; Lewis, J.D.; Conroy, J.P.; Tissue, D.T. Photosynthetic Responses of Two Eucalypts to Industrial-Age Changes in Atmospheric [CO2] and Temperature. Plant Cell Environ. 2010, 33, 1671–1681. [Google Scholar] [CrossRef] [PubMed]
- Duursma, R.A. Plantecophys—An R Package for Analysing and Modelling Leaf Gas Exchange Data. PLoS ONE 2015, 10, e0143346. [Google Scholar] [CrossRef]
- Sharkey, T.D.; Bernacchi, C.J.; Farquhar, G.D.; Singsaas, E.L. Fitting Photosynthetic Carbon Dioxide Response Curves for C3 leaves. Plant Cell Environ. 2007, 30, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Heberling, J.M.; Fridley, J.D. Resource-Use Strategies of Native and Invasive Plants in Eastern North American Forests. New Phytol. 2013, 200, 523–533. [Google Scholar] [CrossRef]
- Marshall, B.; Biscoe, P.V. A Model for C3 Leaves Describing the Dependence of Net Photosynthesis on Irradiance: II. Application to the Analysis of Flag Leaf Photosynthesis. J. Exp. Bot. 1980, 31, 41–48. [Google Scholar] [CrossRef]
- Thornley, J.H.M.; France, J. Mathematical Models in Agriculture: Quantitative Methods for the Plant, Animal and Ecological Sciences. Available online: https://books.google.com.au/books?id=rlwBCRSHobcC&printsec=frontcover#v=onepage&q&f=false (accessed on 21 July 2020).
- Johnson, I.R. DairyMod and the SGS Pasture Model: A Mathematical Description of the Biophysical Model Structure; IMJ Consultants: Dorrigo, NSW, Australia, 2016; Available online: http://imj.com.au/dairymod/ (accessed on 18 June 2020).
- Bender, S.F.; Plantenga, F.; Neftel, A.; Jocher, M.; Oberholzer, H.R.; Köhl, L.; Giles, M.; Daniell, T.J.; Van Der Heijden, M.G.A. Symbiotic Relationships between Soil Fungi and Plants Reduce N2O Emissions from Soil. ISME J. 2014, 8, 1336–1345. [Google Scholar] [CrossRef] [PubMed]
- Zufferey, V.; Spring, J.-L.; Verdenal, T.; Dienes, A.; Belcher, S.; Lorenzini, F.; Koestel, C.; Rösti, J.; Gindro, K.; Spangenberg, J.; et al. The Influence of Water Stress on Plant Hydraulics, Gas Exchange, Berry Composition and Quality of Pinot Noir Wines in Switzerland. Oeno One 2017, 51. [Google Scholar] [CrossRef]
- Medlyn, B.E.; De Kauwe, M.G.; Lin, Y.S.; Knauer, J.; Duursma, R.A.; Williams, C.A.; Arneth, A.; Clement, R.; Isaac, P.; Limousin, J.M.; et al. How Do Leaf and Ecosystem Measures of Water-Use Efficiency Compare? New Phytol. 2017, 216, 758–770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiltpold, I.; Demarta, L.; Johnson, S.N.; Moore, B.D.; Power, S.A.; Mitchell, C. Silicon and other essential element composition in roots using X-ray fluorescence spectroscopy: A high throughput approach. Invertebr. Ecol. Australas. Grassl. 2016, 191–196. [Google Scholar] [CrossRef]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D. Nlme: Linear and Nonlinear Mixed Effects Models; R Package Version 3.1-117. 2014. Available online: http://CRAN.R-project.org/package=nlme (accessed on 21 August 2020).
- Lenth, R.; Singmann, H.; Love, J.; Buerkner, P.; Herve, M. Emmeans: Estimated Marginal Means, Aka Least-Squares Means; R Package Version 1.3. 4. 2019. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 21 August 2020).
