Deacclimation of Winter Oilseed Rape—Insight into Physiological Changes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Growth Conditions, Experimental Design and Sampling
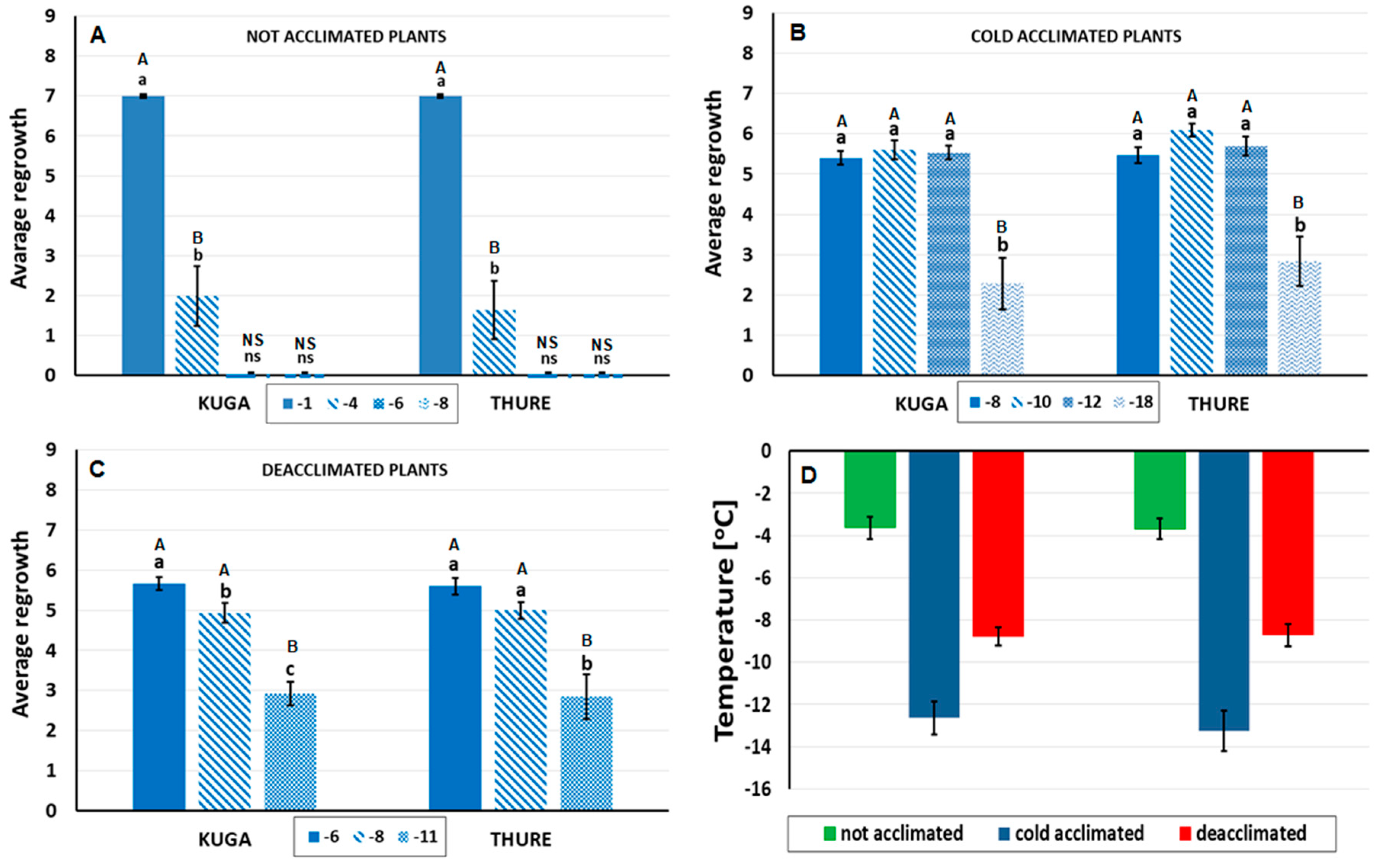
2.2. Testing Frost Tolerance
2.3. Chlorophyll a Fluorescence Measurements
2.4. Analysis of the Photosynthetic Pigment (Chlorophyll a, b and Carotenoid) Content
2.5. Measurements of Leaf Reflectance
2.6. Leaf Gaseous Exchange
2.7. Relative Water Content (RWC)
2.8. Content of Total Soluble Sugars
2.9. Osmotic Potential
2.10. Accumulation of the BnPIP1 Transcript: RNA Isolation, cDNA Synthesis, and Real-Time PCR Reaction
2.11. Protein Concentration in the Crude Leaf Extracts
2.12. Analysis of the Accumulation of the BnPIP1 Aquaporins Using Immunoblotting
2.13. FT-Raman Spectroscopy Measurements
2.14. Statistical Analysis
3. Results
3.1. Frost Tolerance of the Not Acclimated, Cold-Acclimated, and Deacclimated Oilseed Rape Plants
3.2. Physiological/Biochemical Characteristic of Not Acclimated, Cold Acclimated and Deacclimated Oilseed Rape Plants
3.2.1. Photosystem II Efficiency
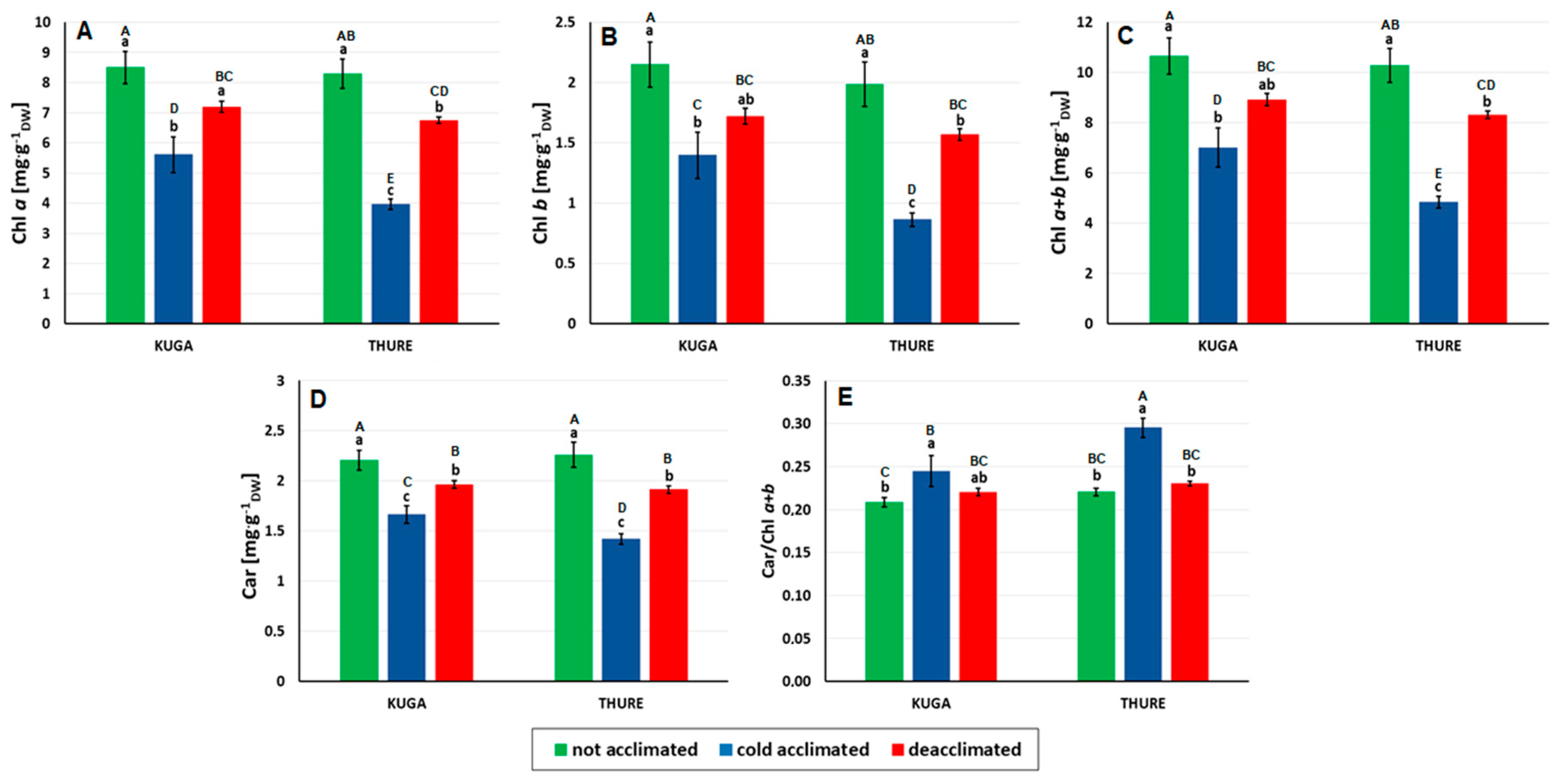
3.2.2. Leaf Pigments
Chlorophyll and Carotenoids
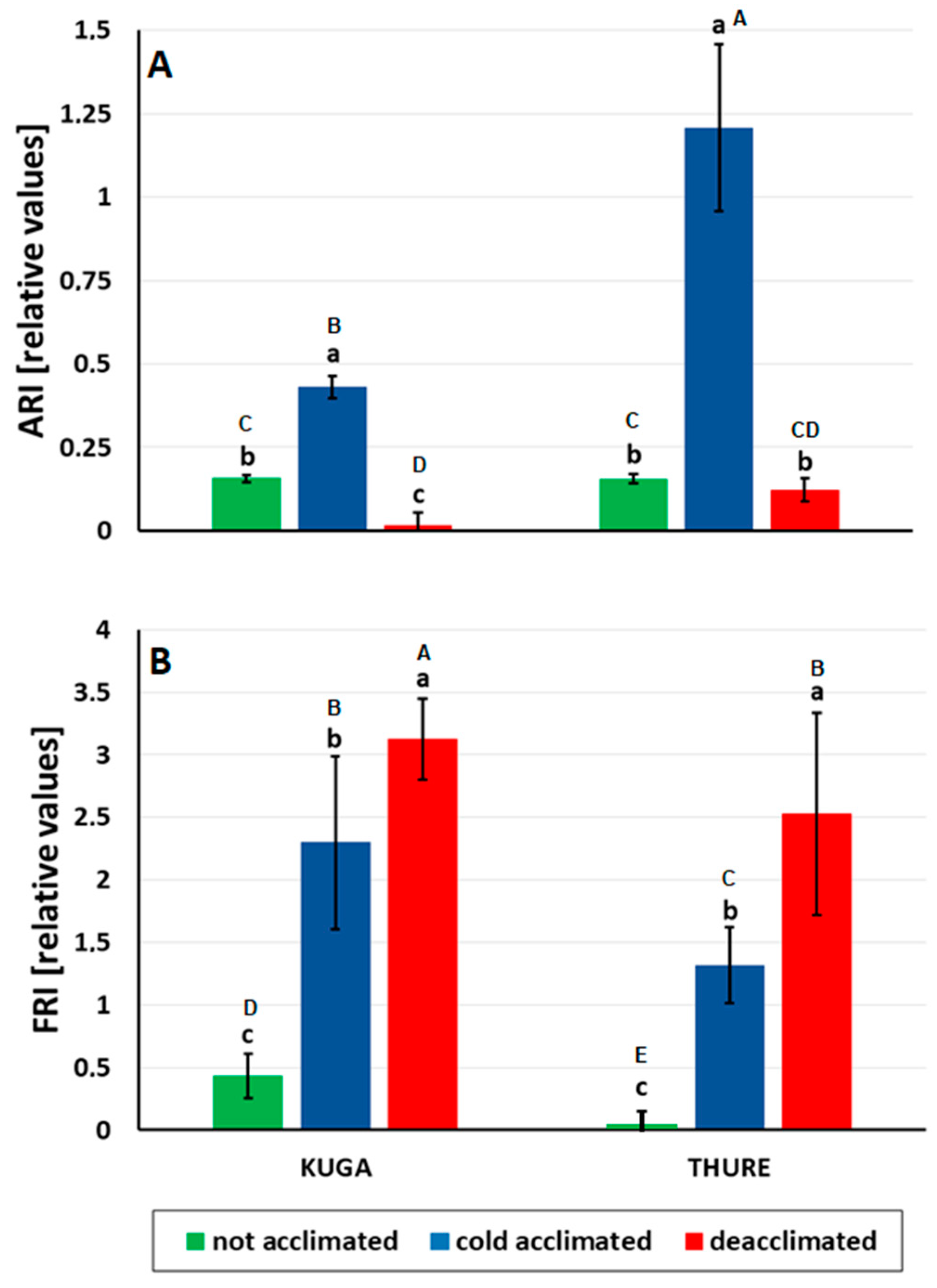
Anthocyanins and Flavonols
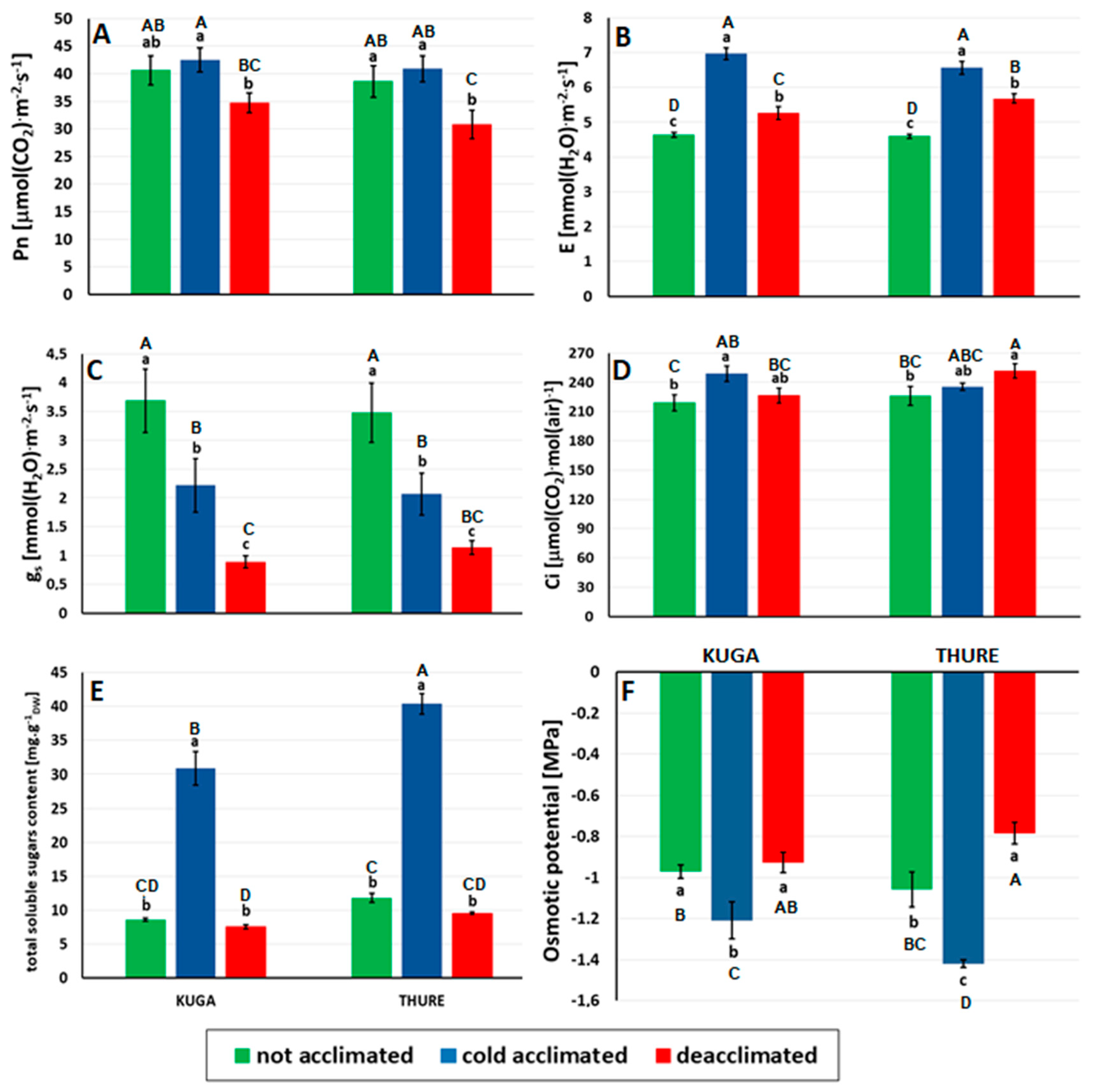
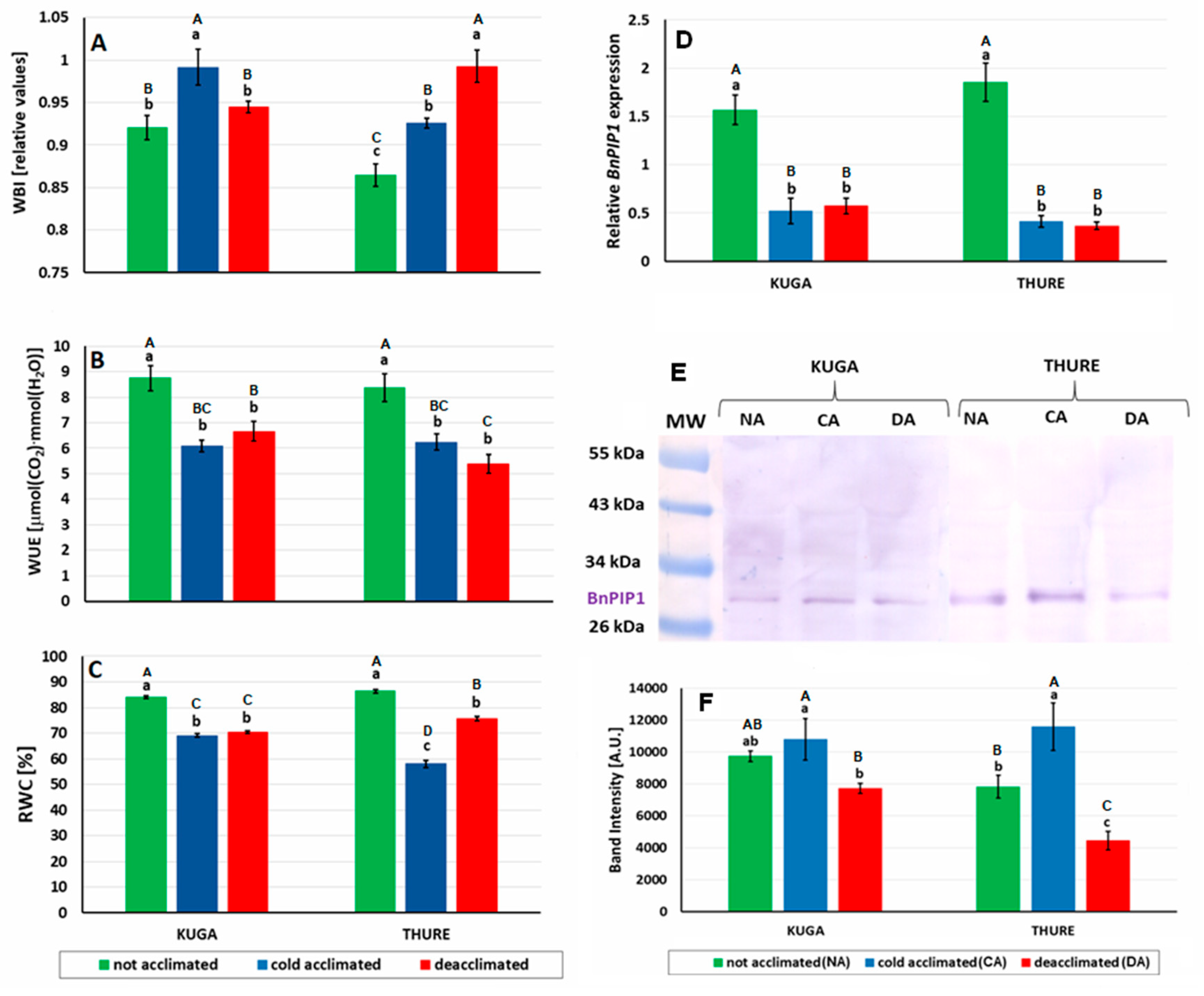
3.2.3. Leaf Gas Exchange
3.2.4. Sugar Accumulation and Osmotic Potential
3.2.5. Leaf Water Relations and Aquaporin Expression
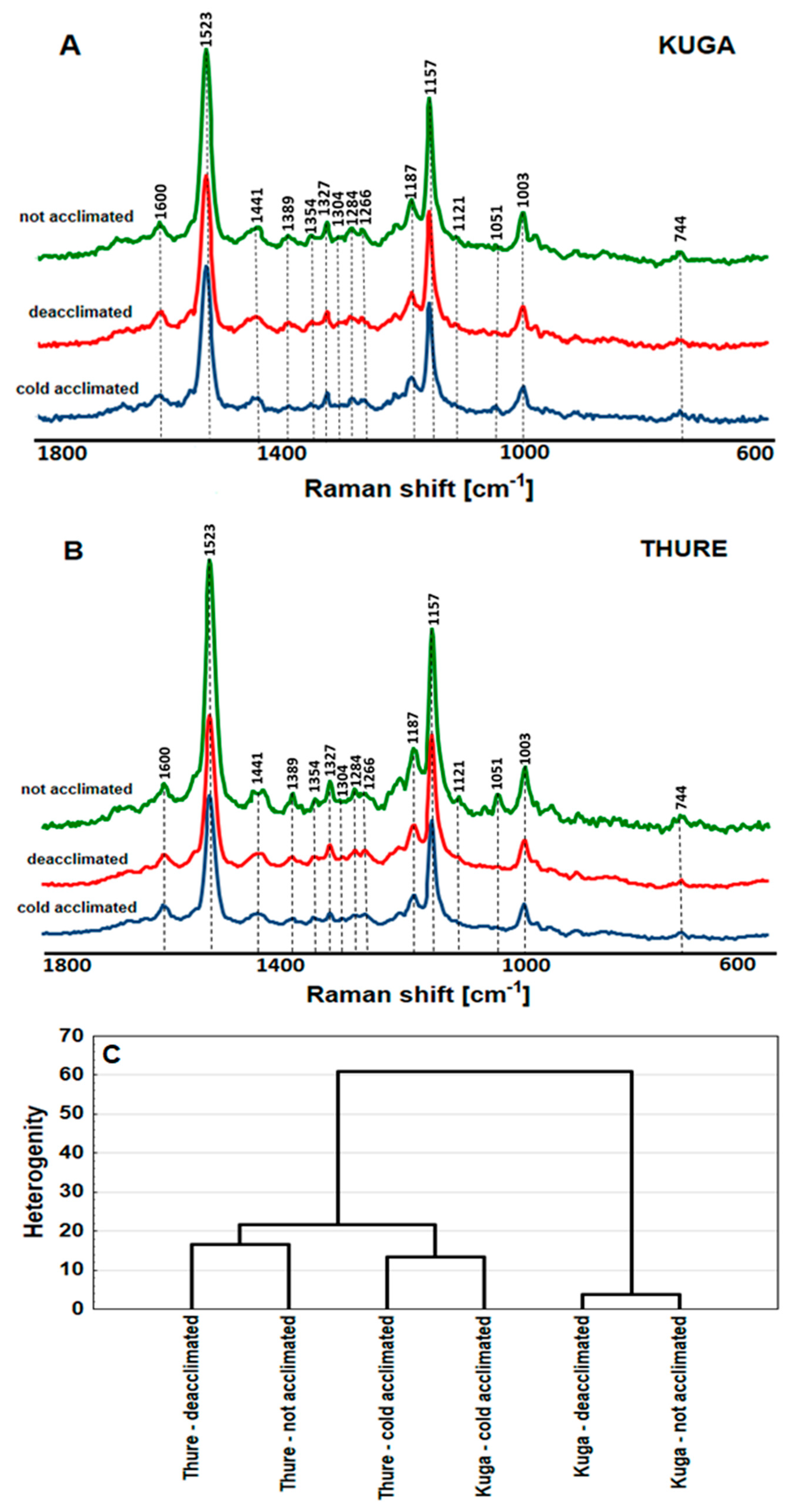
3.2.6. FT-Raman Spectroscopy Measurements
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wałkowski, T. Przezimowanie rzepaku w warunkach klimatycznych Polski—Dobór odmian [Winter survival of oilseed rape in Polish climate]. In Proceedings of the Nauka Doradztwu Rolniczemu, Radzików, Poland, 15–16 September 2016. [Google Scholar]
- Levitt, J. Responses of Plant to Environmental Stress: Water, Radiation, Salt and Other Stresses; Academic Press: New York, NY, USA, 1980. [Google Scholar]
- Li, P.H. Subzero temperature stress physiology of herbaceous plants. Hort. Rev. 1984, 6, 373–416. [Google Scholar]
- McKersie, B.D.; Leshem, Y.Y. Freezing stress. In Stress and Stress Coping in Cultivated Plants; McKersie, B.D., Leshem, Y.Y., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1994; pp. 104–128. [Google Scholar]
- Rapacz, M.; Janowiak, F. Relationship between prehardening photosynthetic activity at cold acclimation temperatures and frost tolerance in Winter Rape Brassica napus var. oleifera. The consequences for the reliability of frost resistance estimation under controlled conditions. J. Agron. Crop Sci. 1999, 182, 57–63. [Google Scholar] [CrossRef]
- Pociecha, E.; Dziurka, M.; Waligórski, P.; Krępski, K.; Janeczko, A. 24-Epibrassinolide pre-treatment modifies cold-induced photosynthetic acclimation mechanisms and phytohormone response of perennial ryegrass in cultivar-dependent manner. J. Plant Growth Regul. 2017, 36, 618–628. [Google Scholar] [CrossRef] [Green Version]
- Janeczko, A.; Dziurka, M.; Pociecha, E. Increased leaf tocopherol and ß-carotene content is associated with the tolerance of winter wheat cultivars to frost. J. Agron. Crop Sci. 2018, 204, 594–602. [Google Scholar] [CrossRef]
