Parameter Selection for the Evaluation of Compost Quality
Abstract
1. Introduction
2. Materials and Methods
2.1. Characterisation of the Raw Materials
2.2. Pile Composition
2.3. Sampling and Variable Analysis
2.4. Data Processing and Analysis
3. Results
3.1. Analysis of Parameters during the Process
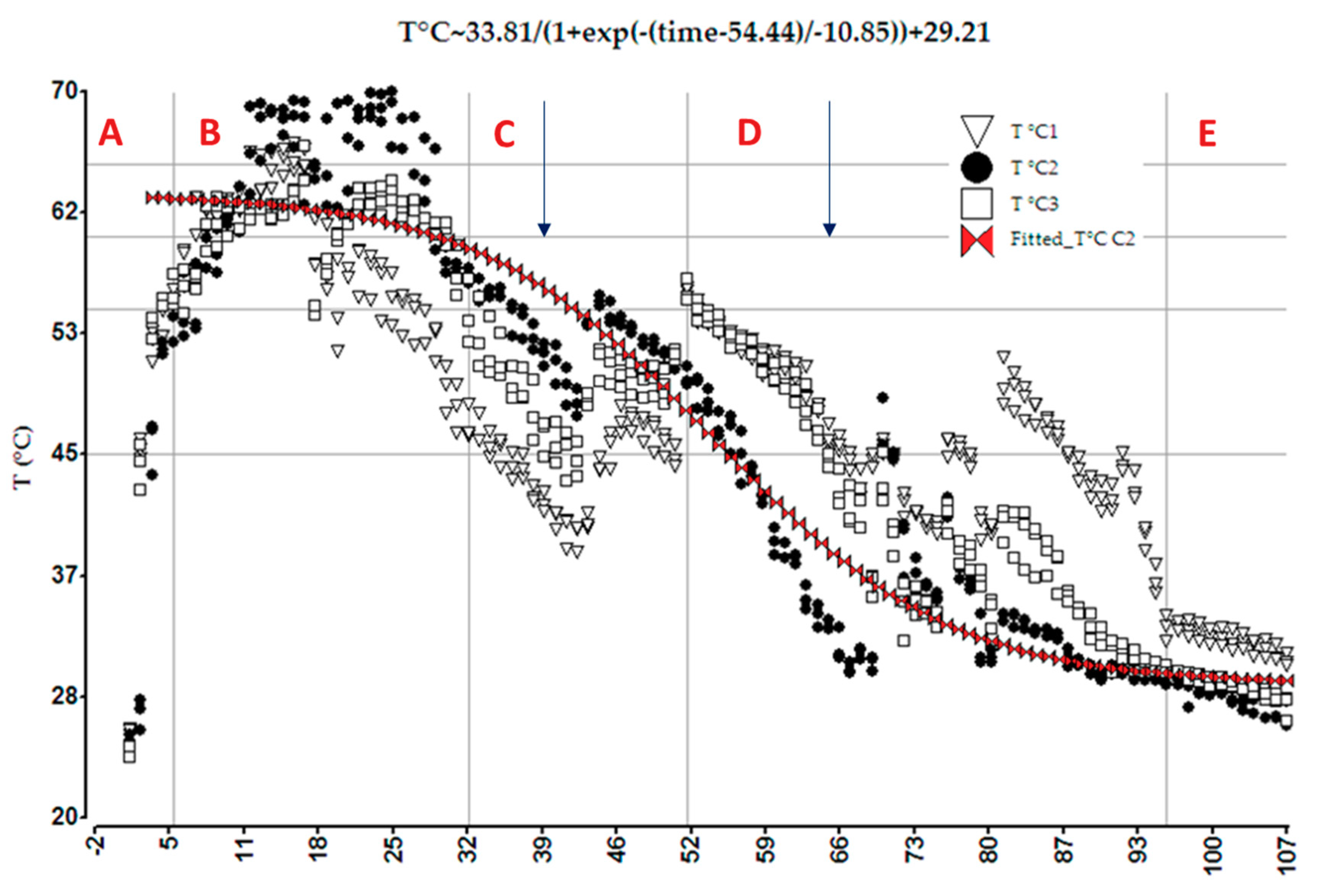
3.1.1. Evolution of Temperature
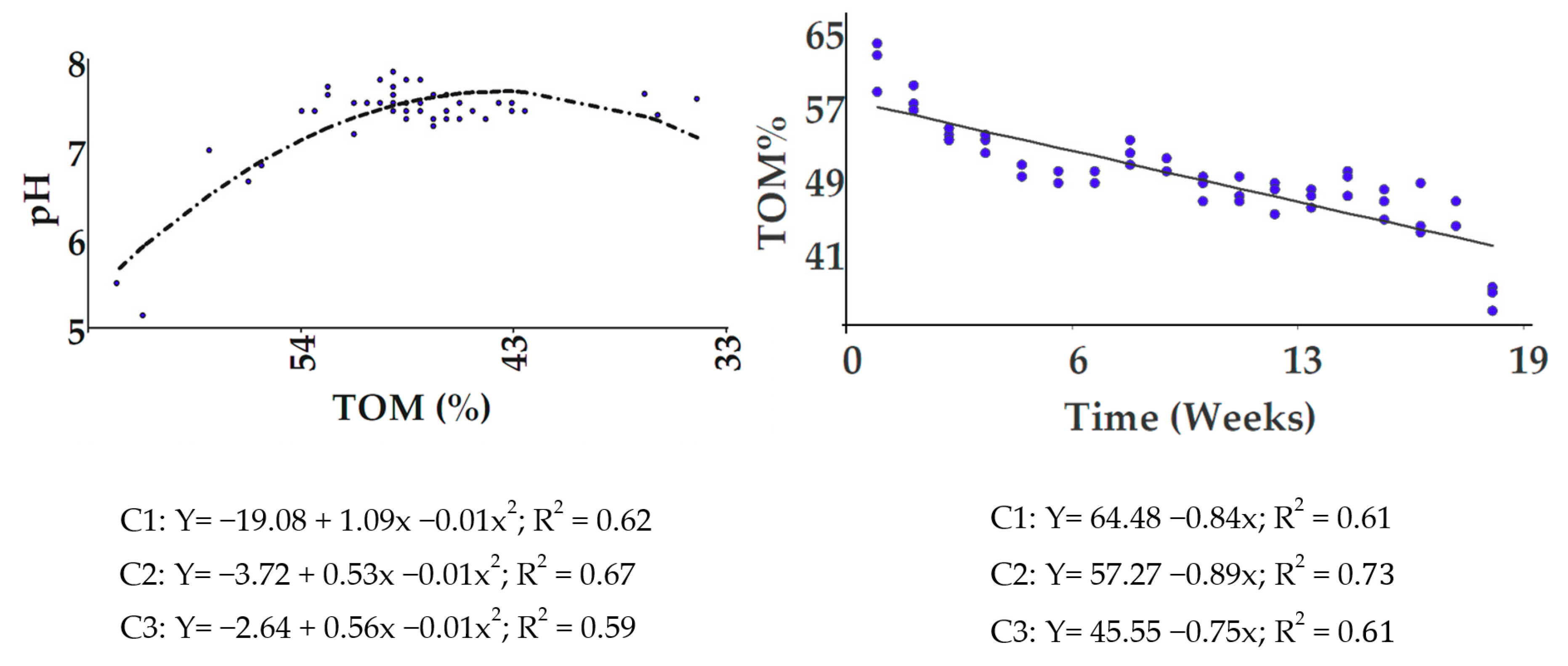
3.1.2. Decomposition of TOM and Evolution of pH Values
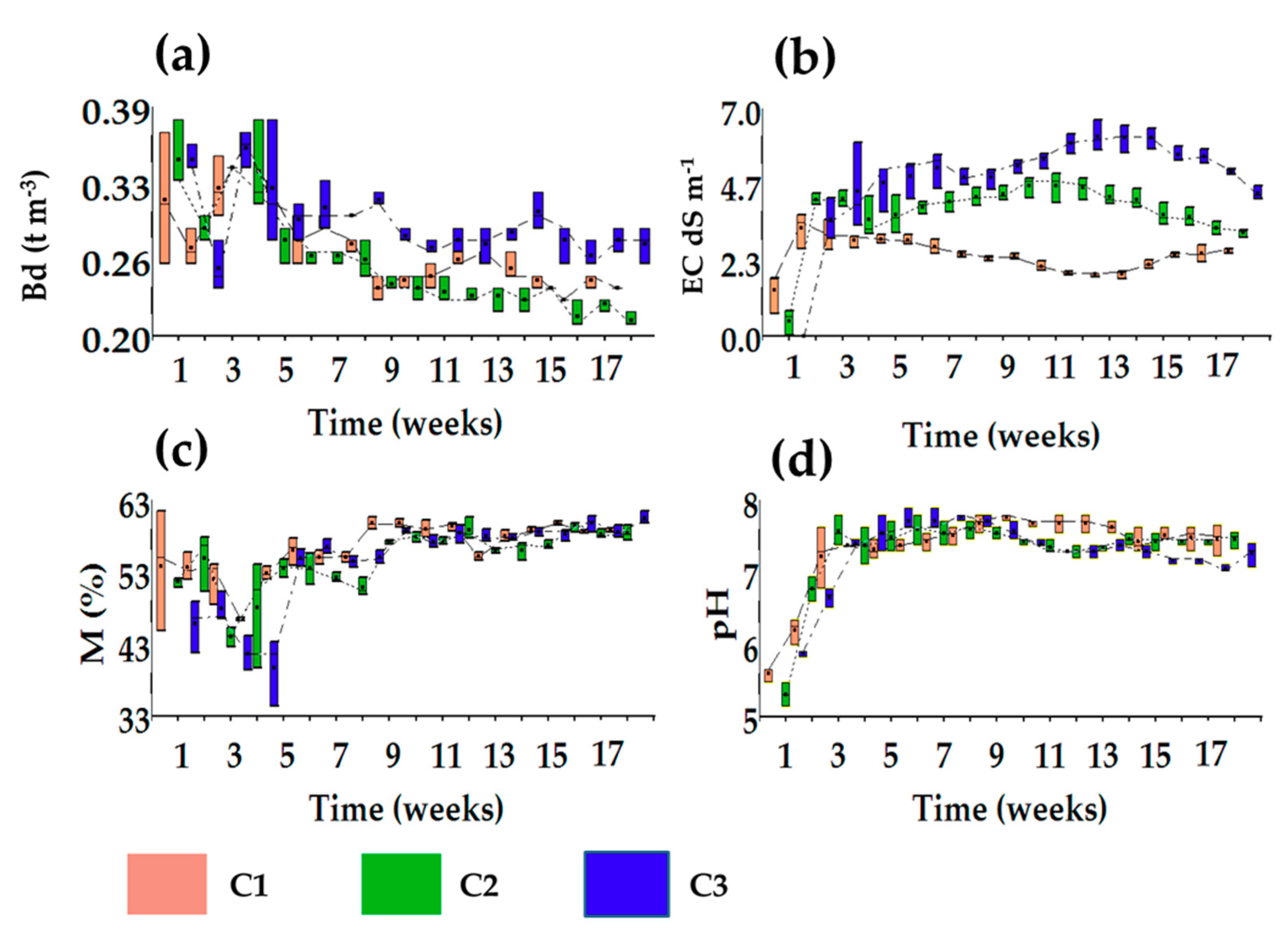
3.1.3. Evolution in Moisture Content
3.1.4. Evolution of Bulk Density
3.1.5. Evolution of Electrical Conductivity Values
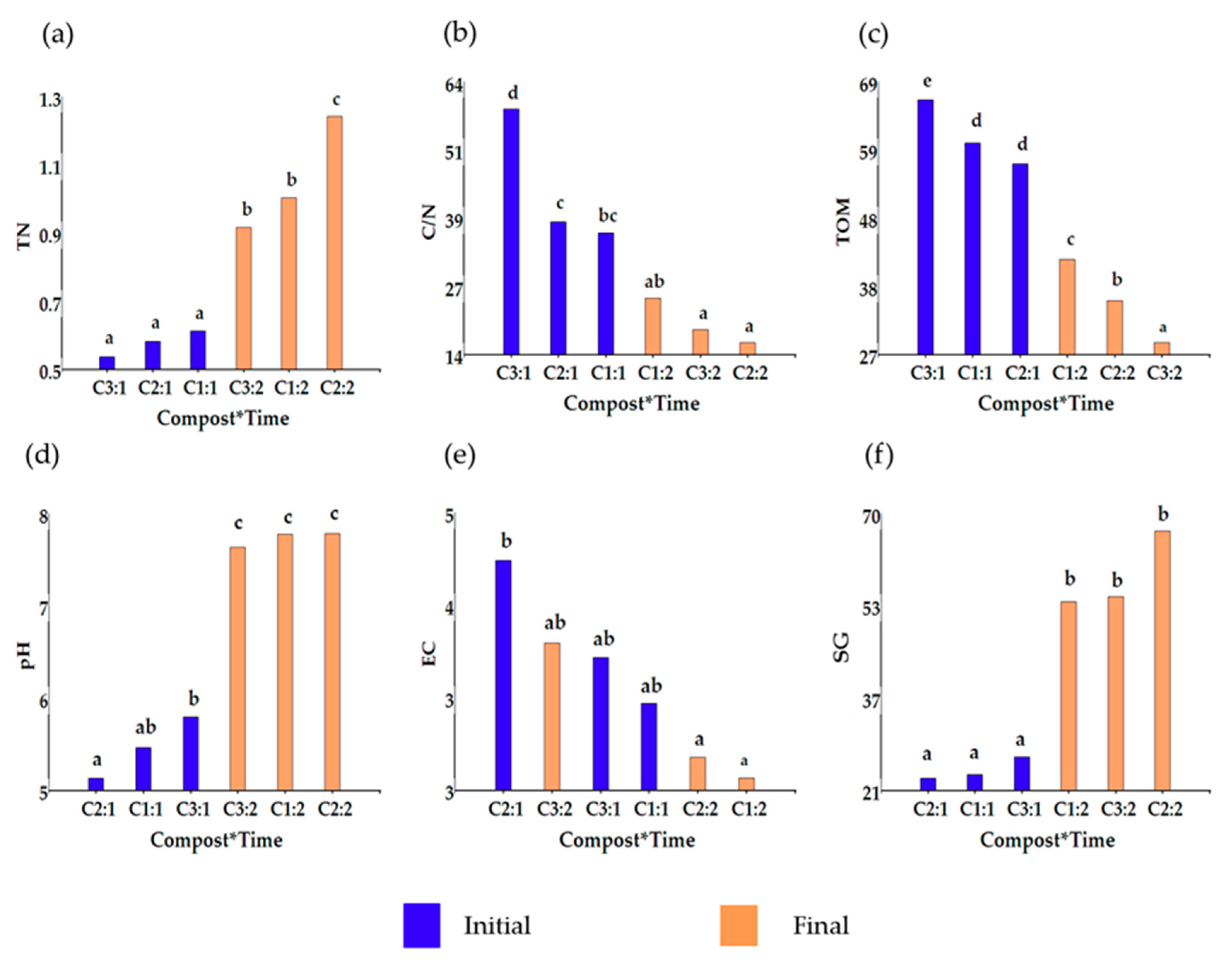
3.2. Characteristics of the Final Products
3.2.1. Resistant Organic and Stability Grade
3.2.2. Biological Response
3.3. Identification of Critical Process Parameters
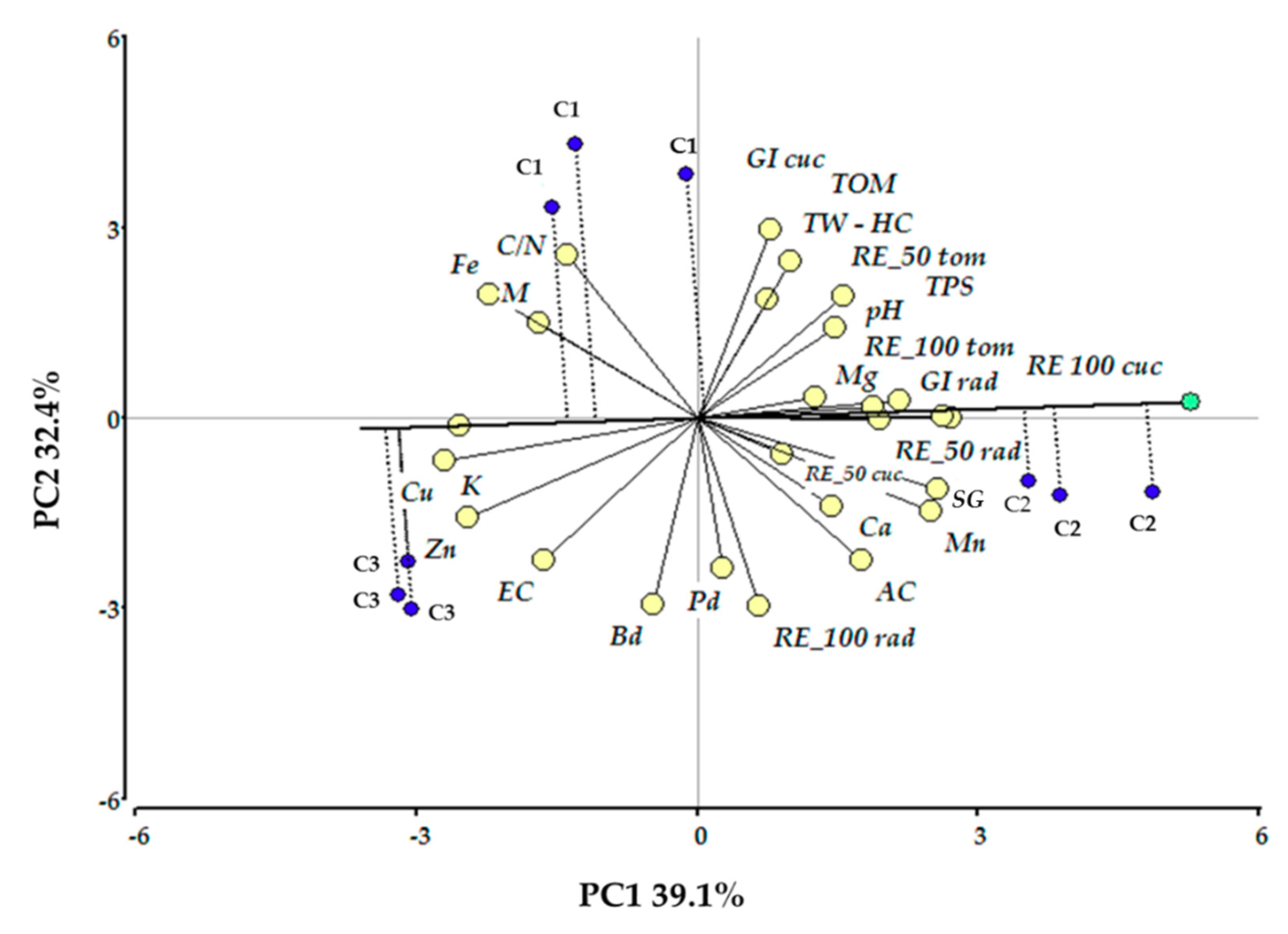
3.3.1. Principal Component Analysis
3.3.2. Evaluation of the Biological Response to Measured Parameters of the Composted Mixtures
4. Discussion
4.1. Evolution of Temperature and Moisture
4.2. Characteristics of the Final Products
4.3. Compost Quality Proposal
