The Effect of Different Doses of Sewage Sludge and Liming on Total Cobalt Content and its Speciation in Soil
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results and Discussion
4. Conclusions
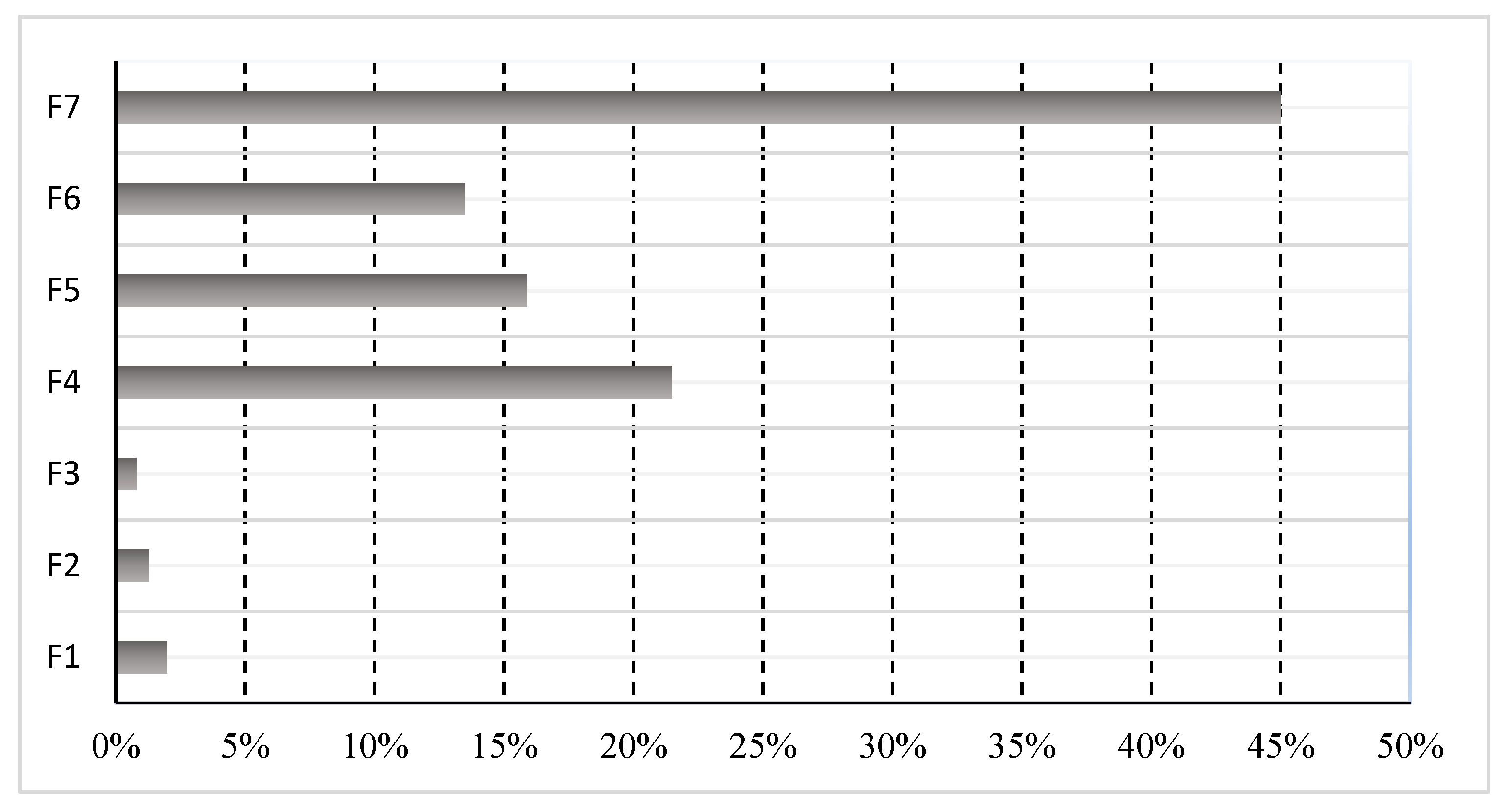
- Percentage of cobalt fractions in municipal sewage sludge can be arranged in a series of increasing percentages: F3(0.80) < F2(1.30) < F1(2.00) < F6(13.5) < F5(15.9) < F4 (21.50) < F7(45.0).
