Carbon Isotope Composition and the NDVI as Phenotyping Approaches for Drought Adaptation in Durum Wheat: Beyond Trait Selection
Abstract
1. Introduction
2. Materials and Methods
2.1. Germplasm Used and Experimental Conditions
2.2. NDVI Measurements
2.3. Dry Biomass Evaluation
2.4. Total Nitrogen and Carbon Content and Carbon Isotope Composition
2.5. Statistical Analysis
3. Results
3.1. General Characteristics of the Elite Durum Panel (EDP)
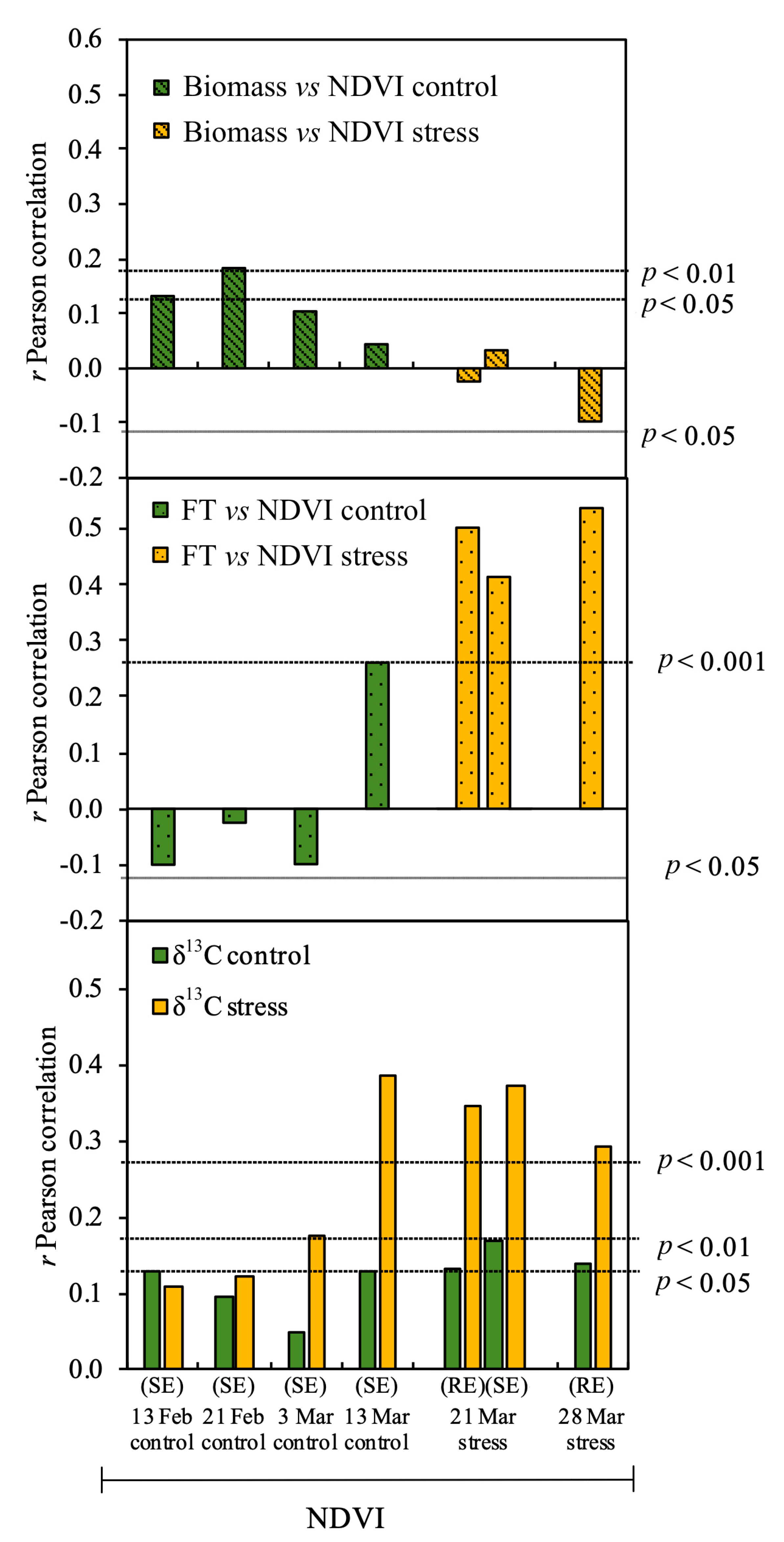
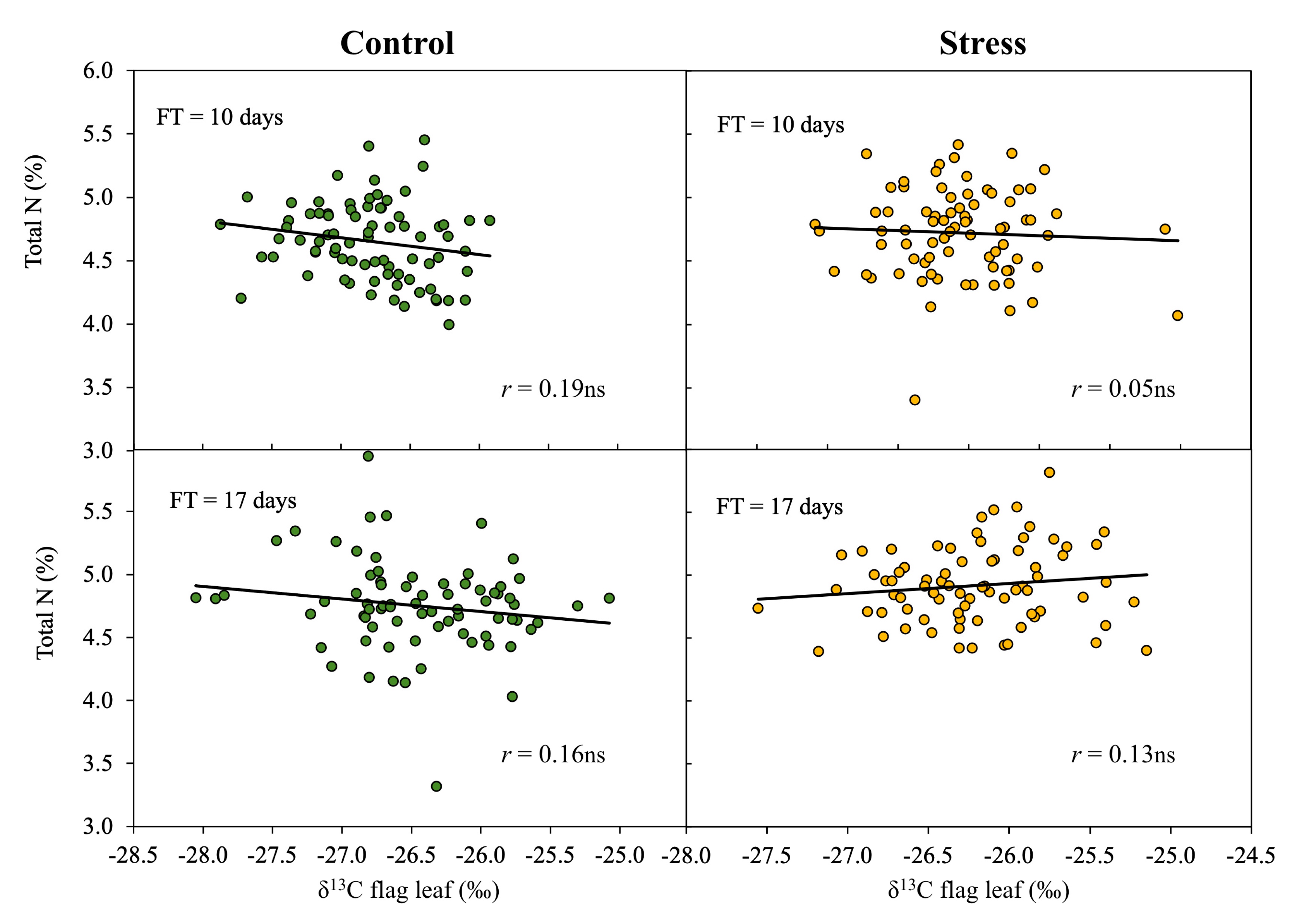
3.2. Relationship between Studied Parameters and Biomass
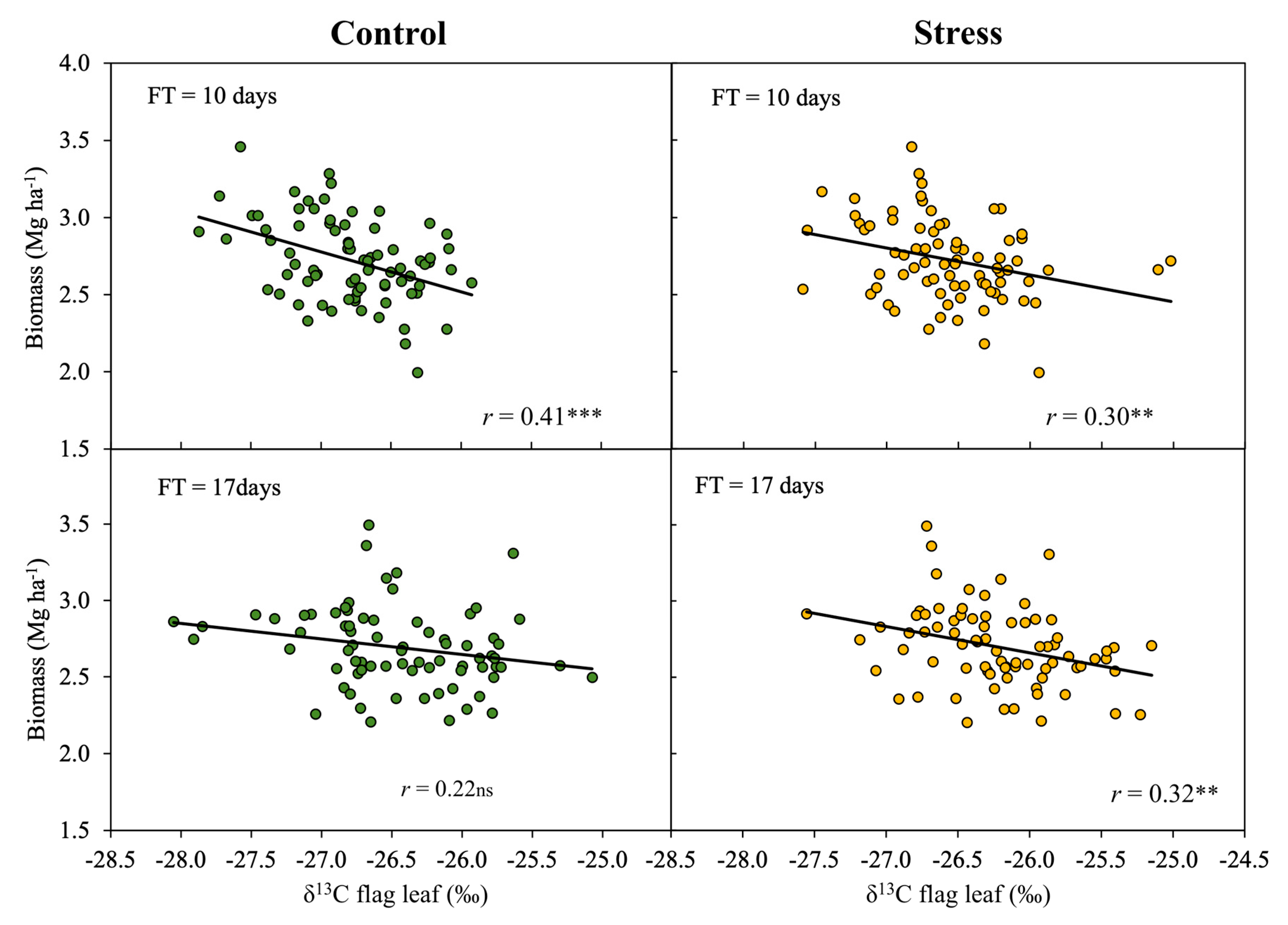
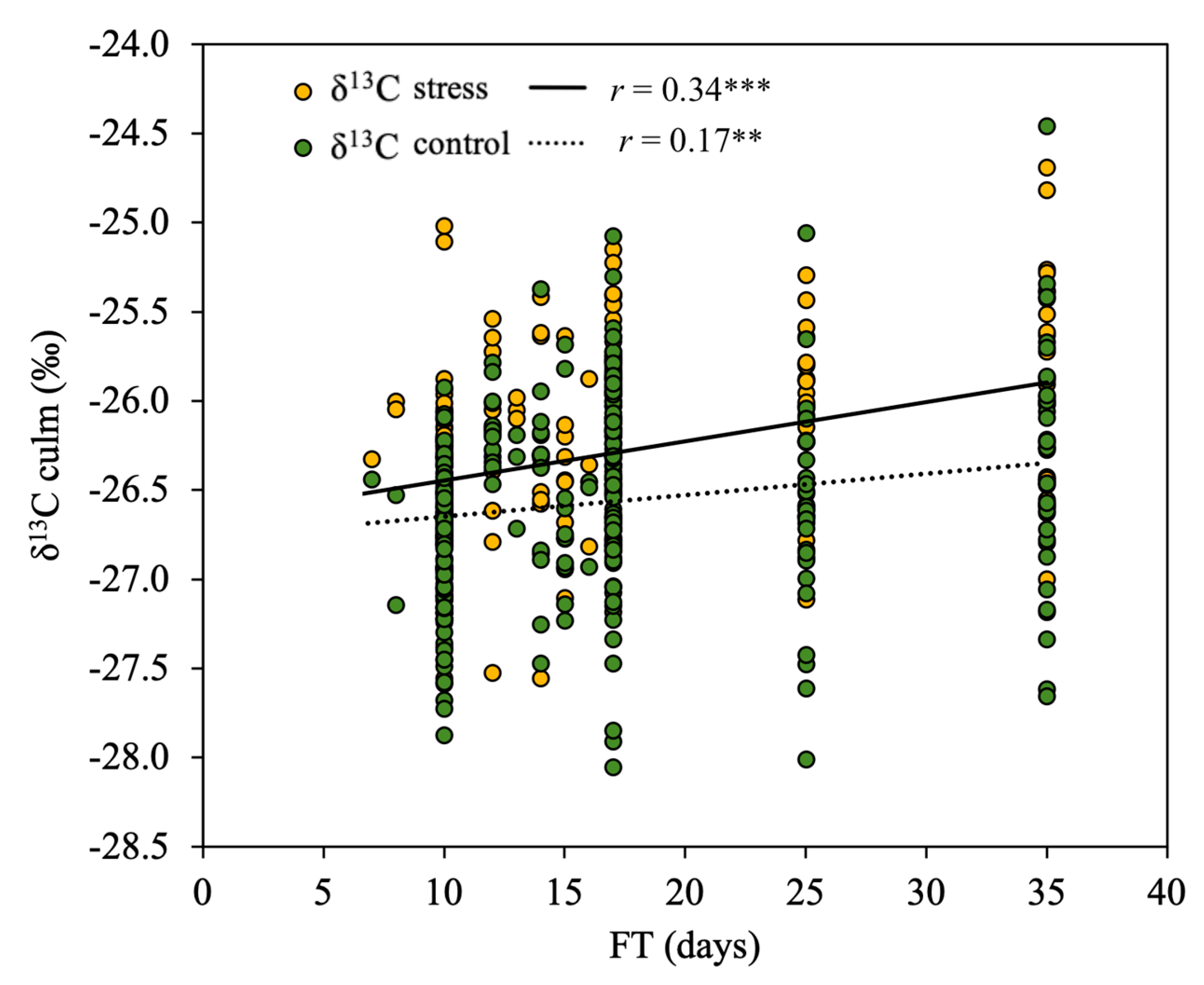
3.3. Effect of Phenology on the Relationship of δ13C to Biomass
4. Discussion
4.1. Effect of Drought in the EDP Based on the NDVI and δ13C
4.2. Biomass Predictions in Early Stages of Crop Development in the EDP Based on the NDVI
4.3. Biomass Prediction During the Late Stages of Crop Development Based on the NDVI
4.4. Biomass Predictions Based on δ13C
4.5. Concluding Remarks on How and When to Use Phenotyping Traits
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lobell, D.B.; Burke, M.B.; Tebaldi, C.; Mastrandrea, M.D.; Falcon, W.P.; Naylor, R.L. Prioritizing climate change adaptation needs for food security in 2030. Science 2008, 319, 607–610. [Google Scholar] [CrossRef]
- Ceccarelli, S.; Grando, S. Drought as a challenge for the plant breeder. Plant Growth Regul. 1996, 20, 149–155. [Google Scholar] [CrossRef]
- Araus, J.L.; Slafer, G.A.; Reynolds, M.P.; Royo, C. Plant breeding and drought in C3 cereals: What should we breed for? Ann. Bot. 2002, 89, 925–940. [Google Scholar] [CrossRef]
- Passioura, J. Grain yield, harvest index, and water use of wheat. J. Aust. Inst. Agric. Sci. 1977, 43, 117–120. [Google Scholar]
- Reynolds, M.P.; Foulkes, M.J.; Slafer, G.A.; Berry, P.; Parry, M.A.J.; Snape, J.W.; Angus, W.J. Raising yield potential in wheat. J. Exp. Bot. 2009, 60, 1899–1918. [Google Scholar] [CrossRef]
- Siddique, K.H.M.; Belford, R.K.; Perry, M.W.; Tennant, D. Growth, development and light interception of old and modern wheat cultivars in a Mediterranean-type environment. Aust. J. Agric. Res. 1989, 40, 473–487. [Google Scholar] [CrossRef]
- Slafer, G.A.; Satorre, E.H.; Andrade, F.H. Increases in grain yield in bread wheat from breeding and associated physiological changes. In Genetic Improvement of Field Crops; Slafer, G.A., Ed.; Marcel Dekker Inc.: New York, NY, USA, 1994; pp. 1–68. ISBN 0-8247-8980-6. [Google Scholar]