- Janeczko, A.; Pociecha, E.; Dziurka, M.; Jurczyk, B.; Libik-Konieczny, M.; Oklestkova, J.; Pilarska, M.; Novak, O.; Filek, M.; Rudolphi-Skórska, E.; et al. Changes in content of steroid regulators during cold hardening of winter wheat—Steroid physiological/biochemical activity and impact on frost tolerance. Plant Physiol. Biochem. 2019, 139, 215–228. [Google Scholar] [CrossRef]
- Cichy, H.; Cichy, A.; Starzycki, M.; Rybiński, W. The effect of plant density on yielding of winter oilseed rape. Biul. Inst. Hod. Aklim. Rośl. 2006, 242, 225. (In Polish) [Google Scholar]
- Lehtonen, I.; Ruosteenoja, K.; Jylhä, K. Projected changes in European extreme precipitation indices on the basis of global and regional climate model ensembles. Int. J. Climatol. 2014, 34, 1208–1222. [Google Scholar] [CrossRef]
- Stupnikova, I.V.; Borovskii, G.B.; Dorofeev, N.V.; Peshkova, A.A.; Voinikov, V.K. Accumulution and disappearance of dehydrins and sugars depending on freezing tolerance of winter wheat plants at different developmental phases. J. Therm. Biol. 2002, 27, 55–60. [Google Scholar] [CrossRef]
- Arora, R.; Rowland, J.J.; Odgen, E.L.; Dhanaraj, A.L. Dehardening kinetics, bud development, and dehydrin metabolism in blueberry cultivars during deacclimation at constant, warm temperatures. J. Am. Soc. Hort. Sci. 2004, 129, 667–674. [Google Scholar] [CrossRef] [Green Version]
- Pagter, M.; Hausman, J.F.; Arora, R. Deacclimation kinetics and carbohydrate changes in stem tissues of Hydrangea in response to an experimental warm spell. Plant Sci. 2011, 180, 140–148. [Google Scholar] [CrossRef]
- In, O.; Berberich, T.; Romdhane, S.; Feierabend, J. Changes in gene expression during dehardening of cold-hardened winter rye (Secale cereal L.) leaves and potential role of a peptide methionine sulfoxide reductase in cold-acclimation. Planta 2005, 220, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Rapacz, M. Cold-deacclimation of oilseed rape (Brassica napus var. oleifera) in response to fluctuating temperatures and photoperiod. Ann. Bot. 2002, 89, 543–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pociecha, E.; Janeczko, A.; Dziurka, M.; Gruszka, D. Disturbances in the Biosynthesis or Signalling of Brassinosteroids That Are Caused by Mutations in the HvDWARF, HvCPD and HvBRI1 Genes Increase the Tolerance of Barley to the Deacclimation Process. J. Plant Growth Regul. 2020. [Google Scholar] [CrossRef]
- Rapacz, M.; Jurczyk, B.; Sasal, M. Deacclimation may be crucial for winter survival of cereals under warming climate. Plant Sci. 2017, 256, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Arora, R.; Taulavuori, K. Increased risk of freeze damage in woody perennials VIS-À-VIS climate change: Importance of deacclimation and dormancy response. Front. Environ. Sci. 2016, 4, 44–50. [Google Scholar] [CrossRef] [Green Version]
- Pagter, M.; Arora, R. Winter survival and deacclimation of perennials under warming climate: Physiological perspectives. Physiol. Plant. 2013, 147, 75–87. [Google Scholar] [CrossRef]
