Effect of Low Temperature on Germination, Growth, and Seed Yield of Four Soybean (Glycine max L.) Cultivars
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
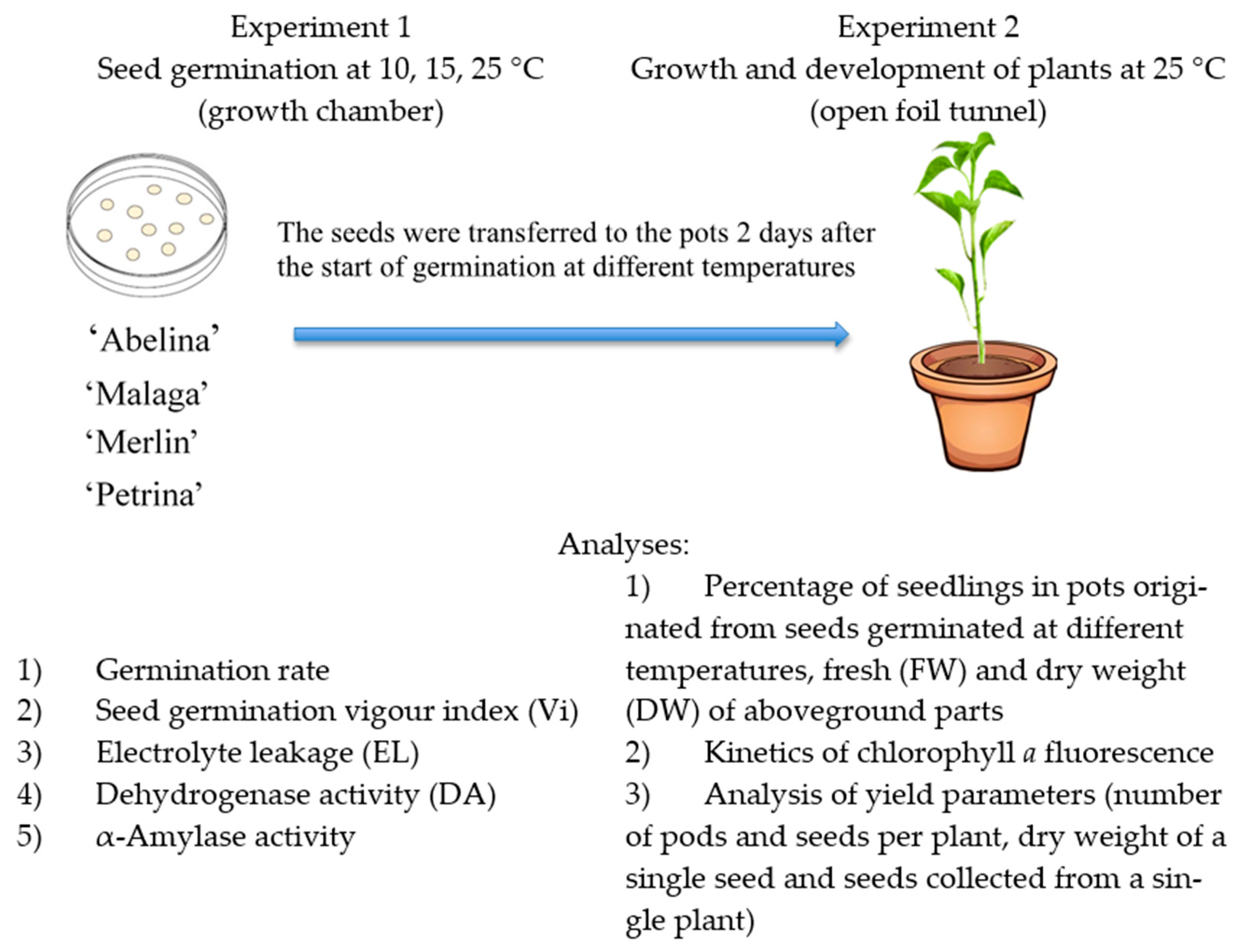
2.2. Study Design
2.3. Experiment 1
2.3.1. Seed Germination Vigor Index (Vi)
2.3.2. Electrolyte Leakage (EL)
2.3.3. Dehydrogenase Activity
2.3.4. α-Amylase Activity Assay
2.4. Experiment 2
2.4.1. Percentage of Seedlings Grown from Seeds Germinated at 10, 15, and 25 °C
2.4.2. Kinetics of Chlorophyll a Fluorescence
2.4.3. Fresh and Dry Weight
2.4.4. Analysis of Yield Parameters
2.4.5. Statistical Analysis
3. Results
3.1. Experiment 1
3.1.1. Seed Germination
3.1.2. Electrolyte Leakage
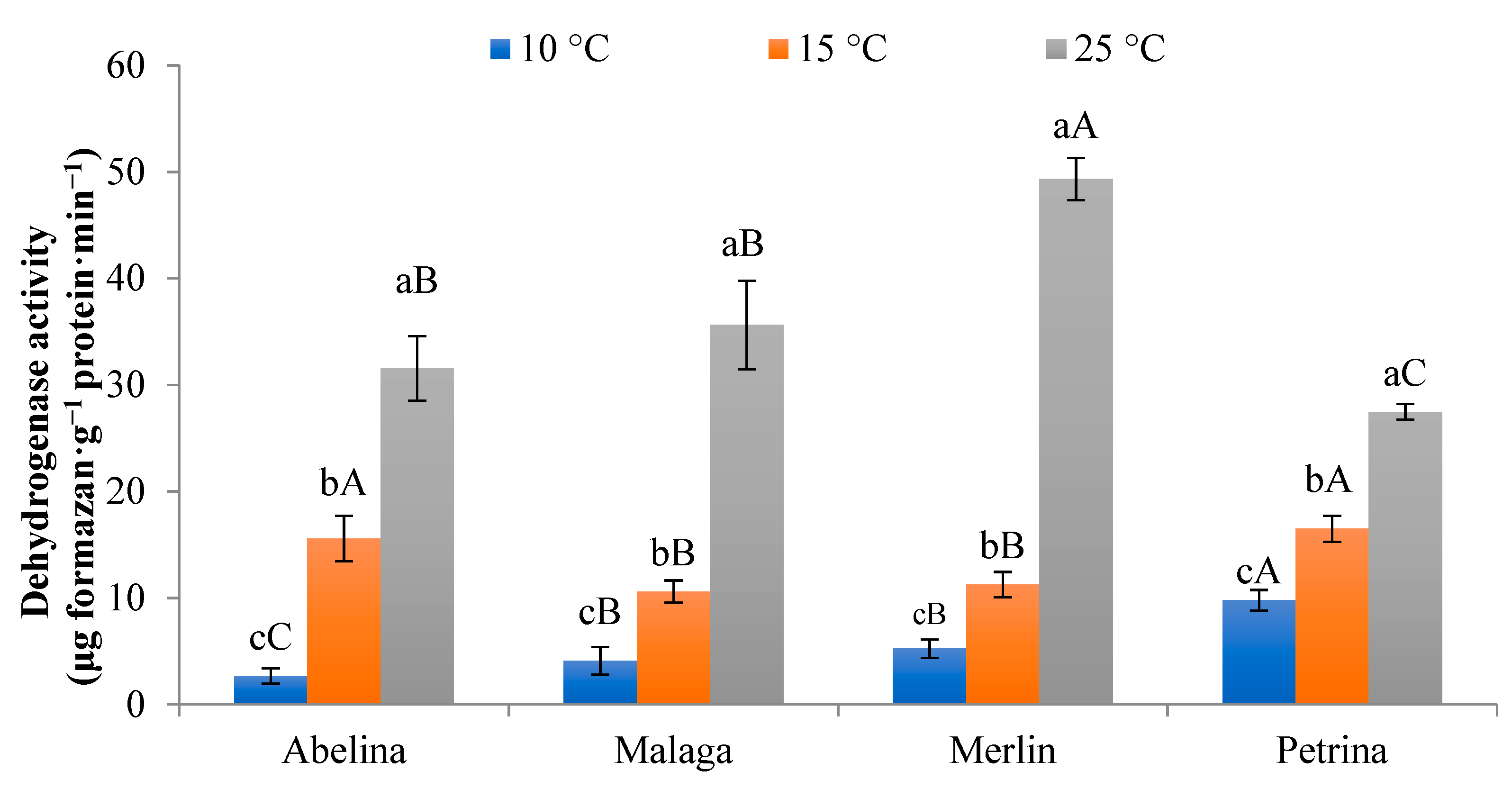
3.1.3. Activity of Dehydrogenase (DA) Pool
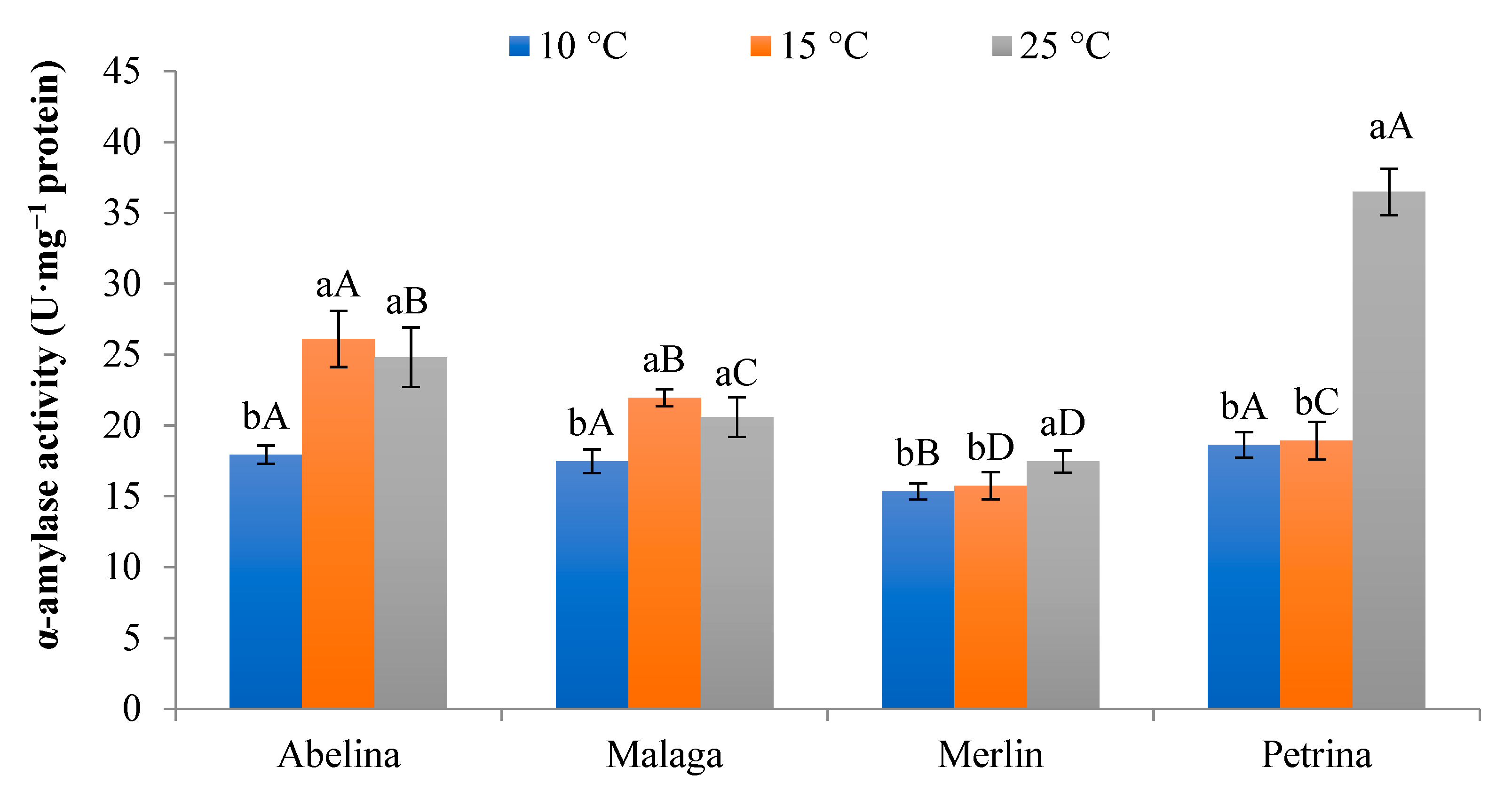
3.1.4. Activity of α-Amylase
3.2. Experiment 2
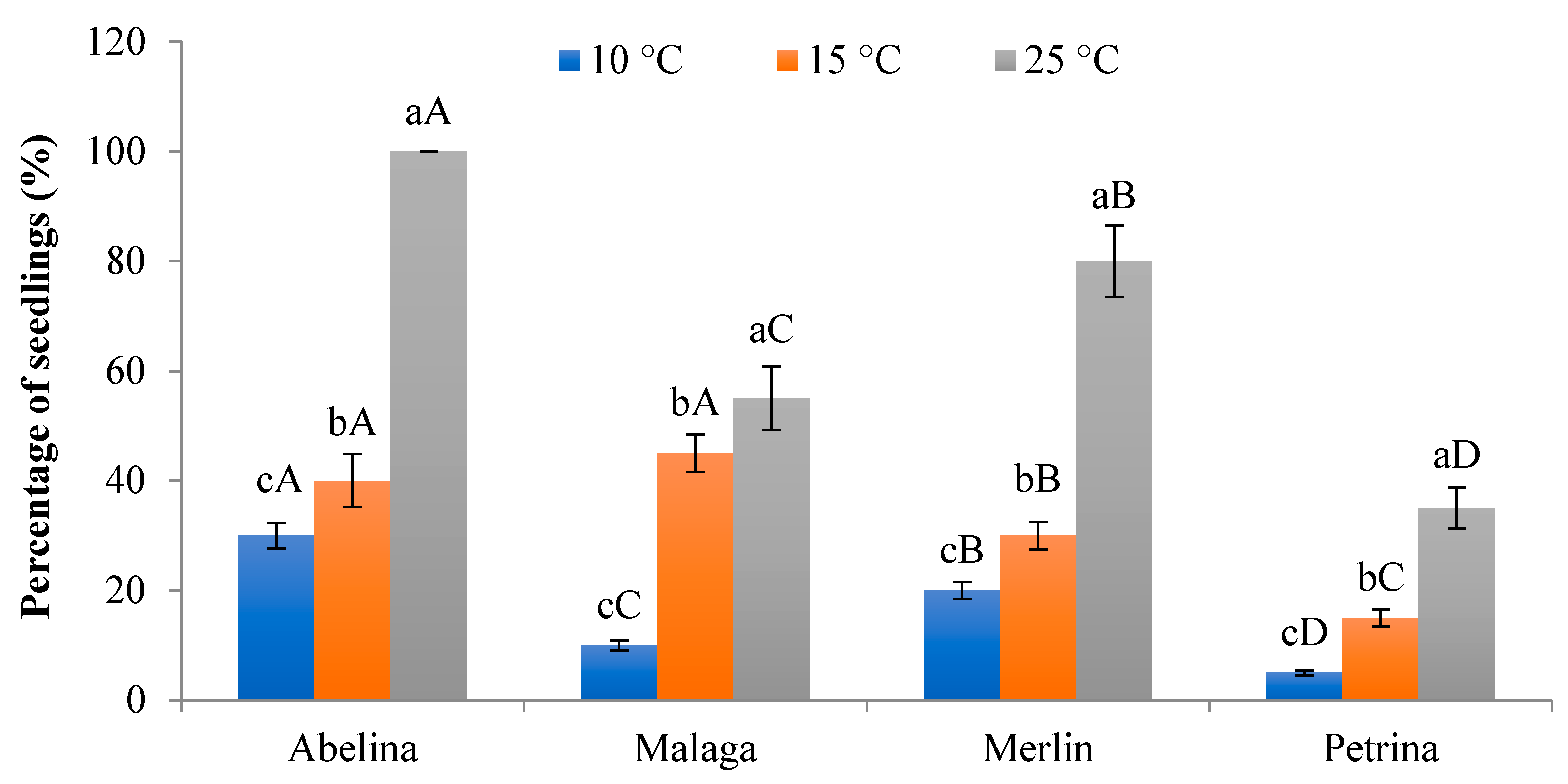
3.2.1. Percentage of Seedlings
3.2.2. Kinetics of Chlorophyll a Fluorescence (Chlf)
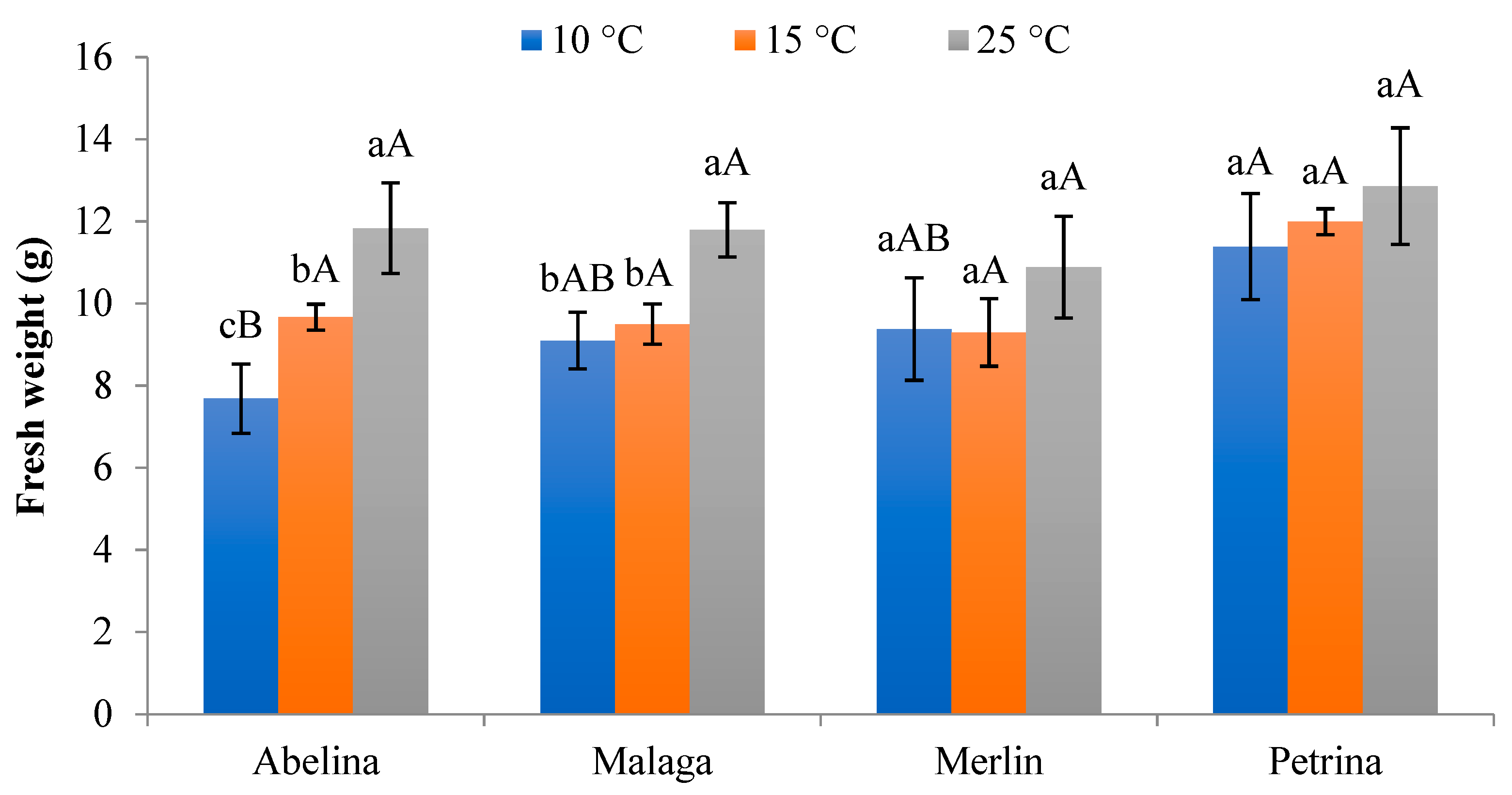
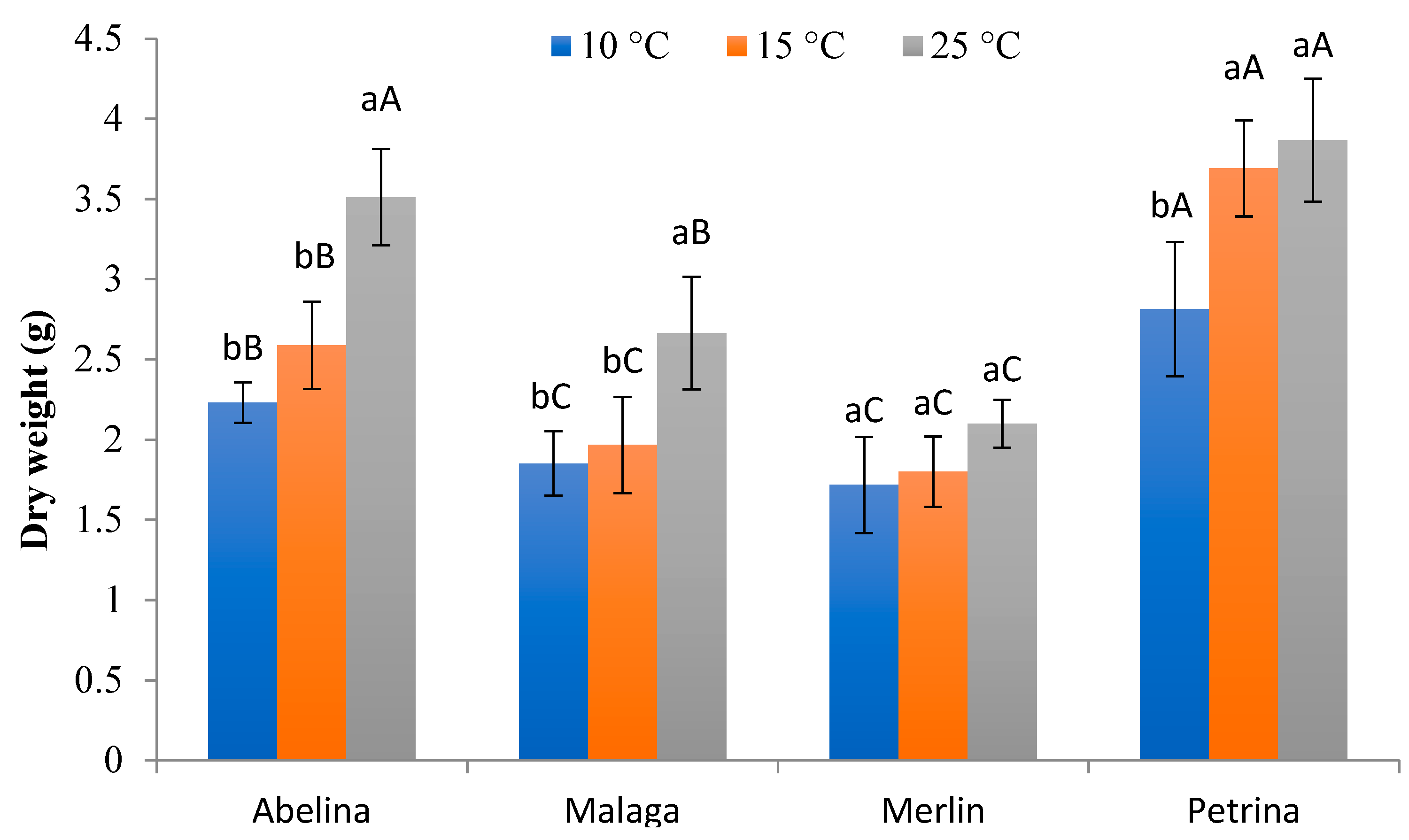
3.2.3. Fresh and Dry Weight of Aboveground Parts
3.2.4. Yield Parameters
4. Discussion
4.1. Consequence of Low Temperature on Seed Germination Process
4.2. Long-Effect of Low Temperature on Soybean Yielding
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Barker, D.G.; Bianchi, S.; Blondon, F.; Dattee, Y.; Duc, G.; Essad, S.; Flament, P.; Gallusci, P.; Genier, G.; Guy, P. Medicago truncatula, a model plant for studying the molecular genetics of the Rhizobium-legume symbiosis. Plant Mol. Biol. Rep. 1990, 8, 40–49. [Google Scholar] [CrossRef]
- Dong, D.; Fu, X.; Yuan, F.; Chen, P.; Zhu, S.; Li, B.; Yang, Q.; Yu, X.; Zhu, D. Genetic diversity and population structure of vegetable soybean (Glycine max (L.) Merr.) in China as revealed by SSR markers. Genet. Resour. Crop Evolution 2014, 61, 173–183. [Google Scholar] [CrossRef]
- Vorobyev, V.; Vorobyev, D.; Zakharkina, N.; Polkovnichenko, A.; Safonov, V. Physiological status of ‘king’ squab pigeon (Columba Livia gm. Cv. ‘king’) in biogeochemical conditions of low iodine, selenium and cobalt levels in the environment. Asia Life Sci. 2019, 28, 99–110. [Google Scholar]