- In the incubation experiment, varied sewage sludge doses had a significant effect on total content of cobalt in the soil. Its amounts increased with higher doses of sewage sludge.
- The percentage of cobalt in separate fractions in the soils of different experimental units was dependent on the dose of sewage sludge and liming. On average, compared to the control, cobalt content increased more than two times as a response to higher doses of sewage sludge (10 and 15% of the weight of the soil).
- Compared to the beginning of the experiment, increasing doses of sewage sludge increased the percentage of cobalt in the organic fraction (F4) after 420 days of soil incubation, while, at the same time, the percentage share of this chemical element bound to manganese oxides (F3) decreased a few times.
- Based on the experiment, it was concluded that the chemical speciation of cobalt is not a permanent phenomenon and that it can be redistributed between the fractions, depending on pH of the soil and its organic matter content.
Author Contributions
Funding
Conflicts of Interest
References
- Statistical Yearbook of the Regions—Poland, Statistics Poland, Warsaw. 2019. Available online: http://stat.gov.pl (accessed on 23 September 2020).
- Waste Act of December 14, 2012 (Dz.U. Nr 0, poz. 21). Available online: http://isap.sejm.gov.pl (accessed on 10 September 2020).
- Antolin, M.; Pascual, I.; Garcia, C.; Polo, A.; Schanez-Diaz, M. Growth, yield and solute content of barley in soils treated with sewage sludge under semiarid Mediterranean conditions. Field Crops Res. 2005, 94, 224–237. [Google Scholar] [CrossRef]
- De Brouwere, K.; Smolders, E. Yield response of crops amended with sewage sludge in the field is more affected by sludge properties than by final soil metal concentration. Eur. J. Soil Sci. 2006, 57, 858–867. [Google Scholar] [CrossRef]
- Diacono, M.; Montemurro, F. Long-term effects of organic amendments on soil fertility. A review. Agron. Sustain. Dev. 2010, 30, 401–422. [Google Scholar] [CrossRef]
- Wong, J.W.; Li, K.; Fang, M.; Su, D.C. Toxicity evaluation of sewage sludges in Hong Kong. Environ. Int. 2001, 27, 373–380. [Google Scholar] [CrossRef]
- Álvarez, E.; Mochón, M.C.; Sanchez, J.; Rodríguez, M. Heavy metal extractable forms in sludge from wastewater treatment plants. Chemosphere 2002, 47, 765–775. [Google Scholar] [CrossRef]
- Hillman, J.P.; Hill, J.; Morgan, J.E.; Wilkinson, J.M. Recycling of sewage sludge to grassland: A review of the legislation to control of the localization and accumulation of potential toxic metals in grazing systems. Grass Forage Sci. 2003, 58, 101–111. [Google Scholar] [CrossRef]
- Gupta, A.K.; Sinha, S. Phytoextraction capacity of the plants growing on tannery sludge dumping sites. Bioresour. Technol. 2007, 98, 1788–1794. [Google Scholar] [CrossRef] [PubMed]
- Gawdzik, J.I. Speciation of heavy metals in sewage sludge: A case study. Ochr. Śr. 2010, 32, 15–19. [Google Scholar]
- Gasco, G.; Martinez-Inigo, M.; Lobo, M. Soil organic matter transformation after a sewage sludge application. EJEAFChe 2004, 3, 716–722. [Google Scholar]
- Czechowska-Kosacka, A. Influence of sewage sludge solidification on immobilisation of heavy metals. Polish J. Environ. Stud. 2007, 16, 625–628. [Google Scholar]
- Hargreaves, J.; Adl, M.; Warman, P. A review of the use of composted municipal solid waste in agriculture. Agric. Ecosyst. Environ. 2008, 123, 1–14. [Google Scholar] [CrossRef]