- Hoffman, L.; DaCosta, M.; Bertrand, A.; Castonguay, Y.; Ebdon, J.S. Comparative assessment of metabolic responses to cold acclimation and deacclimation in annual bluegrass and creeping bentgrass. Environ. Exp. Bot. 2014, 106, 197–206. [Google Scholar] [CrossRef]
- Rapacz, M.; Ergon, A.; Hoglind, M.; Jorgensen, M.; Jurczyk, B.; Ostrem, L.; Rognli, O.A.; Tronsmo, A.M. Overwintering of herbaceous plants in a changing climate. Still more questions than answers. Plant Sci. 2014, 225, 34–44. [Google Scholar] [CrossRef]
- Jørgensen, M.; Torp, T.; Mølmann, J.A.B. Impact of waterlogging and temperature on autumn growth, hardening and freezing tolerance of timothy (Phleum pratense). J. Agron. Crop Sci. 2020, 206, 242–251. [Google Scholar] [CrossRef] [Green Version]
- Pagter, M.; Alpers, J.; Erban, A.; Kopka, J.; Zuther, E.; Hincha, D.K. Rapid transcriptional and metabolic regulation of the deacclimation process in cold acclimated Arabidopsis thaliana. BMC Genom. 2017, 18, 731–747. [Google Scholar] [CrossRef] [Green Version]
- Zuther, E.; Juszczak, I.; Lee, Y.P.; Baier, M.; Hincha, D.K. Time-dependent deacclimation after cold acclimation in Arabidopsis thaliana accessions. Sci. Rep. 2015, 5, 12199–12208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juszczak, I.; Cvetkovic, J.; Zuther, E.; Hincha, D.K.; Baier, M. Natural Variation of Cold Deacclimation Correlates with Variation of Cold-Acclimation of the Plastid Antioxidant System in Arabidopsis thaliana Accessions. Front. Plant Sci. 2016, 7, 305–321. [Google Scholar] [CrossRef] [Green Version]
- Skoczowski, A.; Janeczko, A.; Gullner, G.; Tóbias, I.; Kornas, A.; Barna, B. Response of brassinosteroid-treated oilseed rape cotyledons to infection with the wild type and HR-mutant of Pseudomonas syringae or with P. fluorescence. J. Therm. Anal. Calorim. 2011, 104, 131–139. [Google Scholar] [CrossRef]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. The fluorescence transient as a tool to characterize and screen photosynthetic samples. In Probing Photosynthesis: Mechanism, Regulation and Adaptation; Yunus, M., Pathre, U., Mohanty, P., Eds.; Taylor and Francis: London, UK, 2000; pp. 443–480. [Google Scholar]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. In Current Protocols in Food Analytical Chemistry; Wrolstad, R.E., Acree, T.E., Decker, E.A., Penner, M.H., Reid, D.S., Schwartz, S.J., Shoemaker, C.F., Smith, D., Sporns, P., Eds.; Wiley: New York, NY, USA, 2001. [Google Scholar]
- Gitelson, A.A.; Merzylak, M.N.; Chivkunova, O.B. Optical properties and nondestructive estimation of anthocyanin content in plant leaves. Photochem. Photobiol. 2001, 71, 38–45. [Google Scholar] [CrossRef]
- Merzlyak, M.N.; Solovchenko, A.E.; Smagin, A.I.; Gitelson, A.A. Apple flavonols during fruit adaptation to solar radiation: Spectral features and technique for non-destructive assessment. J. Plant Physiol. 2005, 162, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Peñuelas, J.; Filella, I.; Biel, C.; Serrano, L.; Savé, R. The reflectance at the 950–970 nm region as an indicator of plant water status. Int. J. Remote Sens. 1993, 14, 1887–1905. [Google Scholar] [CrossRef]