- Pratap, A.; Gupta, S.K.; Kumar, J.; Solanki, R. Soybean. In Technological Innovations in Major World Oil Crops; Gupta, S., Ed.; Springer: New York, NY, USA, 2012; Volume 1, pp. 293–321. [Google Scholar] [CrossRef]
- Lorenc-Kozik, A.-M.; Pisulewska, E. Wplyw zroznicowanego nawozenia azotem i mikroelementami na plonowanie wybranych odmian soi. Rośliny Oleiste-Oilseed Crops 2003, 24, 131–142. [Google Scholar]
- Martyniuk, S. Naukowe i praktyczne aspekty symbiozy roślin strączkowych z bakteriami brodawkowymi. Pol. J. Agron. 2012, 9, 17–22. [Google Scholar]
- Filoda, G.; Mrowczynski, M. Metodyka Integrowanej Ochrony Soi dla Producentów; Instytut Ochrony Roślin-Państwowy Instytut Badawczy: Poznań, Polan, 2012. [Google Scholar]
- Khalil, S.K.; Mexal, J.G.; Rehman, A.; Khan, A.Z.; Wahab, S.; Zubair, M.; Khalil, I.H.; Mohammad, F. Soybean mother plant exposure to temperature stress and its effect on germination under osmotic stress. Pak. J. Bot. 2010, 42, 213–225. [Google Scholar]
- Butler, T.J.; Celen, A.E.; Webb, S.L.; Krstic, D.; Interrante, S.M. Temperature affects the germination of forage legume seeds. Crop Sci. 2014, 54, 2846–2853. [Google Scholar] [CrossRef]
- Li, M.; Liu, Y.; Wang, C.; Yang, X.; Li, D.; Zhang, X.; Xu, C.; Zhang, Y.; Li, W.; Zhao, L. Identification of traits contributing to high and stable yields in different soybean varieties across three chinese latitudes. Front. Plant Sci. 2020, 10, 1642. [Google Scholar] [CrossRef]
- Robison, J.; Arora, N.; Yamasaki, Y.; Saito, M.; Boone, J.; Blacklock, B.; Randall, S. Glycine max and Glycine soja are capable of cold acclimation. J. Agron. Crop Sci. 2017, 203, 553–561. [Google Scholar] [CrossRef]
- Vinkovic, T.; Paradikovic, N.; Plavsic, H.; Guberac, V.; Levai, L. Maize and soybean seed vigour under influence of seed age, seed treatment and temperature in cold stress test. Cereal Res. Commun. 2007, 35, 1213–1216. [Google Scholar] [CrossRef]
- Zhao, S.; Xu, X.; Wei, D.; Lin, X.; Qiu, S.; Ciampitti, I.; He, P. Soybean yield, nutrient uptake and stoichiometry under different climate regions of northeast China. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Koester, R.P.; Skoneczka, J.A.; Cary, T.R.; Diers, B.W.; Ainsworth, E.A. Historical gains in soybean (Glycine max Merr.) seed yield are driven by linear increases in light interception, energy conversion, and partitioning efficiencies. J. Exp. Bot. 2014, 65, 3311–3321. [Google Scholar] [CrossRef]