- González, A.; Martín, I.; Ayerbe, L. Barley yield in water-stress conditions. The influence of precocity, osmotic adjustment and stomatal conductance. Field Crops Res. 1999, 62, 23–24. [Google Scholar] [CrossRef]
- Carmo-Silva, E.; Andralojc, P.J.; Scales, J.C.; Driever, S.M.; Mead, A.; Lawson, T.; Raines, C.A.; Parry, M.A.J. Phenotyping of field-grown wheat in the UK highlights contribution of light response of photosynthesis and flag leaf longevity to grain yield. J. Exp. Bot. 2017, 68, 3473–3486. [Google Scholar] [CrossRef]
- Fernandez-Gallego, J.A.; Kefauver, S.C.; Vatter, T.; Aparicio Gutiérrez, N.; Nieto-Taladriz, M.T.; Araus, J.L. Low-cost assessment of grain yield in durum wheat using RGB images. Eur. J. Agron. 2019, 105, 146–156. [Google Scholar] [CrossRef]
- Gracia-Romero, A.; Kefauver, S.C.; Fernandez-Gallego, J.A.; Vergara-Díaz, O.; Nieto-Taladriz, M.T.; Araus, J.L. UAV and ground image-based phenotyping: A proof of concept with durum wheat. Remote Sens. 2019, 10, 1244. [Google Scholar] [CrossRef]
- Van Den Boogaard, R.; Veneklaas, E.J.; Lambers, H. The association of biomass allocation with growth and water use efficiency of two Triticum aestivum cultivars. Aust. J. Plant Physiol. 1996, 23, 751–761. [Google Scholar] [CrossRef]
- Villegas, D.; Aparicio, N.; Blanco, R.; Royo, C. Biomass accumulation and main stem elongation of durum wheat grown under Mediterranean conditions. Ann. Bot. 2001, 88, 617–627. [Google Scholar] [CrossRef]
- Aparicio, N.; Villegas, D.; Casadesus, J.; Araus, J.L.; Royo, C. Spectral vegetation indices as nondestructive tools for determining durum wheat yield. Agron. J. 2000, 91, 83–91. [Google Scholar] [CrossRef]
- Aparicio, N.; Villegas, D.; Araus, J.L.; Casadesús, J.; Royo, C. Relationship between growth traits and spectral vegetation indices in durum wheat. Crop Sci. 2002, 42, 1547–1555. [Google Scholar] [CrossRef]
- Babar, M.A.; Reynolds, M.P.; Van Ginkel, M.; Klatt, A.R.; Raun, W.R.; Stone, M.L. Spectral reflectance to estimate genetic variation for in-season biomass, leaf chlorophyll, and canopy temperature in wheat. Crop Sci. 2006, 46, 1046–1057. [Google Scholar] [CrossRef]
- Tattaris, M.; Reynolds, M.P.; Chapman, S.C. A direct comparison of remote sensing approaches for high-throughput phenotyping in plant breeding. Front. Plant Sci. 2016, 7, 1131. [Google Scholar] [CrossRef]
- Araus, J.L.; Cairns, J.E. Field high-throughput phenotyping: The new crop breeding frontier. Trends Plant Sci. 2014, 19, 52–61. [Google Scholar] [CrossRef]