- Pshinko, G.N. Impact of complexing agents on the processes of sorption treatment of waters containing cobalt. J. Water Chem. Technol. 2008, 30, 197–202. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Pendias, H. Biogeochemistry of Trace Elements; Polish Scientific Publishing Company: Warsaw, Poland, 1999; p. 400. [Google Scholar]
- Cappuyns, V.; Mallaerts, T. Background values of cobalt in Flemish and European soils. Geol. Belg. 2014, 17, 107–114. [Google Scholar]
- Dahlin, S.; Witter, E.; Martensson, A.; Turner, A.; Baath, E. Where’s the limit? Changes in the microbiological properties of agricultural soils at low levels of metal contamination. Soil Biol. Biochem. 1997, 29, 1405–1415. [Google Scholar] [CrossRef]
- McBride, M.B.; Richards, B.K.; Steenhuis, T.; Spiers, G. Long-term leaching of trace elements in a heavily sludge-amended silty clay loam soil. Soil Sci. 1999, 164, 613–623. [Google Scholar] [CrossRef]
- Kirkland, D.; Brock, T.; Haddouk, H.; Hargeaves, V.; Lloyd, M.; Mc Garry, S.; Proudlock, R.; Sarlang, S.; Sewald, K.; Sire, G.; et al. New investigations into the genotoxicity of cobalt compounds and their impact on overall assessment of genotoxic risk. Regul. Toxicol. Pharmacol. 2015, 73, 311–338. [Google Scholar] [CrossRef]
- Merrington, G.; Oliver, I.W.; Smernik, R.J.; McLaughlin, M. The influence of sewage sludge properties on sludge-borne metal availability. Adv. Environ. Res. 2003, 8, 21–36. [Google Scholar] [CrossRef]
- Andersen, M.K.; Raulund-Rasmussen, K.; Hansen, H.C.B.; Strobel, B.W. Distribution and fractionation of heavy metals in pairs of arable and afforested soils in Denmark. Eur. J. Soil Sci. 2002, 53, 491–502. [Google Scholar] [CrossRef]
- Gleyzes, C.; Tellier, S.; Astruc, M. Fractionation studies of trace elements in contaminated soils and sediments: A review of sequential extraction procedures. TrAC Trends Anal. Chem. 2002, 21, 451–467. [Google Scholar] [CrossRef]
- Amir, S.; Hafidi, M.; Merlina, G.; Revel, J.-C. Sequential extraction of heavy metals during composting of sewage sludge. Chemosphere 2005, 59, 801–810. [Google Scholar] [CrossRef]
- Wang, C.; Hu, X.; Chen, M.-L.; Wu, Y.-H. Total concentrations and fractions of Cd, Cr, Pb, Cu, Ni and Zn in sewage sludge from municipal and industrial wastewater treatment plants. J. Hazard. Mater. 2005, 119, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Jamali, M.K.; Kazi, T.G.; Afridi, H.I.; Arain, M.B.; Jalbani, N.; Memon, A.R. Speciation of heavy metals in untreated domestic wastewater sludge by time saving BCR sequential extraction method. J. Environ. Sci. Health Part A 2007, 42, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.R.M.; Sahuquillo, A.; López-Sánchez, J. A Review of the Different Methods Applied in Environmental Geochemistry for Single and Sequential Extraction of Trace Elements in Soils and Related Materials. Water Air Soil Pollut. 2007, 189, 291–333. [Google Scholar] [CrossRef]
- Mench, M.; Tancogne, J.; Gómez, A.; Juste, C. Cadmium bioavailability to Nicotiana tabacum L., Nicotiana rustica L., and Zea mays L. grown in soil amended or not amended with cadmium nitrate. Biol. Fertil. Soils 1989, 8, 48–53. [Google Scholar] [CrossRef]
- Kaplan, M.; Orman, Ş.; Kádár, I.; Koncz, J. Heavy metal accumulation in calcareous soil and sorghum plants after addition of sulphur-containing waste as a soil amendment in Turkey. Agric. Ecosyst. Environ. 2005, 111, 41–46. [Google Scholar] [CrossRef]
- Regulations of the Minister for Environment on soil quality standards and quality standards land 2002. Dz.U. Nr 165, poz. 1359. Available online: http://isap.sejm.gov.pl (accessed on 1 September 2020).