- Damaris, R.N.; Lin, Z.; Yang, P.; He, D. The rice alpha-amylase, conserved regulator of seed maturation and germination. Int. J. Mol. Sci. 2019, 20, 450. [Google Scholar] [CrossRef]
- Bewley, J.D. Seed germination and dormancy. Plant Cell 1997, 9, 1055–1066. [Google Scholar] [CrossRef]
- Fyfield, T.P.; Gregory, P.J. Effects of temperature and water potential on germination, radicle elongation and emergence of mungbean. J. Exp. Bot. 1989, 40, 667–674. [Google Scholar] [CrossRef]
- Plażek, A.; Dubert, F.; Kopeć, P.; Dziurka, M.; Kalandyk, A.; Pastuszak, J.; Wolko, B. Seed hydropriming and smoke water significantly improve low-temperature germination of Lupinus angustifolius L. Int. J. Mol. Sci. 2018, 19, 992. [Google Scholar] [CrossRef]
- Plażek, A.; Dubert, F.; Kopeć, P.; Dziurka, M.; Kalandyk, A.; Pastuszak, J.; Waligórski, P.; Wolko, B. Long-term effects of cold on growth, development and yield of narrow-Leaf lupine may be alleviated by seed hydropriming or butenolide. Int. J. Mol. Sci. 2018, 19, 2416. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, P.R.; Obendorf, R.L. Interaction of initial seed moisture and imbibitional temperature on germination and productivity of soybean. Crop Sci. 1972, 12, 664–667. [Google Scholar] [CrossRef]
- Dubert, F.; Filek, W. T The low temperature drying of soybean (Glycine max) seeds as a factor reducing injuries caused by exposure to chill during subsequent germination. Bull. Pol. Ac. Biol. Sci. 1995, 43, 51–55. [Google Scholar]
- Campos, P.S.; Quartin, V.; Ramalho, J.C.; Nunes, M.A. Electrolyte leakage and lipid degradation account for cold sensitivity in leaves of Coffea sp plants. J. Plant Physiol. 2003, 160, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Plażek, A.; Żur, I. Cold-induced plant resistance to necrotrophic pathogens and antioxidant enzyme activities and cell membrane permeability. Plant Sci. 2003, 164, 1019–1028. [Google Scholar] [CrossRef]