- Araus, J.L.; Kefauver, S.C.; Zaman-Allah, M.; Olsen, M.S.; Cairns, J.E. Translating high-throughput phenotyping into genetic gain. Trends Plant Sci. 2018, 5, 451–466. [Google Scholar] [CrossRef]
- Trapp, J.J.; Urrea, C.A.; Zhou, J.; Khot, L.R.; Sankaran, S.; Miklas, P.N. Selective phenotyping traits related to multiple stress and drought response in dry bean. Crop Sci. 2016, 56, 1460–1472. [Google Scholar] [CrossRef]
- Shi, S.; Azam, F.I.; Li, H.; Chang, X.; Li, B.; Jing, R. Mapping QTL for stay-green and agronomic traits in wheat under diverse water regimes. Euphytica 2017, 213, 1–19. [Google Scholar] [CrossRef]
- Pauli, D.; Andrade-Sanchez, P.; Carmo-Silva, A.E.; Gazave, E.; French, A.N.; Heun, J.; Hunsaker, D.J.; Lipka, A.E.; Setter, T.L.; Strand, R.J.; et al. Field-based high-throughput plant phenotyping reveals the temporal patterns of quantitative trait loci associated with stress-responsive traits in cotton. G3 Genes Genomes Genet. 2016, 6, 865–879. [Google Scholar] [CrossRef]
- Condorelli, G.E.; Maccaferri, M.; Newcomb, M.; Andrade-Sanchez, P.; White, J.W.; French, A.N.; Sciara, G.; Ward, R.; Tuberosa, R. Comparative aerial and ground based high throughput phenotyping for the genetic dissection of NDVI as a proxy for drought adaptive traits in durum wheat. Front. Plant Sci. 2018, 9, 893. [Google Scholar] [CrossRef]
- Rebetzke, G.J.; Jimenez-Berni, J.A.; Bovill, W.D.; Deery, D.M.; James, R.A. High-throughput phenotyping technologies allow accurate selection of stay-green. J. Exp. Bot. 2016, 67, 4919–4924. [Google Scholar] [CrossRef] [PubMed]
- Tucker, C.J. Red and photographic infrared linear combinations for monitoring vegetation. Remote Sens. Environ. 1979, 8, 127–150. [Google Scholar] [CrossRef]
- Lukina, E.V.; Stone, M.L.; Raun, W.R. Estimating vegetation coverage in wheat using digital images. J. Plant Nutr. 1999, 22, 341–350. [Google Scholar] [CrossRef]
- Labus, M.P.; Nielsen, G.A.; Lawrence, R.L.; Engel, R.; Long, D.S. Wheat yield estimates using multi-temporal NDVI satellite imagery. Int. J. Remote Sens. 2002, 23, 4169–4180. [Google Scholar] [CrossRef]
- Ji, L.; Peters, A.J. Assessing vegetation response to drought in the northern Great Plains using vegetation and drought indices. Remote Sens. Environ. 2003, 87, 85–98. [Google Scholar] [CrossRef]