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sztern, D.; Pravia, M. Manual Para la Elaboracion de Compost Bases Conceptuales y Procedimientos; Organizacion Panamericana de la Salud: Washington, DC, USA, 1999; p. 69. [Google Scholar]
- Peña, H.P.; Arias, K.; Santos, M.; Ramírez, B.; Sulbarán, J. Evaluación de la calidad química, física y biológica de tres compost producidos a partir de residuos agroindustriales. Rev. Cient. UNET 2018, 32, 11. [Google Scholar]
- Campitelli, P.; Ceppi, S. Chemical, physical and biological compost and vermicompost characterization: A chemometric study. Chemom. Intell. Lab. 2008, 90, 64–71. [Google Scholar] [CrossRef]
- Bustamante, M.; Suárez-Estrella, F.; Torrecillas, C.; Paredes, C.; Moral, R.; Moreno, J. Use of chemometrics in the chemical and microbiological characterization of composts from agroindustrial wastes. Bioresour. Technol. 2010, 101, 4068–4074. [Google Scholar] [CrossRef] [PubMed]
- Cabeza, M.; Ormeño, M.; Muñoz, L. Generación de propuesta para la estandarización y normalización de la producción y utilización de enmiendas y abonos orgánicos en Venezuela. In Taller de Abonos Orgánicos y Enmiendas; CIDIAT: Mérida, Venezuela, 2012. [Google Scholar]