- Regulations of the Minister for Environment in question sewage sludge 2010. Dz.U. Nr 137, poz. 924. Available online: http://isap.sejm.gov.pl (accessed on 10 September 2020).
- Zeien, H.; Brümmer, G.W. Chemische Extraction zur Bestimmung von Schwermetallbindungsformen in Böden. Mittelign Dtsch Bodenkundl Gesellsch 1989, 59, 505–510. [Google Scholar]
- STATISTICA (Data Analysis Software System); Version 10; StatSoft, Inc.: Palo Alto, CA, USA, 2011; Available online: www.statsoft.com (accessed on 1 September 2020).
- Bojakowska, I.; Dobek, P.; Wołkowicz, S. Impact of biological wastewater treatment ponds on the ground environment. Gór. Ekol. 2012, 7, 59–69. [Google Scholar]
- Malinowska, E. Zinc speciation in soil under various rates of sewage sludge and liming. Environ. Prot. Eng. 2016, 42, 5–15. [Google Scholar] [CrossRef]
- Malinowska, E. The effect of liming and sewage sludge application on heavy metal speciation in soil. Bull. Environ. Contam. Toxicol. 2017, 98, 105–112. [Google Scholar] [CrossRef][Green Version]
- Atkins, P.; Jones, L. Chemia Ogólna. Cząsteczki, Materia, Reakcje (Chemistry: Molecules, Matter, and Change); W. H. Freeman and Company: New York City, NY, USA, 2019. [Google Scholar]
- Bożym, M.; Rajmund, A. The study of cobalt leaching from soils, sewage sludges and composts using a one-step extraction. Environ. Prot. Nat. Resour. 2015, 26, 1–6. [Google Scholar] [CrossRef][Green Version]
- Wang, C.; Li, X.; Wang, P.; Zou, L.-M.; Ma, H.-T. Extractable Fractions of Metals in Sewage Sludges from Five Typical Urban Wastewater Treatment Plants of China. Pedosphere 2006, 16, 756–761. [Google Scholar] [CrossRef]
- Bhattacharyya, P.; Chakrabarti, K.; Chakraborty, A.; Tripathy, S.; Kim, K.; Powell, M.A. Cobalt and nickel uptake by rice and accumulation in soil amended with municipal solid waste compost. Ecotoxicol. Environ. Saf. 2008, 69, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Greinert, A. Kobalt w Środowisku Przyrodniczym i Antropogenicznym (Cobalt in the Natural and Anthropogenic Environment), 1st ed.; Oficyna Wydawnicza Uniwersytetu Zielonogórskiego: Zielona Góra, Poland, 2011; p. 134. ISBN 978-83-7481-437-9. [Google Scholar]
- McLaren, R.G.; Lawson, D.M.; Swift, R.S. Sorption and desorption of cobalt by soils and soil components. J. Soil Sci. 1986, 37, 413–426. [Google Scholar] [CrossRef]
- Czarnowska, K. Total content of heavy metals in parent rocks as references background levels of soils. Soil Sci. Ann. 1996, 47, 43–50. [Google Scholar]