- Bennett, D.; Reynolds, M.; Mullan, D.; Izanloo, A.; Kuchel, H.; Langridge, P.; Schnurbusch, T. Detection of two major grain yield QTL in bread wheat (Triticum aestivum L.) under heat, drought and high yield potential environments. Theor. Appl. Genet. 2012, 125, 1473–1485. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Richards, R.A. Isotopic composition of plant carbon correlates with water-use efficiency of wheat genotypes. Aust. J. Plant Physiol. 1984, 11, 539–552. [Google Scholar] [CrossRef]
- Farquhar, G. Carbon Isotope Discrimination And Photosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 503–537. [Google Scholar] [CrossRef]
- Araus, J.L.; Amaro, T.; Casadesús, J.; Asbati, A.; Nachit, M.M. Relationships between ash content, carbon isotope discrimination and yield in durum wheat. Aust. J. Plant Physiol. 1998, 25, 835–842. [Google Scholar] [CrossRef]
- Richards, R.A.; Rebetzke, G.J.; Condon, A.G.; van Herwaarden, A.F. Breeding opportunities for increasing the efficiency of water use and crop yield in temperate cereals. Crop Sci. 2002, 42, 111–121. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Oleary, M.H.; Berry, J.A. On the relationship between carbon isotope discrimination and the inter-cellular carbon-dioxide concentration in leaves. Aust. J. Plant Physiol. 1982, 9, 121–137. [Google Scholar]
- Condon, A.G.; Richards, R.A.; Rebetzke, G.J.; Farquhar, G.D. Breeding for high water-use efficiency. J. Exp. Bot. 2004, 55, 2447–2460. [Google Scholar] [CrossRef]
- Araus, J.L.; Amaro, T.; Zuhair, Y.; Nachit, M.M. Effect of leaf structure and water status on carbon isotope discrimination in field-grown durum wheat. Plant Cell Environ. 1997, 20, 1484–1494. [Google Scholar] [CrossRef]
- Richards, R.A. Defining selection criteria to improve yield under drought. Plant Growth Regul. 1996, 20, 157–166. [Google Scholar] [CrossRef]
- Voltas, J.; Romagosa, I.; Lafarga, A.; Armesto, A.P.; Araus, J.L.; Sombrero, A. Genotype by environment interaction for grain yield and carbon isotope discrimination of barley in Mediterranean Spain. Aust. J. Agric. Res. 1999, 50, 1263–1271. [Google Scholar] [CrossRef]
- Rebetzke, G.J.; Condon, A.G.; Richards, R.A.; Farquhar, G.D. Selection for reduced carbon isotope discrimination increases aerial biomass and grain yield of rainfed bread wheat. Crop Sci. 2002, 42, 739–745. [Google Scholar] [CrossRef]
- Condon, A.G.; Richards, R.A.; Rebetzke, G.J.; Farquhar, G.D. Improving intrinsic water-use efficiency and crop yield. Crop Sci. 2002, 42, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Araus, J.L.; Villegas, D.; Aparicio, N.; del Moral, L.F.G.; El Hani, S.; Rharrabti, Y.; Ferrio, J.P.; Royo, C. Environmental factors determining carbon isotope discrimination and yield in durum wheat under Mediterranean conditions. Crop Sci. 2003, 43, 170–180. [Google Scholar] [CrossRef]