- Haug, R. The Practical Handbook of Compost Engineering, 1st ed.; CRC Press: Boca Raton, FL, USA, 1993; ISBN 978-0-203-73623-4. [Google Scholar]
- Bueno, P.; Diaz, M.; Cabrera, F. Factores que afectan el proceso de compostaje. In Compostaje; Casco, J.M., Herrero, R.M., Eds.; Mundi-Prensa: Madrid, Spain, 2008; p. 570. [Google Scholar]
- Day, M.; Shaw, K. Biological, chemical and physical processes of composting. In Compost Utilization in Horticultural Cropping Systems; Informa UK Limited: London, UK, 2001; p. 414. [Google Scholar]
- Miyatake, F.; Iwabuchi, K. Effect of compost temperature on oxygen uptake rate, specific growth rate and enzymatic activity of microorganisms in dairy cattle manure. Bioresour. Technol. 2006, 97, 961–965. [Google Scholar] [CrossRef]
- Liang, C.; Das, K.C.; McClendon, R.W. The influence of temperature and moisture contents regimes on the aerobic microbial activity of a biosolids composting blend. Bioresour. Technol. 2003, 86, 131–137. [Google Scholar] [CrossRef]
- Tognetti, C.; Laos, F.; Mazzarino, M.J.; Hernandez, M.T. Composting vs. vermicomposting: A comparison of end product quality. Compos. Sci. Util. 2005, 13, 6–13. [Google Scholar] [CrossRef]