- Kalembasa, D.; Malinowska, E. Bioaccumulation of zinc under the influence of sewage sludge and liming and its speciation in soil. Fresenius Environ. Bull. 2013, 22, 3359–3369. [Google Scholar]
- Speir, T.W.; Van Schaik, A.P.; Percival, H.J.; Close, M.E.; Pang, L.P. Heavy metals in soil, plants and groundwater following high-rate sewage sludge application to land. Water Air Soil Pollut. 2003, 150, 319–358. [Google Scholar] [CrossRef]
- Filipek, T.; Skowrońska, M. Current dominant causes and effects of acidification of soils under agricultural use in Poland. Acta Agrophys. 2013, 20, 283–294. [Google Scholar]
- Malinowska, E. The effects of soil liming and sewage sludge application on dynamics of cooper fractions and total cooper concentration. Environ. Monit. Assess. 2017, 188, 597. [Google Scholar] [CrossRef]
- Domańska, J. Changes of the content of total and extractable forms of lead (Pb) in soil affected by organic matter and lime. Adv. Agric. Sci. Prob. 2006, 512, 91–97. [Google Scholar]
- Zhai, M.; Kampunzu, H.A.B.; Modisi, M.P.; Totolo, O. Distribution of heavy metals in Gaborone urban soils (Botswana) and its relationship to soil pollution and bedrock composition. Environ. Earth Sci. 2003, 45, 171–180. [Google Scholar] [CrossRef]
- Blake, L.; Goulding, K.; Mott, C.J.B.; Johnston, A. Changes in soil chemistry accompanying acidification over more than 100 years under woodland and grass at Rothamsted Experimental Station, UK. Eur. J. Soil Sci. 1999, 50, 401–412. [Google Scholar] [CrossRef]
- Laudicina, V.A.; Palazzolo, E.; Badalucco, L. Natural Organic Compounds in Soil Solution: Potential Role as Soil Quality Indicators. Curr. Org. Chem. 2013, 17, 2991–2997. [Google Scholar] [CrossRef]
- Bakkaus, E.; Gouget, B.; Gallien, J.-P.; Khodja, H.; Carrot, F.; Morel, J.; Collins, R. Concentration and distribution of cobalt in higher plants: The use of micro-PIXE spectroscopy. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2005, 231, 350–356. [Google Scholar] [CrossRef]
- Pinkerton, B.W.; Brown, K.W. Plant Accumulation and Soil Sorption of Cobalt from Cobalt-Amended Soils1. Agron. J. 1907, 77, 634–638. [Google Scholar] [CrossRef]
| pH | DM % | Organic Carbon Corg | Total Nitrogen Ntot | C:N | Co | Pb | Cd | Cr | Cu | Zn | Ni |
|---|---|---|---|---|---|---|---|---|---|---|---|
| g kg−1 | mg kg−1 | ||||||||||
| 6.8 | 25.0 | 345 | 40 | 8.6:1 | 3.80 | 50.23 | 0.165 | 19.85 | 85.0 | 1120 | 50.14 |
| Fraction | Name | Extraction Reagent | Extraction Time | pH |