- Uemura, M.; Tominaga, Y.; Nakagawara, C.; Shigematsu, S.; Minami, A.; Kawamura, Y. Responses of the plasma membrane to low temperatures. Physiol. Plant. 2006, 126, 81–89. [Google Scholar] [CrossRef]
- Lee, S.C.; Cheng, H.; King, K.E.; Wang, W.F.; He, Y.W.; Hussain, A.; Lo, J.; Harberd, N.P.; Peng, J.R. Gibberellin regulates Arabidopsis seed germination via RGL2, a GAI/RGA-like gene whose expression is up-regulated following imbibition. Genes Dev. 2002, 16, 646–658. [Google Scholar] [CrossRef]
- Buchanan, B.B.; Gruissem, W.; Jones, R.L. Biochemistry and Molecular Biology of Plants; John Wiley & Sons: Chichester, UK, 2015. [Google Scholar]
- Yang, L.; Wang, X.; Chang, N.; Nan, W.; Wang, S.; Ruan, M.; Sun, L.; Li, S.; Bi, Y. Cytosolic glucose-6-phosphate dehydrogenase is involved in seed germination and root growth under salinity in Arabidopsis. Front. Plant Sci. 2019, 10, 182. [Google Scholar] [CrossRef] [PubMed]
- Saatbau. Soja-Odmiany. Available online: https://www.saatbau.pl/asp/soja,53,,1 (accessed on 8 April 2021).
- Danko. Katalogi Odmian. Available online: https://danko.pl/pliki-do-pobrania/katalogi/ (accessed on 8 April 2021).
- Steponkus, P.L.; Lanphear, F.O. Refinement of the triphenyl tetrazolium chloride method of determining cold injury. Plant Physiol. 1967, 42, 1423–1426. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Bernfeld, P. Amylases, α and β. Methods Enzymol. 1955, 1, 149–158. [Google Scholar]
- Hoagland, D.; Arnon, D. The water-culture method forgrowing plants without soil. Univ. Calif. Agric. Exp. Stn. Circ. 1938, 347, 29–32. [Google Scholar]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. The fluorescence transient as a tool to characterize and screen photosynthetic samples. In Probing Photosynthesis: Mechanisms, Regulation and Adaptation; Yunus, M., Pathre, U., Mohanty, P., Eds.; Taylor and Francis: London, UK; New York, NY, USA, 2000; pp. 445–483. [Google Scholar]
- Hatfield, J.; Egli, D. Effect of temperature on the rate of soybean hypocotyl elongation and field emergence. Crop Sci. 1974, 14, 423–426. [Google Scholar] [CrossRef]
- Alm, D.M.; Stoller, E.W.; Wax, L.M. An index model for predicting seed germination and emergence rates. Weed Technol. 1993, 7, 560–569. [Google Scholar] [CrossRef]