- Janowiak, F.; Markowski, A. Changes in leaf water relations and injuries in maize seedlings induced by different chilling conditions. J. Agron. Crop Sci. 1994, 172, 19–28. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.; Hamilton, J.K.; Rebers, P.A.; Smith, F. A colorimetric method for the determination of sugars. Nature 1951, 168, 167–168. [Google Scholar] [CrossRef]
- Bach, A.; Kapczyńska, A.; Dziurka, K.; Dziurka, M. Phenolic compounds and carbohydrates in relation to bulb formation in Lachenalia “Ronina” and “Rupert” in vitro cultures under different lighting environments. Sci. Horti. 2015, 188, 23–29. [Google Scholar] [CrossRef]
- Jurczyk, B.; Rapacz, M.; Budzisz, K.; Barcik, W.; Sasal, M. The effects of cold, light and time of day during low-temperature shift on the expression of CBF6, FpCor14b and LOS2 in Festuca pratensis. Plant Sci. 2012, 183, 143–148. [Google Scholar] [CrossRef]
- Ma, L.; Wu, J.; Qi, W.; Coulter, J.A.; Fang, Y.; Li, X.; Liu, L.; Jin, J.; Niu, Z.; Yue, J.; et al. Screening and verification of reference genes for analysis of gene expression in winter rapeseed (Brassica rapa L.) under abiotic stress. PLoS ONE 2020, 15, e0236577. [Google Scholar] [CrossRef]
- Sadura, I.; Libik-Konieczny, M.; Jurczyk, B.; Gruszka, D.; Janeczko, A. Plasma membrane ATPase and the aquaporin HvPIP1 in barley brassinosteroid mutants acclimated to high and low temperature. J. Plant Physiol. 2020, 244, 153090–153097. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Schulz, H.; Baranska, M. Identification and Quantification of Valuable Plant Substances by IR and Raman Spectroscopy. Vib. Spectrosc. 2007, 43, 13–25. [Google Scholar] [CrossRef]
- Schrader, B.; Klump, H.H.; Schenzel, K.; Schulz, H. Non-destructive NIR FT Raman analysis of plants. J. Mol. Struct. 1999, 509, 201–212. [Google Scholar] [CrossRef]
- Edwards, H.G.M.; Moody, C.D.; Villar, S.E.J.; Wynn-Williams, D.D. Raman spectroscopic detection of key biomarkers of cyanobacteria and lichen symbiosis in extreme Antarctic habitats: Evaluation for Mars lander missions. Icarus 2005, 174, 560–571. [Google Scholar] [CrossRef]
- Kula, M.; Rys, M.; Saja, D.; Tys, J.; Skoczowski, A. Far-red dependent changes in the chemical composition of Spirulina platensis. Eng. Life Sci. 2016, 16, 777–785. [Google Scholar] [CrossRef]
- Moravcová, S.; Tůma, J.; Dučaiová, Z.K.; Waligórski, P.; Kula, M.; Saja, D.; Słomka, A.; Bąba, W.; Libik-Konieczny, M. Influence of salicylic acid pretreatment on seeds germination and some defence mechanisms of Zea mays plants under copper stress. Plant Physiol. Biochem. 2018, 122, 19–30. [Google Scholar] [CrossRef]
- Rapacz, M. Regulation of frost resistance during cold de-acclimation and re-acclimation in oilseed rape. A possible role of PSII redox state. Physiol. Plant. 2002, 115, 236–243. [Google Scholar] [CrossRef]
- Pomeroy, M.K.; Andrews, C.J.; Fedak, G. Cold hardening and dehardening responses in winter wheat and winter barley. Can. J. Plant Sci. 1975, 55, 529–535. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Han, Q.; Mukai, Y. Cold acclimation and photoinhibition of photosynthesis accompanied by needle color changes in Cryptomeria japonica during the winter. J. For. Res. 1999, 4, 229–234. [Google Scholar] [CrossRef]