|---|---|---|---|---|
| F1 | easily soluble | 1 mol NH4NO3 dm−3 | 24 h | neutral |
| F2 | exchangeable | 1 mol CH3COONH4 dm−3 | 24 h | 6.0 |
| F3 | bound to MnOx | 1 mol NH2OH HCl dm−3 + 1 mol CH3COONH4 dm−3 | 0.5 h | 6.0 |
| F4 | bound to organic matter | 0.025 mol C10H22N4O8 dm−3 | 1.5 h | 4.6 |
| F5 | bound to amorphous FeOx | 0.2 mol (NH4)2C2O4 dm−3 + 0.2 mol H2C2O4 dm−3 | 4 h | 3.25 |
| F6 | bound to crystalline FeOx | 0.2 mol (NH4)2C2O4 dm−3 + 0.2 mol H2C2O4 dm−3 + 0.1 mol C6H8O6 dm−3 | 0.5 h | 3.25 |
| F7 | residual | Calculated as the difference between the total content of cobalt and the sum of the above determined fractions | - | - |
| Days | 0 | 5% | 10% | 15% | Mean | 0 | 5% | 10% | 15% | Mean |
|---|---|---|---|---|---|---|---|---|---|---|
| Without Liming | Liming | |||||||||
| 30 | 1.02 | 1.68 | 2.33 | 2.76 | 1.95 | 0.999 | 1.52 | 2.41 | 2.87 | 1.95 |
| 60 | 1.11 | 1.55 | 2.52 | 2.71 | 1.97 | 1.04 | 1.45 | 2.36 | 2.69 | 1.89 |
| 90 | 1.03 | 1.69 | 2.74 | 2.87 | 2.08 | 1.10 | 1.36 | 2.71 | 2.59 | 1.94 |
| 120 | 0.998 | 1.76 | 2.81 | 2.78 | 2.09 | 0.949 | 1.69 | 2.51 | 2.55 | 1.93 |
| 360 | 0.987 | 1.74 | 2.68 | 2.71 | 2.03 | 0.955 | 1.54 | 2.66 | 2.78 | 1.98 |
| 420 | 1.02 | 1.63 | 2.60 | 2.66 | 1.98 | 1.00 | 1.50 | 2.58 | 2.81 | 1.97 |
| mean | 1.03 | 1.68 | 2.61 | 2.75 | 2.02 | 1.01 | 1.51 | 2.54 | 2.72 | 1.94 |
| LSD0.05 for: A = 0.12; B = 0.065; C = n.s.; A/B = n.s.; B/A = n.s.; A/C = n.s.; C/A = n.s.; B/C = n.s.; C/B = n.s. | ||||||||||
| Fertilization Object | F1 | F2 | F3 | F4 | F5 | F6 | F7 | pH * |
|---|---|---|---|---|---|---|---|---|
| 30 days | ||||||||
| without liming | ||||||||
| control | 1.82 | 1.10 | 2.36 | 25.45 | 19.05 | 20.21 | 30.01 | 4.30 |
| 5% | 1.81 | 0.991 | 1.35 | 28.00 | 20.03 | 15.01 | 32.81 | 4.55 |
| 10% | 1.98 | 1.20 | 1.31 | 35.04 | 11.00 | 14.38 | 35.09 | 5.05 |
| 15% | 2.09 | 1.21 | 1.34 | 37.01 | 10.60 | 10.80 | 37.01 | 5.17 |
| mean | 1.93 | 1.13 | 1.59 | 31.38 | 15.17 | 15.10 | 33.73 | |
| liming | ||||||||
| control | 1.08 | 0.981 | 0.301 | 25.05 | 16.32 | 20.26 | 36.01 | 6.30 |
| 5% | 1.40 | 0.995 | 0.309 | 32.01 | 18.94 | 14.51 | 31.84 | 6.32 |
| 10% | 1.52 | 1.00 | 0.321 | 29.09 | 18.12 | 13.44 | 36.51 | 6.35 |
| 15% | 1.55 | 1.08 | 0.311 | 28.95 | 19.37 | 10.70 | 38.04 | 6.48 |
| mean | 1.39 | 1.01 | 0.311 | 28.78 | 18.19 | 14.73 | 35.60 | |
| 60 days | ||||||||
| without liming | ||||||||
| control | 1.05 | 1.70 | 2.20 | 20.16 | 17.23 | 22.61 | 35.05 | 4.38 |
| 5% | 2.59 | 1.95 | 2.93 | 25.11 | 17.96 | 20.72 | 28.84 | 4.80 |