- Araus, J.L.; Cabrera-Bosquet, L.; Serret, M.D.; Bort, J.; Nieto-Taladriz, M.T. Comparative performance of δ13C, δ18O and δ15N for phenotyping durum wheat adaptation to a dryland environment. Funct. Plant Biol. 2013, 40, 595–608. [Google Scholar] [CrossRef]
- Condon, A.; Richards, R. Broad sense heritability and genotype × environment interaction for carbon isotope discrimination in field-grown wheat. Aust. J. Agric. Res. 1992, 43, 921–934. [Google Scholar] [CrossRef]
- Rebetzke, G.J.; Condon, A.G.; Farquhar, G.D.; Appels, R.; Richards, R.A. Quantitative trait loci for carbon isotope discrimination are repeatable across environments and wheat mapping populations. Theor. Appl. Genet. 2008, 118, 123–137. [Google Scholar] [CrossRef]
- Foster, B.P.; Ellis, R.P.; Moir, J.; Talame, V.; Sanguineti, M.C.; Tuberosa, R.; This, D.; Teulat-Merah, B.; Ahmed, I.; Mariy, S.A.E.E.; et al. Genotype and phenotype associations with drought tolerance in barley tested in North Africa. Ann. Appl. Biol. 2004, 144, 157–168. [Google Scholar] [CrossRef]
- Laza, M.R.; Kondo, M.; Ideta, O.; Barlaan, E.; Imbe, T. Identification of quantitative trait loci for δ13C and productivity in irrigated lowland rice. Crop Sci. 2006, 46, 763–773. [Google Scholar] [CrossRef]
- Araus, J.L.; Slafer, G.A.; Royo, C.; Serret, M.D. Breeding for yield potential and stress adaptation in cereals. CRC Crit. Rev. Plant Sci. 2008, 27, 377–412. [Google Scholar] [CrossRef]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Coplen, T.B. Explanatory glossary of terms used in expression of relative isotope ratios and gas ratios. In Comission on Isotopic Abundances and Atomic Weights; IUPAC Provisional Recommendations; U.S. Geological Survey: Reston, VA, USA, 2008. [Google Scholar]
- Mahey, R.K.; Singh, R.; Sidhu, S.S.; Narang, R.S. The use of remote sensing to assess the effects of water stress on wheat. Exp. Agric. 1991, 27, 423–429. [Google Scholar] [CrossRef]
- Cabrera-Bosquet, L.; Molero, G.; Stellacci, A.; Bort, J.; Nogués, S.; Araus, J. NDVI as a potential tool for predicting biomass, plant nitrogen content and growth in wheat genotypes subjected to different water and nitrogen conditions. Cereal Res. Commun. 2011, 39, 147–159. [Google Scholar] [CrossRef]
- Richards, R.A.; Lukacs, Z. Seedling vigour in wheat—Sources of variation for genetic and agronomic improvement. Aust. J. Agric. Res. 2002, 53, 41–50. [Google Scholar] [CrossRef]