- Janeczko, A.; Gullner, G.; Skoczowski, A.; Dubert, F.; Barna, B. Effects of brassinosteroid infiltration prior to cold treatment on ion leakage and pigment contents in rape leaves. Biol. Plant. 2007, 51, 355–358. [Google Scholar] [CrossRef]
- Hajihashemi, S.; Noedoost, F.; Geuns, J.M.C.; Djalovic, I.; Siddique, K.H.M. Effect of Cold Stress on Photosynthetic Traits, Carbohydrates, Morphology, and Anatomy in Nine Cultivars of Stevia rebaudiana. Front Plant Sci. 2018, 9, 1430–1441. [Google Scholar] [CrossRef] [Green Version]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Bioch. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Rapacz, M.; Gąsior, D.; Zwierzykowski, Z.; Leśniewska-Bocianowska, A.; Humphreys, M.W.; Gay, A.P. Changes in cold tolerance and the mechanisms of acclimatation of photosystem II to cold hardening generated by anther culture of Festuca pratensis × Lolium multiflorum cultivars. New Phytol. 2004, 161, 105–114. [Google Scholar] [CrossRef]
- Ensminger, I.; Busch, F.; Huner, N.P.A. Photostasis and cold acclimation: Sensing low temperature through photosynthesis. Physiol. Plant. 2006, 126, 28–44. [Google Scholar] [CrossRef]
- Rapacz, M. Chlorophyll a fluorescence transient during freezing and recovery in winter wheat. Photosynthetica 2007, 5, 409–418. [Google Scholar] [CrossRef]
- Maciejewska, U.; Tomczyk, J.; Kacperska, A. Effects of cold on CO2 exchange in winter rape leaves. Physiol. Plant. 1984, 62, 315–320. [Google Scholar] [CrossRef]
- Solovchenko, A.E.; Chivkunova, O.B.; Gitelson, A.A.; Merzylak, M.N. Non-Destructive Estimation Pigment Content Ripening Quality and Damage in Apple Fruit with Spectral Reflectance in the Visible Range. Fresh Prod. 2010, 4, 91–102. [Google Scholar]
- Gitelson, A.; Solovchenko, A. Non-invasive quantification of foliar pigments: Possibilities and limitations of reflectance- and absorbance-based approaches. J. Photochem. Photobiol. 2018, 178, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Chalker-Scott, L. Environmental significance of anthocyanins in plant stress responses. Photochem. Photobiol. 1999, 70, 1–9. [Google Scholar] [CrossRef]
- Merzylak, M.N.; Chivkunova, O.B. Light stress induced pigment changes and evidence for anthocyanin photoprotection in apple fruit. Photochem. Photobiol. 2000, 55, 154–162. [Google Scholar]
- Oliwa, J.; Skoczowski, A. Different response of photosynthetic apparatus to high-light stress in sporotrophophyll and nest leaves of Platycerium bifurcatum. Photosynthetica 2019, 57, 147–159. [Google Scholar] [CrossRef]
- Anderson, R.; Ryser, P. Early autumn senescence in red maple (Acer rubrum L.) is associated with high leaf anthocyanin content. Plants 2015, 4, 505–522. [Google Scholar] [CrossRef] [Green Version]
- Pietrini, F.; Iannelli, M.A.; Massacci, A. Anthocyanin accumulation in the illuminated surface of maize leaves enhances protection from photo-inhibitory risks at low temperature, without further limitation to photosynthesis. Plant Cell Environ. 2002, 25, 1251–1259. [Google Scholar] [CrossRef]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef]