- Desalegn, G.; Binner, E.; Lechner, P. Humification and Degradability Evaluation During Composting of Horse Manure and Biowaste. Compos. Sci. Util. 2008, 16, 90–98. [Google Scholar] [CrossRef]
- Tognetti, C.; Mazzarino, M.J.; Laos, F. Improving the quality of municipal organic waste compost. Bioresour. Technol. 2007, 98, 1067–1076. [Google Scholar] [CrossRef]
- Campitelli, P. Calidad de Compost y Vermicompuestos para su Uso como Enmiendas Orgánicas en Suelos Agrícolas. Ph.D. Thesis, Universidad Nacional de Córdoba, Córdoba, Argentina, 2010. [Google Scholar]
- Thompson, W.H. Biological assays. In Test Methods for the Examination of Composting and Compost; Thompson, W.H., Ed.; U.S. Composting Council: Raleigh, NC, USA, 2001; pp. 1177–1183. [Google Scholar]
- Zucconi, F.; Pera, A.; Forte, M. Evaluating toxicity of immature compost. BioCycle 1981, 22, 54–57. [Google Scholar]
- Emino, E.R.; Warman, P.R. Biological assay for compost quality. Compos. Sci. Util. 2004, 12, 342–348. [Google Scholar] [CrossRef]
- Iglesias, E.; Barral, M.; Marhuenda-Egea, F. Indicadores de la estabilidad y madurez del compost. In Compostaje; Casco, J.M., Herrero, R.M., Eds.; Mundi-Prensa: Madrid, Spain, 2008; pp. 244–283. [Google Scholar]