- Govaerts, B.; Verhulst, N. The Normalized Difference Vegetation Index (NDVI) Greenseeker (TM) Handheld Sensor: Toward Integrated Evaluation of Crop Management; Part B: User Guide; Cimmyt: El Batán, Mexico, 2010. [Google Scholar]
- Sirault, X.R.R.; Condon, A.G.; Wood, J.T.; Farquhar, G.D.; Rebetzke, G.J. “Rolled-upness”: Phenotyping leaf rolling in cereals using computer vision and functional data analysis approaches. Plant Methods 2015, 11. [Google Scholar] [CrossRef] [PubMed]
- Marti, J.; Bort, J.; Slafer, G.A.; Araus, J.L. Can wheat yield be assessed by early measurements of Normalized Difference Vegetation Index? Ann. Appl. Biol. 2007, 150, 253–257. [Google Scholar] [CrossRef]
- Slafer, G.A.; Araus, J.L.; Royo, C.; Moral, L.F.G. Promising eco-physiological traits for genetic improvement of cereal yields in Mediterranean environments. Ann. Appl. Biol. 2005, 146, 61–70. [Google Scholar] [CrossRef]
- Duan, T.; Chapman, S.C.; Guo, Y.; Zheng, B. Dynamic monitoring of NDVI in wheat agronomy and breeding trials using an unmanned aerial vehicle. Field Crops Res. 2017, 210, 71–80. [Google Scholar] [CrossRef]
- Magney, T.S.; Eitel, J.U.H.; Huggins, D.R.; Vierling, L.A. Proximal NDVI derived phenology improves in-season predictions of wheat quantity and quality. Agric. For. Meteorol. 2016, 217, 46–60. [Google Scholar] [CrossRef]
- Fischer, R.A. The effect of duration of the vegetative phase in irrigated semi-dwarf spring wheat on phenology, growth and potential yield across sowing dates at low latitude. Field Crops Res. 2016, 198, 188–199. [Google Scholar] [CrossRef]
- Royo, C.; Aparicio, N.; Villegas, D.; Casadesus, J.; Monneveux, P.; Araus, J.L. Usefulness of spectral reflectance indices as durum wheat yield predictors under contrasting Mediterranean conditions. Int. J. Remote Sens. 2003, 24, 4403–4419. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Hubick, K.T.; Condon, A.G.; Richards, R.A. Carbon Isotope fractionation and plant water-use efficiency. In Stable Isotopes in Ecological Research; Springer: Berlin/Heidelberg, Germany, 1989; ISBN 146128127X. [Google Scholar]
- Fischer, R.A.; Rees, D.; Sayre, K.D.; Lu, Z.-M.; Condon, A.G.; Saavedra, A.L. Wheat yield progress associated with higher stomatal conductance and photosynthetic rate, and cooler canopies. Crop Sci. 1998, 38, 1467–1475. [Google Scholar] [CrossRef]