- Jouve, L.; Jacques, D.; Douglas, G.C.; Hoffmann, L.; Hausman, J.-F. Biochemical characterization of early and late bud flushing in common ash (Fraxinus excelsior L.). Plant Sci. 2007, 172, 962–969. [Google Scholar] [CrossRef]
- Patton, A.J.; Cunningham, S.M.; Volenec, J.J.; Reicher, Z.J. Differences in freeze tolerance of zoysiagrass: II. Carbohydrate and proline accumulation. Crop Sci. 2007, 47, 2170–2181. [Google Scholar] [CrossRef]
- Hoffman, L.; DaCosta, M.; Ebdon, J.S.; Watkins, E. Physiological changes during cold acclimation of perennial ryegrass accessions differing in freeze tolerance. Crop Sci. 2010, 50, 1037–1047. [Google Scholar] [CrossRef]
- Pagter, M.; Jensen, C.R.; Petersen, K.K.; Liu, F.; Arora, R. Changes in carbohydrates, ABA and bark proteins during seasonal cold acclimation and deacclimation in Hydrangea species differing in cold hardiness. Physiol. Plant. 2008, 134, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhou, H.; Cao, S.; Xia, Y.-P.; Arora, R. Comparative Physiology of Natural Deacclimation in Ten Azalea Cultivars. HortScience 2017, 52, 1451–1457. [Google Scholar] [CrossRef] [Green Version]
- Thomashow, M.F. Plant cold acclimation: Freezing tolerance genes and regulatory mechanisms. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 571–599. [Google Scholar] [CrossRef] [Green Version]
- Shin, H.; Oh, Y.; Kim, D. Differences in cold hardiness, carbohydrates, dehydrins and related gene expressions under an experimental deacclimation and reacclimation in Prunus persica. Physiol. Plant. 2015, 154, 485–499. [Google Scholar] [CrossRef]
- Ouyang, L.; Leus, L.; De Keyser, E.; Van Labeke, M.-C. Cold acclimation and deacclimation of two garden rose cultivars under controlled daylength and temperature. Front. Plant Sci. 2020, 11, 327–341. [Google Scholar] [CrossRef]
- Sasaki, H.; Ichimura, K.; Oda, M. Changes in Sugar Content during Cold Acclimation and Deacclimation of Cabbage Seedlings. Ann. Bot. 1996, 78, 365–369. [Google Scholar] [CrossRef] [Green Version]
- Guy, C.L. Cold Acclimation and Freezing Stress Tolerance: Role of Protein Metabolism. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1990, 41, 187–223. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef] [Green Version]
- Guy, C.L. Freezing tolerance of plants: Current understanding and selected emerging concepts. Can. J. Biol. 2003, 81, 1216–1223. [Google Scholar] [CrossRef]
- Li, P.H.; Palta, J.P. Frost hardening and freezing stress in tuber-bearing solanum species. In Plant Cold Hardiness and Freezing Stress; Li, P.H., Sakai, A., Eds.; Academic Press: New York, NY, USA, 1978; pp. 49–71. [Google Scholar]
- Araus, J.L.; Slafer, G.A.; Royo, C.; Serret, M.D. Breeding for Yield Potential and Stress Adaptation in Cereals. Crit. Rev. Plant Sci. 2001, 27, 377–412. [Google Scholar] [CrossRef]