- Jagadabhi, P.S.; Wani, S.P.; Kaushal, M.; Patil, M.; Vemula, A.K.; Rathore, A. Physico-chemical, microbial and phytotoxicity evaluation of composts from sorghum, finger millet and soybean straws. Int. J. Recycl. Org. Waste Agric. 2019, 8, 279–293. [Google Scholar] [CrossRef]
- MARNR Ministerio del ambiente y los recursos renovables. Atlas Del Estado Táchira Zona 6; San Cristóbal Cartografía Nacional: Caracas, Venezuela, 1986. [Google Scholar]
- TMECC. Nitrogen. Method 04.02. In Test. Methods for the Examination of Composting and Compost; Thompson, W., Ed.; The Composting Council Research and Education Foundation: Bethesda, MD, USA, 2002; p. 04.02-01. [Google Scholar]
- Sadzawka, A.; Carrasco, M.A.; Grez, R.; Mora, M.M. Métodos de Análisis de Compost; Instituto de Investigaciones Agropecuarias: Santiago, Chile, 2005; ISBN 0717-4810. [Google Scholar]
- Pire, R.; Pereira, A. Propiedades físicas de componentes de sustratos de uso común en la horticultura del estado lara, venezuela. Propuesta metodológica. Bioagro 2003, 15, 55–64. [Google Scholar]
- Richards, T. Compost Mixture Calculation Spreadsheets; Cornell Composting Science & Engineering: Ithaca, NY, USA, 2014; Available online: http://compost.css.cornell.edu/download.html (accessed on 6 October 2020).