| 10% | 1.90 | 0.991 | 1.31 | 24.75 | 18.04 | 13.00 | 40.11 | 5.02 |
| 15% | 1.52 | 1.02 | 2.31 | 27.04 | 17.00 | 10.05 | 41.08 | 5.00 |
| mean | 1.77 | 1.42 | 2.19 | 24.27 | 17.56 | 16.60 | 36.27 | |
| liming | ||||||||
| control | 1.11 | 1.58 | 1.25 | 23.85 | 19.01 | 20.05 | 33.17 | 6.25 |
| 5% | 1.38 | 0.709 | 1.39 | 30.49 | 18.88 | 17.20 | 29.95 | 6.30 |
| 10% | 1.45 | 0.985 | 1.14 | 35.45 | 14.56 | 11.96 | 34.48 | 6.25 |
| 15% | 1.80 | 0.846 | 1.85 | 36.21 | 12.15 | 11.08 | 36.08 | 6.50 |
| mean | 1.44 | 1.03 | 1.41 | 31.50 | 16.15 | 15.07 | 33.42 | |
| 90 days | ||||||||
| without liming | ||||||||
| control | 2.60 | 2.05 | 1.21 | 21.05 | 14.02 | 29.07 | 30.01 | 4.26 |
| 5% | 2.51 | 3.70 | 1.19 | 25.29 | 13.26 | 22.54 | 31.51 | 5.15 |
| 10% | 2.85 | 3.00 | 2.24 | 25.06 | 18.45 | 19.41 | 29.00 | 5.10 |
| 15% | 3.13 | 2.78 | 2.24 | 24.08 | 18.14 | 19.00 | 30.63 | 5.12 |
| mean | 2.77 | 2.88 | 1.72 | 23.87 | 15.97 | 22.51 | 30.29 | |
| liming | ||||||||
| control | 2.21 | 1.55 | 1.22 | 22.50 | 15.20 | 28.90 | 28.42 | 6.25 |
| 5% | 1.74 | 2.70 | 1.19 | 21.91 | 14.19 | 28.35 | 29.91 | 6.34 |
| 10% | 2.11 | 2.69 | 2.05 | 28.08 | 17.36 | 18.96 | 28.72 | 6.40 |
| 15% | 2.99 | 2.68 | 2.22 | 27.04 | 17.21 | 14.81 | 33.05 | 6.45 |
| mean | 2.26 | 2.41 | 1.67 | 24.88 | 15.99 | 22.76 | 30.03 | |
| 120 days | ||||||||
| without liming | ||||||||
| control | 1.75 | 1.30 | 0.918 | 23.00 | 14.01 | 30.11 | 29.01 | 4.30 |
| 5% | 1.76 | 2.39 | 1.20 | 26.00 | 16.12 | 25.60 | 27.02 | 5.17 |
| 10% | 2.80 | 2.70 | 3.19 | 21.56 | 18.23 | 24.72 | 26.80 | 5.20 |
| 15% | 2.84 | 2.66 | 3.16 | 24.01 | 17.98 | 12.63 | 36.72 | 5.21 |
| mean | 2.29 | 2.26 | 2.12 | 23.64 | 16.59 | 23.27 | 29.89 | |
| liming | ||||||||
| control | 1.50 | 1.49 | 1.11 | 25.00 | 20.41 | 19.99 | 30.50 | 6.31 |
| 5% | 1.57 | 1.96 | 1.60 | 28.90 | 16.58 | 17.39 | 32.00 | 6.40 |
| 10% | 1.70 | 1.60 | 1.18 | 25.42 | 16.01 | 23.05 | 31.04 | 6.45 |
| 15% | 1.79 | 2.62 | 1.19 | 23.08 | 17.02 | 15.17 | 38.95 | 6.37 |
| mean | 1.64 | 1.92 | 1.27 | 25.60 | 17.55 | 18.90 | 33.12 | |
| Fertilization Objects | F1 | F2 | F3 | F4 | F5 | F6 | F7 | pH * |
|---|---|---|---|---|---|---|---|---|
| 360 days | ||||||||
| without liming | ||||||||
| control | 2.50 | 2.30 | 2.19 | 18.80 | 12.96 | 39.45 | 21.79 | 4.40 |
| 5% | 1.60 | 1.38 | 1.11 | 24.20 | 10.25 | 31.57 | 29.89 | 4.80 |
| 10% | 1.63 | 1.40 | 0.325 | 28.91 | 13.69 | 23.05 | 31.00 | 5.15 |
| 15% | 1.68 | 1.50 | 0.500 | 30.42 | 13.44 | 18.47 | 34.05 | 5.05 |