- Peñuelas, J.; Piñol, J.; Ogaya, R.; Filella, I. Estimation of plant water concentration by the reflectance water index WI (R900/R970). Int. J. Remote Sens. 1997, 18, 2869–2875. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Dold, C. Water Use Efficiency: Advances and Challenges in a Changing Climate. Front. Plant Sci. 2019, 10, 61–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johansson, I.; Karlsson, M.; Johanson, U.; Larsson, C.; Kjellbom, P. The role of aquaporins in cellular and whole plant water balance. Biochim. Biophys. Acta Biomembr. 2000, 1465, 324–342. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Arora, R. Understanding the cellular mechanism of recovery from freeze–thaw injury in spinach: Possible role of aquaporins, heat shock proteins, dehydrin and antioxidant system. Physiol. Plant. 2014, 150, 374–387. [Google Scholar] [CrossRef]
- Gerbeau, P.; Amodeo, G.; Henzler, T.; Santoni, V.; Ripoche, P.; Maurel, C. The water permeability of Arabidopsis plasma membrane is regulated by divalent cations and pH. Plant J. 2002, 30, 71–81. [Google Scholar] [CrossRef]
- Yu, X.; Peng, Y.H.; Zhang, M.H.; Shao, Y.J.; Su, W.A.; Tang, Z.C. Water relations and an expression analysis of plasma membrane intrinsic proteins in sensitive and tolerant rice during chilling and recovery. Cell Res. 2006, 16, 599–608. [Google Scholar] [CrossRef] [Green Version]
- Pawłowicz, I.; Rapacz, M.; Perlikowski, D.; Gondek, K.; Kosmala, A. Abiotic stresses influence the transcript abundance of PIP and TIP aquaporins in Festuca species. J. Appl. Genet. 2017, 58, 421–435. [Google Scholar] [CrossRef] [Green Version]
- Aroca, R.; Amodeo, G.; Fernandez-Illescas, S.; Herman, E.M.; Chaumont, F.; Chrispeels, M.J. The Role of Aquaporins and Membrane Damage in Chilling and Hydrogen Peroxide Induced Changes in the Hydraulic Conductance of Maize Roots. Plant Physiol. 2005, 137, 341–353. [Google Scholar] [CrossRef] [Green Version]
- Miki, Y.; Takahashi, D.; Kawamura, Y.; Uemura, M. Temporal proteomics of Arabidopsis plasma membrane during cold- and de-acclimation. Proteomics 2019, 197, 71–81. [Google Scholar] [CrossRef]
- Withnall, R.; Chowdhry, B.Z.; Silver, J.; Edwards, H.G.; de Oliveira, L.F. Raman spectra of carotenoids in natural products. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2003, 59, 2207–2212. [Google Scholar] [CrossRef]
- Baranska, M.; Schulz, H.; Joubert, E.; Manley, M. In Situ Flavonoid Analysis by FT-Raman Spectroscopy: Identification, Distribution, and Quantification of Aspalathin in Green Rooibos (Aspalathus Linearis). Anal. Chem. 2006, 78, 7716–7721. [Google Scholar] [CrossRef] [PubMed]
- Baranska, M.; Baranski, R.; Schulz, H.; Nothnagel, T. Tissue-specific accumulation of carotenoids in carrot roots. Planta 2006, 224, 1028–1037. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, E.H.; Liakopoulou-Kyriakides, M.; Christofilos, D.; Arvanitidis, I.; Kourouklis, G. Raman Spectroscopy for Intracellular Monitoring of Carotenoid in Blakeslea Trispora. Appl. Biochem. Biotechnol. 2009, 159, 478–487. [Google Scholar] [CrossRef]
- Rys, M.; Szaleniec, M.; Skoczowski, A.; Stawoska, I.; Janeczko, A. FT-Raman spectroscopy as a tool in evaluation the response of plants to drought stress. Open Chem. 2015, 13, 1091–1100. [Google Scholar] [CrossRef]
- Saja, D.; Rys, M.; Stawoska, I.; Skoczowski, A. Metabolic response of cornflower (Centaurea cyanus L.) exposed to tribenuron-methyl: One of the active substances of sulfonylurea herbicides. Acta Physiol. Plant. 2016, 38, 168–180. [Google Scholar] [CrossRef] [Green Version]