- Blum, A. Plant Breeding for Stress Environments; CRC Press Inc.: Boca Raton, FL, USA, 1988; p. 223. [Google Scholar]
- Bajji, M.; Kinet, J.-M.; Lutts, S. Osmotic and ionic effects of NaCl on germination, early seedling growth, and ion content of Atriplex halimus (Chenopodiaceae). Can. J. Bot. 2002, 80, 297–304. [Google Scholar] [CrossRef]
- Demidchik, V.; Straltsova, D.; Medvedev, S.S.; Pozhvanov, G.A.; Sokolik, A.; Yurin, V. Stress-induced electrolyte leakage: The role of K+-permeable channels and involvement in programmed cell death and metabolic adjustment. J. Exp. Bot. 2014, 65, 1259–1270. [Google Scholar] [CrossRef] [PubMed]
- Duke, S.H.; Schrader, L.E.; Miller, M.G. Low temperature effects on soybean (Glycine max [L.] Merr. cv. Wells) mitochondrial respiration and several dehydrogenases during imbibition and germination. Plant Physiol. 1977, 60, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Airaki, M.; Leterrier, M.; Mateos, R.M.; Valderrama, R.; Chaki, M.; Barroso, J.B.; Del Rio, L.A.; Palma, J.M.; Corpas, F.J. Metabolism of reactive oxygen species and reactive nitrogen species in pepper (Capsicum annuum L.) plants under low temperature stress. Plant Cell Environ. 2012, 35, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Saruyama, H.; Tanida, M. Effect of chilling on activated oxygen-scavenging enzymes in low temperature-sensitive and-tolerant cultivars of rice (Oryza sativa L.). Plant Sci. 1995, 109, 105–113. [Google Scholar] [CrossRef]
- Van Heerden, P.D.; Tsimilli-Michael, M.; Krüger, G.H.; Strasser, R.J. Dark chilling effects on soybean genotypes during vegetative development: Parallel studies of CO2 assimilation, chlorophyll a fluorescence kinetics O-J-I-P and nitrogen fixation. Physiol. Plant. 2003, 117, 476–491. [Google Scholar] [CrossRef]
- Revilla, P.; Malvar, R.; Cartea, M.; Butron, A.; Ordás, A. Inheritance of cold tolerance at emergence and during early season growth in maize. Crop Sci. 2000, 40, 1579–1585. [Google Scholar] [CrossRef]
- Sharma, P.; Sharma, N.; Deswal, R. The molecular biology of the low-temperature response in plants. Bioessays 2005, 27, 1048–1059. [Google Scholar] [CrossRef]