- Roche, D. Stomatal conductance is essential for higher yield potential of C3 crops. CRC Crit. Rev. Plant Sci. 2015. [Google Scholar] [CrossRef]
- Evans, J.R. Nitrogen and photosynthesis in the flag leaf of wheat (Triticum aestivum L.). Plant Physiol. 1983, 72, 297–302. [Google Scholar] [CrossRef]
- Richards, A.R.; Condon, G.A. Challenges ahead in using carbon isotope discrimination in plant-breeding programs. In Stable Isotopes and Plant Carbon-Water Relations; James, R.E., Anthony, E.H., Graham, D.F., Eds.; Academic Press: London, UK, 1993; pp. 451–462. ISBN 978-0-12-233380-4. [Google Scholar]
- Cooper, P.J.M.; Gregory, P.J. Soil water management in the rain-fed farming systems of the Mediterranean region. Soil Use Manag. 1987, 3, 57–62. [Google Scholar] [CrossRef]
- Cantero-Martinez, C.; Villar, J.M.; Romagosa, I.; Fereres, E. Growth and yield responses of two contrasting barley cultivars in a Mediterranean environment. Eur. J. Agron. 1995, 4, 317–326. [Google Scholar] [CrossRef]
- Condon, A.; Richards, R.; Farquhar, G. Relationships between carbon isotope discrimination, water use efficiency and transpiration efficiency for dryland wheat. Aust. J. Agric. Res. 1993, 44, 1693–1711. [Google Scholar] [CrossRef]
- Pennacchi, J.P.; Carmo-Silva, E.; Andralojc, P.J.; Feuerhelm, D.; Powers, S.J.; Parry, M.A.J. Dissecting wheat grain yield drivers in a mapping population in the UK. Agronomy 2018, 8, 94. [Google Scholar] [CrossRef]
- Tuberosa, R. Phenotyping for drought tolerance of crops in the genomics era. Front. Physiol. 2012, 3, 347. [Google Scholar] [CrossRef]
- Tardieu, F. Any trait or trait-related allele can confer drought tolerance: Just design the right drought scenario. J. Exp. Bot. 2012, 63, 25–31. [Google Scholar] [CrossRef]



| Agronomic Variables | AVG | SE | ||
|---|---|---|---|---|
| Biomass 2017 (Mg ha−1) | 2.67 | 0.02 | ||
| Flowering time range (days) | 17.08 | 0.52 | ||
| NDVI (Unmanned Aerial Vehicles) | Zadoks (GS) | |||
| NDVI (SE) 13 Feb control | 31 | 0.548 | g | 0.002 |
| NDVI (SE) 21 Feb control | 0.706 | e | 0.002 | |
| NDVI (SE) 3 Mar control | 0.656 | f | 0.002 | |
| NDVI (SE) 13 Mar control | 53 | 0.714 | d | 0.002 |
| NDVI (RE) 21 Mar stress | 0.829 | b | 0.001 | |
| NDVI (SE) 21 Mar stress | 0.879 | a | 0.001 | |
| NDVI (RE) 28 Mar stress | 58 | 0.772 | c | 0.002 |
| Mean Squares | ||||
| NDVI measurements | 3.02 *** |
| Control | Stress | ANOVA | |||
|---|---|---|---|---|---|
| AVG | SE | AVG | SE | Mean Squares | |
| Total N (%) | 4.74 | 0.03 | 4.87 | 0.02 | 1.89 ** |
| Total C (%) | 43.69 | 0.21 | 44.35 | 0.11 | 31.98 ** |