- Baranski, R.; Baranska, M.; Schulz, H. Changes in carotenoid content and distribution in living plant tissue can be observed and mapped in situ using NIR-FT-Raman spectroscopy. Planta 2005, 222, 448–457. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |

| Gene Name | GenBank ID | Forward Primer | Reverse Primer | TaqMan MGB Probe |
|---|---|---|---|---|
| PIP1 | AF118382.1 | TGTCGTTGGTTAGAGCCATATTGT | CTTTGACGAACCCAACTCCACATA | FAM-TCGCACCCAAACACTG-MGB |
| Actin | AF111812.1 | ACTCTGGTGATGGTGTGTCTCA | GCGTGAGGAAGAGCATAACCTT | FAM-CCGTGCCGATCTACG-MGB |
| Parametrs | cv. KUGA | cv. THURE | ||||
|---|---|---|---|---|---|---|
| Not Acclimated | Cold Acclimated | Deacclimated | Not Acclimated | Cold Acclimated | Deacclimated | |
| Fv/Fm | 0.823 b (B) | 0.810 c (C) (−1.6) | 0.840 a (A) (+2.1) | 0.829 b (B) | 0.810 c (C) (−2.3) | 0.838 a (A) (+1.1) |
| ABS/RC | 1.155 a (A) | 1.168 a (A) (+1.1) | 1.034 b (B) (−10.5) | 1.157 a (A) | 1.007 b (B) (−13.0) | 1.085 ab (AB) (−6.2) |
| DIo/RC | 0.204 a (B) | 0.223 a (A) (+9.3) | 0.166 b (D) (−18.6) | 0.198 a (B) | 0.192 a (BC) (−3.0) | 0.176 a (CD) (−11.1) |
| TRo/RC | 0.951 a (A) | 0.945 a (A) (−0.6) | 0.868 b (BC) (−8.7) | 0.958 a (A) | 0.815 b (C) (−14.9) | 0.909 a (AB) (−5.1) |
| ETo/RC | 0.609 a (AB) | 0.590 a (B) (−3.1) | 0.610 a (AB) (+0.1) | 0.625 a (A) | 0.503 b (C) (−19.5) | 0.634 a (A) (+1.4) |
| ABS/CSm | 1621.8 a (A) | 1674.2 a (A) (+3.2) | 857.9 b (B) (−47.1) | 1638.5 a (A) | 1638.3 a (A) (−0.01) | 844.3 b (B) (−48.5) |
| DIo/CSm | 285.9 b (B) | 318.5 a (A) (+11.4) | 137.2 c (C) (−52.0) | 279.9 b (B) | 309.5 a (A) (+10.6) | 136.4 c (C) (−51.3) |
| TRo/CSm | 1335.8 a (A) | 1355.7 a (A) (+1.5) | 720.7 b (B) (−46.0) | 1358.6 a (A) | 1328.7 a (A) (−2.2) | 707.9 b (B) (−47.9) |
| ETo/CSm | 857.0 a (AB) | 848.4 a (AB) (−1.0) | 508.9 b (C) (−40.6) | 889.5 a (A) | 823.4 b (B) (−7.4) | 496.0 c (C) (−44.2) |
| RC/CSm | 1404.2 a (B) | 1433.4 a (A) (+2.1) | 829.7 b (C) (−40.9) | 1416.2 b (B) | 1626.9 a (A) (+14.9) | 778.2 c (C) (−45.1) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rys, M.; Pociecha, E.; Oliwa, J.; Ostrowska, A.; Jurczyk, B.; Saja, D.; Janeczko, A. Deacclimation of Winter Oilseed Rape—Insight into Physiological Changes. Agronomy 2020, 10, 1565. https://doi.org/10.3390/agronomy10101565
Rys M, Pociecha E, Oliwa J, Ostrowska A, Jurczyk B, Saja D, Janeczko A. Deacclimation of Winter Oilseed Rape—Insight into Physiological Changes. Agronomy. 2020; 10(10):1565. https://doi.org/10.3390/agronomy10101565
Chicago/Turabian StyleRys, Magdalena, Ewa Pociecha, Jakub Oliwa, Agnieszka Ostrowska, Barbara Jurczyk, Diana Saja, and Anna Janeczko. 2020. "Deacclimation of Winter Oilseed Rape—Insight into Physiological Changes" Agronomy 10, no. 10: 1565. https://doi.org/10.3390/agronomy10101565
APA StyleRys, M., Pociecha, E., Oliwa, J., Ostrowska, A., Jurczyk, B., Saja, D., & Janeczko, A. (2020). Deacclimation of Winter Oilseed Rape—Insight into Physiological Changes. Agronomy, 10(10), 1565. https://doi.org/10.3390/agronomy10101565