- Ladror, U.; Dyck, R.; Silbernagel, M. Effects of oxygen and temperature during imbibition on seeds of two bean lines at two moisture levels. J. Am. Soc. Hortic. Sci. 1986, 111, 572–577. [Google Scholar]
- Zaiter, H.; Baydoun, E.; Sayyed-Hallak, M. Genotypic variation in the germination of common bean in response to cold temperature stress. Plant Soil 1994, 163, 95–101. [Google Scholar] [CrossRef]
- Wang, L.-J.; Zhang, P.; Wang, R.-N.; Pu, W.; Huang, S.-B. Effects of variety and chemical regulators on cold tolerance during maize germination. J. Integr. Agric. 2018, 17, 2662–2669. [Google Scholar] [CrossRef]
- Sharkey, T.D.; Schrader, S.M. High temperature stress. In Physiology and Molecular Biology of Stress Tolerance in Plants; Rao, K.V.M., Raghavendra, A.S., Reddy, K.J., Eds.; Springer: Berlin, Germany, 2006; pp. 101–129. [Google Scholar]
- Mathur, S.; Agrawal, D.; Jajoo, A. Photosynthesis: Response to high temperature stress. J. Photochem. 2014, 137, 116–126. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Lukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant 2016, 38, 102. [Google Scholar] [CrossRef]
- Wawrzyniak, M.; Ogrodowczyk, M. Badanie związku potrzeb jaryzacyjnych z zimotrwałością roślin rzepaku ozimego o zmodyfikowanych cechach jakościowych. Rośliny Oleiste Oilseed Crops 2003, Tom XXIV, 283–293. [Google Scholar]
- Bolhar-Nordenkampf, H.R.; Oquist, G. Chlorophyll fluorescence as a tool in photosynthesis research. In Photosynthesis and Production in a Changing Environment; Hall, D.O., Scurlock, J.M.O., Bolhàr-Nordenkampf, H.R., Leegood, R.C., Long, S.P., Eds.; Springer: Berlin, Germany, 1993; pp. 193–206. [Google Scholar]
- Wuebker, E.F.; Mullen, R.E.; Koehler, K. Flooding and temperature effects on soybean germination. Crop Sci. 2001, 41, 1857–1861. [Google Scholar] [CrossRef]
- Kurosaki, H.; Yumoto, S. Effects of low temperature and shading during flowering on the yield components in soybeans. Plant Prod. Sci. 2003, 6, 17–23. [Google Scholar] [CrossRef]
- Mattson, M.P. Hormesis defined. Ageing Res. Rev. 2008, 7, 1–7. [Google Scholar] [CrossRef]
- Pastuszak, J.; Kopeć, P.; Plażek, A.; Gondek, K.; Szczerba, A.; Hornyák, M.; Dubert, F. Antioxidant activity as a response to cadmium pollution in three durum wheat genotypes differing in salt-tolerance. Open Chem. 2020, 18, 1230–1241. [Google Scholar] [CrossRef]