- Thomas, M.; Hill, G. Photosynthesis under Field Conditions. In Photosynthesis in Plants; Iowa State College Press: Ames, IA, USA, 1949; pp. 19–52. [Google Scholar]
- Charles-edwards, D.A.; Charles-edwards, J.; Cooper, J.P. The Influence of Temperature on Photosynthesis and Transpiration in Ten Temperate Grass Varieties Grown in Four Different Environments. J. Exp. Bot. 1971, 22, 650–662. [Google Scholar] [CrossRef]
- Farfan-Vignolo, E.R.; Asard, H. Effect of Elevated CO2 and Temperature on the Oxidative Stress Response to Drought in Lolium perenne L. and Medicago sativa L. Plant Physiol. Biochem. 2012, 59, 55–62. [Google Scholar] [CrossRef]
- Sasaki, H.; FuKuyama, M.; Toko, O.; Suyama, T.; Shoji, A. Effects of Increasing CO2 Concentration and Leaf Temperature on the Photosynthesis of Tall Fescue: Festuca arundinacea Schreb. Jpn. J. Grassl. Sci. 2002, 48, 12–16. [Google Scholar] [CrossRef]
- Jiang, Y.; Huang, B. Physiological Responses to Heat Stress Alone or in Combination with Drought: A Comparison between Tall Fescue and Perennial Ryegrass. HortScience 2001, 36, 682–686. [Google Scholar] [CrossRef] [Green Version]
- Murata, Y.; Iyama, J.; Honma, T. Studies on the Photosynthesis of Forage Crops: IV. Influence of Air-Temperature upon the Photosynthesis and Respiration of Alfalfa and Several Southern Type Forage Crops. Jpn. J. Crop Sci. 1965, 34, 154–158. [Google Scholar] [CrossRef] [Green Version]
- Brown, R.H.; Radcliffe, D.E. A Comparison of Apparent Photosynthesis in Sericea lespedeza and Alfalfa 1. Crop Sci. 1986, 26, 1208–1211. [Google Scholar] [CrossRef]
- Al-hamdani, S.; Todd, G.W. Effect of Temperature Regimes on Photosynthesis, Respiration, and Growth in Alfalfa. Proc. Okla. Acad. Sci. 1990, 70, 1–4. [Google Scholar]
- Pearson, C.J.; Hunt, L.A. Effects of Pretreatment Temperature on Carbon Dioxide Exchange in Alfalfa. Can. J. Bot. 1972, 50, 1925–1930. [Google Scholar] [CrossRef]
- Ziska, L.H.; Bunce, J.A. Direct and Indirect Inhibition of Single Leaf Respiration by Elevated CO2 Concentrations: Interaction with Temperature. Physiol. Plant. 1994, 90, 130–138. [Google Scholar] [CrossRef]
- Bond-Lamberty, B.; Thomson, A. Temperature-Associated Increases in the Global Soil Respiration Record. Nature 2010, 464, 579–582. [Google Scholar] [CrossRef]
- Rustad, L.E.; Campbell, J.L.; Marion, G.M.; Norby, R.J.; Mitchell, M.J.; Hartley, A.E.; Cornelissen, J.H.C.; Gurevitch, J.; Alward, R.; Beier, C.; et al. A Meta-Analysis of the Response of Soil Respiration, Net Nitrogen Mineralization, and Aboveground Plant Growth to Experimental Ecosystem Warming. Oecologia 2001, 126, 543–562. [Google Scholar] [CrossRef]