- TMECCa. Field sampling of compost materials. In Test Methods for the Examination of Composting and Compost; Thompson, W., Ed.; The Composting Council Research and Education Foundation and Agriculture: Bethesda, MD, USA, 2002; p. 26. [Google Scholar]
- Zucconi, F.; Monaco, A.; Forte, M.; De Bertoldi, M. Phytotoxins during the stabilizatión of organic matter. In Composting of Agricultural and Other Wastes; Gasser, J., Ed.; Elsevier Applied Science Publishers: Essex, London, UK, 1984; p. 336. ISBN 0-85334-357-8. [Google Scholar]
- McKean, S. Manual de Análisis de Suelos y Tejido Vegetal. Una Guia Teórica y Práctica de Metodologias; Centro Internacional de Agricultura Tropical: Cali, Colombia, 1993. [Google Scholar]
- Sadzawka, R.A. Requisitos Analíticos del Compost y de las Materias Primas para Compostaje Según la Norma Chilena NCh 2880; Instituto de Investigaciones Agropecuarias: Santiago, Chile, 2004; p. 27. [Google Scholar]
- He, Z.; Yang, X.; Kahn, B.; Stoffella, P.; Calvert, D. Plant Nutrition Benefits of Phosphorus, Potassium, Calcium, Magnesium, and Micronutrients from Compost Utilization. In Compost Utilization in Horticultural Cropping Systems; Stofella, P.J., Khan, B.A., Eds.; CRC Press: Boca Raton, FL, USA, 2001; p. 431. ISBN 1-56670-460-X. [Google Scholar]
- Abad, M.; Noguera, P.; Burés, S. National inventory of organic wastes for use as growing media for ornamental potted plant production: Case study in Spain. Bioresour. Technol. 2001, 77, 197–200. [Google Scholar] [CrossRef]
- López, M.; Huerta-Pujol, O.; Martínez-Farré, F.X. Resources, Conservation and Recycling Approaching compost stability from Klason lignin modified method: Chemical stability degree for OM and N quality assessment. Resour. Conserv. Recy. 2010, 55, 171–181. [Google Scholar] [CrossRef]
- Soliva, M.; López, M. Calidad del Compost: Influencia del Tipo de Material Tratado y de las Condiciones del Proces; Escuela Superior de Agricultura: Barcelona, Spain, 2004; Available online: https://ruralcat.gencat.cat/documents/20181/81510/Altres3_Calidad+del+compost_+influencia+del+tipo+de+material+tratado+y+delas+condiciones+del+procesopdf/80b5b931-0521-426b-a733-6be0ac2d3a68 (accessed on 6 October 2020).
- Bundesgütegemeinschaft Kompost. Merkblatt zum RAL-Prüfzeugnis Kompost RAL-GZ 251. 2010, pp. 8–11. Available online: https://www.kompost.de/fileadmin/docs/guetesicherung/Merkblatt_Kompost.pdf (accessed on 6 October 2020).
- Rienzo, J.A.D. Análisis de Regresión; Facultad de Ciencias Agropecuarias: Córdoba, Argentina, 2011; Available online: http://sites.google.com/site/dirienzojulio (accessed on 6 October 2020).
- Nielsen, H.; Berthelsen, L. A Model for Temperature Dependency of Thermophilic Composting Process Rate. Compost Sci. Util. 2013, 10, 249–257. [Google Scholar] [CrossRef]
- Moreno, J.; Mormeneo, B. Microbiología y Bioquímica del proceso de compostaje. In Compostaje; Casco, J.M., Herrero, R.M., Eds.; Mundi-Prensa: Madrid, Spain, 2008; pp. 111–140. [Google Scholar]
- Sánchez, M.A.; Roig, A.; Paredes, C.; Bernal, M. Nitrogen transformation during organic waste composting by the Rutgers system and its efects on pH, EC and maturity of the composting mixtures. Bioresour. Technol. 2001, 78, 301–308. [Google Scholar] [CrossRef]
- Lewis, N.G.; Yamamoto, E. Lignin: Occurrence, biogenesis and biodegradation. Annu. Rev. Plant. Physiol. Plant. Mol. Biol. 1990, 41, 455–496. [Google Scholar] [CrossRef]
- Acosta, Y.; Zauahre, M.E.; Rodríguez, L. Indicadores de calidad bioquímica y estabilidad de la materia orgánica durante el proceso de compostaje de residuos orgánicos. Univ. Zulia Venez. 2012, 12, 10. [Google Scholar]
- Balzarini, M.; Bruno, C.; Córdoba, M.; Teich, I. Herramientas en el Análisis Estadístico Multivariado; Escuela Virtual Internacional CAVILA, Facultad de Ciencias Agropecuarias, Universidad Nacional de Córdoba: Córdoba, Argentina, 2015; Available online: https://www.researchgate.net/publication/286931204 (accessed on 6 October 2020).