| Temperature/Cultivar | 10 °C | 15 °C | 25 °C |
|---|---|---|---|
| Abelina | 0 cA | 26.7 ± 3.7 bB | 100 ± 0 aA |
| Malaga | 0 cA | 16.7 ± 2.8 bC | 100 ± 0 aA |
| Merlin | 0 cA | 19.9 ± 2.4 bC | 97.5 ± 1.3 aA |
| Petrina | 0 cA | 52.5 ± 5.8 bA | 98.5 ± 1.2 aA |
| Temperature/Cultivar | 15 °C | 25 °C |
|---|---|---|
| Abelina | 0.35 ± 0.08 bB | 3.30 ± 0.28 aA |
| Malaga | 0.35 ± 0.1 bB | 2.88 ± 0.22 aB |
| Merlin | 0.28 ± 0.08 bB | 3.13 ± 0.54 aA |
| Petrina | 1.05 ± 0.10 bA | 2.80 ± 0.39 aB |
| Temperature/Cultivar | 10 °C | 15 °C | 25 °C |
|---|---|---|---|
| Abelina | 26.72 ± 2.70 aD | 26.15 ± 2.82 aB | 21.60 ± 2.01 bB |
| Malaga | 52.22 ± 1.13 aA | 35.98 ± 0.70 bA | 26.12 ± 1.42 cA |
| Merlin | 32.09 ± 2.49 aC | 26.68 ± 2.94 bB | 21.26 ± 2.94 cB |
| Petrina | 37.25 ± 1.94 aB | 26.74 ± 4.64 bB | 17.34 ± 1.93 cC |
| Cultivar | Temp. (°C) | ABS/CSm | TRo/CSm | ETo/CSm | DIo/CSm | RC/CSm | PIABS |
|---|---|---|---|---|---|---|---|
| Abelina | 10 | 312 ± 19 aA | 259 ± 13 aA | 109 ± 3 bB | 57 ± 6 aB | 793 ± 61 cB | 2.54 ± 0.24 aAB |
| 15 | 312 ± 18 aA | 259 ± 14 aA | 111 ± 8 bB | 53 ± 4 aA | 922 ± 83 bB | 1.88 ± 0.26 bB | |
| 25 | 324 ± 21 aB | 266 ± 18 aB | 120 ± 2 aB | 52 ± 3 aB | 1057 ± 129 aB | 1.42 ± 0.13 cD | |
| Malaga | 10 | 320 ± 18 aA | 264 ± 13 aA | 114 ± 7 aAB | 60 ± 7 aB | 954 ± 73 bA | 2.43 ± 0.25 aB |
| 15 | 327 ± 21 aA | 270 ± 18 aA | 118 ± 9 aAB | 57 ± 4 aA | 996 ± 78 bA | 2.19 ± 0.08 abAB | |
| 25 | 336 ± 28 aB | 276 ± 14 aB | 125 ± 11 aB | 55 ± 5 aA | 1178 ± 110 aA | 2.11 ± 0.1 bB | |
| Merlin | 10 | 297 ± 15 aA | 250 ± 15 aA | 121 ± 6 aA | 52 ± 2 aC | 971 ± 77 bA | 2.68 ± 0.22 aA |
| 15 | 309 ± 24 aA | 257 ± 19 aA | 122 ± 7 aAB | 50 ± 2 abB | 1050 ± 84 aA | 2.92 ± 0.38 aA | |
| 25 | 308 ± 24 aB | 257 ± 17 aB | 126 ± 9 aB | 46 ± 3 bC | 1063 ± 83 aB | 2.62 ± 0.05 aA | |
| Petrina | 10 | 311 ± 21 bA | 256 ± 12 bA | 118 ± 8 bAB | 89 ± 4 aA | 891 ± 71 bAB | 2.74 ± 0.18 aA |
| 15 | 323 ± 22 bA | 267 ± 16 bA | 131 ± 10 aA | 55 ± 6 bA | 957 ± 83 abAB | 2.96 ± 0.17 aA | |
| 25 | 382 ± 30 aA | 292 ± 12 aA | 135 ± 12 aA | 55 ± 5 bA | 1111 ± 88 aA | 1.82 ± 0.08 bC |