- Ryan, E.M.; Ogle, K.; Zelikova, T.J.; Lecain, D.R.; Williams, D.G.; Morgan, J.A.; Pendall, E. Antecedent Moisture and Temperature Conditions Modulate the Response of Ecosystem Respiration to Elevated CO2 and Warming. Glob. Chang. Biol. 2015, 21, 2588–2602. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Wan, S.; Hui, D.; Wallace, L.L. Acclimatization of Soil Respiration to Warming in a Tall Grass Prairie. Nature 2001, 413, 622–625. [Google Scholar] [CrossRef]
- Pendall, E.; Heisler-White, J.L.; Williams, D.G.; Dijkstra, F.A.; Carrillo, Y.; Morgan, J.A.; LeCain, D.R. Warming Reduces Carbon Losses from Grassland Exposed to Elevated Atmospheric Carbon Dioxide. PLoS ONE 2013, 8, e71921. [Google Scholar] [CrossRef]
- Yin, H.; Li, Y.; Xiao, J.; Xu, Z.; Cheng, X.; Liu, Q. Enhanced Root Exudation Stimulates Soil Nitrogen Transformations in a Subalpine Coniferous Forest under Experimental Warming. Glob. Chang. Biol. 2013, 19, 2158–2167. [Google Scholar] [CrossRef]
- Yin, H.; Xiao, J.; Li, Y.; Chen, Z.; Cheng, X.; Zhao, C.; Liu, Q. Warming Effects on Root Morphological and Physiological Traits: The Potential Consequences on Soil C Dynamics as Altered Root Exudation. Agric. Meteorol. 2013, 180, 287–296. [Google Scholar] [CrossRef]
- Zhang, Z.; Qiao, M.; Li, D.; Yin, H.; Liu, Q. Do Warming-Induced Changes in Quantity and Stoichiometry of Root Exudation Promote Soil N Transformations via Stimulation of Soil Nitrifiers, Denitrifiers and Ammonifiers? Eur. J. Soil Biol. 2016, 74, 60–68. [Google Scholar] [CrossRef]
- Feltner, K.C.; Massengale, M.A. Influence of Temperature and Harvest Management on Growth, Level of Carbohydrates in the Roots, and Survival of Alfalfa (Medicago sativa L.). Crop Sci. 1965, 5, 585–588. [Google Scholar] [CrossRef]
- Smith, D. Influence of Temperature on the Yield and Chemical Composition of ‘Vernal’ Alfalfa at First Flower. Agron. J. 1969, 61, 470–472. [Google Scholar] [CrossRef]
- Cooper, J.; Taiton, N. Light and Temperature Requirements for the Growth of Tropical and Temperate Grasses. Herb. Abstr. 1968, 38, 167–176. [Google Scholar]
- Sinclair, T.; Fiscus, E.; Wherley, B.; Durham, M.; Rufty, T. Atmospheric Vapor Pressure Deficit Is Critical in Predicting Growth Response of “Cool-Season” Grass Festuca arundinacea to Temperature Change. Planta 2007, 227, 273–276. [Google Scholar] [CrossRef]
- Nelson, C.J.; Asay, K.H.; Horst, G.L. Relationship of Leaf Photosynthesis to Forage Yield of Tall Fescue. Crop Sci. 1975, 15, 476–478. [Google Scholar] [CrossRef]
- Kiniry, J.R.; Kim, S. A Review of Modeled Water Use Efficiency of Highly Productive Perennial Grasses Useful for Bioenergy. Agronomy 2020, 10, 328. [Google Scholar] [CrossRef] [Green Version]
- Chapin, F.S.; Van Cleve, K. (Eds.) Approaches to Studying Nutrient Uptake, Use and Loss in Plants. In Plant Physiological Ecology; Springer: Dordrecht, The Netherlands, 2000; pp. 185–207. [Google Scholar] [CrossRef]
- Duursma, R.A.; Payton, P.; Bange, M.P.; Broughton, K.J.; Smith, R.A.; Medlyn, B.E.; Tissue, D.T. Near-Optimal Response of Instantaneous Transpiration Efficiency to Vapour Pressure Deficit, Temperature and [CO2] in Cotton (Gossypium hirsutum L.). Agric. Meteorol. 2013, 168, 168–176. [Google Scholar] [CrossRef]