- Siles-Castellano, A.B.; López, M.J.; López-González, J.A.; Suárez-Estrella, F.; Jurado, M.M.; Estrella-González, M.J.; Moreno, J. Comparative analysis of phytotoxicity and compost quality in industrial composting facilities processing different organic wastes. J. Clean. Prod. 2020, 252, 119820. [Google Scholar] [CrossRef]
- Masaguer, A.; Benito, M. Evaluación de la calidad del compost. In Compostaje; Casco, J.M., Herrero, R.M., Eds.; Mundi-Prensa: Madrid, Spain, 2008; pp. 285–304. [Google Scholar]
- Defrieri, R.L.; Jimenez, M.P.; Effron, D.; Palma, M. Utilización de parámetros químicos y microbiológicos como criterios de madurez durante el proceso de compostaje. AgriScientia 2005, 22. [Google Scholar] [CrossRef]
- Gao, Y.; Yu, H.; Liu, P.; Ma, C.; Li, Q.; Jiang, W. Ending composting during the thermophilic phase improves cultivation substrate properties and increasing winter cucumber yield. Waste Manag. 2018, 79, 260–272. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.; Bhattacharyya, P. Effect of municipal solid waste compost on seed germination of rice, wheat and cucumber. Arch. Agron. Soil Sci. 2003, 49, 407–414. [Google Scholar] [CrossRef]
- Miao-miao, H.E.; Guang-ming, T.; Xing-qiang, L.; Yi-tong, Y.U. Effects of two sludge application on fractionation and phytotoxicity of zinc and copper in soil. J. Environ. Sci. 2007, 19, 1482–1490. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
| Material | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Fruit Waste | Sludge | Debris | Rice Hulls | Sawdust | Dairy Manure | FSC | |||
| Bd (kg m−3) | 0.8 | 0.9 | 0.8 | 0.1 | 0.1 | 0.3 | 0.4 | |||
| M (%) | 80.3 | 77.0 | 75.1 | 2.0 | 54.1 | 5.0 | 52.3 | |||
| TOC (%) | 55.2 | 48.3 | 57.2 | 46.5 | 56.4 | 32.6 | 12.2 | |||
| TN (%) | 1.9 | 1.8 | 0.9 | 0.4 | 0.7 | 3.7 | 0.5 | |||
| Treatment | Amount (kg) | Total (kg) | C/N * | M * (%) | ||||||
| C1 | 2600 | 400 | 40 | 400 | 3400 | 36.5 | 51.8 | |||
| C2 | 2500 | 800 | 100 | 1000 | 4400 | 38.4 | 57.0 | |||
| C3 | 2900 | 600 | 150 | 1000 | 4650 | 59.0 | 53.8 | |||
| Initial | Final | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Reference Values | C1 | C2 | C3 | C1 | C2 | C3 |
| SG% * | >50 | 23.42 a | 22.75 a | 26.46 a | 54.09 a | 66.55 a | 54.91 a |
| TN% * | >0.5 | 0.58 b | 0.55 ab | 0.5 a | 1.00 ab | 1.26 b | 0.91 a |
| C/N [28] | <25 | 36.45 a | 38.42 a | 59.03 b | 24.48 b | 16.40 a | 18.7 a |
| pH [28] | 5–8.5 | 5.53 ab | 5.2 a | 5.87 b | 7.87 a | 7.88 a | 7.73 a |
| EC dSm−1 [28] | ≤3a ≤ 8b | 3.32 a | 4.22 a | 3.61 a | 2.86 a | 2.99 a | 3.70 b |
| TOM % [28] | >20 | 60.04 b | 56.82 a | 66.74 c | 42.0 c | 35.56 b | 29.11 a |
| M (%) [28] | 50–60 | 51.75 a | 46.04 a | 53.84 a | 56.09 b | 52.79 a | 54.9 ab |
| GI rad% [16] | >50 | 49.40 a | 60.60 a | 41.43 a | |||
| GI cuc% * [16] | >50 | 90.23 a | 65.20 ab | 40.27 b | |||
| RE 100 rad% [16] | >50 | 75.90 a | 103.50 a | 67.87 a | |||
| RE 50 cuc [16] | >50 | 112.5 a | 128.50 a | 115.40 a | |||
| RE 100 cuc [16] | >50 | 75.03 a | 135.70 b | 46.20 a | |||