| δ13C (‰) | −26.57 | 0.04 | −26.29 | 0.03 | 9.35 *** |
| Variable | df | r | Mean Squares | F | Significance (p) | ||
|---|---|---|---|---|---|---|---|
| Global | (−) δ13C stress | 235 | 0.29 | 1.60 | 21.26 | 0.000 | |
| (−) δ13C stress, (+) NDVI (SE) 21 Feb control | 234 | 0.36 | 1.20 | 16.85 | 0.000 | ||
| Flowering time | 10 days | (−) δ13C control | 75 | 0.38 | 0.78 | 12.93 | 0.001 |
| (−) δ13C control, (+) NDVI (SE) 21 Feb control | 74 | 0.44 | 0.51 | 8.75 | 0.000 | ||
| 12 days | (+) Total N stress | 8 | 0.64 | 0.39 | 5.50 | 0.047 | |
| 15 days | (−) δ13C control | 8 | 0.74 | 0.25 | 9.64 | 0.015 | |
| 17 days | (−) δ13C stress | 62 | 0.32 | 0.49 | 7.12 | 0.010 | |
| 25 days | (−) NDVI (SE) 28 Mar stress | 24 | 0.50 | 0.50 | 7.84 | 0.010 | |
| (−) NDVI (RE) 28 Mar stress, (+) NDVI (SE) 21 Mar stress | 23 | 0.71 | 0.52 | 11.97 | 0.000 | ||
| (−) NDVI (RE) 28 Mar stress, (+) NDVI (SE) 21 Mar stress, (−) δ13C stress | 22 | 0.79 | 0.42 | 11.95 | 0.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanchez-Bragado, R.; Newcomb, M.; Chairi, F.; Condorelli, G.E.; Ward, R.W.; White, J.W.; Maccaferri, M.; Tuberosa, R.; Araus, J.L.; Serret Molins, M.D. Carbon Isotope Composition and the NDVI as Phenotyping Approaches for Drought Adaptation in Durum Wheat: Beyond Trait Selection. Agronomy 2020, 10, 1679. https://doi.org/10.3390/agronomy10111679
Sanchez-Bragado R, Newcomb M, Chairi F, Condorelli GE, Ward RW, White JW, Maccaferri M, Tuberosa R, Araus JL, Serret Molins MD. Carbon Isotope Composition and the NDVI as Phenotyping Approaches for Drought Adaptation in Durum Wheat: Beyond Trait Selection. Agronomy. 2020; 10(11):1679. https://doi.org/10.3390/agronomy10111679
Chicago/Turabian StyleSanchez-Bragado, Rut, Maria Newcomb, Fadia Chairi, Giuseppe Emanuele Condorelli, Richard W. Ward, Jeffrey W. White, Marco Maccaferri, Roberto Tuberosa, Jose Luis Araus, and Maria Dolors Serret Molins. 2020. "Carbon Isotope Composition and the NDVI as Phenotyping Approaches for Drought Adaptation in Durum Wheat: Beyond Trait Selection" Agronomy 10, no. 11: 1679. https://doi.org/10.3390/agronomy10111679
APA StyleSanchez-Bragado, R., Newcomb, M., Chairi, F., Condorelli, G. E., Ward, R. W., White, J. W., Maccaferri, M., Tuberosa, R., Araus, J. L., & Serret Molins, M. D. (2020). Carbon Isotope Composition and the NDVI as Phenotyping Approaches for Drought Adaptation in Durum Wheat: Beyond Trait Selection. Agronomy, 10(11), 1679. https://doi.org/10.3390/agronomy10111679