| mean | 1.85 | 1.65 | 1.04 | 25.58 | 12.59 | 28.14 | 29.18 | |
| liming | ||||||||
| control | 1.45 | 0.800 | 0.118 | 24.70 | 13.01 | 38.40 | 21.52 | 6.10 |
| 5% | 1.45 | 1.311 | 0.310 | 27.00 | 12.69 | 28.24 | 29.00 | 6.25 |
| 10% | 1.59 | 1.40 | 0.545 | 30.56 | 14.52 | 25.74 | 25.65 | 6.28 |
| 15% | 1.60 | 1.41 | 0.630 | 32.86 | 14.03 | 25.47 | 24.00 | 6.35 |
| mean | 1.52 | 1.23 | 0.401 | 28.78 | 13.56 | 29.46 | 25.04 | |
| 420 days | ||||||||
| without liming | ||||||||
| control | 1.35 | 1.14 | 0.101 | 23.00 | 11.23 | 43.13 | 20.05 | 4.35 |
| 5% | 1.40 | 1.20 | 0.341 | 20.50 | 12.03 | 37.53 | 27.00 | 4.90 |
| 10% | 1.45 | 1.24 | 0.552 | 29.00 | 12.45 | 34.60 | 20.71 | 5.20 |
| 15% | 2.47 | 2.20 | 0.450 | 29.42 | 11.56 | 22.84 | 31.06 | 5.08 |
| mean | 1.57 | 1.45 | 0.361 | 25.48 | 11.82 | 34.53 | 24.71 | |
| liming | ||||||||
| control | 1.20 | 0.901 | 0.100 | 25.00 | 11.02 | 40.78 | 21.00 | 6.00 |
| 5% | 1.33 | 1.19 | 0.250 | 35.09 | 14.11 | 27.94 | 20.09 | 6.30 |
| 10% | 1.39 | 1.20 | 0.356 | 30.01 | 12.36 | 29.61 | 25.07 | 6.18 |
| 15% | 1.89 | 1.82 | 0.273 | 45.68 | 13.02 | 9.27 | 28.05 | 6.28 |
| mean | 1.45 | 1.28 | 0.245 | 33.95 | 12.63 | 26.90 | 23.55 | |
| Fraction | pH | Co | ||
|---|---|---|---|---|
| −Ca | +Ca | −Ca | +Ca | |
| F1 | 0.279 | 0.460 * | 0.304 | 0.432 * |
| F2 | −0.223 | 0.374 | −0.236 | 0.251 |
| F3 | 0.037 | 0.564 * | 0.100 | 0.201 |
| F4 | 0.489 * | 0.138 | 0.535 * | 0.503 * |
| F5 | −0.037 | 0.453 * | 0.081 | −0.072 |
| F6 | −0.425 * | −0.719 * | −0.568 * | −0.513 * |
| F7 | 0.257 | 0.634 * | 0.405 * | 0.265 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malinowska, E.; Jankowski, K. The Effect of Different Doses of Sewage Sludge and Liming on Total Cobalt Content and its Speciation in Soil. Agronomy 2020, 10, 1550. https://doi.org/10.3390/agronomy10101550
Malinowska E, Jankowski K. The Effect of Different Doses of Sewage Sludge and Liming on Total Cobalt Content and its Speciation in Soil. Agronomy. 2020; 10(10):1550. https://doi.org/10.3390/agronomy10101550
Chicago/Turabian StyleMalinowska, Elżbieta, and Kazimierz Jankowski. 2020. "The Effect of Different Doses of Sewage Sludge and Liming on Total Cobalt Content and its Speciation in Soil" Agronomy 10, no. 10: 1550. https://doi.org/10.3390/agronomy10101550
APA StyleMalinowska, E., & Jankowski, K. (2020). The Effect of Different Doses of Sewage Sludge and Liming on Total Cobalt Content and its Speciation in Soil. Agronomy, 10(10), 1550. https://doi.org/10.3390/agronomy10101550