| Cultivar | Temp. (°C) | Number of Pods Per Plant | Number of Seeds Per Plant | DW of Seeds Per Plant (g) | DW of a Single Seed (g) |
|---|---|---|---|---|---|
| Abelina | 10 | 1.8 ± 0.2 bB | 15.7 ± 1.7 aB | 0.23 ± 0.04 aB | 0.019 ± 0.002 cC |
| 15 | 2.3 ± 0.3 aB | 5.0 ± 1.1 bB | 0.21 ± 0.02 aC | 0.033 ± 0.003 bD | |
| 25 | 2.1 ± 0.1 aB | 5.6 ± 1.4 bC | 0.25 ± 0.04 aC | 0.047 ± 0.005 aB | |
| Malaga | 10 | 1.4 ± 0.2 bC | 25.7 ± 2.5 aA | 0.15 ± 0.03 cC | 0.007 ± 0.001 bD |
| 15 | 2.4 ± 0.5 abB | 4.8 ± 0.6 cB | 0.19 ± 0.02 bD | 0.039 ± 0.004 aC | |
| 25 | 2.6 ± 0.5 aB | 9.0 ± 0.9 bB | 0.32 ± 0.03 aB | 0.036 ± 0.004 aC | |
| Merlin | 10 | 2.1 ± 0.4 bA | 13.8 ± 1.4 aB | 0.37 ± 0.05 aA | 0.024 ± 0.002 bA |
| 15 | 2.6 ± 0.2 aB | 11.1 ± 1.3 aA | 0.36 ± 0.04 aB | 0.051 ± 0.005 aA | |
| 25 | 2.4 ± 0.1 abB | 6.7 ± 1.4 bC | 0.35 ± 0.05 aB | 0.054 ± 0.005 aA | |
| Petrina | 10 | 2.0 ± 0.3 cA | 4.0 ± 0.5 bC | 0.08 ± 0.02 bD | 0.021 ± 0.002 cA |
| 15 | 5.9 ± 0.2 aA | 5.6 ± 1.4 bB | 0.57 ± 0.07 aA | 0.041 ± 0.004 aB | |
| 25 | 5.0 ± 0.5 bA | 12.8 ± 1.4 aA | 0.56 ± 0.08 aA | 0.045 ± 0.005 aB |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szczerba, A.; Płażek, A.; Pastuszak, J.; Kopeć, P.; Hornyák, M.; Dubert, F. Effect of Low Temperature on Germination, Growth, and Seed Yield of Four Soybean (Glycine max L.) Cultivars. Agronomy 2021, 11, 800. https://doi.org/10.3390/agronomy11040800
Szczerba A, Płażek A, Pastuszak J, Kopeć P, Hornyák M, Dubert F. Effect of Low Temperature on Germination, Growth, and Seed Yield of Four Soybean (Glycine max L.) Cultivars. Agronomy. 2021; 11(4):800. https://doi.org/10.3390/agronomy11040800
Chicago/Turabian StyleSzczerba, Anna, Agnieszka Płażek, Jakub Pastuszak, Przemysław Kopeć, Marta Hornyák, and Franciszek Dubert. 2021. "Effect of Low Temperature on Germination, Growth, and Seed Yield of Four Soybean (Glycine max L.) Cultivars" Agronomy 11, no. 4: 800. https://doi.org/10.3390/agronomy11040800
APA StyleSzczerba, A., Płażek, A., Pastuszak, J., Kopeć, P., Hornyák, M., & Dubert, F. (2021). Effect of Low Temperature on Germination, Growth, and Seed Yield of Four Soybean (Glycine max L.) Cultivars. Agronomy, 11(4), 800. https://doi.org/10.3390/agronomy11040800