- Allen, L.H.; Pan, D.; Boote, K.J.; Pickering, N.B.; Jones, J.W. Carbon Dioxide and Temperature Effects on Evapotranspiration and Water Use Efficiency of Soybean. Agron. J. 2003, 95, 1071–1081. [Google Scholar] [CrossRef]
- Nakagawa, H.; Hone, T.; Kim, H.Y.; Ohnishi, H.; Homma, K. Rice Responses to Elevated CO2 Concentrations and High Temperatures. J. Agric. Meteorol. 1997, 52, 797–800. [Google Scholar] [CrossRef]




| Variable # | S | T | S:T | |
|---|---|---|---|---|
| Photosynthetic Capacity | Asat | 35.77 *** | 3.01 | 5.59 * |
| gs | 8.91 ** | 0.03 | 0.46 | |
| E | 20.56 *** | 2.05 | 0.06 | |
| Topt | 3.13 | 0.13 | 0.7 | |
| Aopt | 48.77 *** | 2.74 | 0.76 | |
| Amax | 27.63 *** | 0.25 | 4.65 | |
| Vcmax ^ | 33.89 *** | 0.77 | 3.96 | |
| Jmax ^ | 35.1 *** | 0.16 | 5.59 * | |
| Jmax:Vcmax | 2.63 | 1.31 | 0.07 | |
| Vcmax25 ^ | 35.37 *** | 6.63 | 3.24 | |
| Jmax25 ^ | 34.57 *** | 2.59 | 5.36 * | |
| Jmax25:Vcmax25 | 4.13 | 2.28 | 0.21 | |
| Respiration | Rdark | 8.22 * | 8.26 | 2.03 |
| Reco ^ | 11.95 ** | 5.91 | 2.46 | |
| Carbon Balance | Rdark:Asat | 0.07 | 11.08 | 3.75 |
| WUE | Asat/E | 1.78 | 2.33 | 0.74 |
| Asat/gs | 1.97 | 0.95 | 1.06 | |
| Biomass | Shoot Biomass | 579.58 *** | 112.26 ** | 30.01 *** |
| Root biomass ^ | 236.34 *** | 44.49 * | 2.67 | |
| Root:Shoot | 39.53 *** | 5.24 | 1.84 | |
| Total Biomass | 602.31 *** | 107.86 * | 30.51 *** | |
| Leaf Stoichiometry | N ^ | 487.9 *** | 17.77 | 0.214 |
| P | 13.17 *** | 0.99 | 7.49 * | |
| N:P | 76.66 *** | 5.69 | 6.07 * | |
| C:N | 848.6 *** | 36.3 * | 0.4 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jacob, V.; Zhang, H.; Churchill, A.C.; Yang, J.; Choat, B.; Medlyn, B.E.; Power, S.A.; Tissue, D.T. Warming Reduces Net Carbon Gain and Productivity in Medicago sativa L. and Festuca arundinacea. Agronomy 2020, 10, 1601. https://doi.org/10.3390/agronomy10101601
Jacob V, Zhang H, Churchill AC, Yang J, Choat B, Medlyn BE, Power SA, Tissue DT. Warming Reduces Net Carbon Gain and Productivity in Medicago sativa L. and Festuca arundinacea. Agronomy. 2020; 10(10):1601. https://doi.org/10.3390/agronomy10101601
Chicago/Turabian StyleJacob, Vinod, Haiyang Zhang, Amber C. Churchill, Jinyan Yang, Brendan Choat, Belinda E. Medlyn, Sally A. Power, and David T. Tissue. 2020. "Warming Reduces Net Carbon Gain and Productivity in Medicago sativa L. and Festuca arundinacea" Agronomy 10, no. 10: 1601. https://doi.org/10.3390/agronomy10101601
APA StyleJacob, V., Zhang, H., Churchill, A. C., Yang, J., Choat, B., Medlyn, B. E., Power, S. A., & Tissue, D. T. (2020). Warming Reduces Net Carbon Gain and Productivity in Medicago sativa L. and Festuca arundinacea. Agronomy, 10(10), 1601. https://doi.org/10.3390/agronomy10101601