| RE 50 tom [16] | >50 | 78.60 a | 85.70 a | 72.43 a | |||
| RE 100 tom [16] | >50 | 78.60 a | 64.30 a | 37.93 a | |||
| TPS% v/v [29] | ≥85 | 54.91 b | 43.73 a | 48.01 a | 47.95 a | 46.31 a | 38.46 a |
| AC% v/v [29] | 20–30 | 29.68 b | 23.09 a | 18.51 a | 12.27 a | 15.85 b | 14.29 b |
| TW-HC% v/v [29] | 55–70 | 25.23 b | 20.64 a | 29.5 b | 35.68 a | 30.46 ab | 24.17 a |
| Bd * t m−3 [29] | 0.2–0.4 | 0.31 a | 0.35 a | 0.35 a | 0.24 a | 0.21 a | 0.28 a |
| Pd t m−3 [29] | 1.4–2.0 | 0.76 b | 0.57 a | 0.78 b | 0.55 a | 0.7 a | 0.73 a |
| Ca % [29] | >11.21 | 39.94 a | 43.82 a | 41.59 a | 51.34 a | 56.52 b | 52.23 a |
| Mg % [29] | >1.46 | 1.62 a | 1.81 a | 1.51 a | 2.03 a | 2.67 b | 2.70 b |
| K% [29] | >5.06 | 5.90 ab | 5.2 a | 6.23 b | 8.08 a | 7.55 a | 11.16 b |
| Fe * mg kg−1 [30] | <9300 | 13100 b | 6000 a | 10400 ab | 17900 b | 11200 a | 13500 ab |
| Mn * mg kg−1 [30] | 431–600 | 127.33 a | 224.83 a | 147.00 ab | 226.83 a | 273.17 ab | 303.83 b |
| Cu mg kg−1 [30] | <100 | 41.00 b | 30.33 a | 50.67 c | 62.00 b | 48.50 a | 68.83 c |
| Zn * mg kg−1 [30] | <200 | 76.17 ab | 52.00 a | 151.00 b | 160.17 a | 170.67 ab | 247.83 b |
| Variables | Principal Component 1 | Principal Component 2 |
|---|---|---|
| TN | 0.95 | −0.002 |
| RE 100 cuc | 0.92 | 0.01 |
| SG | 0.89 | −0.35 |
| Mn | 0.87 | −0.47 |
| GI rad | 0.75 | 0.08 |
| Fe | −0.77 | 0.61 |
| Zn | −0.85 | −0.5 |
| K | −0.89 | −0.04 |
| Cu | −0.94 | −0.21 |
| TW-HC | 0.35 | 0.78 |
| GI cuc | 0.28 | 0.94 |
| TOM | 0.27 | 0.94 |
| RE_100 rad | 0.23 | −0.94 |
| Bd | −0.16 | −0.93 |
| AC | 0.61 | −0.71 |
| C/N | −0.48 | 0.81 |
| EC | −0.57 | −0.71 |
| Variable (1) | Variable (2) | Spearman | p-Value |
|---|---|---|---|
| GI cuc | TPS | 0.75 | 0.0339 |
| GI cuc | Bd | −0.88 | 0.0017 |
| GI cuc | TOM | 0.90 | 0.0109 |
| GI cuc | EC | −0.78 | 0.0122 |
| RE 100 cuc | TN | 0.87 | 0.0025 |
| RE 100 cuc | K | −0.74 | 0.0214 |
| RE 100 cuc | Cu | −0.93 | 0.0003 |
| RE 100 cuc | Zn | −0.95 | 0.0001 |
| RE_50 rad | K | −0.69 | 0.0399 |
| RE_100 rad | AC | 0.81 | 0.0081 |
| RE_100 rad | Bd | 0.86 | 0.0028 |
| RE_100 rad | TOM | −0.84 | 0.0049 |
| RE_100 rad | C/N | −0.78 | 0.0138 |
| RE_100 rad | Mn | 0.84 | 0.0046 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peña, H.; Mendoza, H.; Diánez, F.; Santos, M. Parameter Selection for the Evaluation of Compost Quality. Agronomy 2020, 10, 1567. https://doi.org/10.3390/agronomy10101567
Peña H, Mendoza H, Diánez F, Santos M. Parameter Selection for the Evaluation of Compost Quality. Agronomy. 2020; 10(10):1567. https://doi.org/10.3390/agronomy10101567
Chicago/Turabian StylePeña, Haydee, Heysa Mendoza, Fernando Diánez, and Mila Santos. 2020. "Parameter Selection for the Evaluation of Compost Quality" Agronomy 10, no. 10: 1567. https://doi.org/10.3390/agronomy10101567
APA StylePeña, H., Mendoza, H., Diánez, F., & Santos, M. (2020). Parameter Selection for the Evaluation of Compost Quality. Agronomy, 10(10), 1567. https://doi.org/10.3390/agronomy10101567






